Introduction
Intraductal papillary mucinous neoplasms (IPMNs) are distinguished from conventional pancreatic ductal adenocarcinoma (PDAC) by their macrocystic appearance, papillary architecture, and mucin secreting epithelium. However, like PDAC and many extra-pancreatic tumors, invasive cancer develops in IPMNs over many years in the context of a dysplasia-to-carcinoma histologic progression.1, 2 Invasive carcinoma may arise within an IPMN (IPMN-associated carcinoma), and is defined as extension of neoplastic cells through the basement membrane of the involved ducts. IPMNs with associated carcinoma are staged like conventional PDAC using the standard American Joint Committee on Cancer (AJCC), 7th ed., criteria for pancreatic cancer.3
IPMNs that harbor foci of invasive carcinoma are at risk for recurrence after resection, in contrast to benign IPMNs which are typically considered curable, although there remains residual risk of additional IPMNs forming in the remnant pancreas in some patients due to a presumed “field defect.”4–7 Although the risk of recurrence has not been completely characterized, the prognosis is generally believed to be more favorable as compared to conventional PDAC because IPMN-associated carcinomas are frequently diagnosed at an earlier stage.8, 9 Roughly one-quarter of IPMN-associated carcinomas are detected and resected at the T1 stage (≤ 20 mm in diameter and confined to the pancreas), and 50% are resected at the T1 or T2 stage (> 20 mm, yet still confined to the pancreas)8. In contrast, 5% of PDACs are resected at the T1 stage, and 15% at the T1 or T2 stage.8, 10 Moreover, some IPMN-associated carcinomas are of the colloid type, which is typically less aggressive than the conventional tubular type carcinoma.9
While IPMN-associated carcinoma and conventional PDAC are considered distinct pathologic entities, it is important to note that they share many clinical, pathologic, and molecular features. For instance, survival after resection of IPMN-associated carcinoma and conventional PDAC are actually similar after adjusting for pathologic features and tumor stage.8, 9 In addition, the most common subtype of IPMN-associated carcinoma (the tubular or ductal subtype) is indistinguishable from PDAC microscopically.11 At the molecular level, KRAS mutations are a common feature of both tubular type IPMN-associated carcinoma and conventional pancreatic ductal adenocarcinoma,12, 13 although colloid type invasive carcinomas more commonly contain GNAS mutations (personal communication, PJA). Finally, familial pancreatic cancer syndromes (e.g., Peutz-Jeghers and Hereditary Breast and Ovarian Cancer syndromes) carry an increased risk for developing both PDAC and IPMN-associated carcinoma, suggesting a common molecular mechanism for tumor initiation.14–17 Although IPMN-associated carcinoma and conventional PDAC are clearly separate pathologic entities, it stands to reason that small IPMN-associated carcinomas represent perhaps the best available opportunity to study the biology of early pancreatic cancer.
Beyond biologic insights, a focused analysis of clinical and pathologic outcomes related to small IPMN-associated carcinoma has a more practical purpose. Currently, decisions related to adjuvant therapy of small IPMN-associated carcinoma are rendered in the absence of any reliable data on recurrence risk and long-term survival. Treatment recommendations may be based on experiences with localized and resected conventional ductal adenocarcinoma (PDAC) with a higher T-stage that are not truly generalizable to this cohort 18–20 or even outcome data of early non-pancreatic cancers. For instance, other common cancer types (e.g., breast, lung) are typically cured by resection alone when the lesions are under 10 mm in size, and experience with these diseases may perhaps bias oncologists against adjuvant therapy in the setting of small IPMN-associated carcinoma.21, 22 Studies that specifically profile the clinical outcomes of patients with small IPMN-associated carcinoma may therefore inform oncologists on the utility of adjuvant therapy for this diagnosis.
The present study was designed to better understand the clinical and pathologic outcomes in patients with IPMN-associated carcinoma containing foci of invasion ≤ 20 mm. Data were collected from four high volume pancreatic cancer programs, and all included cases were re-reviewed by expert pancreatic cancer pathologists.
Methods
Patients
This study was approved by the institutional review boards at the participating centers. Pancreatic surgery databases were queried for all resected IPMN (n=1122) at Thomas Jefferson University (between years 2006–2012, 186 resected IPMN), Massachusetts General Hospital (1993–2012, 434 resected IPMN), Memorial Sloan Kettering Cancer Center (1998–2012, 289 resected IPMN), and the University of Pennsylvania (2007–2013, n=213). From this cohort, IPMN-associated carcinomas were identified from each institution: 36, 110, 96, and 38, respectively (n=280, 25% of all resected IPMN). Based on pathology reports and data recorded in institutional databases, the cohort was further refined to include IPMN with invasive foci ≤ 20 mm in diameter (small IPMN-associated carcinoma). These included both T1 and T3 lesions according to AJCC staging criteria 3. The size threshold allowed for unambiguous inclusion criteria and was selected because of the criteria used in current TNM staging. No T2 lesions were included since these invasive lesions exceed 20 mm, and T3 lesions were included when size criteria were satisfied but the cancer extended beyond the pancreas. Multifocal lesions were categorized according to the largest focus. The size of the benign (or non-invasive) cystic component was recorded, but was not a criterion for study inclusion.
Data collection and pathology review
Hematoxylin and eosin stained slides from each of the identified IPMN-associated carcinomas were collected and retrospectively reviewed at each institution by an expert pancreatic cancer pathologist (W.J., O.B, M.M.K., E.E.F.). Cases were excluded after pathologic review if the findings were not conclusive for an IPMN, no invasive component was noted, or invasive foci measured >20 mm. Challenging cases were reviewed under a multi-headed microscopic and a consensus reached by at least two pancreatic pathologists from separate participating sites. Specific pathologic features and definitions used in this study are provided in Table 1.
Table 1.
Recorded pathologic features
| Pathologic feature | Categories and definitions |
|---|---|
| Non-invasive component | |
| Size of total IPMN lesion | Size in mm |
| Epithelial subtype of IPMN* | Intestinal, pancreatobiliary, gastric-foveolar, oncocytic |
| Type of duct involvement | Side-branch or main duct involvement (main duct includes mixed side-branch/main duct)** |
| Invasive component | |
| Size of invasive component (mm) | Vertical microscopic distance in mm between the deepest focus of invasion and the involved duct; ≤ 20 mm was inclusion criteria |
| Histology of the invasive component | Tubular (ductal), colloid (mucinous), oncocytic, signet ring (>50% cancer cells with signet ring features), or mixed*** |
| Multifocality of invasive component | Yes or no; size was based on the diameter of the largest focus |
| Growth pattern of the invasive component | Infiltrative (neoplastic cells/glands embedded within desmoplastic stroma) or pushing/expansile (passive growth related to ductal dilitation from intraductal hypertension or intraductal spread of cancer cells) |
| T-stage3 | T1, T2, T3, T4 |
| Grade | Well or moderate or poorly differentiated |
| Resected lymph nodes | Total number |
| Lymph node metastases | Total number |
| Microscopic vascular invasion | Yes or no |
| Microscopic lymphatic invasion | Yes or no |
| Microscopic perineural invasion | Yes or no |
| Resected pancreatic margin status | For dysplasia, this refers specifically to the transected margin, while for invasive cancer this may include any recorded margin, including the bile duct, duodenal, uncinate or posterior margins. The degree of dysplasia was categorized as low-, intermediate-, or high- grade; dysplasia type included IPMN or PanIN. The conventional designation of R-status is only applicable to IPMNs without or with invasive disease at the resection margin (R0 or R1, respectively) |
In the case of IPMN exhibiting heterogeneous epithelium, histology was recorded as mixed.
In the case of pure main duct IPMN, cyst size was measured grossly based on the diameter of the main pancreatic duct.
When more than one histologic subtype was present, the histology of the invasive component was recorded as mixed.
Clinical data were captured from institutional databases and patient records, and included gender, age, adjuvant chemotherapy, adjuvant radiation therapy, time to first recurrence, recurrence pattern and overall survival. Recurrences were defined as convincing clinical or radiographic evidence of disease. A tissue diagnosis was not required. Sites of recurrence are detailed, and different sites are counted separately when multifocal recurrences were observed. The type of recurrence was categorized as a local-only recurrence (retroperitoneal or surgical bed), distant-only recurrence, or both local and distant recurrence. Attempts were made to capture all cancer recurrences. Records were thoroughly reviewed in an attempt to distinguish true recurrences from metachronous pancreatic cancer whenever possible. Phone calls to patients and families were also utilized to obtain follow-up data in some instances.
Statistical analysis
Categorical variables were analyzed with a chi-squared test or logistic regression, while comparisons of continuous outcome variables were made using the Mann-Whitney rank sum test. Multivariate logistic regression including statistically significant univariate predictors was used to assess the effects of independent variables on outcomes. Estimates of central tendency are reported as median values. The log rank test and Cox proportional hazards model were used for survival analyses. Statistical significance was accepted for a p-value < 0.05. Statistics were performed using Stata, version 12.0 (College Station, TX).
Results
Demographic and perioperative data
A total of 70 small IPMN-associated carcinomas (≤ 20 mm invasive component) were identified after pathologic review, which comprised 6.2% of all resected IPMN and 25.0% of all resected IPMN-associated carcinoma at the four participating institutions. Clinical data are summarized in Table 2. The operations included 42 (60.0%) pancreaticoduodenectomies, 14 (20.0%) distal pancreatectomies, 11 (15.7%) total pancreatectomies, and 3 (4.3%) middle pancreatectomies. The patients were evenly split between genders, and the median age was 69 years (range, 47 to 91). Preoperative CA 19-9 levels were measured in 49 patients, and 13 (26.5%) had elevated values (>37 U/mL). There were six patients (8.6%) with CA19-9 levels above 100 U/mL, and one patient had a value above 1000 U/mL (1057 U/mL). These patients all had lesions in the head of the pancreas, and several were believed to have had elevated serum levels due to biliary obstruction.
Table 2.
Demographic and perioperative data
| Small IPMN-associated carcinoma, n=70 | |
|---|---|
| Institution | |
| MGH (1993–2012) | 28 |
| MSKCC (1998–2012) | 21 |
| TJU (2006–2012) | 15 |
| U Penn (2007–2013) | 6 |
| Procedure and demographics | |
| Operation, n (%) | |
| Pancreaticoduodenectomy | 42 (60.0) |
| Distal pancreatectomy | 14 (20.0) |
| Total pancreatectomy | 11 (15.7) |
| Middle pancreatectomy | 3 (4.3) |
| Gender, n (%) | |
| Male | 35 (50.0) |
| Female | 35 (50.0) |
| Age (years), median (range) | 69 (47–91) |
MGH (Massachusetts General Hospital); MSKCC (Memorial Sloan-Kettering Cancer Center); TJU (Thomas Jefferson University); U Penn (University of Pennsylvania)
Pathologic data
The macroscopic size of the entire lesion measured at resection (including the invasive and intraductal components), ranged from 5 to 150 mm, with a median IPMN size of 30 mm (pathologic data for the cohort are summarized in Table 3). Nearly all IPMNs involved the main pancreatic duct (n=67, 95.7%), while only 3 (4.3%) were entirely limited to a side- branch duct. The distribution of non-IPMN-associated carcinoma epithelial subtypes was as follows: 32 (45.7%) intestinal, 14 (20.0%) gastric-foveolar, 14 (20.0%) pancreatobiliary, 6 (8.6%) oncocytic, and 4 (5.7%) mixed (Supplemental Figure 1).
Table 3.
Pathologic data
| Small IPMN-associated carcinoma, n=70 | |
|---|---|
| Non-invasive component | |
| * IPMN lesion size (mm), median (range) | 30 (5–150) |
| Epithelial subtype, n (%) | |
| Intestinal | 32 (45.7) |
| Gastric/foveolar | 14 (20.0) |
| Pancreatobiliary | 14 (20.0) |
| Oncocytic | 6 (8.6) |
| Mixed | 4 (5.7) |
| Duct involvement | |
| Main duct | 67 (95.7) |
| Side-branch | 3 (4.3) |
| Invasive component | |
| Size of invasive component (mm), median, range | 5 (0.5–20) |
| Size distribution, categorized by diameter, n (%) | |
| 0 to 4 mm | 35 (50.0) |
| 5 to 9 mm | 16 (22.9) |
| 10 to 14 mm | 7 (10.0) |
| 15 to 19 mm | 12 (17.1) |
| Histology of invasive component, n (%) | |
| Tubular (ductal) | 40 (57.1) |
| Colloid (mucinous) | 20 (28.6) |
| Oncocytic | 7 (10.0) |
| Signet ring | 1 (1.4) |
| Mixed | 2 (2.9) |
| Multiple invasive foci, n (%) | 46 (65.7) |
| Growth pattern, n (%) | |
| Infiltrative | 48 (68.6) |
| Pushing/expansile | 22 (31.6) |
| Lymph node metastases, n (%) | 13 (18.6) |
| T-stage | |
| T1 | 54 (77.1) |
| T3 | 16 (22.9) |
| Microscopic vessel invasion, n (%) | 2 (2.9) |
| Microscopic lymphatic invasion, n (%) | 10 (14.3) |
| Microscopic perineural invasion, n (%) | 14 (20.0) |
| Grade | |
| Well | 22 (31.9) |
| Moderate | 39 (56.5) |
| Poor | 8 (11.6) |
| Unknown | 1 (1.4) |
| Neoplastic cells at resection margin, n (%) | 17 (24.3) |
| Margin details, n (%) | |
| Type of neoplastic cells | |
| PanIN | 6 (8.6) |
| IPMN | 8 (11.4) |
| Invasive cancer | 3 (4.3) |
| Degree of dysplasia | |
| Low-grade dysplasia | 2 (2.9) |
| Intermediate-grade dysplasia | 3 (4.3) |
| High-grade dysplasia | 9 (12.9) |
| Invasive cancer | 3 (4.3) |
the entire IPMN, including invasive and non-invasive components
The size of the invasive component was ≤ 20 mm for all cases in the study based on inclusion criteria, but less than 10 mm in 73% of patients; the median size of the invasive component in the series was 5 mm (range, 0.5 mm to 20 mm). The cohort was sub-categorized by size of the invasive component at 5 mm intervals (0 to 4 mm, 5 to 9 mm, 10 to 14 mm, and 15 to 20 mm) with the following distribution: 35 (50.0%), 16 (22.9%), 7 (10.0%) and 12 (17.1%), respectively. Histologic subtypes of invasive cancer included tubular (n=40, 57.1%), colloid (n=20, 28.6%), oncocytic (n=7, 10.0%), signet ring (n=1, 1.4%), and mixed (n=2, 2.9%)(Figure 1). Multiple foci of invasive disease were observed in two-thirds of the cohort.
Figure 1.
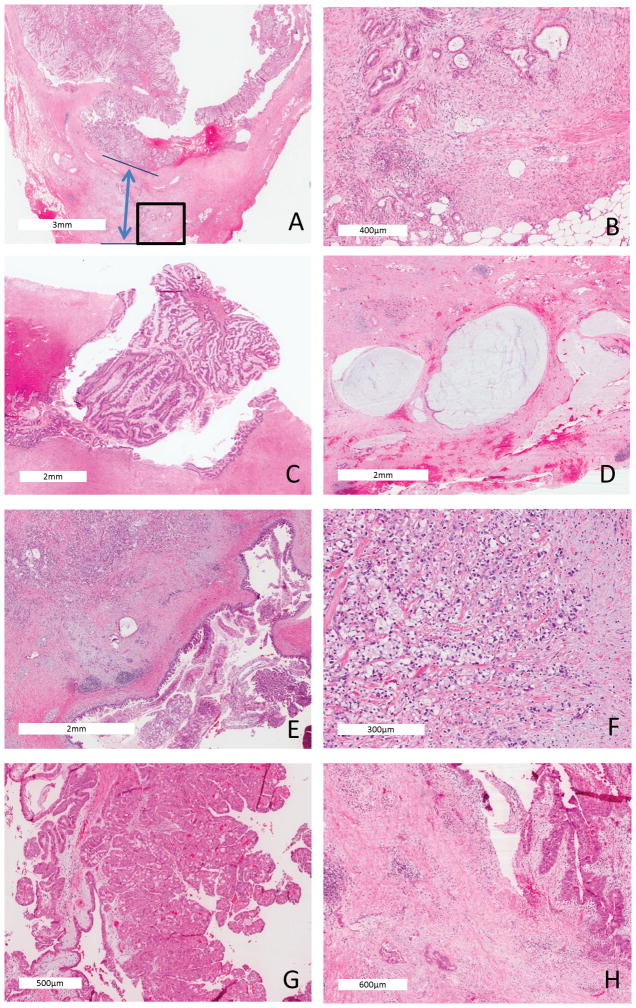
Small IPMN-associated carcinoma (less than 2 cm invasive component) (hematoxylin and eosin stained). (A) Tubular adenocarcinoma (20X, similar to conventional ductal adenocarcinoma with a fibrotic stroma) arising in a gastric-foveolar IPMN (the double arrow demonstrates the distance from non-IPMN-associated carcinoma, top line, to the deepest aspect of invasion, lower line); (B) Higher power (100X) of the invasive focus bounded by the box in (A); (C) Intestinal type IPMN (40X) giving rise to (D) invasive colloid carcinoma (40X), characterized by extensive mucin pools and floating neoplastic epithelia within the mucin; (E) Gastric-foveolar type IPMN (40X) giving rise to (F) signet ring adenocarcinoma (200X); (G) Oncocytic type IPMN (100X) (G) giving rise to (H) an oncocytic type carcinoma (100X).
Regional lymph node metastases were identified in 13 (18.8%) specimens. Five patients had regional lymph node metastases identified in more than 1 node (2 positive nodes in one patient, 4 positive nodes in two patients, and 5 positive nodes in two patients). Microscopic vessel invasion was observed in just two cancers (2.9%), microscopic lymphatic invasion in 10 patients (14.3%), and microscopic perineural invasion in 14 patients (19.0%). The invasive component was well differentiated in 22 patients (31.9%), moderately differentiated in 39 (56.5%), and poorly differentiated in 8 patients (11.6%).
Neoplastic cells were observed at a resection margin in 17 (24.3%) cases. Specifically, the findings were noted to be PanIN lesions at the margin in 6 (8.6%) patients, IPMN in 8 patients (11.4%), and invasive cancer in 3 patients (4.3%). The degree of dysplasia (PanIN and IPMN lesions grouped together) at the margin was low grade in 2 patients (2.9%), intermediate grade in 3 patients (4.3%), and high grade in 9 patients (12.9%).
Recurrence and survival
Recurrent cancer was identified in 17 patients (24.3%), and sites of recurrence included the retroperitoneum (including the surgical bed and/or pancreatic remnant), peritoneum, liver, lung, and spleen (Table 4). There were four patients who recurred in the remnant pancreas, raising the possibility that the presumed recurrence was a metachonous (i.e., separate primary) pancreatic cancer in these cases. Based on careful review of patient records, one of these cases was particularly suspicious for a second primary (as opposed to a true intraductal recurrence of the original cancer), since the disease free interval was more than 10 years, and the original IPMN-associated carcinoma was a colloid carcinoma without lymph node spread (i.e., presumably a low-risk lesion). In contrast, the other three patients experienced early recurrences (7, 12 and 14 months), and had high risk IPMN-associated carcinomas found within the original pancreatic resection specimen. The first patient had perineural invasion and a single regional lymph node metastasis at first resection, the second patient had a poorly differentiated invasive focus with perineural and lymphatic invasion, and the third patient had carcinoma in situ at the resection margin.
Table 4.
Recurrence, survival and adjuvant therapy data
| Small IPMN-associated carcinoma n=70 | |
|---|---|
| Recurrence, n (%) | 17 (24.3) |
| Time to recurrence, months, median (range) | 16.2 (4 to 132) |
| Sites of recurrence*, n (%)† | |
| Retroperitoneum | 9 (52.9) |
| Peritoneal (peritoneal surface or mesentery) | 6 (35.2) |
| Liver | 4 (23.4) |
| Lung | 3 (17.6) |
| Type of recurrence, n(%)* | |
| Local | 6 (35.2) |
| Distant | 8 (47.1) |
| Both distant and local | 3 (17.7) |
| Overall survival | |
| Median, months | 99 |
| 1-year, % | 90 |
| 5-year,% | 59 |
| Adjuvant chemotherapy | 15 (21.4) |
| Adjuvant radiation | 11 (15.7) |
the provided percentages are in reference to patients who had a recurrence
percentages add up to greater than 100%, as some patients recurred at multiple sites
Approximately one-third of the patients who had a recurrence experienced a local recurrence only, while two-thirds experienced a distant recurrence (either in isolation or in association with a local recurrence). In this small cohort, there was no specific pathologic feature that associated with a particular recurrence pattern. The median time to recurrence was 16.2 months (range 4 to 132) in the cohort. The time of first recurrence was greater than four years following resection in 6 patients (approximately 1/3 of recurrences).
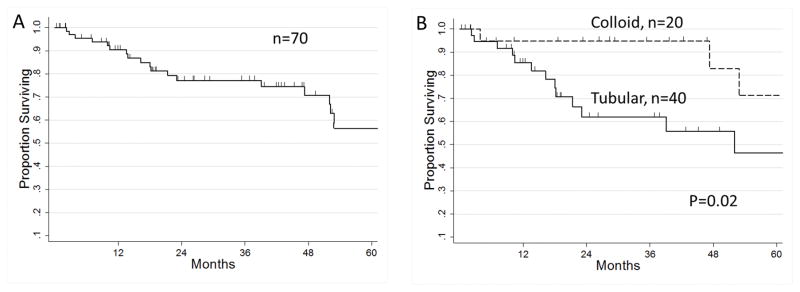
The overall median follow-up in the entire cohort was 26.2 months (range 0.2–179 months). There were 22 deaths (31.4%) during the study period, while 48 (68.6%) patients were still alive at last follow-up. Among the living patients, the median length of follow-up was 28.7 months. For much of the living cohort, the follow-up was short; overall survival was censored within 10 months in 12.9% of this subgroup, and within five months in 8.6%. The median estimated survival in the whole cohort of small IPMN-associated carcinomas was 99 months, the 1-year-survival was 90%, and the 5-year survival was 59% (Figure 2A). In a subgroup analysis of the two most common adenocarcinoma subtypes, patients with colloid carcinoma had superior overall survival to those with tubular adenocarcinoma (Figure 2B, 2 year survival 95% and 62% respectively, p=0.02). Adjuvant chemotherapy was given to a minority of patients (n=15, 21.4%), with 11 of these patients additionally receiving chemoradiation (15.7% of the cohort).
Figure 2.
Kaplan-Meier survival curves. (A) Overall survival of all patients with small IPMN invasive (n=70). Median survival= 99 months, 1-year survival 90%, 2-year survival 77%, and the 5-year survival 59%. (B) Overall survival of patients with small IPMN-associated carcinoma, comparing the two most common histologic subtypes: tubular (n=40, 2-year survival 62%) and colloid (n=20, 2-year survival 95%, p=0.02).
Risk factors for recurrence and survival
Clinicopathologic features were analyzed to identify risk factors for cancer recurrence. The univariate analysis is presented in Supplemental Table 1. The presence of regional lymph node metastases (53.9% recurrence rate vs. 17.5% recurrence rate in the absence of nodal metastases, chi-square p=0.006), microscopic lymphatic invasion (50.0% vs. 20.0%, p=0.04), and T3-stage (50.0% vs. 16.7%, p=0.006) were statistically significant univariate risk factors for recurrence. Notably, adjuvant chemotherapy use was not associated with recurrence risk (40.0% recurrence with chemotherapy vs. 20.0% without chemotherapy, p=0.11). There were no predictors of recurrence in a multivariate logistic regression model including these univariate risk factors (positive nodes, T3-stage, lymphatic invasion). However, this is very likely related to the strong overlapping variance in this small dataset among the independent significant univariate variables. Both T3-stage (7.4% T1-stage cancers had regional lymph node metastases vs. 56.3% T3-stage) and lymphatic invasion (10.0% without lymphatic invasion vs. 70.0% with lymphatic invasion, p<0.001) were highly associated with regional lymph node metastases.
Recurrence risk was analyzed in greater detail across various subgroups to determine if clusters of pathologic features may be identified with an especially low recurrence risk, such that patients meeting specified criteria may reliably be considered to be cured by resection. As indicated above, size was not predictive of recurrence in this cohort. IPMN-associated carcinoma with invasive foci < 5 mm in fact had a statistically similar recurrence rate (17.1%, 6 out of 35) to larger invasive lesions (31.4%, p=0.2, Table 5). Regional lymph node involvement was also observed in the smallest cancers (0–4 mm diameter, 3 out of 35, 8.6%), providing further evidence of the metastatic potential of early IPMN-associated carcinoma. Notably, the rate of lymph node spread was directly related to invasive component size (linear regression, slope coefficient 10.9, p=0.002). Importantly, even the invasive foci in the smallest size range (foci under 5 mm) and without apparent lymph node spread still carried a substantial recurrence risk (12.4%, 4 of 32). Colloid carcinomas represent a particularly favorable subtype of IPMN-associated carcinoma 9, and in the present series, none of the cases in this subgroup (n=20) exhibited regional lymph node spread. Two recurrences were noted in this subgroup (10%, 2 out of 20), although in one instance, the recurrence occurred after a very long disease free interval (10 years, previously highlighted) and most likely represented a metachronous PDA primary, as already mentioned.
Table 5.
Risk of recurrence and specimen lymph node involvement based on size of invasive component (n=70)
| Invasive component size | No recurrence | Recurrence | No lymph node metastases | Lymph node metastases |
|---|---|---|---|---|
| 0 to 4 mm | 29 (82.9%) | 6 (17.1%) | 32 (91.4) | 3 (8.6) |
| 5 to 9 mm | 12 (75.0%) | 4 (25.0%) | 13 (81.3) | 3 (18.8) |
| 10 to 14 mm | 3 (42.9%) | 4 (57.1%) | 5 (71.4) | 2 (28.6) |
| 15 to 19 mm | 9 (75.0%) | 3 (25.0%) | 7 (58.3) | 5 (41.7) |
| Total | 53 (75.7%) | 17 (24.3%) | 57 (81.4) | 13 (18.6) |
| P=0.2, chi-square | P=0.002, linear regression |
In the absence of adverse univariate risk factors for recurrence (regional lymph node metastases, lymphatic invasion, T3-stage), IPMN-associated carcinomas still had a recurrence rate of 12.5% (6 of 42). Tubular carcinoma with this cluster of pathologic features had an 8.3% recurrence risk (2 of 22), while colloid carcinoma meeting these criteria had a recurrence rate of 5.9% (1 of 16). Again, the single recurrence in the latter group occurred in the previously described patient with a presumed metachronous primary lesion, suggesting that this group of patients may indeed have a uniquely favorable biology.
Pathologic features were also evaluated as predictors of overall survival. In univariate analyses, tubular adenocarcinoma (vs. colloid carcinoma, p=0.02), intermediate-grade dysplasia (or greater) at the transected pancreatic margin (p=0.004), and T3-stage (0.03) were associated with a poor outcome. In a multivariate Cox proportional hazards model including these three variables, tubular adenocarcinoma (hazards ratio=3.7, p=0.04) remained statistically significant. In summary, lymphatic spread of disease and T3-stage were predictive of recurrence, while tubular subtype (vs., colloid) was most predictive of worse overall survival.
Discussion
In the modern era of high-resolution cross-sectional imaging, IPMNs are encountered with increased frequency and are often asymptomatic. A study of more than 2000 CT scans performed in the general population for a multitude of indications identified asymptomatic pancreatic cysts in 2% of cases, and a large number of these lesions were likely IPMNs.23 Most of these incidentally discovered IPMNs are indolent, and unlikely to be clinically relevant during an individual’s lifetime. However, a minority will grow and transform over time into lethal cancers if left untreated. Recent surgical series suggest that 5% to 10% of pancreatic resections at high-volume centers are now performed for IPMNs.20 There has been a strong focus in the surgical literature towards understanding which IPMNs are at highest risk for malignant transformation and should be resected at a curable stage. International Consensus Guidelines have been recently published on this subject,24, 25 and suggest that IPMNs be resected if the cyst causes symptoms, contains mural nodules, or involves the main pancreatic duct, after considering the age and performance status of the patient.24 IPMNs that measure over 3 cm on cross-sectional imaging without these high-risk stigmata may also be considered for resection, particularly in younger patients.24
In light of the growing number of detected IPMNs, high volume centers are encountering a growing number of lesions that have small foci of invasive cancer. Importantly, these early IPMN-associated carcinomas provide a unique lens into the natural history and biology of pancreatic cancer. To our knowledge, outcome studies that focus on small IPMN-associated carcinomas have been limited to a handful of small studies from East Asia.26–28 In one study by Nara et al., 104 cases of resected IPMNs were analyzed over a 20 year period.27 There were 51 IPMN-associated carcinomas that were sub-grouped into four pathologic categories: 1) “infiltrative growth,” 2) “mucous rupture”, 3) “expansile growth”, and 4) “intra-abdominal rupture.” Criteria for ‘minimal invasion’ were separately applied to each of the four phenotypes, and size of the invasive component (≤ 5mm) was only considered for the “infiltrative growth” subtype. Of note, definitions were complex and some categories (mucous rupture and expansile growth) are not generally accepted as representing true invasion.29 In the overall group of 26 ‘minimally invasive’ IPMN-associated carcinomas, just 2 (7.7%) recurrences were reported. None of the minimally invasive IPMN-associated carcinomas spread to regional lymph nodes in the study, and the 10-year disease-specific survival in these patients was 100%. When size was used to stratify patients, 23 IPMN-associated carcinomas harbored an invasive component under 20 mm; there were 5 recurrences in this group, yielding a comparable recurrence rate to the findings reported herein. A South Korean study using the aforementioned Japanese criteria for minimal invasion reported 2 recurrences out of 24 cases (8.3%),28 while a separate Japanese study identified two recurrences in five patients with minimally invasive IPMN-associated carcinoma.26
The current multi-institutional study of 70 patients with small IPMN-associated carcinoma represents the largest study on the subject to date. The cohort was almost entirely comprised of IPMN involving the main duct on pathologic review, suggesting that small IPMN-associated carcinomas that exclusively involve side branch ducts are extremely rare. The data reveal a recurrence rate after resection for small IPMN-associated carcinoma (≤ 20 mm invasive component) of 24.3%, at a median follow-up of just over two years (26.1 months). Regional lymph node metastases, microscopic lymphatic invasion, and T3-stage were identified as unadjusted predictors of recurrence. Importantly, tubular-type carcinomas were still at risk for recurrence, even when these three adverse features were absent. In contrast, the findings from this study suggest that small colloid carcinomas (≤ 20 mm invasive component) lacking these three risk factors are likely to be cured by resection. Due to strong interdependence of the three univariate predictor variables of recurrence, none of the univariate risk factors retained statistical significance in the multivariate regression model.
It is worth highlighting a counter-intuitive (statistically insignificant) trend towards increased recurrence observed with adjuvant chemotherapy use, which was likely attributable to the selection bias of high risk cancers in the chemotherapy group. For instance, patients undergoing adjuvant chemotherapy had a higher rate of lymph node metastases (46.7 vs. 10.9%, p=0.002). Finally, tubular adenocarcinoma subtype was the single adverse prognostic factor for survival in a multivariate Cox model. This adverse risk factor failed to predict recurrence (p=0.12), possibly related to the underpowered nature of the small sample size.
There are several unique aspects to this study worth highlighting. First, data from four separate high volume institutions were collated in order to generate a modest sample size necessary for meaningful analyses. In addition, inclusion of patients from multiple datasets reduces potential institutional biases present in many published series (such as referral patterns, quality of follow-up in institutional databases, etc.). Importantly, recurrence rates at the four participating centers were consistent, and ranged between 20 and 33% (p=0.9). Every case included in the present analysis was reexamined by an expert pancreatic cancer pathologist. All challenging cases from each site were reviewed by at least one additional pancreatic pathologist from a separate participating institution, and a consensus was reached between pathologists regarding the pathologic features in question. The active of involvement of pancreatic pathologists from each site is a particular strength of the study. Numerous conversations and emails were performed by pathologists to ensure the integrity and consistency of the pathologic review and data analysis.
The relative small sample size and retrospective aspect of this study are clear limitations that should be considered when interpreting these data. However, it is likely that the retrospective nature of the data collection may in fact bias the results towards an underestimation of the true recurrence rate for small IPMN-associated carcinoma. There are several lines of evidence that support this interpretation. First, the long-term follow-up in the study was incomplete. The median time to recurrence in the cohort was 16 months, yet 25% of recurrences occurred beyond 5 years. The median follow up period for living patients was just over 2 years in the study, suggesting that roughly 1/3 of recurrences have yet to manifest clinically or are unknown to the study nvestigators. Second, there was uncertainty regarding the cause of death for many of the patients in the study. A total of 22 deaths were recorded (31.9% of the cohort), yet only 9 deaths (40.9% of deaths) occurred in patients with documented disease recurrence. It is quite conceivable, and in fact probable, that at least some of the remaining deaths (13 of 22, 59.1%) are attributable to cancer recurrence, unbeknownst to the study investigators.
An additional limitation of this retrospective study relates to the challenge of distinguishing true recurrences from metachronous pancreatic cancer (i.e., a second independent pancreatic cancer). With the exception of one instance highlighted above (a “recurrence” 10 years after a resection of a 5 mm colloid carcinoma), all of the recurrences in the present series were presumed to represent true recurrences after a rigorous review of electronic records. Nevertheless, we cannot definitively make this claim without genomic sequencing and genotyping respective tumors (i.e., comparing the molecular profile of the recurrence to the corresponding resected primary 2, 30). We therefore did not exclude cases from this study when the distinction was unclear. The prior literature provides some support that questionable cases, particularly ones that involve the pancreatic remnant, more likely represent true intraductal recurrences than second primary cancers. For instance, the incidence of PDAC after resection of benign IPMN disease, or in patients with low-risk IPMN followed by surveillance imaging, is reportedly much lower (4%) than the recurrence rate observed in this study (24%).4–6 Additionally, metachronous PDAC in patients with benign IPMN disease typically occurs after a longer disease-free interval than the time-to-recurrence reported in this study. Only 15% of metachronous PDAC occur by 15 months as reported in the literature,4, 5 while half of the patients in this study recurred by that same time point (suggesting invasive cancer recurrence). Other limitations of the study include: difficulty in determining which factors led surgeons’ to recommend resection, an unclear understanding of why patients did not receive adjuvant therapy, and a lack of understanding of the natural history of unresected small IPMN-associated carcinoma.
The very real and substantial recurrence risk of small IPMN-associated carcinoma runs counter to experiences with other common solid tumors, and may reflect intrinsic biologic differences between pancreatic adenocarcinoma and these other solid tumor types. For instance, breast cancers under 2 cm have a relative 5-year survival rate in excess of 95% (as compared to 59% in the present cohort).22 Similar findings have been reported for non-small cell lung cancer; 21 in the absence of lymph node metastases, resection of these small, non-pancreatic cancers is typically curative.21, 31 While the recurrence rate reported in the present study may be surprising in the context of these other tumor types, the data are actually consistent with recent molecular and pre-clinical studies of pancreatic cancer. Mounting evidence suggests that malignant pancreatic cells disseminate at a very early stage. Yachida et al. performed genomic sequencing in paired primary and metastatic pancreatic ductal adenocarcinoma from seven individuals enrolled in a rapid autopsy program.2 Using a ‘molecular clock’ concept based on passenger mutation rates, the authors determined that metastatic subclones emerge in primary pancreatic tumors an average of 2.7 years (or 31 months) prior to the death of the patient. Since patients with localized and resectable PDAC typically present clinically 18 months prior to this terminal outcome (i.e., the median survival after resection),20 one can logically infer that micrometastases frequently predate a patient’s operative resection date. This inference is supported by clinical experience, since more than 90% of patients with PDAC ultimately recur after a complete resection for PDAC.20 Considering a typical cancer doubling time of roughly 3 months,32 one can even extrapolate backwards from these data to estimate the size of an invasive lesion capable of metastatic spread. Roughly 3 to 4 doubling times occur over the 13 months that separate the calculated emergence of metastatic clones and resection of a 3 cm PDAC (the median size at resection).20 A “back of the envelope” calculation suggests then that metastases occur in patients harboring invasive carcinoma foci measuring just 2 to 4 mm in size.
In an experimental mouse study, Rhim et al. used a sophisticated cell lineage-labeling transgenic mouse to track pancreatic cancer cells geographically.33 In this mouse model, all pre-invasive pancreas cells containing mutant KRAS and TP53 genes expressed a gene encoding a fluorescent reporter protein, enabling investigators to trace pre-malignant cells thoughout the animal. Fluorescently labeled cells were detected in the blood and liver tissue in mice that only harbored noninvasive PanIN lesions in their pancreas by histologic review. In this model, cells considered as ‘non-invasive’ actually demonstrated metastatic potential, suggesting that conventional histologic classification schemes are inconsistent with disease biology in this context.
Conclusions
This study revealed a recurrence risk of 24.3% for resected small IPMN-associated carcinoma (≤ 20 mm invasive component), at a median follow-up interval of 26.2 months. Due to incomplete follow-up and unknown causes of death in several patients, the true recurrence rate of these early invasive lesions may in fact be higher, which has important implications on the post-operative management and surveillance of patients with small IPMN-associated carcinoma. In light of the real and substantial recurrence risk observed in this cohort of patients, adjuvant chemotherapy (with or without radiation) should be considered, and may be the preferred approach in younger patients with a good performance status, particularly to treat the tubular (ductal) subtype. These patients should be followed post-operatively with close surveillance plans, similar to patients with resected conventional PDAC (e.g., imaging, physical exam, and serum tumor markers every 3 to 6 months for two years, then annually or semiannually).34 These data also suggest that effective early detection strategies for pancreatic cancer must achieve detection capabilities that are superior to available technologies in order to identify invasive lesions in the sub-centimeter range, prior to systemic dissemination. Finally, for IPMN-associated carcinomas, the size of the invasive component should be indicated on pathology reports separately from the overall cyst size when possible, since tumor staging is based on the extent of invasive disease.
Supplementary Material
Footnotes
An earlier version of these data was presented at the Annual Meeting of the Pancreas Club, Chicago, IL, May 3, 2014
References
- 1.Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol. 2008;1:306–16. [PMC free article] [PubMed] [Google Scholar]
- 2.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edge SE, Byrd DR. AJCC Cancer Staging Manual. 7. New York: Springer; 2009. [Google Scholar]
- 4.He J, Cameron JL, Ahuja N, et al. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J Am Coll Surg. 2013;216:657–65. doi: 10.1016/j.jamcollsurg.2012.12.026. discussion 665–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafemina J, Katabi N, Klimstra D, et al. Malignant progression in IPMN: a cohort analysis of patients initially selected for resection or observation. Ann Surg Oncol. 2013;20:440–7. doi: 10.1245/s10434-012-2702-y. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsuka T, Kono H, Tanabe R, et al. Follow-up study after resection of intraductal papillary mucinous neoplasm of the pancreas; special references to the multifocal lesions and development of ductal carcinoma in the remnant pancreas. Am J Surg. 2012;204:44–8. doi: 10.1016/j.amjsurg.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Shi C, Hruban RH. Intraductal papillary mucinous neoplasm. Hum Pathol. 2012;43:1–16. doi: 10.1016/j.humpath.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470–6. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yopp AC, Katabi N, Janakos M, et al. Invasive carcinoma arising in intraductal papillary mucinous neoplasms of the pancreas: a matched control study with conventional pancreatic ductal adenocarcinoma. Ann Surg. 2011;253:968–74. doi: 10.1097/SLA.0b013e318214bcb4. [DOI] [PubMed] [Google Scholar]
- 10.House MG, Gonen M, Jarnagin WR, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–55. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 11.Mino-Kenudson M, Fernandez-del Castillo C, Baba Y, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut. 2011;60:1712–20. doi: 10.1136/gut.2010.232272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011;108:21188–93. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su GH, Hruban RH, Bansal RK, et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154:1835–40. doi: 10.1016/S0002-9440(10)65440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato N, Rosty C, Jansen M, et al. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001;159:2017–22. doi: 10.1016/S0002-9440(10)63053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 17.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. quiz e14–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–81. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 19.Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–26. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210–1. [DOI] [PubMed] [Google Scholar]
- 21.Lee PC, Korst RJ, Port JL, et al. Long-term survival and recurrence in patients with resected non-small cell lung cancer 1 cm or less in size. J Thorac Cardiovasc Surg. 2006;132:1382–9. doi: 10.1016/j.jtcvs.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 22.Elkin EB, Hudis C, Begg CB, et al. The effect of changes in tumor size on breast carcinoma survival in the U.S: 1975–1999. Cancer. 2005;104:1149–57. doi: 10.1002/cncr.21285. [DOI] [PubMed] [Google Scholar]
- 23.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–7. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–9. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 26.Nakagohri T, Asano T, Kenmochi T, et al. Long-term surgical outcome of noninvasive and minimally invasive intraductal papillary mucinous adenocarcinoma of the pancreas. World J Surg. 2002;26:1166–9. doi: 10.1007/s00268-002-6254-3. [DOI] [PubMed] [Google Scholar]
- 27.Nara S, Shimada K, Kosuge T, et al. Minimally invasive intraductal papillary-mucinous carcinoma of the pancreas: clinicopathologic study of 104 intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2008;32:243–55. doi: 10.1097/PAS.0b013e3181484f1e. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Jang KT, Mo Park S, et al. Prognostic relevance of pathologic subtypes and minimal invasion in intraductal papillary mucinous neoplasms of the pancreas. Tumour Biol. 2011;32:535–42. doi: 10.1007/s13277-010-0148-z. [DOI] [PubMed] [Google Scholar]
- 29.Stelow EB, Pambuccian SE, Bauer TW, et al. Mucus rupture (extrusion) and duct expansion/expansive growth are not diagnostic of minimal invasion when seen with intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2009;33:320–1. doi: 10.1097/PAS.0b013e3181861bcd. author reply 321–2. [DOI] [PubMed] [Google Scholar]
- 30.Matthaei H, Norris AL, Tsiatis AC, et al. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255:326–33. doi: 10.1097/SLA.0b013e3182378a18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg. 2005;129:87–93. doi: 10.1016/j.jtcvs.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 32.Friberg S, Mattson S. On the growth rates of human malignant tumors: implications for medical decision making. J Surg Oncol. 1997;65:284–97. doi: 10.1002/(sici)1096-9098(199708)65:4<284::aid-jso11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Network NCC. NCCN Clinical Practice Guidelines in Oncology [Pancreatic Adenocarcinoma web site] 2014 Available at: https://www.nccn.org/store/login/login.aspx?ReturnURL=http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.