Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is an independent risk factor for hypertension (HTN). Increasing evidence from animal and human studies suggests that HTN exacerbates OSA. We performed a systematic review and meta-analysis of studies evaluating the effect of anti-hypertensive medications on the severity of OSA.
Methods:
A literature search of PubMed and Embase was done using search concepts of OSA, HTN, and drug classes used to treat HTN. Studies that reported changes in the severity of OSA objectively by using apnea-hypopnea index (AHI) or respiratory disturbance index (RDI) were included. Pooled mean difference estimates were calculated. Tests for heterogeneity, publication bias, and subgroup sensitivity analysis were conducted.
Results:
Of 27,376 studies screened, only 11 met inclusion criteria, including 5 randomized controlled trials and 6 single-arm prospective trials. The pooled mean difference estimate (95% confidence interval [CI]), based on a random-effects model, was −5.69 (95% CI −10.74 to −0.65), consistent with an overall decrease in AHI or RDI attributable to antihypertensive medications. The effect size was even more pronounced, −14.52 (95% CI −25.65 to −3.39), when only studies using diuretics were analyzed. There was no significant heterogeneity or publication bias among the studies. Meta-regression indicated neither age, baseline AHI, nor change in systolic/diastolic blood pressure influenced the results.
Conclusions:
Collectively, findings from these relatively small, short-term studies tend to support the contention that treatment with antihypertensive agents confers a statistically significant, albeit small, reduction in the severity of OSA, which may be more pronounced with the use of diuretics.
Citation:
Khurshid K, Yabes J, Weiss PM, Dharia S, Brown L, Unruh M, Jhamb M. Effect of antihypertensive medications on the severity of obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med 2016;12(8):1143–1151.
Keywords: antihypertensive medications, hypertension, obstructive sleep apnea
INTRODUCTION
Hypertension (HTN) and obstructive sleep apnea (OSA) coexist in millions of people and have been associated with heart disease, stroke, and premature death.1 The prevalence of HTN has been increasing in the United States and is particularly high in older adults, with up to 60% of those older than 60 y and 90% of those in the eighth and ninth decades of life having elevated blood pressure (BP).2 The causal relationship between elevated BP and cardiovascular mortality appears consistent across age groups, with those with elevated BP in every decade of life at increased risk of death.3 OSA is present in 1 of 5 adults in the United States and is particularly common among adults with HTN4,5; 30% of patients with HTN and 80% of those with resistant HTN exhibit significant OSA.6
BRIEF SUMMARY
Current Knowledge/Study Rationale: Increasing evidence suggests that hypertension (HTN) exacerbates obstructive sleep apnea (OSA). It is thus plausible that intensive treatment of HTN may improve OSA severity, either by volume control (by promoting diuresis or suppressing aldosterone), stabilization of upper airway muscles, or perhaps because of some direct effect of antihypertensive medications
Study Impact: Out of more than 27,000 studies screened, only 11 evaluated the effect of antihypertensive treatment on objective OSA severity, thus revealing a critical need for more research in this area. Our meta-analysis suggests that treatment with antihypertensive agents confers a statistically significant, albeit small, reduction in the severity of OSA, which may be more pronounced with the use of diuretics.
OSA leads to repetitive episodes of hypercapnia, sleep disruption, hypoxemia, and activation of the sympathetic nervous system, and in recent years the last two factors have been strongly implicated as mechanisms contributing to the development or worsening of HTN.7 Prospective studies have demonstrated that OSA has a causal relationship to cardiovascular disease, including stroke, myocardial infarction, and congestive heart failure, even after adjustment for obesity and other common potential confounders.1,8 Additionally, OSA has been shown to be independently associated with resistant HTN, one compelling study found OSA was present in 83% of patients with resistant HTN.6 These findings have led national committees to consider OSA as a cause of HTN and, importantly, resistant HTN.9 Despite strong observational data relating OSA to HTN, treatment of OSA with continuous positive airway pressure (CPAP) has demonstrated only modest improvement in daytime BP in randomized controlled trials (RCT)10 calling into question whether the observed association between HTN and OSA may in fact be bidirectional.
Hypothetically, intensive BP control may influence OSA by stabilizing the upper airway, because genioglossus activity is adversely affected by increases in BP.11 Furthermore, there is mounting evidence for the role of volume status in affecting the severity of OSA. In part, these data consist of experimental manipulation of volume or its distribution in normal subjects that demonstrate changes in upper airway resistance, cross-sectional area, and collapsibility in directions that would promote OSA when fluid accumulates caudally,12,13 and similar effects in patients with resistant HTN14 and coexisting congestive heart failure and OSA have been observed.15 Also compelling are comparisons between dialysis techniques in patients with coexisting end-stage renal disease (ESRD) and OSA; regimens that promote reduced volume status are associated with reductions in OSA severity16,17 and a relationship has been reported between the degree of rostral fluid shift during nighttime recumbency and OSA severity in patients with ESRD.18 Lastly, emerging evidence suggests the role of aldosterone excess, especially among resistant hypertensives, in worsening OSA severity, likely by promoting fluid retention and subsequent parapharyngeal edema.19
Clearly, antihypertensive therapy may have differing effects on volume status, depending on the agent employed. However, based on the increasing evidence suggesting that elevated BP may play a role in the pathogenesis of OSA, it is at least plausible that intensive treatment of HTN may bring about improvement in the severity of the OSA, either by volume control (either directly by promoting diuresis or suppressing aldosterone), stabilization of upper airway muscles, or perhaps because of some direct effect of antihypertensive medications. A handful of small RCTs and single-arm prospective studies, each with methodological limitations, have investigated this possibility. The aim of our study was therefore to conduct a systematic review and meta-analysis to determine whether these studies, analyzed as a whole, support an effect of anti-hypertensive medications on OSA severity. Because existing literature supports the association of nocturnal hypoxia with cardiovascular outcomes,20,21 we additionally evaluated the effect of antihypertensive medications on nocturnal oxygenation.
METHODS
Literature Search
Literature searches were done using the National Library of Medicine's PubMed search engine (http://www.ncbi.nlm.nih.gov/pubmed/) and Elsevier B.V.'s Embase.com search engine (http://www.embase.com). Search strategies were developed by an experienced librarian (PW) and included the concepts of HTN, antihypertensive classes, and sleep apnea (see Appendix in the supplemental material). Additional sources were identified from the reference lists of articles retrieved. Searches were first run in May (PubMed) and December (Embase.com) of 2012, revised and re-run in March 2013 and subsequently in March 2014.
Study Eligibility and Selection
Two reviewers (MJ, KK) independently evaluated articles for eligibility in a two-stage procedure. In the first stage, all identified abstracts were reviewed. In the second stage, full texts of those that met the inclusion criteria or those for which there was uncertainty as to eligibility were independently reviewed by both reviewers. Inclusion criteria consisted of (1) study population— adults age 18 y or older with HTN; (2) intervention—use of anti-hypertensive medications; (3) outcome—OSA severity measured objectively and reported as apnea-hypopnea index (AHI, number of events of apnea or hypopnea per hour of sleep) or respiratory disturbance index (RDI, total number of apneas, hypopneas, and respiratory effort-related arousals per hour of sleep). Studies with primarily central sleep apnea as the outcome were excluded.22–24 Studies reporting OSA severity in any other unit of measurement such as apnea index25 or static charge-sensitive bed26,27 or oxygenation level were excluded; and (4) study design—RCTs or prospective single arm studies. Cross-sectional studies were excluded. Studies were eligible for inclusion whether published or unpublished and irrespective of language. Studies on OSA due to obesity–hypoventilation syndrome, high altitude sickness, or involving surgery or benzodiazepines as an intervention, or including children were excluded. Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for conduct of systematic reviews were followed (Figure 1).
Figure 1. Systematic literature: review process.
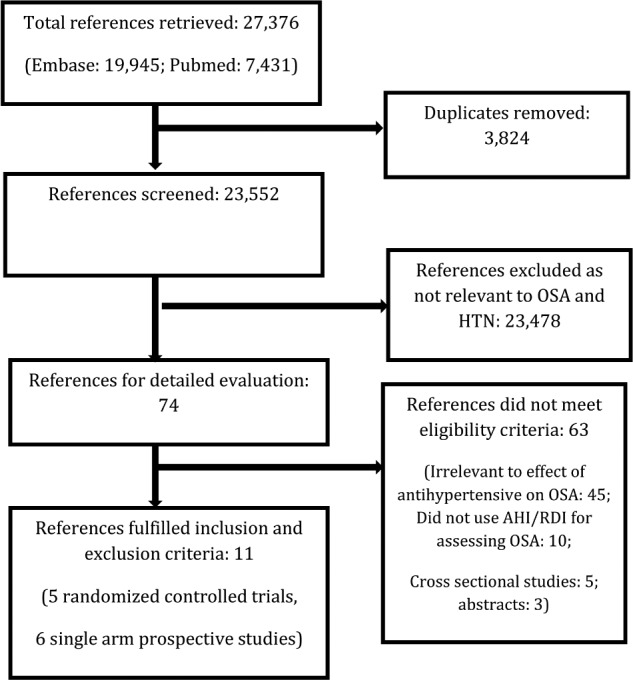
Flow chart of study selection process. AHI, apnea-hypopnea index; HTN, hypertension; OSA, obstructive sleep apnea; RCT, randomized controlled trial; RDI, respiratory disturbance index.
Data Extraction
Data from the final selected articles was abstracted independently by two reviewers (KK, MJ) and verified by a third reviewer (JY). Means and standard deviations (SD) for the OSA outcomes were extracted from preintervention and postintervention assessments. Medians were used to impute missing means and range/4 to impute missing SD28,29 as prescribed in the meta-analysis literature.30 A within-subject correlation of 0.4 was assumed between pre-post outcome measurements to approximate the SD of pre-post differences if not reported. The severity of sleep apnea was reported as RDI in two trials5,28 and AHI in the rest of the trials. Two trials reported posttreatment AHI or RDI for rapid eye movement (REM) and non-REM sleep separately; however, sufficient information was available to allow for computation of AHI or RDI based on total sleep time, which was then used in subsequent analyses.5,29 There were large inconsistencies in the measures of nocturnal oxygenation used across the studies. Of the 11 studies that reported prenocturnal and post-nocturnal oxygenation measurement values, 7 reported lowest oxygen saturation22,28,29,31–34 and 4 reported mean oxygen saturation,22,29,32,35 but only 3 reported oxygen desaturation index22,28,34 and only 2 reported hypoxic index.35,36 Given the extent of missing data for these variables, we limited further analysis to the two variables with most complete data: lowest oxygen saturation and mean oxygen saturation. Mean and standard deviation (SD) for change in systolic and diastolic blood pressure was extracted using office/in-hospital BP measurements for all but one study,33 in which only ambulatory BP data were reported. Study quality for RCTs using Jadad scoring guidelines was assessed independently by two reviewers (KK, MJ).37
Statistical Analysis
Both fixed- and random-effects models were used. We used the maximum likelihood method to estimate the between study variance (τ2) in the random-effects model. Forest plots were constructed to show the estimates and 95% confidence intervals (CI) for individual study and the summary effect sizes. We assessed heterogeneity in the effect sizes via a formal Q-statistic and quantification heterogeneity metric (I2), and visually through forest plots. Exploratory meta-regression analysis was done using study-specific covariates including age, baseline AHI, and systolic/diastolic BP. We also conducted a leave-one-out method sensitivity analysis, whereby we re-ran the meta-analysis iteratively, removing studies one by one, to identify suspected outliers. To investigate publication bias, we constructed funnel plots and tested for asymmetry using the Egger regression test33and the trim and fill method.34 All statistical analyses were carried out in R (version 13.1) using the metafor package (http://www.jstatsoft.org/v36/i03/ and http://www.R-project.org/).
RESULTS
Characteristics of Included Studies
Our search resulted in 11 studies with total of 299 subjects that met our inclusion and exclusion criteria. Of these, five were RCTs and six were prospective single arm trials. Details of individual studies are presented in Table 1 (RCTs) and Table 2 (single-arm prospective trials). Of the five RCTs, three had a crossover design28,33,38 and two had parallel designs.5,35 Two of the RCTs were placebo controlled5,33 whereas the other three were double blind.28,35,38 Apart from the study by Heit-mann et al.,35 the trials were generally of poor quality. Of the six single-arm prospective studies, two were done using acetazolamide.29,31 Although acetazolamide is an antihypertensive agent, it was primarily used as a respiratory stimulant in these studies. Thus, these studies were excluded from the main analysis, but included in sensitivity analyses.
Table 1.
Characteristics of randomized controlled trials.
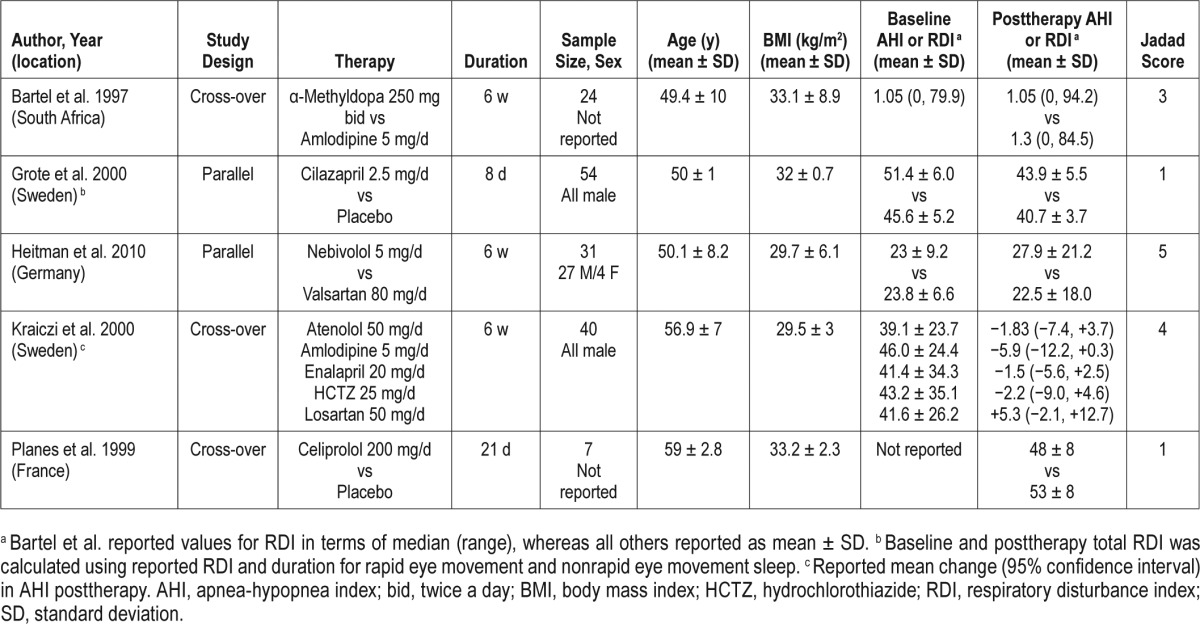
Table 2.
Characteristics of single arm prospective studies.
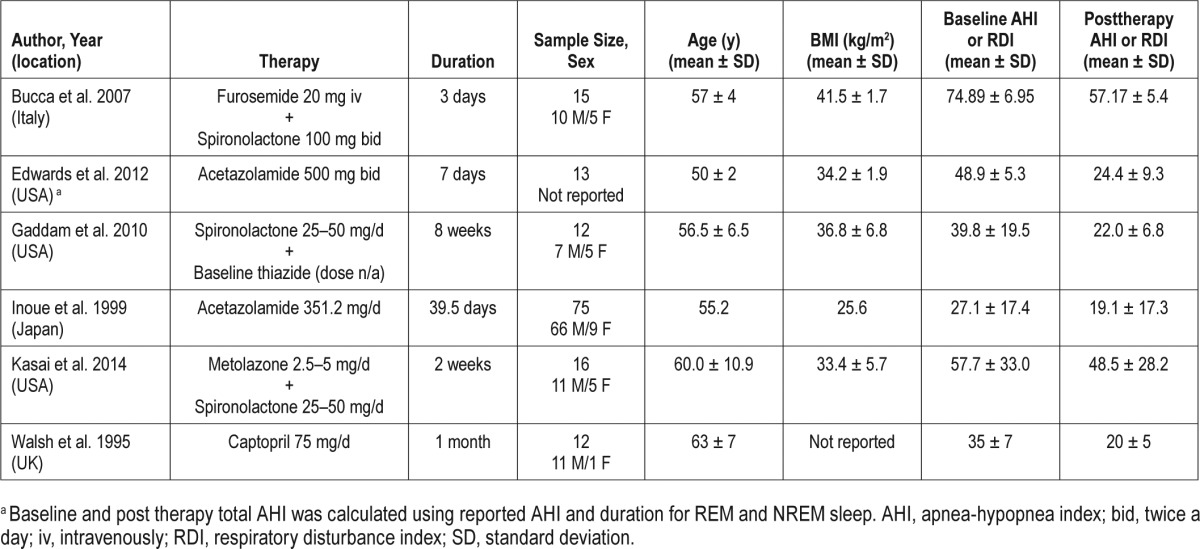
The studies varied in the duration of follow-up from 3 to 56 days. Different anti-hypertensive drug classes were used in the trials, including renin-angiotensin-aldosterone system inhibitors, beta-blockers, diuretics, calcium channel blockers, and ganglion blockers. The trials also differed in terms of the patient populations enrolled. The sample size ranged from 7 to 75 subjects. Among the studies that reported sex of the participants, only 11.8% (n = 29) were females and race was reported only by Gaddam et al.36 (six white, six black). There were three studies5,22,36 that confined enrollment to patients with severe OSA (AHI ≥ 30) whereas others included a full range of mild to severe OSA. Of note, in the trial conducted by Bartel et al.,28 all but one participant had RDI < 20 with a median RDI (1.05 events/h) that was normal at baseline.
Main Outcomes
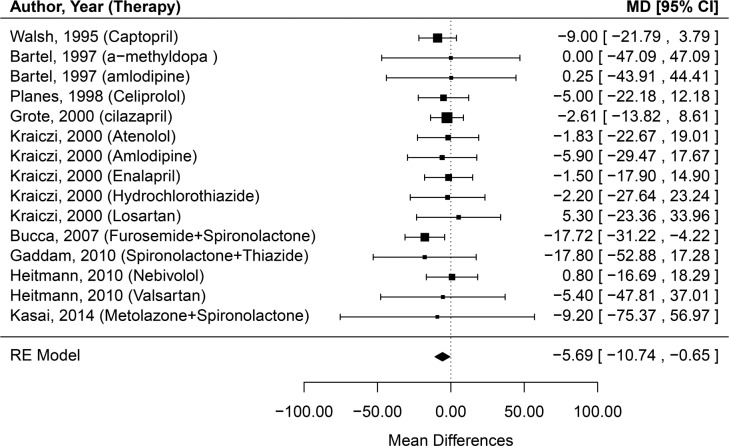
Initially, a fixed-effects model was used to estimate the effect of antihypertensive medications on change in severity of OSA. The pooled estimate (95% CI) of mean differences in AHI of was −5.69 (−10.74, −0.65), which was same using the random-effects model (Figure 2). No evidence of heterogeneity was found (Q = 5.75, p = 0.97; I2 = 0%). Because the study by Bartel et al.28 mostly included patients without OSA, we conducted additional analysis excluding this study. This revealed similar results with the effect size - pooled estimate of mean difference in AHI being −5.84 [−10.94, −0.74] in both fixed- and random-effects models. All the aforementioned analytic methods support a finding that antihypertensive medications, as a general class, may have a modest clinically meaningful effect in improving OSA severity.
Figure 2. Forest plot of effect of antihypertensive treatment on change in severity of obstructive sleep apnea (mean change in apnea-hypopnea index).
The effect size estimate is given for each study expressed in terms of mean differences (MD), with the corresponding 95% confidence internal (CI) indicated by the error bars. The pooled estimate is shown according to the random-effects (RE) model.
Effect of Diuretics on Change in OSA Severity
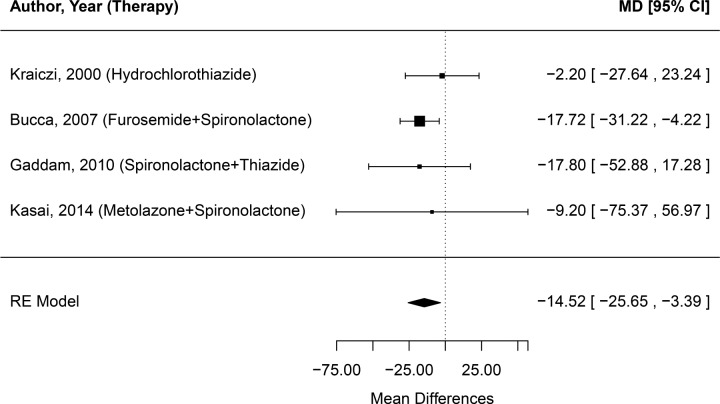
Because volume overload, rostral fluid shifts, and upper airway edema may play a role in OSA severity, we analyzed the change in AHI preintervention and postintervention in the four studies that used diuretics (Figure 3). The random-effects model pooled estimate of effect size (95% CI) in these studies was −14.52 (−25.65, −3.39) indicating that there was significant improvement from the use of diuretics in OSA severity. Notably, the total number of subjects for this analysis was only 57, thus the wide CIs.
Figure 3. Forest plot of effect of diuretic treatment on change in severity of obstructive sleep apnea (mean change in apneahypopnea index).
The effect size estimate is given for each study expressed in terms of mean differences (MD), with the corresponding 95% confidence internal (CI) indicated by the error bars. The pooled estimate is shown according to the random- effects (RE) model.
Effect of Age and Baseline OSA Severity
An exploratory meta-regression was performed using a random-effects model with study mean age and mean baseline AHI or RDI values as moderators in order to identify if the effect of antihypertensives on mean change in AHI varied by age or baseline OSA severity. Omnibus testing (QM = 1.20, p = 0.55) indicated that neither age nor initial AHI or RDI influenced the impact of antihypertensives on OSA severity.
Effect of Change in Blood Pressure
We further examined if there was any relation in the amount of blood pressure reduction by antihypertensive treatment and the effect size on OSA severity. All but one study reported preintervention and postintervention BP.34 The mean change in systolic BP was −5.53 (−10.29, −0.76) and diastolic BP was −4.34 (−7.07, −1.61). Meta-regression results indicated that neither systolic nor diastolic BP change had any significant influence on the effect of antihypertensives on mean change in AHI (Omnibus QM = 0.90, p = 0.34 and QM = 0.80, p = 0.37, respectively).
Effect of Antihypertensive Treatment on Nocturnal Oxygenation
There was no significant effect of antihypertensive treatment on lowest nocturnal oxygenation saturation. The pooled estimate (95% CI) of mean differences in lowest oxygen saturation was 2.01 (−0.72, 4.73), using both the fixed- and random-effects models. However, there was a statistically significant increase in the mean nocturnal oxygenation saturation after antihypertensive treatment, although the magnitude was very small and not likely to be clinically meaningful (pooled estimate [95% CI] of mean differences 1.19 (0.39, 1.99) using the random effects model). For all these models, there was no evidence of heterogeneity or publication bias
Publication Bias
The regression test for funnel plot asymmetry was not significant (z = 0.42, p = 0.68), indicating low likelihood of publication bias (Figure S1 in the supplemental material). This finding was supported by the application of Trim and Fill method, which estimated the number of missing NULL studies.
Sensitivity Analyses
Because in addition to its action as a respiratory stimulant acetazolamide may cause reduction in OSA severity due to its antihypertensive effect, we conducted sensitivity analyses by including two studies that used acetazolamide.29,31 The random-effects model pooled effect size (95% CI) showed even further decrease in mean change in AHI at −7.23 (−12.02, −2.43) indicating that there was a significant and clinically meaningful reduction in OSA severity (Figure S2 in the supplemental material). We also conducted sensitivity analysis for the effect on nocturnal oxygenation by excluding these studies. The pooled effect on lowest nocturnal oxygenation remained statistically insignificant. Moreover, the pooled estimate (95% CI) of mean differences in mean oxygen saturation was no longer significant and was 0.24 (−1.38, 1.85) using both the fixed- and random-effects models, with no evidence of heterogeneity or publication bias.
Most studies included in the analysis reported AHI whereas two reported RDI.5,28 Raw mean differences of the measured RDI or AHI were used in the primary analysis. However, to account for the possibility that these two measures could have different variability, we also conducted a meta-analysis using standardized mean differences. Hedges g was calculated for each study and used as an effect size measure. The fixed-model effect size for standardized mean change in AHI was −0.53 (−0.73, −0.33) and random-model effect size was −1.46 (−2.52, −0.40) (data not shown). These results, although statistically significant, are not clinically meaningful, and thus very similar to the primary analysis results.
DISCUSSION
In this study, we systematically reviewed and conducted a meta-analysis of data from RCTs as well as prospective single-arm trials to quantify the effects of antihypertensive medications on the severity of OSA. Of more than 27,000 abstracts that were screened, only 11 studies (including only 5 RCTs), met the inclusion criteria, highlighting the dearth of evidence in this area. Altogether, the results revealed that there is a decrease in AHI or RDI after treatment with antihypertensive medications, which reached statistical significance but with a magnitude that may not be clinically significant. This effect was more pronounced in studies using diuretics. Subject age, baseline AHI or RDI, or change in systolic or diastolic BP did not seem to play a role in the degree of improvement in OSA severity. There was no clinically meaningful improvement in nocturnal oxygenation after antihypertensive treatment.
The trials varied in their use of antihypertensive medication class, dose, and the number of drugs prescribed. The duration of intervention was not uniform, and the participants varied in terms of age, sex, and OSA severity. Females and minority racial/ethnic groups were severely underrepresented, the subjects studied were almost exclusively Caucasian men. The quality of RCTs was low, largely due to inadequate description of randomization methods, small sample size, and subject dropout.
The mechanisms by which antihypertensive medications may affect severity of OSA are poorly understood. We view the mounting evidence relating volume overload in ESRD to OSA as a model for this novel mechanism in the larger population of those with HTN and OSA.16,18,39 In healthy subjects, fluid displacement from the legs by application of lower body positive pressure has been shown to increase neck circumference and upper airway airflow resistance and collapsibility, and reduce upper airway caliber.40 In a study of 12 patients with OSA and chronic venous insufficiency, compression stockings were used to minimize the reservoir of fluid available for redistribution and reduced the severity of OSA by 36%, supporting the proposition that displacement of fluid from the legs to the neck exacerbates OSA.41 In a study of 25 patients with resistant HTN, increased rostral fluid redistribution was significantly associated with more severe OSA.14 However, Jafari and Mohsenin42 reported on obese subjects with and without OSA who did not exhibit worsening of their AHI or RDI during the course of the night despite significant rostral fluid shifts. For methodological reasons, these investigators did not include OSA severity during REM sleep in their analysis. We believe changes in upper airway characteristics from fluid shifts would be expected to have a more profound effect on OSA severity during this sleep state. This is because the tone of the dilator muscles responsible for maintaining upper airway patency is reduced more in REM sleep than in non-REM sleep due to the phenomena of REM-related skeletal muscle hypotonia.43 More recently, lower fluid overload after hemodialysis has been shown to be correlated with lower obstructive AHI,44 thus suggesting fluid overload as a possible mechanism in pathogenesis of OSA. In fact, improved ultrafiltration by nocturnal hemodialysis or continuous peritoneal ambulatory dialysis has been shown to improve OSA severity.17,45 In nondialysis-dependent hypertensive patients, the plasma and extracellular fluid volume usually decreases by 10% to 12% in the initial few weeks of treatment with diuretics, which may potentially improve OSA severity. However, evidence supporting this is limited. We found a more pronounced clinically meaningful improvement in AHI severity when analyzing studies using diuretics only, thus again reinforcing the role of volume overload in OSA.
In patients with resistant hypertension, growing evidence suggests that aldosterone excess contributes to greater severity of OSA by promoting fluid retention, and subsequently parapharyngeal edema.19,46 Patients with resistant hypertension have been shown to have higher aldosterone levels and intravascular volume expansion, as compared to controls.47 Thus, treatment with aldosterone blockers, such as spironolactone, may improve OSA severity.22,32,36 However, the evidence supporting this is limited by nonrandomized observational study designs.
Evidence also suggests a role of sympathetic tone on OSA severity. A recent meta-analysis showed renal sympathetic denervation was associated with a significant reduction in mean AHI [mean difference −9.61 (95% CI −15.43 to −3.79), p = 0.001] 6 month postdenervation.48 It is hypothesized that denervation reduces salt avidity and perhaps affects venous capacitance and blood pooling, thereby reducing peripharyngeal fluid accumulation and OSA severity. However, these results are based on observational data from only 5 studies and 49 patients, and warrant further investigation.
Hypertension may negatively affect upper airway muscle tone. Acute increases in systolic BP have an inhibitory effect on upper airway muscles in sleeping intact cats,49 decerebrate cats,50 and anesthetized dogs,51 and if confirmed in humans could represent a mechanism by which uncontrolled BP could worsen OSA. In fact, Garpestad et al.11 demonstrated in five young adult men who were screened to be free of sleep disorders that when the patients were awake, mean genioglossal electromyography activity decreased with higher mean arterial pressure. However, average upper airway resistance (Rua) remained unchanged when Wilson et al.52 acutely increased BP in nine sleeping young adults without sleep apnea, although Rua did increase further in those with higher baseline values. Thus, individuals with preexisting upper airway attributes that put them at risk for OSA may have that risk amplified with the development of HTN.
The strengths of our study include its methodology, broad search strategy using multiple search engines, and use of rigorous methods to account for heterogeneity and publication bias. Only those trials that used objective measures of OSA severity derived from overnight polysomnography rather than from static charge-sensitive bed or pulse oximetry were included. Inclusion of non-English articles and unpublished studies was done in order to decrease bias. Our limitations are primarily due to the generally poor quality of the studies that met inclusion criteria. However, despite the methodological limitations of these studies, to the best of our knowledge, this is the only evidence are available in the literature on this topic. Considering the strength of the observed association and robust statistical parameters, such as lack of heterogeneity or publication bias, it may be reasonable to consider our study hypothesis generating and warranting further research in this area. In addition, although we did subgroup analysis of only those studies including diuretics, our conclusions are limited due to the small number of subjects in these studies and lack of objective measures of volume status such as bioimpedance analysis.
CONCLUSIONS
Because OSA and HTN coexist in a population numbered in the millions, innovative treatment approaches that treat the interactions between these two entities could profoundly affect the health and well-being of these patients. It is evident from these small, short-term follow-up studies that there is a beneficial effect of antihypertensive medications on sleep apnea severity, although this effect may or may not prove to be clinically important. If intensive BP control improves OSA in patients with HTN, then treatment of sleep apnea in these individuals could include greater efforts at BP control in addition to using positive airway pressure or other modalities such as oral appliances or surgery. Larger studies with longer follow-up and that inclusion of minorities and women are surely warranted to increase our understanding of the effect of BP treatment and individual antihypertensive medication class on OSA severity. Moreover, one population particularly stands out as potentially benefiting from such research: patients with chronic kidney disease experience a four times higher incidence of OSA than the general population53 and the prevalence of HTN in these individuals is virtually a given.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by National Institutes of Health NIDDK Grant P30-DK-079307 and American Heart Association grant 11FTF7520014 (Jhamb). Dr. Brown has consulted for Considine & Associates Inc.; receives royalties from UpToDate; has received honoraria from Current Opinion in Pulmonary Medicine and Sleep Medicine Clinics for editing; and has received honorarium for attending a focus group for Phillips Respironics. Dr. Unruh has recieved grant support from Dialysis Clinic Inc. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- OSA
obstructive sleep apnea
- HTN
hypertension
- AHI
apnea-hypopnea index
- RDI
respiratory disturbance index
- CI
confidence interval
- BP
blood pressure
- CPAP
continuous positive airway pressure
- RCT
randomized controlled trials
- ESRD
end-stage renal disease
- REM
rapid eye movement
- SD
standard deviation
REFERENCES
- 1.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 2.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension. 2008;52:818–27. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 4.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 5.Grote L, Wutkewicz K, Knaack L, Ploch T, Hedner J, Peter JH. Association between blood pressure reduction with antihypertensive treatment and sleep apnea activity. Am J Hypertens. 2000;13:1280–7. doi: 10.1016/s0895-7061(00)01207-3. [DOI] [PubMed] [Google Scholar]
- 6.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–7. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Caples SM, Kara T, Somers VK. Cardiopulmonary consequences of obstructive sleep apnea. Semin Respir Crit Care Med. 2005;26:25–32. doi: 10.1055/s-2005-864208. [DOI] [PubMed] [Google Scholar]
- 8.Seif F, Patel SR, Walia HK, et al. Obstructive sleep apnea and diurnal nondipping hemodynamic indices in patients at increased cardiovascular risk. J Hypertens. 2014;32:267–75. doi: 10.1097/HJH.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–26. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Cao Q, Guo Z, Dai Q. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens. 2016;18:153–8. doi: 10.1111/jch.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garpestad E, Basner RC, Ringler J, et al. Phenylephrine-induced hypertension acutely decreases genioglossus EMG activity in awake humans. J Appl Physiol. 1992;72:110–5. doi: 10.1152/jappl.1992.72.1.110. [DOI] [PubMed] [Google Scholar]
- 12.White LH, Motwani S, Kasai T, Yumino D, Amirthalingam V, Bradley TD. Effect of rostral fluid shift on pharyngeal resistance in men with and without obstructive sleep apnea. Respir Physiol Neurobiol. 2014;192:17–22. doi: 10.1016/j.resp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Shiota S, Ryan CM, Chiu KL, et al. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. 2007;62:868–72. doi: 10.1136/thx.2006.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman O, Bradley TD, Logan AG. Influence of lower body positive pressure on upper airway cross-sectional area in drug-resistant hypertension. Hypertension. 2013;61:240–5. doi: 10.1161/HYPERTENSIONAHA.112.203547. [DOI] [PubMed] [Google Scholar]
- 15.Kasai T, Motwani SS, Yumino D, et al. Contrasting effects of lower body positive pressure on upper airways resistance and partial pressure of carbon dioxide in men with heart failure and obstructive or central sleep apnea. J Am Coll Cardiol. 2013;61:1157–66. doi: 10.1016/j.jacc.2012.10.055. [DOI] [PubMed] [Google Scholar]
- 16.Beecroft JM, Hoffstein V, Pierratos A, Chan CT, McFarlane P, Hanly PJ. Nocturnal haemodialysis increases pharyngeal size in patients with sleep apnoea and end-stage renal disease. Nephrol Dial Transplant. 2008;23:673–9. doi: 10.1093/ndt/gfm598. [DOI] [PubMed] [Google Scholar]
- 17.Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344:102–7. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
- 18.Elias RM, Bradley TD, Kasai T, Motwani SS, Chan CT. Rostral overnight fluid shift in end-stage renal disease: relationship with obstructive sleep apnea. Nephrol Dial Transplant. 2012;27:1569–73. doi: 10.1093/ndt/gfr605. [DOI] [PubMed] [Google Scholar]
- 19.Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens. 2012;26:281–7. doi: 10.1038/jhh.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147:266–74. doi: 10.1378/chest.14-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi M, Fujimoto K, Urushibata K, Uchikawa S, Imamura H, Kubo K. Nocturnal oxygen desaturation correlates with the severity of coronary atherosclerosis in coronary artery disease. Chest. 2003;124:936–41. doi: 10.1378/chest.124.3.936. [DOI] [PubMed] [Google Scholar]
- 22.Bucca CB, Brussino L, Battisti A, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. 2007;132:440–6. doi: 10.1378/chest.07-0311. [DOI] [PubMed] [Google Scholar]
- 23.Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173:234–7. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 24.Tamura A, Kawano Y, Kadota J. Carvedilol reduces the severity of central sleep apnea in chronic heart failure. Circ J. 2009;73:295–8. doi: 10.1253/circj.cj-08-0678. [DOI] [PubMed] [Google Scholar]
- 25.Mayer J, Weichler U, Herres-Mayer B, Schneider H, Marx U, Peter JH. Influence of metoprolol and cilazapril on blood pressure and on sleep apnea activity. J Cardiovasc Pharmacol. 1990;16:952–61. doi: 10.1097/00005344-199012000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Kantola I, Rauhala E, Erkinjuntti M, Mansury L. Sleep disturbances in hypertension: a double-blind study between isradipine and metoprolol. J Cardiovasc Pharmacol. 1991;18(Suppl 3):S41–5. [PubMed] [Google Scholar]
- 27.Pelttari LH, Hietanen EK, Salo TT, Kataja MJ, Kantola IM. Little effect of ordinary antihypertensive therapy on nocturnal high blood pressure in patients with sleep disordered breathing. Am J Hypertens. 1998;11(3 Pt 1):272–9. doi: 10.1016/s0895-7061(97)00469-x. [DOI] [PubMed] [Google Scholar]
- 28.Bartel PR, Loock M, Becker P, Robinson E, van der Meyden C, Rossouw S. Short-term antihypertensive medication does not exacerbate sleep-disordered breathing in newly diagnosed hypertensive patients. Am J Hypertens. 1997;10:640–5. doi: 10.1016/s0895-7061(96)00507-9. [DOI] [PubMed] [Google Scholar]
- 29.Edwards BA, Sands SA, Eckert DJ, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590(Pt 5):1199–211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue Y, Takata K, Sakamoto I, Hazama H, Kawahara R. Clinical efficacy and indication of acetazolamide treatment on sleep apnea syndrome. Psychiatry Clini Neurosci. 1999;53:321–2. doi: 10.1046/j.1440-1819.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 32.Kasai T, Bradley TD, Friedman O, Logan AG. Effect of intensified diuretic therapy on overnight rostral fluid shift and obstructive sleep apnoea in patients with uncontrolled hypertension. J Hypertens. 2014;32:673–80. doi: 10.1097/HJH.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 33.Planes C, Foucher A, Leroy M, et al. Effect of celiprolol treatment in hypertensive patients with sleep apnea. Sleep. 1999;22:507–13. doi: 10.1093/sleep/22.4.507. [DOI] [PubMed] [Google Scholar]
- 34.Walsh JT, Andrews R, Starling R, Cowley AJ, Johnston ID, Kinnear WJ. Effects of captopril and oxygen on sleep apnoea in patients with mild to moderate congestive cardiac failure. Br Heart J. 1995;73:237–41. doi: 10.1136/hrt.73.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heitmann J, Greulich T, Reinke C, et al. Comparison of the effects of nebivolol and valsartan on BP reduction and sleep apnoea activity in patients with essential hypertension and OSA. Curr Med Res Opin. 2010;26:1925–32. doi: 10.1185/03007995.2010.497326. [DOI] [PubMed] [Google Scholar]
- 36.Gaddam K, Pimenta E, Thomas SJ, et al. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2010;24:532–7. doi: 10.1038/jhh.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88:156–75. doi: 10.2522/ptj.20070147. [DOI] [PubMed] [Google Scholar]
- 38.Kraiczi H, Hedner J, Peker Y, Grote L. Comparison of atenolol, amlodipine, enalapril, hydrochlorothiazide, and losartan for antihypertensive treatment in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1423–8. doi: 10.1164/ajrccm.161.5.9909024. [DOI] [PubMed] [Google Scholar]
- 39.Lyons OD, Bradley TD, Chan CT. Hypervolemia and Sleep Apnea in Kidney Disease. Semin Nephrol. 2015;35:373–82. doi: 10.1016/j.semnephrol.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Chiu KL, Ryan CM, Shiota S, et al. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006;174:1378–83. [Google Scholar]
- 41.Redolfi S, Arnulf I, Pottier M, Bradley TD, Similowski T. Effects of venous compression of the legs on overnight rostral fluid shift and obstructive sleep apnea. Respir Physiol Neurobiol. 2011;175:390–3. doi: 10.1016/j.resp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Jafari B, Mohsenin V. Overnight rostral fluid shift in obstructive sleep apnea: does it affect the severity of sleep-disordered breathing? Chest. 2011;140:991–7. doi: 10.1378/chest.11-0044. [DOI] [PubMed] [Google Scholar]
- 43.Casey KR, Cantillo KO, Brown LK. Sleep-related hypoventilation/hypoxemic syndromes. Chest. 2007;131:1936–48. doi: 10.1378/chest.06-2334. [DOI] [PubMed] [Google Scholar]
- 44.Ogna A, Forni Ogna V, Mihalache A, et al. Obstructive sleep apnea severity and overnight body fluid shift before and after hemodialysis. Clin J Am Soc Nephrol. 2015;10:1002–10. doi: 10.2215/CJN.08760914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang SC, Lam B, Lai AS, et al. Improvement in sleep apnea during nocturnal peritoneal dialysis is associated with reduced airway congestion and better uremic clearance. Clin J Am Soc Nephrol. 2009;4:410–8. doi: 10.2215/CJN.03520708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzaga CC, Gaddam KK, Ahmed MI, et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6:363–8. [PMC free article] [PubMed] [Google Scholar]
- 47.Gaddam KK, Nishizaka MK, Pratt-Ubunama MN, et al. Characterization of resistant hypertension: association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Arch Intern Med. 2008;168:1159–64. doi: 10.1001/archinte.168.11.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shantha GP, Pancholy SB. Effect of renal sympathetic denervation on apneahypopnea index in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2015;19:29–34. doi: 10.1007/s11325-014-0991-z. [DOI] [PubMed] [Google Scholar]
- 49.Marks JD, Harper RM. Differential inhibition of the diaphragm and posterior cricoarytenoid muscles induced by transient hypertension across sleep states in intact cats. Exper Neurol. 1987;95:730–42. doi: 10.1016/0014-4886(87)90312-8. [DOI] [PubMed] [Google Scholar]
- 50.Mayor AH, Schwartz AR, Rowley JA, et al. Effect of blood pressure changes on air flow dynamics in the upper airway of the decerebrate cat. Anesthesiology. 1996;84:128–34. doi: 10.1097/00000542-199601000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Van Lunteren E, Van de Graaff WB, Parker DM, et al. Activity of upper airway muscles during augmented breaths. Respir Physiol. 1983;53:87–98. doi: 10.1016/0034-5687(83)90018-x. [DOI] [PubMed] [Google Scholar]
- 52.Wilson CR, Manchanda S, Crabtree D, Skatrud JB, Dempsey JA. An induced blood pressure rise does not alter upper airway resistance in sleeping humans. J Appl Physiol. 1998;84:269–76. doi: 10.1152/jappl.1998.84.1.269. [DOI] [PubMed] [Google Scholar]
- 53.Unruh ML, Sanders MH, Redline S, et al. Sleep apnea in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the Sleep Heart Health Study. J Am Soc Nephrol. 2006;17:3503–9. doi: 10.1681/ASN.2006060659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.