Abstract
Study Objectives:
The aim of this study was to investigate the prevalence and characteristics of periodic limb movements during sleep (PLMS) in Korean patients with restless legs syndrome (RLS).
Methods:
Unmedicated adult patients with idiopathic RLS (n = 354) who underwent polysomnography at three major sleep centers in tertiary hospitals were included. Characteristics of PLMS in RLS were analyzed using the time structure of polysomnographically recorded leg movements and periodicity indices (PIs). RLS severity and subjective sleep quality were assessed.
Results:
Out of 354 patients with idiopathic RLS (mean age: 52.9 ± 12.0 years), 150 patients (42.3%) had RLS with a PLMS index greater than 15 events/h, and 204 (57.9%) had a PLMS index greater than 5 events/h. The distribution of inter-LM intervals was bimodal, and high PIs (0.86 ± 0.10) were observed in patients with RLS and PLMS (PLMS index > 15 events/h). The PLMS index was positively correlated with age (r = 0.228; p < 0.001), the periodic limb movements in wakefulness index (r = 0.455, p < 0.001) and arousal index (r = 0.174, p = 0.014), but not with RLS severity and parameters of sleep quality. In multivariate analysis, age and male gender were independently associated with PLMS > 15 events/h.
Conclusions:
The prevalence of PLMS in Korean patients with RLS was lower than that observed in Western countries, but the characteristics of PLMS were not different. Ethnic differences and/or different genetic backgrounds may contribute to the varying prevalence of PLMS in RLS.
Citation:
Shin JW, Koo YS, Lee BU, Shin WC, Lee SK, Cho YW, Jung KY. Prevalence and characteristics of periodic limb movements during sleep in Korean adult patients with restless legs syndrome. J Clin Sleep Med 2016;12(8):1089–1097.
Keywords: restless legs syndrome, periodic limb movements in sleep, periodicity index, prevalence, sleep quality
INTRODUCTION
Periodic limb movements during sleep (PLMS) are a sleep-related phenomenon, with periodic episodes of repetitive stereotypical movements of the extremities.1 Based on a study in North America, 80% to 90% of patients with restless legs syndrome (RLS) have PLMS2; hence, PLMS is closely associated with RLS.3 The therapeutic effects of dopamine agonists on RLS and PLMS support the hypothesis that RLS and PLMS are associated with similar alterations in the dopaminergic system.4,5 Therefore, PLMS may be an endophenotype of RLS, and the presence of PLMS may improve the diagnostic accuracy of RLS.6
The prevalence of PLMS has been reported to differ between ethnic groups; indeed, PLMS has been shown to be significantly more prevalent among Caucasian children than among African American children.7 A genome-wide association study showed a significant association between an intron of BTBD9 on chromosome 6p and PLMS in the Icelandic population.8 These data suggest that the presence of PLMS in RLS may be influenced by ethnic or genetic factors. In a small study involving Korean adult patients with RLS, we found that only 41.8% of subjects had a PLMS index greater than 15;9 this prevalence is lower than that previously reported.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Although it is generally known that restless legs syndrome (RLS) is associated with periodic limb movements during sleep (PLMS) in up to 90%, current knowledge of prevalence of PLMS in RLS is limited, especially in Asia. Therefore, we investigated prevalence and characteristics of PLMS in Korean patients with RLS.
Study Impact: The prevalence of PLMS in Korean patients was lower than that reported in patients from western countries, whereas independent factors associated with PLMS and the characteristics of periodic limb movement structure were not different from those in patients from western countries. The low prevalence of PLMS in the Korean population may be explained by genetic factors, including ethnic and gender differences.
We hypothesized that the prevalence of PLMS in Korean patients with RLS differs from those in patients from western countries. To address this issue, we investigated the prevalence of PLMS in a large sample of Korean adult patients with RLS, and determined the characteristics of motor phenomena with night distribution of PLMS in RLS. Additionally, we assessed whether PLMS affected the quality of sleep using the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), Insomnia Severity Index (ISI), and polysomnography (PSG) parameters.
METHODS
Patients
This was a retrospective review of unmedicated adult patients with idiopathic RLS who underwent one-night polysomnography (PSG) at 3 major sleep centers in tertiary Hospitals from July 2006 to January 2014. The study was approved by the institutional review board of each institution. Patients were included in the analysis if they (1) met the International Restless Legs Syndrome Study Group (IRLSSG) diagnostic criteria,4 (2) were ≥ 18 years of age at the time of diagnosis, and (3) were examined by PSG when not under any treatment for RLS. Patients were excluded if they had (1) obstructive sleep apnea on PSG (apnea-hypopnea index [AHI] > 10 events/h); (2) other specific sleep disorders (e.g., narcolepsy, REM sleep behavior disorder) associated with PLMS; (3) secondary causes of RLS including iron deficiency, pregnancy, neuropathy, multiple sclerosis, or renal failure, or took medications that caused or exacerbated RLS; or (4) disorders with symptoms similar to RLS, such as positional discomfort, leg cramps, essential tremor, parkinson disease, neuroleptic-induced akathisia, vascular claudication, neurogenic claudication, myelopathy, and arthritis. We confirmed if the RLS patients had exclusion criteria (3) and (4) through examining previous electronic medical records (EMRs). We defined those who had RLS with a PLMS index > 15 events/h as a patient with RLS and PLMS, which was consistent with ICSD-3 criteria.10 To investigate the characteristics of the RLS with PLMS group, we compared the data with a control group, including patients who had RLS without PLMS (PLMS index ≤ 15 events/h), and age- and gender-matched non-RLS patients (insomnia, n = 136; sleep breathing disorder, n = 224; REM sleep behavior disorder, n = 2) who underwent a PSG at 3 sleep centers were analyzed.
PSG records and EMRs were used to acquire baseline demographics, including age, the family history of RLS, onset age, gender, body mass index, initial serum ferritin levels, and sleep parameters of each patient. However, clinical details such as the family history of RLS, onset age and serum ferritin levels were not available from some of the patients.
Polysomnography
PSG assessments included electroencephalogram (EEG; C3/ A2, C4/A1, F3/A2, F4/A1, O1/A2, and O2/A1), left and right electro-oculograms, submental electromyogram (EMG), superficial EMG of both the anterior tibialis muscles, and electrocardiogram. Airflow (nasal flow pressure sensor and oronasal thermistor), chest and abdominal breathing efforts, and trans-cutaneous oximetry were monitored in all patients in the single overnight session.
Sleep stage was scored in 30-s epochs according to the standard criteria described by the American Academy of Sleep Medicine manual for scoring sleep.11 Periodic leg movements were scored according to World Association of Sleep Medicine/IRLSSG criteria.12 In brief, limb movements were scored if they were 0.5–10 s long and had an EMG amplitude ≥ 8 μV above the resting EMG; periodic limb movements were scored if the limb movements occurred as part of a series of ≥ 4, with 5–90 s between each movement in a series. As per the International Classification of Sleep Disorders, a PLM index (PLMI) > 15 events/h was considered abnormal.13 PSG parameters were calculated as follows: total sleep time (TST), time spent in any sleep stage during the sleep period; sleep latency, time from “lights out” until the first epoch of any sleep stage excluding sleep stage 1; REM sleep latency, time from sleep onset until the first epoch of REM sleep; sleep efficiency (SE), percentage of TST during time in bed; Total arousal index, number of arousals per hour of TST; PLMS index, number of PLM during sleep per hour of TST; and periodic limb movement during wakefulness (PLMW) index, number of PLM during wakefulness per hour of wake time; PLM-arousal index, PLM associated with arousals per hour.
Analysis of Leg Movement (LM) Architecture
We analyzed the LMs in detail, including the periodicity of the motor phenomenon, using methods described by Ferri et al.14 with all PSG records from one hospital. LMs were detected by experienced technicians using the World Association of Sleep Medicine/IRLSSG criteria.12 As mentioned above, LMs were included when the EMG amplitude increased to 8 μV, and the endpoint was when the amplitude decreased to less than 2 μV above the resting level and remained below that value for 0.5 s. LM interval was defined as the time between onsets of 2 subsequent LMs. The number of inter-movement intervals that were 10–90 s long and in sequences of ≥ 3 was divided by the total number of intervals to yield the periodicity index (PI); this index can vary between 0 (absence of periodicity, with none of the intervals between 10 and 90 s long) and 1 (complete periodicity, with all intervals between 10 and 90 s long). In addition, we investigated the distribution of PLM per hour of sleep during the first 8-h sleep period using PSG records of 10% of patients randomly chosen from each group (RLS with and without PLMS index > 15 events/h) using the “Randomized” program (http://www.randomized.com/) with the above PSG records.
Scales
For assessing the severity of RLS, we used the Korean version of the International Restless Legs Syndrome Rating Scale (IRLS) scores.15 We used all Korean versions of the PSQI,16 ISI,17 and ESS18 for evaluating the sleep quality and daytime sleepiness. We identified good sleepers and poor sleepers via PSQI using a cutoff value > 5 points.19
Statistical Analysis
Statistical analyses were performed using SPSS for Windows (version 21.0; SPSS Inc., Armonk, NY, USA). Categorical data were compared using the χ2 test and Fisher exact test. Continuous variables were presented as mean ± standard deviation or median ± interquartile range and they were compared using the independent Student t-test and the Mann-Whitney U test. The logistic regression model was used to assess the independent factors between demographics and PLMS index > 15 events/h. Associations between the variables were determined using nonparametric partial correlation with Spearman rank correlation. Differences or associations with p values < 0.05 were considered statistically significant.
RESULTS
Demographic Characteristics
Table 1 shows the characteristics and sleep parameters of all patients. A total of 440 patients were diagnosed with unmedicated idiopathic RLS, and 354 patients satisfied all the inclusion criteria. Of 440 patients, 83 patients who had an AHI > 10 were excluded. Additionally, 3 children were excluded. The mean age at onset was 52.9 ± 12.0 years, and the proportion of women (66.1%) was higher than that of men (33.9%). A total of 173 of 327 (52.9%) patients were classified as having the early onset form of RLS (age of onset < 45 years), and 87 of 291 (29.9%) patients had a family history of RLS. There was no statistical difference in basal characteristics in the three sleep centers (data not shown).
Table 1.
Comparison of clinical characteristics and sleep parameters between the RLS only group and the RLS with PLMS group.
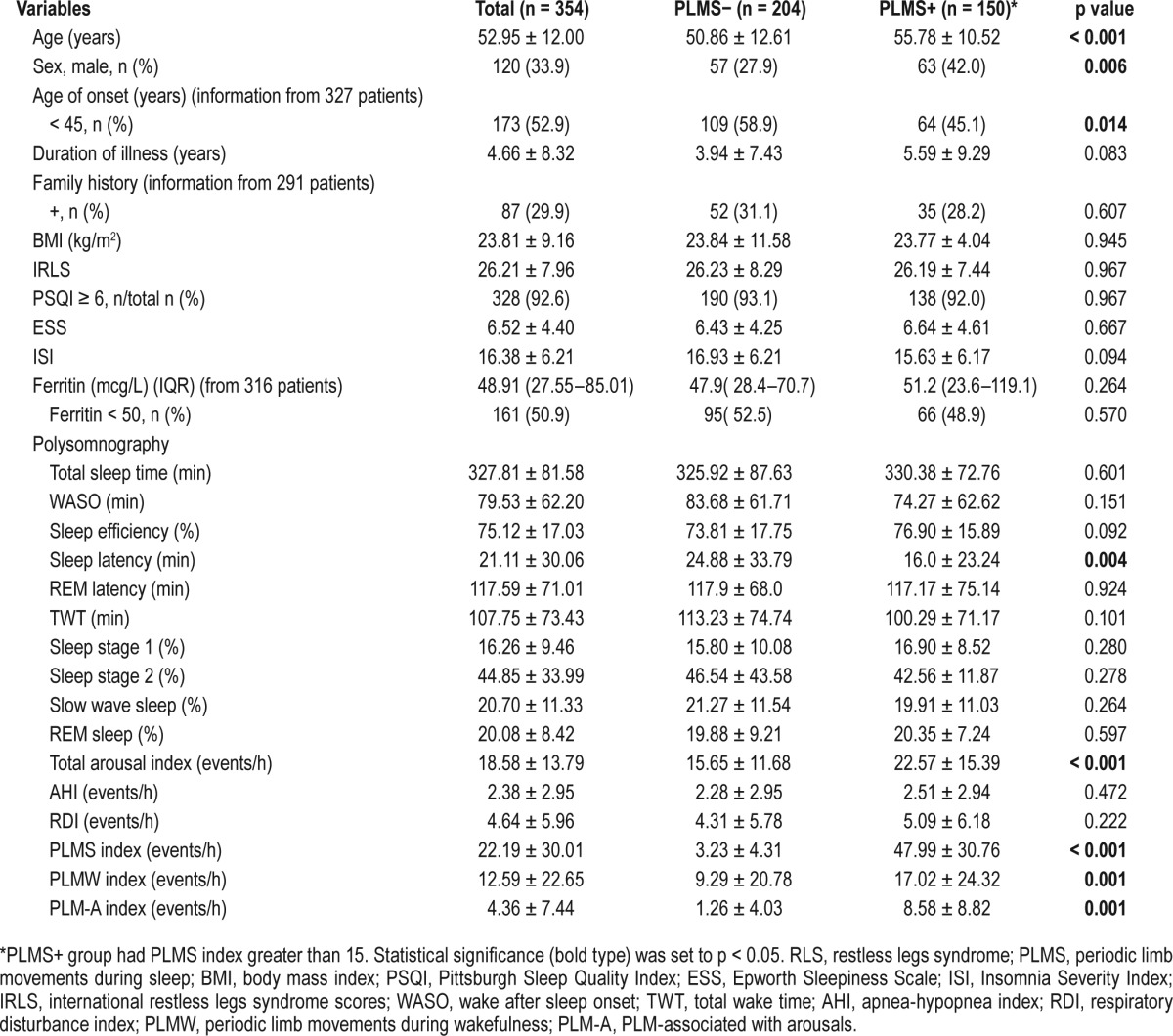
Out of 354 patients, 62.5% had moderate to severe insomnia (ISI ≥ 11), 77% had severe RLS (IRLS ≥ 21), and 92.6% were poor sleepers. The PSG parameters showed low mean sleep efficiency (75.12% ± 17.03%) and high values of mean wake after sleep onset (WASO; 79.53 ± 62.20 min).
Prevalence and Clinical Features of PLMS > 15 events/h in RLS
Two hundred five (57.9%) patients had PLMS indices > 5, and 150 (42.3%) met the criterion of > 15. With the cutoff value of PLMS set as > 15, patients with PLMS were older (55.78 ± 10.52 years in patients with PLMS versus 50.86 ± 12.61 years in patient without PLMS; p < 0.001), with a higher proportion of the late onset phenotype (54.9% in patients with PLMS versus 41.1% in patients without PLMS; p = 0.014), and a higher proportion of men (42.0% in patients with PLMS versus 27.9% in patients without PLMS; p = 0.006). The duration of illness was longer in the group with PLMS, although this difference was not statistically significant. The median ferritin levels and the proportion of patients with low ferritin levels (serum ferritin < 50 mcg/L) were not different between in the group with and without PLMS in RLS patients (Table 1).
In addition, we investigated the association of a PLMS index > 15 events/h in RLS patients and demographics using the multiple variable logistic regression analysis, and the significantly independent factors for PLMS in RLS were age (OR: 1.035 (1.00–1.06), p = 0.010) and male gender (OR 2.107 [1.14–3.87], p = 0.017) (Table S1 in the supplemental material).
In non-RLS control patients (122 men, 240 women, mean age 53.75 ± 11.33 years), 19.6 % had PLMS indices greater than 5 and 11.9 % had indices > 15, and age was positively correlated with PLMS index (r = 0.167, p = 0.001). However, gender difference was not seen in controls with a PLMS index > 15 events/h.
Characteristics of LM Structure and Night Distribution
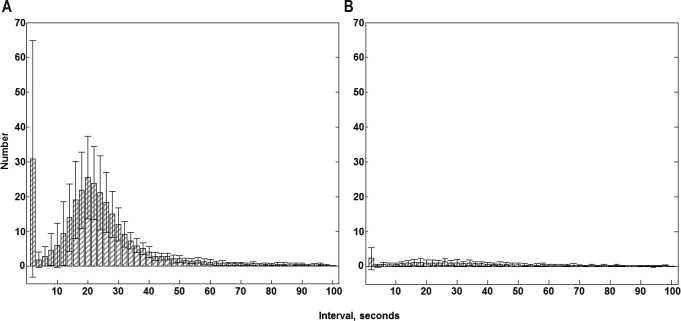
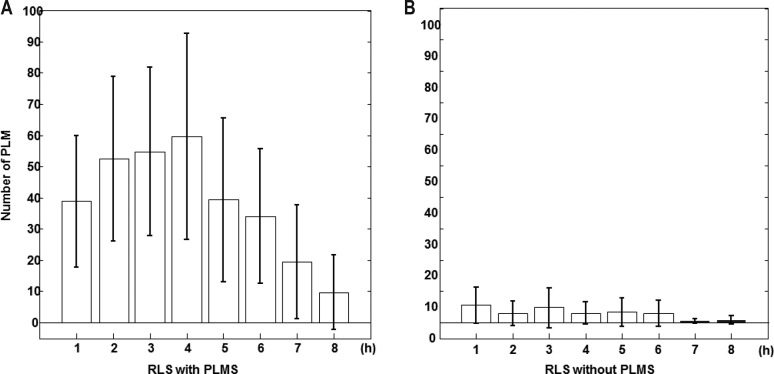
The distribution of inter-LM intervals in patients with and without PLMS is shown in Figure 1. In patients with PLMS, a bimodal distribution of intervals was observed, with peak occurrences at intervals of 1–2 s and 18–22 s. In contrast, in patients without PLMS, there were no patterns, and the inter-LMs intervals were widely distributed. As we compared the distribution of intervals between patients with and without PLMS, we investigated the median value of inter-LM intervals in the PSG data of each patient with weighted statistics that gave equal weight to each patient and used the Mann-Whitney U test, and found a statistically significant difference between the two groups (p = 0.028) (Figure S1 in the supplemental material). Moreover, we found that the mean PI was significantly higher for patients with PLMS than patients without PLMS (0.86 ± 0.10 in patients with PLMS versus 0.37 ± 0.29 in patients without PLMS; p < 0.0001; Figure 2). Additionally, we investigated the distribution of the number of PLM per hour of sleep for the first 8 h in patients with and without PLMS. Patients with PLMS were most likely to have PLM during the first half of the sleep period and showed a progressively decreasing number of PLM per hour of sleep throughout the night (Figure 3). However, patients with a PLM index < 15 showed an even distribution throughout the night. Using a repeated-measures ANOVA and the Bonferroni method, the difference between the 2 groups showed a significant effect for both Group (p = 0.001) and Hour factors (p < 0.001); their interaction was also significant (p < 0.0001).
Figure 1. Comparison of distributions of inter-leg movement intervals in patients with restless legs syndrome.
Comparison of the distributions of inter-leg movement intervals in patients with restless legs syndrome (A) with PLMS (PLMS index > 15 events/h, n = 36) and (B) without PLMS (PLMS index ≤ 15 events/h, n = 48). Values are shown as mean (columns) and standard error of the mean (whiskers). PLMS, periodic limb movements during sleep.
Figure 2.
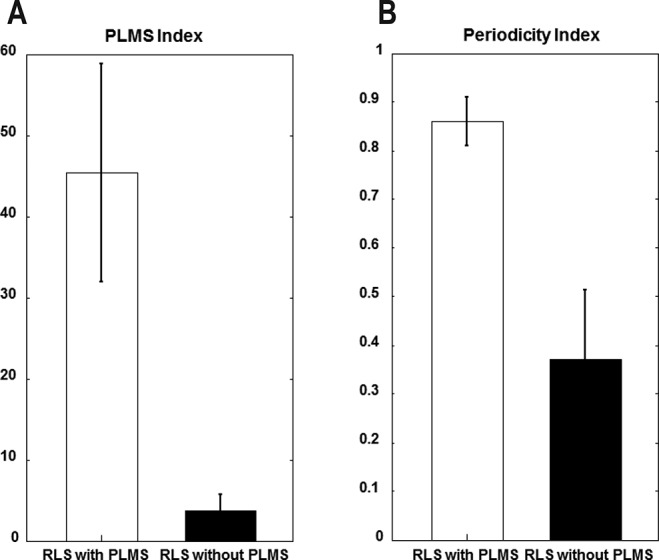
(A) PLMS index and (B) periodicity index in patients with restless legs syndrome with PLMS (PLMS index greater > 15 events/h, n = 36) and without PLMS (PLMS index ≤ 15 events/h, n = 48). Values are shown as mean (columns) and standard error of the mean (whiskers). RLS, restless legs syndrome; PLMS, periodic limb movements during sleep.
Figure 3. Comparison of the distributions of the number of periodic limb movements per hour in patients with restless legs syndrome.
Comparison of the distributions of the number of periodic limb movements per hour of sleep for the first 8 h in patients with restless legs syndrome (A) with PLMS (PLMS index > 15 events/h n = 47) and (B) without PLMS (PLMS index ≤ 15 events/h, n = 61). Values are shown as mean (columns) and standard error of the mean (whiskers). RLS, restless legs syndrome; PLMS, periodic limb movements during sleep.
Relationship between Sleep Parameters and PLMS Severity
Patients who had PLMS had a shorter sleep latency and higher total arousal indices, including PLM-arousal indices, compared with those without PLMS. ESS and ISI scores did not differ significantly between the 2 groups (Table 1).
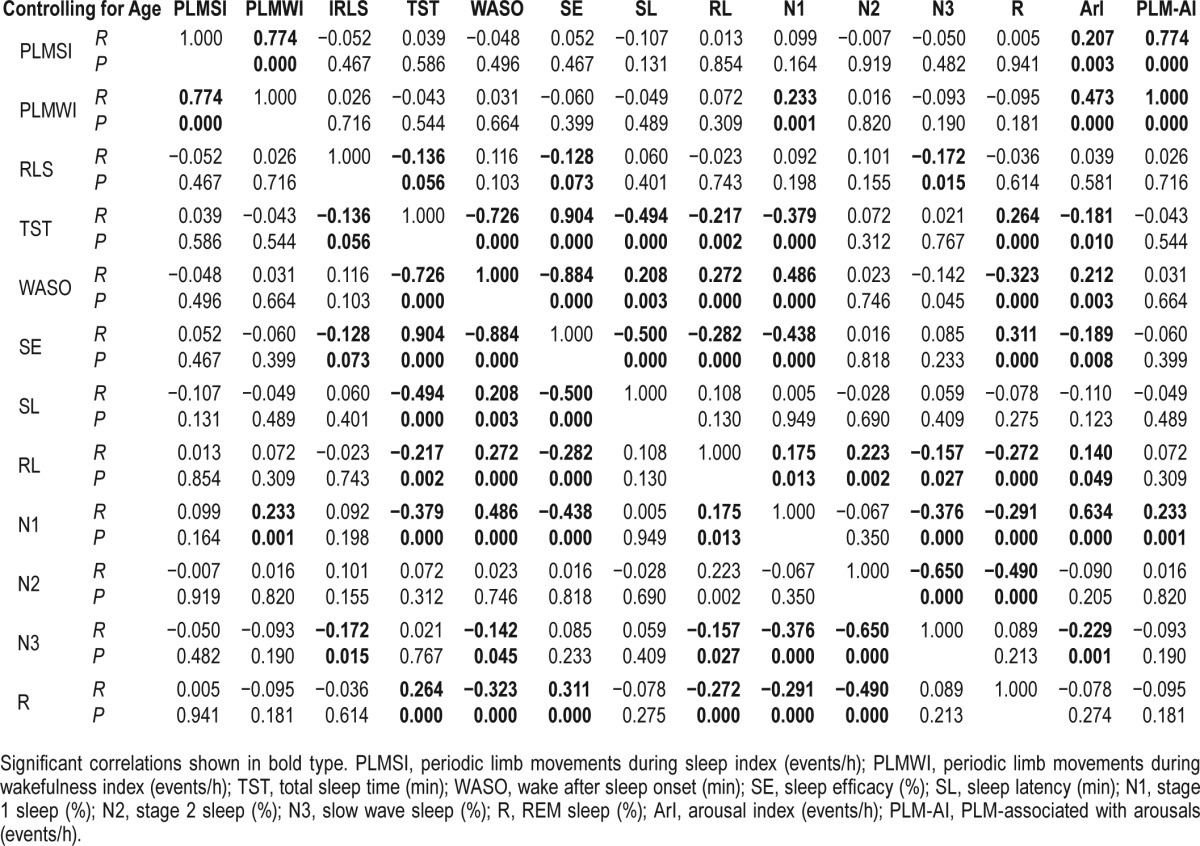
Tables 2 and 3 show correlations between the PLMS index and demographics with sleep parameters, including IRLS. After adjusting for age, the PLMS index was positively correlated with arousal (total arousal index, r = 0.207, p = 0.003; PLM-arousal index, r = 0.774, p < 0.001) and PLMW index (r = 0.774, p < 0.001). However, the PLMS index was not correlated with parameters representing sleep quality or quantity, and severity of RLS. On the other hand, the IRLS score was positively correlated with the ESS and ISI. In addition, as the IRLS score increased, the proportion of slow wave sleep decreased significantly, and TST and SE tended to decrease.
Table 2.
Spearman rank correlation between the clinical characteristics and PLMS index.
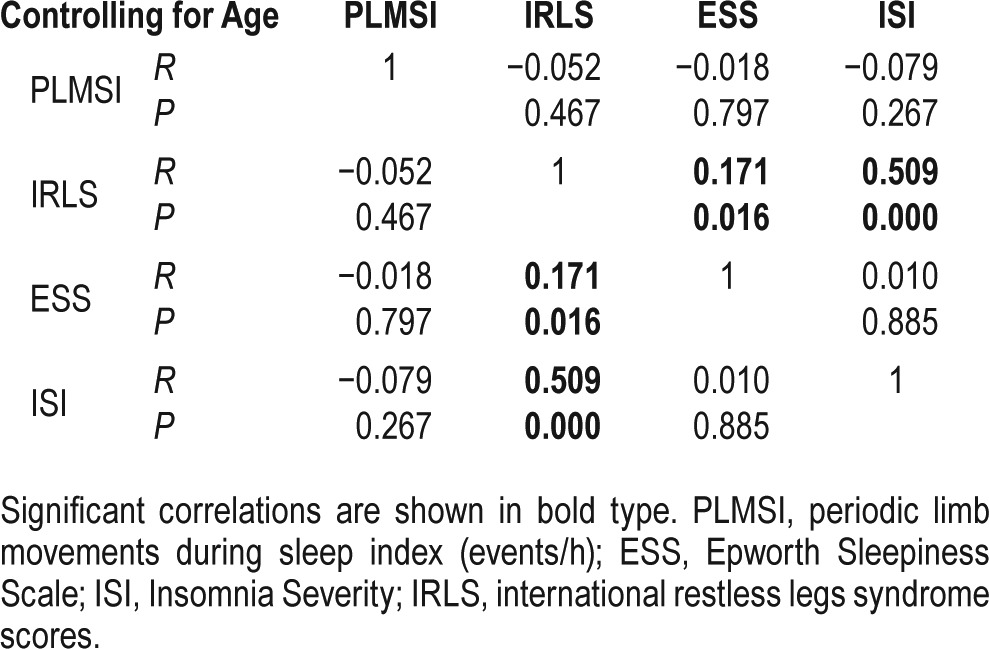
Table 3.
Partial correlation with Spearman rank correlation between the polysomnographic findings and PLMS index.
DISCUSSION
In this study, we showed that the prevalence of PLMS in Korean adult patients with RLS was lower than that reported in patients from western countries through the data of three major sleep centers in different areas of Korea. The proportion of patients having RLS with a PLMS index greater than 5 was 57.9%, while that for patients with a PLMS index greater than 15 was 42.3%. This is the first study investigating the prevalence of PLMS in a large number of adult patients with RLS in Asia.
Montplaisir et al. reported the characteristics of PLMS in 133 patients with RLS from PSG recordings; 82.2% of their patients had a PLMS index greater than 5 in a one-night PSG2; thus, the prevalence of RLS was much higher than that in our study. While we did not observe any difference in the mean age in our study, the proportion of men was higher than that in our study (47.3% in the study by Montplaisir versus 33.9% in this study). Therefore, the lower proportion of men in our study compared with that in the study by Montplaisir may have contributed to the lower prevalence of PLMS in our study. In general, the number of women suffering from RLS is twice as high as the number of men suffering from RLS for all population groups and ages.20 However, in various epidemiological studies on RLS from different countries, the proportion of men with RLS has been variable: 31.7% to 41.2% in some European studies and 35.5% to 41% in South Korea and Japan.21 Moreover, higher proportions of men have been reported in the USA and Canada (50% and 43.3%, respectively).21 Considering the different proportion of men and a gender as independent factor for PLMS index > 15 events/h, gender itself may be one of the factors contributing to the prevalence of PLMS in RLS patients.
Recent studies have reported significant ethnic differences in the prevalence of PLMS in patients with RLS. Koo et al. evaluated the associations of the incidence of cardiovascular disease with PLMS in 2,911 men, reporting that high-PLMI categories were associated with Caucasian race.22 A study investigating the sequence variants contributing to RLS found that an allele of rs3923809 in an intron of BTBD9 on chromosome 6p21.2 was associated with susceptibility to PLMS. This mutation showed no association with patients having RLS without PLMS but was strongly associated with patients having PLMS without RLS symptoms.8 Interestingly, the number of Icelandic subjects who had the A allele of rs3923809 was 873 out of 943 (92.5%), while the number of Korean subjects who had this variant was 235 of 317 (74.2%).23 In addition, considering the allele frequency of rs3923809 in BTBD9, Icelandic patients with RLS have a much higher allele frequency than patients from other European countries.24 An another recent study showed stronger association with polymorphisms in TOX3/BC034767, MEISI1, MAP2K5/SKOR1, and PTPRD as well as BTBD9 in subjects with PLM without RLS symptoms.25 Thus, there was greater genetic association with PLMS rather than RLS, and this genetic difference may contribute to the lower prevalence of PLMS in Korean patients with RLS.
We analyzed the motor phenomenon of PLM in RLS using PI and inter-LM intervals, and found that the pathologic PLMS (defined as PLMI > 15 events/h) in RLS had high periodicity. Moreover, we showed that the number of PLM was detected mostly during the first half of the sleep period. These results were similar to those reported by Ferri et al.14,26 Although the prevalence of PLMS in patients with RLS differed among countries and was affected by genetic factors, the characteristics of PLMS in patients with RLS were not different. In addition, the characteristics of PLM in patients having RLS without PLMS (PLMI ≤ 15) were similar to those of PLM in normal subjects, as reported by Ferri.14 Therefore, the pathogenic mechanisms in patients having RLS with pathologic PLMS may differ from those in patients having RLS without PLMS.
The relationship between sleep disturbance and PLMS in patients with RLS is controversial.27,28 In our study, PLMS was associated with increased arousal index and shorter sleep latency. As the ESS, ISI, and PSQI were not associated with the PLMS index, we suggest that PLMS is not an important factor contributing to sleep disturbance in RLS. However, we believe the trend towards increased PLM related arousal events suggests the reduced sleep latency in patients with PLMS may reflect a slight increase in sleep pressure caused by sleep fragmentation, although it did not have a clinical effect. The severity of RLS (i.e., IRLS score) was associated with sleep quality including insomnia and decreased slow wave sleep in our data. A recent study using proton magnetic resonance spectroscopy showed that a significant increase in the thalamic glutamatergic activity could produce hyperarousal in patients with RLS, and increased glutamatergic activity was correlated significantly with sleep parameters representing arousal sleep disturbance, with the exception of the PLMS index.29 Therefore, sleep problems in patients with RLS may be due to RLS severity rather than PLMS.
In our study, age and male gender were independent factor for PLMS index > 15 events/h in RLS patients. This result consistent with two recent studies that investigated the prevalence and determinants of PLM in a general population.30,31 Ferritin did not show any correlation with PLMS in our results. In the abovementioned two studies, results of relationship between ferritin and PLMS index were different from each other. One study showed a similar result to our study in multiple variable logistic analysis.30 The other study showed that low ferritin was associated with PLMS index > 15 events/h, after adjusting C-reactive protein levels, age, and gender. However, the effect size was small, predicting an increase of 0.0034 PLM events/h for the decrease of every 1 ng/mL of ferritin.31 Another genetic study has demonstrated that serum ferritin levels were decreased by 13% per allele of the at-risk variant in PLMS.8 Considering conflicting results, further clinical studies are needed.
RLS severity was not an independent factor for PLMS > 15 events/h in the patients with RLS and didn't show correlation between PLMS index and IRLS in our study. PLMS is considered as an important biomarker of RLS and is closely associated with RLS. However, relationship between two factors was controversial in some studies.2,32–34 Therefore, this issue is also an important problem to be solved in the future. Although PLMS index was not correlated with RLS severity, the PLMS index was positively correlated with the PLMW index in our data. This finding could support that the multiple suggested immobilization test would be useful in the future to confirm diagnosis and identify daytime symptoms in RLS patients.35
Considering our results that PLMS is less prevalent in the Korean RLS population, and there are no effects on sleep parameters and no relationship with the severity of RLS, we postulate that PLMS is a “genuine” disorder with an ascertainable phenotype. PLMS might simply represent a marker of increased sympathetic output.36,37 This can explain why other sleep disorders including sleep apnea, which generate autonomic instability, are risk factors for PLMS.38 Recent studies have suggested PLMS with or without RLS as a marker of autonomic stress and a risk factor for cardiovascular disorders.22,39 Considering the low prevalence of PLMS in Korean RLS population, Korean patients with RLS might have lower cardiovascular risk than those in Western countries.
Our study has some limitations. First, as this is a retrospective study, we had no choice but to apply the 2003 IRLSSG diagnostic criteria for RLS in our inclusion criteria. Most recent IRLSSG diagnostic criteria additionally include “The occur-rence of the above features is not solely accounted for as symptoms primary to another medical or a behavioral condition (e.g. myalgia, venous stasis, leg edema, arthritis, leg cramps, positional discomfort, habitual foot tapping.” Therefore, we created exclusion criteria to overcome this limitation tried to provide differential diagnoses using previous EMRs. Second, all patients underwent only one night of PSG. When considering the night-to-night variability of the PLMS index,40 the index may change if patients are examined for a second night with PSG. However, the large number of patients included in our study may mitigate the night-to-night variability of PLMS. Additionally, in a study by Ferri et al.,26 PI was shown to be more stable than the PLMS index, which has higher night-tonight variability. Therefore, the effects of the characteristics of PLM itself on sleep quality and quantity may be less affected by night-to-night variability. Third, because the PSG data of two sleep centers had only checked periodic limb movements, we analyzed the inter-movement interval of all LMs from the data of only one sleep center, which had recorded all LMs. However, we supposed this data would not have differed from the other two centers, as there was no statistical difference among the basal characteristics of the three sleep centers.
In summary, the prevalence of PLMS in Korean patients was lower than that reported in patients from western countries. The PLMS index was not related to the severity of RLS in Korean adult patients with RLS. However, the characteristics of LM periodicity and time structure were not different from those in patients from western countries. The low prevalence of PLMS in the Korean population may be explained by genetic factors, including ethnic and gender differences. Further studies using large populations from different ethnic backgrounds are required to confirm our conclusions.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2011-0029740, NRF-2014R1A2A2A04003858, and No. 2014R1A5A2010008). The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- EMG
electromyogram
- EMRs
electronic medical records
- ESS
Epworth Sleepiness Scale
- IRLS
International Restless Legs Syndrome Rating Scale
- IRLSSG
International Restless Legs Syndrome Study Group
- ISI
Insomnia Severity Index
- LM
leg movement
- PIs
periodicity indices
- PLMS
periodic limb movements during sleep
- PLMW
periodic limb movement during wakefulness
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- RLS
restless legs syndrome
- SE
sleep efficiency
- TST
total sleep time in bed
REFERENCES
- 1.Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 2.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–5. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 3.Berry RB. Fundamentals of sleep medicine. Elsevier Saunders. 2012 [Google Scholar]
- 4.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 5.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–97. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkelman JW. Periodic limb movements in sleep--endophenotype for restless legs syndrome? N Engl J Med. 2007;357:703–5. doi: 10.1056/NEJMe078129. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien LM, Holbrook CR, Faye Jones V, Gozal D. Ethnic difference in periodic limb movements in children. Sleep Med. 2007;8:240–6. doi: 10.1016/j.sleep.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Stefansson H, Rye DB, Hicks A, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357:639–47. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 9.Eun MY, HY, Kim JB, Jung KY. Comparison of sleep quality and polysomnographic findings in patients with RLS according to the presence of periodic limb movements during sleep. J Korean Sleep Res Soc. 2011;8:4–8. [Google Scholar]
- 10.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 11.Walters AS, Lavigne G, Hening W, et al. The scoring of movements in sleep. J Clin Sleep Med. 2007;3:155–67. [PubMed] [Google Scholar]
- 12.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146:1387–94. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 14.Ferri R, Zucconi M, Manconi M, et al. Different periodicity and time structure of leg movements during sleep in narcolepsy/cataplexy and restless legs syndrome. Sleep. 2006;29:1587–94. doi: 10.1093/sleep/29.12.1587. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Kim D, Lee J, et al. The reliability and validity of the Korean versions of the International Restless Legs Scale and the Restless Legs Syndrome Quality of Life Questionnaire. J Korean Neurol Assoc. 2010;28:263–9. [Google Scholar]
- 16.Sohn SI, Kim DH, Lee MY, Cho YW. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012;16:803–12. doi: 10.1007/s11325-011-0579-9. [DOI] [PubMed] [Google Scholar]
- 17.Cho YW, Song ML, Morinc CM. Validation of a korean version of the insomnia severity index. J Clin Neurol. 2014;10:210–5. doi: 10.3988/jcn.2014.10.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho YW, Lee JH, Son HK, Lee SH, Shin C, Johns MW. The reliability and validity of the Korean version of the Epworth sleepiness scale. Sleep Breath. 2011;15:377–84. doi: 10.1007/s11325-010-0343-6. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Int Med. 2011;171:887–95. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohayon MM, O'Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283–95. doi: 10.1016/j.smrv.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath. 2012;16:987–1007. doi: 10.1007/s11325-011-0606-x. [DOI] [PubMed] [Google Scholar]
- 22.Koo BB, Blackwell T, Ancoli-Israel S, et al. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011;124:1223–31. doi: 10.1161/CIRCULATIONAHA.111.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MK, Cho YW, Shin WC, et al. Association of restless legs syndrome variants in Korean patients with restless legs syndrome. Sleep. 2013;36:1787–91. doi: 10.5665/sleep.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemlink D, Polo O, Frauscher B, et al. Replication of restless legs syndrome loci in three European populations. J Med Genet. 2009;46:315–8. doi: 10.1136/jmg.2008.062992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore Ht, Winkelmann J, Lin L, Finn L, Peppard P, Mignot E. Periodic leg movements during sleep are associated with polymorphisms in BTBD9, TOX3/BC034767, MEIS1, MAP2K5/SKOR1, and PTPRD. Sleep. 2014;37:1535–42. doi: 10.5665/sleep.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferri R, Fulda S, Manconi M, et al. Night-to-night variability of periodic leg movements during sleep in restless legs syndrome and periodic limb movement disorder: comparison between the periodicity index and the PLMS index. Sleep Med. 2013;14:293–6. doi: 10.1016/j.sleep.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Inoue Y, Nanba K, Honda Y, Takahashi Y, Arai H. Subjective sleep quality and suggested immobilization test in restless leg syndrome and periodic limb movement disorder. Psychiatry Clin Neurosci. 2002;56:293–4. doi: 10.1046/j.1440-1819.2002.00966.x. [DOI] [PubMed] [Google Scholar]
- 28.Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10:169–77. doi: 10.1016/j.smrv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Allen RP, Barker PB, Horska A, Earley CJ. Thalamic glutamate/glutamine in restless legs syndrome: increased and related to disturbed sleep. Neurology. 2013;80:2028–34. doi: 10.1212/WNL.0b013e318294b3f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haba-Rubio J, Marti-Soler H, Marques-Vidal P, et al. Prevalence and determinants of periodic limb movements in the general population. Ann Neurol. 2016;79:464–74. doi: 10.1002/ana.24593. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Moore H, Lin L, et al. Association of low ferritin with PLM in the Wisconsin Sleep Cohort. Sleep Med. 2015;16:1413–8. doi: 10.1016/j.sleep.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Borreguero D, Larrosa O, de la Llave Y, Granizo JJ, Allen R. Correlation between rating scales and sleep laboratory measurements in restless legs syndrome. Sleep Med. 2004;5:561–5. doi: 10.1016/j.sleep.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Allen RP, Earley CJ. Validation of the Johns Hopkins restless legs severity scale. Sleep Med. 2001;2:239–42. doi: 10.1016/s1389-9457(00)00080-0. [DOI] [PubMed] [Google Scholar]
- 34.Hornyak M, Hundemer HP, Quail D, Riemann D, Voderholzer U, Trenkwalder C. Relationship of periodic leg movements and severity of restless legs syndrome: a study in unmedicated and medicated patients. Clin Neurophysiol. 2007;118:1532–7. doi: 10.1016/j.clinph.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Borreguero D, Kohnen R, Boothby L, Tzonova D, Larrosa O, Dunkl E. Validation of the multiple suggested immobilization test: a test for the assessment of severity of restless legs syndrome (Willis-Ekbom Disease) Sleep. 2013;36:1101–9. doi: 10.5665/sleep.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52:786–91. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- 37.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 38.Manconi M, Ferri R, Zucconi M, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012;71:834–44. doi: 10.1002/ana.23565. [DOI] [PubMed] [Google Scholar]
- 39.Stevens MS. Restless legs syndrome/Willis-Ekbom disease morbidity: burden, quality of life, cardiovascular aspects, and sleep. Sleep Med Clin. 2015;10:369–73. xv–xvi. doi: 10.1016/j.jsmc.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Sforza E, Haba-Rubio J. Night-to-night variability in periodic leg movements in patients with restless legs syndrome. Sleep Med. 2005;6:259–67. doi: 10.1016/j.sleep.2004.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.