Abstract
Typically, time-consuming standard toxicological assays using the zebrafish (Danio rerio) embryo model evaluate mortality and teratogenicity after exposure during the first 2 days post-fertilization. Here we describe an automated image-based high content screening (HCS) assay to identify the teratogenic/embryotoxic potential of compounds in zebrafish embryos in vivo. Automated image acquisition was performed using a high content microscope system. Further automated analysis of embryo length, as a statistically quantifiable endpoint of toxicity, was performed on images post-acquisition. The biological effects of ethanol, nicotine, ketamine, caffeine, dimethyl sulfoxide and temperature on zebrafish embryos were assessed. This automated developmental toxicity assay, based on a growth-retardation endpoint should be suitable for evaluating the effects of potential teratogens and developmental toxicants in a high throughput manner. This approach can significantly expedite the screening of potential teratogens and developmental toxicants, thereby improving the current risk assessment process by decreasing analysis time and required resources. Published 2014. This article is a U.S. Government work and is in the public domain in the USA.
Keywords: zebrafish embryo, high content screening, automated imaging, integrated morphometric analysis, ethanol, nicotine, ketamine
Introduction
The zebrafish (Danio rerio) model offers several advantages for screening a large number of compounds in drug discovery and toxicological studies. Because its genetic and physiologic makeup is comparable to that of mammals, this model has continued to gain popularity since its emergence in the 1990s (Gorge and Nagel, 1990). The toxicities of drugs and environmental pollutants are well conserved between humans and zebrafish (Van den Belt et al., 2000; Spitsbergen and Kent, 2003; Teraoka et al., 2003; Hill et al., 2005; Zon and Peterson, 2005; Reimers et al., 2006; Kari et al., 2007; Parng et al., 2007; Brittijn et al., 2009).
There are several inherent advantages of utilizing zebrafish for drug screening [reviewed in (Kanungo et al., 2014)]. They are easily bred in large numbers year-round, with a single spawning producing ~200 eggs. Additionally, embryos can live up to a few days after fertilization without external feeding. Drugs can diffuse through the skin and gills of embryos/larvae, and enter the body orally when zebrafish begin to swallow at around 72 h post-fertilization (hpf). In addition, the transparency of zebrafish for several days post-fertilization (dpf) enables the in vivo observation of internal organ development. The embryos develop rapidly ex utero, with most organs becoming fully functional at 2–5 dpf (Stainier and Fishman, 1994). Furthermore, the organization of the genome and the genetic pathways controlling signal transduction and development are highly conserved between zebrafish and humans (Granato and Nusslein-Volhard, 1996; Postlethwait et al., 2000).
Another very attractive feature of zebrafish embryo assays for toxicity screening is its amenability to medium-to-high-throughput experiments. Zebrafish embryos can survive for a few days in multi-well plates and therefore can be used to assess drug effects in a high throughput manner (MacRae and Peterson, 2003). Image-based high content assays for toxicity screening using transgenic zebrafish embryos with morphology and behavioral endpoints are fast emerging (Yozzo et al., 2013; Raftery et al., 2014). Zebrafish are also tolerant to low concentrations of dimethyl sulfoxide (DMSO), which is frequently used as a vehicle for many test compounds (Goldsmith, 2004).
Although conventional in vitro assays using cultured cells can be used to evaluate some aspects of drug toxicities, results are frequently not predictive of results in vivo because of the increased complexity found in whole organisms such as drug absorption, distribution, metabolism and excretion (ADME). In zebrafish embryos, complex cell-cell and cell-matrix interactions remain intact, in contrast to most cell-based in vitro screening models. To date, the zebrafish model is the only vertebrate model used for chemical genetics and large-scale in vivo drug screens (Driever and Fishman, 1996; Haffter et al., 1996; Vogt et al., 2009). Standard toxicological assays using the zebrafish model include evaluation of lethality and teratogenicity upon exposure to chemicals during the first few days post-fertilization. Morphological defects such as edema and altered body length/curvature, heart rate and locomotion are common toxicological endpoints for testing compounds.
There are many reports emphasizing the zebrafish as a useful model for research on drugs of abuse [reviewed in (Stewart et al., 2011)]. Zebrafish larvae have been used as a model vertebrate in large-scale genetic analyses to study biological effects of ethanol (Lockwood et al., 2004). When embryos are exposed to ethanol, several abnormalities that include delay in motor and reflex development, pre- and post-natal growth with organ-specific deformities in the cranium, face, joints and heart become obvious (Jones et al., 1973; Staisey and Fried, 1983; Driscoll et al., 1990). Using the zebrafish embryo as a model, alcohol exposure studies have detailed developmental malformations (Bilotta et al., 2004; Carvan et al., 2004; Arenzana et al., 2006; Dlugos and Rabin, 2007), although high doses of ethanol are required to induce defects that are similar to those seen in mammalian models (Cudd, 2005; Matsui et al., 2006). Neurotoxicity in zebrafish embryos caused by exposure to ethanol has been reported for a single dose of ethanol and one time point (5-h embryo exposed to 2.5% ethanol for 1 h; Parng et al., 2007). In another study, a 24-h ethanol treatment induced central nervous system (CNS) neuron death and skeletal dysmorphogenesis in zebrafish embryos in a dose-dependent (3–100 mM) manner (Carvan et al., 2004). Additionally, zebrafish embryos exposed to ethanol for 16 h (between 8 and 24 hpf) develop defective motor neurons, motor axons and muscle fibers (Sylvain et al., 2010). We previously reported adverse effects of ethanol on zebrafish embryo axonogenesis using an image-based, high content assay (Kanungo et al., 2011).
Nicotine, another compound of considerable continued interest, is a notable tobacco product constituent. Exposure of the developing human fetus to nicotine from maternal serum has been linked to a number of abnormalities, including spontaneous abortions, low birth weight, sudden infant death (DiFranza and Lew, 1995), and significant cognitive, intellectual and behavioral impairments in offspring (Sexton et al., 1990; Olds et al., 1994).
Ketamine [2-(2-chlorophenyl)-2-(methylamino)-cyclohexanone], a pediatric general anesthetic, is a non-competitive antagonist of NMDA receptors (Kohrs and Durieux, 1998). NMDA receptors are a subclass of ionotropic glutamate receptors that mediate excitatory transmission throughout the CNS (Dingledine, 1983). Previous studies found that ketamine modulates sensory-motor gating in zebrafish larvae (Burgess and Granato, 2007). Additionally, ketamine exposure was shown to reduce motor neuron numbers, axon length in transgenic zebrafish embryos (Kanungo et al., 2011), and cytochrome p450 aromatase mRNA and estradiol-17β (E2) levels in wild-type (WT) embryos (Trickler et al., 2013).
It has been reported that caffeine, an anxiogenic drug, at 2 mM caused a significant decrease in locomotor activity, with most zebrafish larvae also presenting morphological defects (Pruvot et al., 2012). Although it is unclear at present whether the observed decrease in locomotor activity was as a result of the observed morphological defects or additional effects on neural development, it was suggested that there might be a correlation between altered morphology and locomotor defects (Pruvot et al., 2012). In separate studies, caffeine resulted in a kinked tail upon acute exposure in 2- to 3-day-old zebrafish embryos (Rana et al., 2010), whereas a shorter body length and reduced tactile reflex was observed in 24–48 hpf embryos after prolonged exposure (Chen et al., 2008). Caffeine also induced shorter tails at 0.25 mM and dorsal curvature at 0.5 mM in all (96.8%) surviving larvae with additional edema and hemorrhages observed in about 30% of surviving larvae at 3 mM; at higher concentrations, all survivors were edematous (Pruvot et al., 2012).
Temperature is an important environmental factor, having a strong effect on all developmental processes in fish, including morphology (Pakkasmaa et al., 2006; Janhunen et al., 2010): body shape is significantly affected by changes in temperature during the first stages of development (Sfakianakis et al., 2011).
In the present study, we report an in vivo method for a developmental toxicity assay based on morphology using high content analysis of zebrafish embryos exposed to ethanol, nicotine, ketamine, caffeine, DMSO and different temperatures. Here, hb9-GFP transgenic zebrafish embryos (Flanagan-Steet et al., 2005) were used. The transcription factor hb9 is found in developing motor neurons of both mammals (William et al., 2003) and zebrafish (Cheesman et al., 2004; Park et al., 2004). In the hb9-GFP transgenic fish, motor neurons and their axons are labeled with strong neuron-specific expression of GFP under the control of the regulatory elements of the zebrafish hb9 gene (Flanagan-Steet et al., 2005). Here, it is demonstrated that high content analysis of a single morphological endpoint (body length) is advantageous in designing a high throughput approach to the risk assessment of chemicals, benefitting from exceptional rapidity and cost-efficiency.
Materials and Methods
Animals
Adult transgenic (hb9:GFP) zebrafish (AB strain) were obtained from the Zebrafish International Resource Center at the University of Oregon (Eugene, OR, USA). The fish were kept in fish tanks (Aquatic Habitats) at the NCTR/FDA zebrafish facility containing buffered water (pH 7.5) at 28.5 °C and were fed live brine shrimp and Zeigler-dried fiake food daily (Zeiglers Bros., Inc., Gardeners, PA, USA). Handling and maintenance of zebrafish were in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the NCTR/FDA IACUC. The day–night cycle was maintained at 14 : 10 h and spawning and fertilization were stimulated by the onset of light. Fertilized zebrafish embryos were collected from the bottom of the tank. The eggs/embryos were placed in Petri dishes and washed thoroughly with buffered embryo water [reverse osmosis water containing 60 mg sea salt (Crystal Sea®, Aquatic Eco-systems, Inc., Apopka, FL, USA) per liter of water (pH 7.5)] and then allowed to develop in an incubator at 28.5 °C for further experiments.
Embryo Dechorionation
For experimental ease of handling embryos are often manipulated with chorions intact (Murphey and Zon, 2006) and most zebrafish embryo toxicity studies have been conducted using embryos without dechorionation (Khersonsky et al., 2003; Jung et al., 2005; Anderson et al., 2007; Choi et al., 2007; Hultman et al., 2008). However, there are discrepancies in findings owing to differential uptake/metabolism of chemicals between embryos and adults, which is reportedly resolved when embryos are dechorionated prior to treatment [Wendler et al. unpublished data discussed in (Lammer et al., 2009)]. Therefore, at 24 hpf, we manually dechorionated all embryos used in the present studies under a dissecting microscope using a pair of watchmaker’s forceps.
Reagents
Ethanol (EtOH; 200 Proof, Cat # 2716 GEA) was purchased from Decon Labs, Inc. (King of Prussia, PA, USA). Dimethyl sulfoxide (DMSO) (Cat # 41639), nicotine (Cat # N5260) and caffeine (Cat # C0750) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ketamine (ketamine HCl, Cat # NDC 57319-542-02) was purchased from Phoenix (St. Joseph, MO, USA). The nicotine stock solution was prepared by dissolving nicotine hydrogen tartrate (powder) in buffered embryo water. The ketamine HCl solution was diluted with buffered embryo water to the desired concentrations. DMSO and EtOH were added directly to the embryo water. The caffeine stock solution was prepared by dissolving caffeine in embryo water. Tricaine, anesthetic for the zebrafish embryos, was purchased from Sigma-Aldrich. All stock solutions were made fresh in glass vials.
Treatment of Zebrafish Embryos with Drugs and Arraying of Embryos in Multi-Well Plates
Ethanol, nicotine, ketamine, caffeine and DMSO were placed in Petri dishes with embryos (100 embryos per 30 ml of buffered egg water) for chemical exposure. Various concentrations of ethanol (1%, 1.5%. 2% and 2.5% v/v), nicotine (30 and 50 μM), ketamine (2 mM), caffeine (0.05–5.0 mM) and DMSO (0.1%) were used for the treatments. In addition to drug treatments, dechorionated embryos were also allowed to grow at 22 or 28.5 °C for 24 h. Untreated controls were examined in parallel. Post-treatment, zebrafish embryos were manually placed (one embryo per well) in black 384-well plates (BD Falcon, Cat # 353270) and 25 μl of fish water containing 0.016% tricaine was added to each well. After 20 min at room temperature, plates were centrifuged at 200 rpm (32xg) for 2 min to orient the embryos flat on the bottom of the wells.
High-Throughput Image Acquisition
After drug exposure and positioning in the multi-well plates, live automated imaging of zebrafish embryos using the ImageXpress XL system (Molecular Devices, Sunnyvale, CA, USA) was performed (Fig. 1). A fluorescent image of each embryo was captured automatically using a 2× PlanApo objective (binning2, gain2) and a FITC filter. In the MetaXpress software, under Acquisition Loops, laser-based focusing and shading correction were enabled. Under Autofocus, the equipment was directed to focus on the plate bottom, and offset by the bottom thickness. Safe working distance operations were enabled. Under Wavelength, FITC was selected with an exposure of 100 ms. The autofocus was set to Laser with Z-offset. Proper image acquisition was confirmed in several wells to ensure that gain and exposure levels did not result in images with saturated regions. Plate acquisition then commenced. The instrument automatically acquired images from all wells and archived them in a server database as low resolution montages of the entire plate as well as individual high-resolution TIFF files. A 384-well plate required ~12 min for image acquisition and storage. During the entire image acquisition period, the internal temperature within the IXM system was maintained at 28 °C with the temperature controlling system turned on and set to 28 °C.
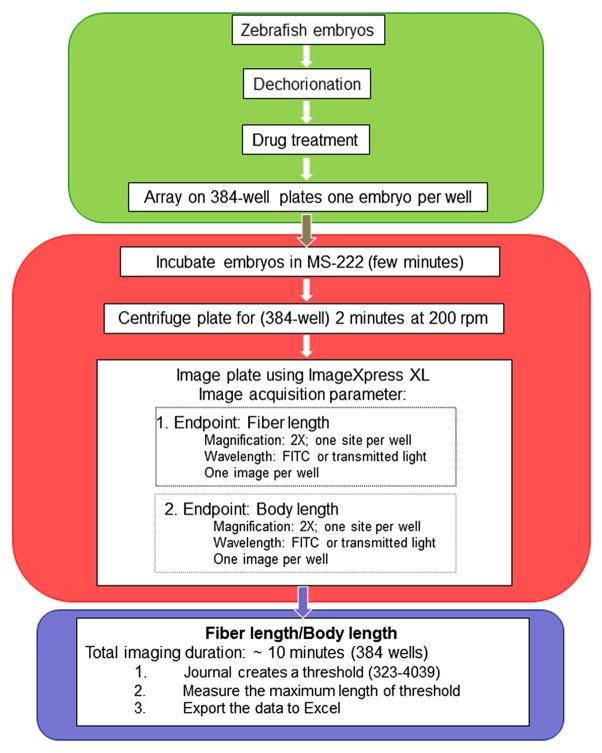
Figure 1.
Flow chart summarizing the steps used for the high content screening (HCS) assay of zebrafish embryos that include compound exposure, image acquisition from multi-well plates, endpoint selection and data analysis.
Data Analysis
After image acquisition, the body length and fiber length (Fig. 2) of zebrafish embryos were detected via the MetaXpress v5.1.0.46 by utilizing a custom journal that integrated the Integrated Morphometry Analysis (IMA) module (Molecular Devices, Sunnyville, CA, USA). Body length (called length for the duration of this manuscript) was defined as the straight line distance between the embryo head and tail. However, as indicated in Fig. 2, fiber length takes embryo curvature into account. The custom journal files consisted of a setup journal (Fig. S1) and an analysis journal (Fig. S2). The setup journal directed the user to select a directory for saving the images that had been analyzed using the IMA and thresholded. The analysis journal successively opened the high resolution TIFF files associated with each well, maximized them, applied the predefined inclusive threshold (file name: fish length.GTH) and IMA settings (file name: fish length. IMA), measured and logged the length and fiber length of each embryo (Fig. 2), and saved each image with its thresholded and IMA overlays. IMA was set to detect thresholded embryos with an area ≥ 500 000um2. The thresholds and IMA setting excluded debris from being included in data analysis.
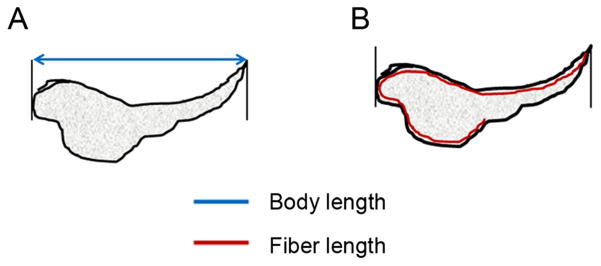
Figure 2.
Schematic diagram explaining the difference between the two morphological endpoints the Integrated Morphometry Analysis (IMA) module quantifies, fiber length (A) and body length (B), obtained from the high content screening (HCS) assay of zebrafish embryos. Fiber length is , where ‘p’ is the perimeter and ‘a’ is the area of the image.
Statistical Analysis
Data from images processed using the custom journal (Fig. S1 and S2), were automatically exported to Microsoft Excel. Data were then pasted into Sigma Plot 9.0/Sigma Stat 3.1 for statistical analysis. One-way ANOVA and Holm–Sidak pair-wise multiple comparison post-hoc tests were used to determine statistical significance with P <0.05. For two-group comparisons, a t-test was used to determine statistical significance with P <0.05. For data failing Shaprio-Wilk normality testing, Kruskal–Wallis ANOVA on ranks with Tukey’s post-hoc tests were alternatively performed with P <0.05.
Results
In an attempt to establish a method to accurately measure developmental toxicity in a fast and robust manner, we describe here an image-based high content screening (HCS) assay using zebrafish embryos based on a single parameter as an endpoint. In order to determine the reliability of this automated imaging system-based assay, we tested a number of known teratogens using two different endpoints, linear axial length and fiber length that takes into account the curved contour of the embryos, a morphological anomaly that often occurs with toxicant exposure.
Here, we chose to study the effects of the different compounds after exposures between 28 and 52 hpf, a critical period for organ-ogenesis in the developing larva. Although the major processes taking place during early embryo development (e.g. gastrulation, conversion-extension, epiboly, segmentation, etc.) are completed by this stage, organogenesis and further growth continues. We have quantified the endpoints (body length and fiber length) as measures of teratogenicity (growth retardation) in embryos and larvae up to 52 hpf. In all of our experiments, we used 24–28 hpf embryos as the initial age of exposure. This age was chosen for exposure because 24 hpf is the earliest stage at which the rudiments of all organ systems are already developed.
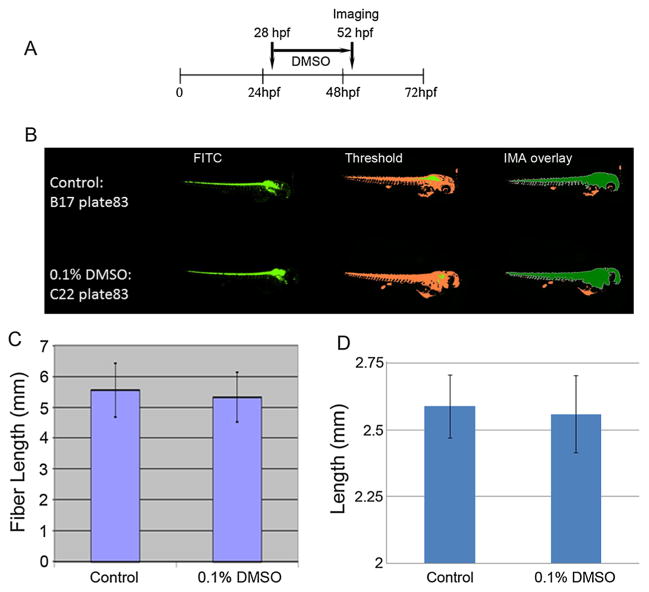
DMSO was used as a solvent/vehicle for E2 (estradiol-17β) in our earlier work in which we obtained consistent results using zebrafish embryos: E2 altered the expression of specific target genes (Kanungo et al., 2012; Trickler et al., 2013). Those studies demonstrated that E2 dissolved in DMSO (final DMSO concentration 0.1%) as a stock solution was biologically active when added to the zebrafish water but showed no toxicity in terms of lethality or malformations. For the present high content assays, we arrayed the hb9-GFP Tg embryos in 384-well plates (Fig. 3) and for most of our exposures (ethanol, nicotine, ketamine and caffeine), zebrafish embryo water served as the vehicle. As in our previous E2 studies, the maximum concentration of DMSO utilized was 0.1%. Embryos at 28 hpf were exposed for 24 h (Fig. 4A) and their lengths obtained from their archived images using the IMA software (Fig. 4B). There were no significant differences between the untreated controls and the DMSO-treated embryos in either fiber length (Fig. 4C) or length (Fig. 4D). Such data serve to demonstrate the reliability of the automated image-based high content assay. However, note that the measurements for fiber length are double that of length. Although neither differed significantly from the control, the data for length were exactly as predicted or observed with manual measurements of body length for embryos of this age. These results suggest that when using transgenic zebrafish, length, therefore, may be the more reliable endpoint when screening compounds to determine their developmental toxicological potencies. Although no additional time is required to measure fiber length, limiting data analyses to the most predictive endpoint proves more relevant.
Figure 3.
A low resolution montage of a region of a 384-well plate showing zebrafish embryos arrayed in the wells. Post-exposure to drugs and chemicals, embryos are manually placed into the wells of the plate using a transfer pipet. A multi-channel pipet is used to aspirate excess water from the wells and 50 μl fish water containing tricaine (0.016%) is added to each well for anesthesia.
Figure 4.
Representative images from a 384-well plate used for high content automatic image capture and analysis of embryos (n = 30/each): treatment schedule shown in (A). A panel of control (untreated) and dimethyl sulfoxide (DMSO)-treated embryos [48 h post-fertilization (hpf)] is presented (B). Representative images of embryos captured using a FITC filter (green), threshold images (orange) and integrated morphometric analysis Integrated Morphometry Analysis (IMA) overlay images (orange plus green) are shown, respectively, for control (upper panel), and 0.1% DMSO (lower panel). Fiber lengths (C) and lengths (D) of the embryos in the control and 0.1% DMSO-treated groups are presented as means ± SDs.
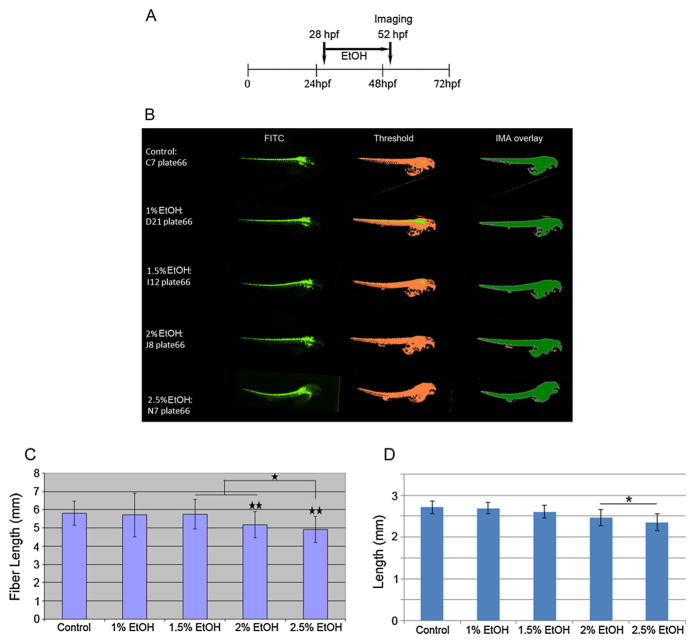
Ethanol (alcohol) is a known teratogenic agent. When pregnant mothers consume alcohol, they can give birth to children with fetal alcohol syndrome (FAS), which is characterized by intrauterine growth retardation, craniofacial malformations, physical and mental retardation, and cardiac septal defects (Jones and Smith, 1973). Here, we exposed 28-hpf embryos for 24 h to various concentrations of ethanol (Fig. 5A). Automated imaging of the treated embryos was used to measure embryo length (Fig. 5B). Our results showed significant growth retardation in zebrafish embryos after 24 h of static exposure to 2–2.5% ethanol compared with the control (Fig. 5C–D). Ehanol at 2.5% significantly retarded fiber length but not body length in the zebrafish embryos compared with both 1.5% and 2% ethanol-treated embryos (Fig. 5C).
Figure 5.
Images (as described for Fig. 5) of control and ethanol (EtOH)-treated embryos from a 384-well plate captured by the high content automatic imager. Treatment schedule is shown in A. Representative images for control and EtOH-treated embryos are shown (B). Data for control and ethanol-treated embryos (n = 37/each) from a 384-well plate captured by the high content automatic imager. Fiber lengths and lengths were calculated from the archived images and representative images from the control, 1%, 1.5%, 2% and 2.5% EtOH exposure groups: values are presented as means ± SDs for fiber length (C) and length (D). * = P <0.05; ** = P <0.01.
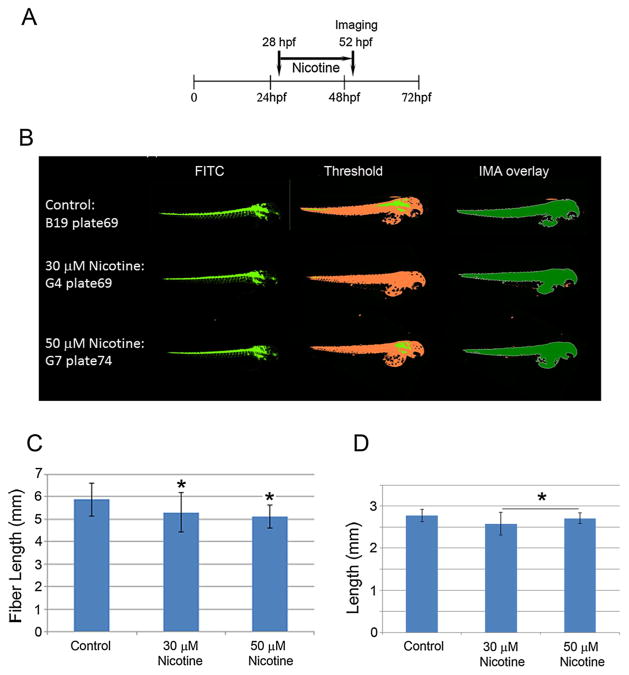
Nicotine (one of the major alkaloids found in tobacco) is highly addictive and has both stimulant and sedative effects on the brain. It can induce analgesic effects and enhance cognitive functions in mice (Damaj et al., 1994; Biala and Kruk, 2009). A dose range of 0.5–1.0 mg kg–1 can be lethal for adult humans, whereas 0.1 mg kg–1 can be lethal in children (Okamoto et al., 1994). In the present studies, we treated 28-hpf zebrafish embryos for 24 h with 30 and 50 μM nicotine (Fig. 6A). Automated high content analyses of the images of these embryos (Fig. 6B) showed a significant reduction in length (Fig. 6D) and fiber length (Fig. 6C). Again, the measurements for fiber length are double those of length which were as predicted for embryos of this age. These results indicate that, with respect to length, the high content assay is reproducible and predictive for nicotine effects.
Figure 6.
Nicotine exposure schedule is shown in (A). Representative images (as described for Fig. 5) of control and nicotine-treated (30 and 50 μM) embryos (n = 51/group) from a 384-well plate captured by the high content automatic imager are presented in panel (B). Fiber lengths (C) and lengths (D) were calculated from the archived images and are presented as means ± SDs (B). * = P <0.05.
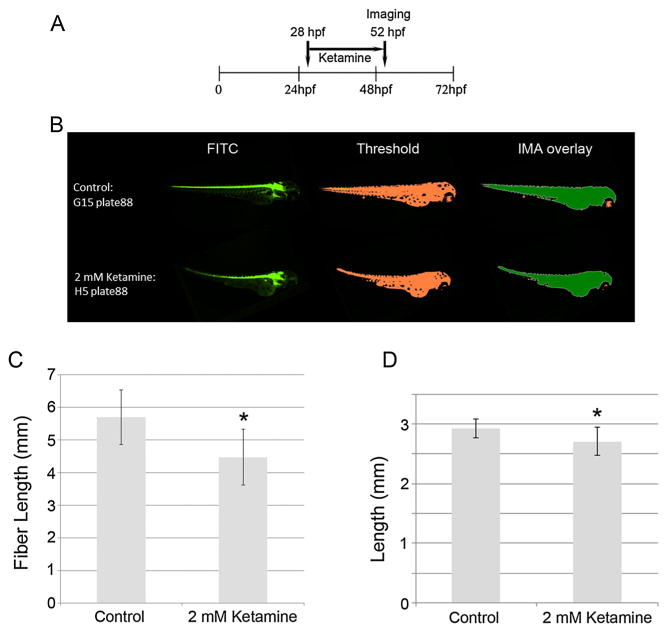
Ketamine [2-(o-chlorophenyl)-2-(methylamino)-cyclohexanone or K], an N-methyl D-aspartate (NMDA) receptor antagonist, is an anesthetic often used in animals and was initially commercialized by Parke, Davis and Company in 1962 to replace phencyclidine and provide a safer anesthetic alternative for humans which avoided hallucinations, neurotoxicity and seizures [reviewed in (Sinner and Graf, 2008)]. Although generally considered a pediatric anesthetic in humans, ketamine is one of the most commonly abused recreational drugs worldwide, making it an emerging topic of clinical interest for pediatricians and neonatologists (Mickley et al., 2004). Our results (Fig. 7A–D) showed that ketamine caused significant growth retardation at 2 mM: this resulted in an internal dose (i.e. that was actually absorbed by the embryo) of 2.2 μg ml–1 (Cuevas et al., 2013; Trickler et al., 2013), a dose equivalent to the lower range of human anesthetic doses. The measurements for fiber length were again double those of length and consistent with those expected for embryos of this age. These data also indicate that, with respect to length, the high content assay is reproducible and predictive for the effects of ketamine and suggest that this assay can identify the developmental toxicological potential of compounds not previously known or established as developmental toxicants, possibly because the effects are not detectable using the naked eye.
Figure 7.
Treatment schedule for ketamine (A) and representative images (as described for Fig. 5) of control and 2 mM ketamine-treated embryos (n = 17/group) from a 384-well plate captured by the high content automatic imager (B). Fiber lengths (C) and lengths (D) were calculated from the archived images for control and ketamine-treated embryos and are presented as means ± SDs. * = P <0.05.
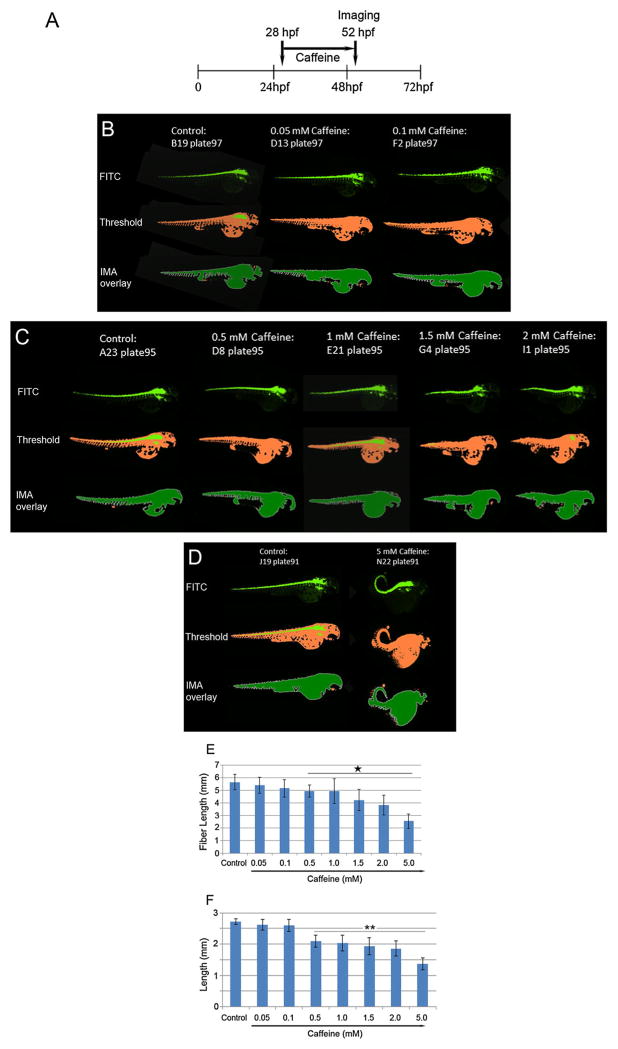
Caffeine is the most widely used CNS stimulant worldwide. Maternal caffeine intake during pregnancy is associated with a lower birth weight but not with a shortened gestation (Sengpiel et al., 2013). In the present study, we exposed 28-hpf embryos for 24 h (Fig. 8A) to concentrations of caffeine ranging from 0.05 to 5 mM. Automated high content analyses of embryo images (Fig. 8B–D) showed a dose-dependent effect on development (morphology). Reductions in fiber length (Fig. 8E) and length (Fig. 8F) occurred starting at 0.1 mM and increased with dose with statistically significant changes in body shape emerging at 0.5 mM. As with other treatments, the measurements for fiber length were double those of length that were embryo age appropriate. These results indicate that, with respect to caffeine exposures, the high content assay paradigm using zebrafish embryos is reproducible and potentially useful for risk assessment. Although we do not report mortality in the current study, it is likely that deformed embryos would not survive.
Figure 8.
Schedule for caffeine treatment (A) and representative images of control and caffeine-treated embryos from a 384-well plate captured by the high content automatic imager. Images and representative images for control, 0.05 mM, 0.1 mM caffeine (B), control, 0.5 mM, 1.0 mM, 1.5 mM and 2 mM caffeine (C), and control and 5 mM caffeine (D), are shown. Values for fiber lengths (E) and lengths (F) of control and caffeine-treated zebrafish embryos (n = 39/group) are presented as means ± SDs obtained from multiple experiments. *P = <0.05; ** = P <0.01.
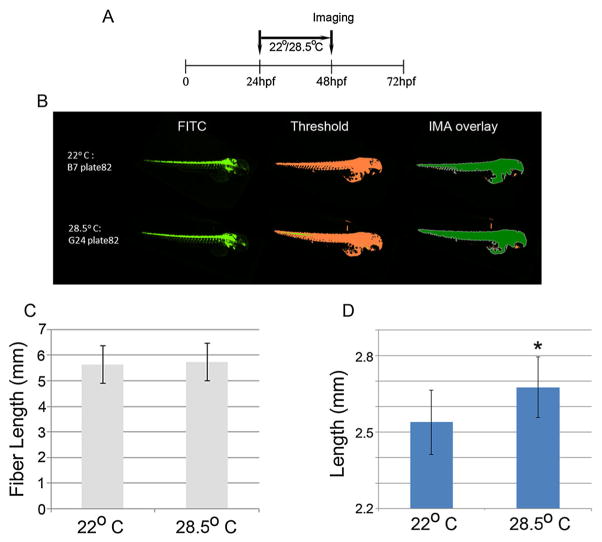
In fish, the appearance of morpho-anatomical deformities can be associated with temperature (Wang and Tsai, 2000; Koumoundouros et al., 2009; Sfakianakis and Kentouri, 2010). There is a lack of published data on the influence of temperature on fish body shape and especially on specimens reared under laboratory conditions. In our study, we precisely determined body length using objective automated imaging procedures and discovered that zebrafish embryos reared at a lower temperature (22 °C) are significantly smaller than those reared at the optimal temperature of 28.5 °C (Fig. 9A–D). As with other treatments, fiber length is double that of length, which is the predicted length for embryos of this age. Here again, the high content assay using the endpoint of length in early zebrafish embryos is reproducible and sensitive to effect. The noted effect of temperature is confirmed by the observation that, at lower temperatures, zebrafish embryos grow more slowly and this effect can be seen with the naked eye. With transgenic embryos, there is a large possibility of only portions of the yolk sac being detected (e.g. in the IMA overlay image on Fig. 9B, the yolk sac is not entirely detected because of the convoluted dark pockets owing to pigmentation; that can skew the measurement), leading to an exaggeration of reported lengths. For this reason, when imaging transgenic zebrafish in a high content manner, body length (length) is the most accurate and reproducible endpoint.
Figure 9.
Representative images (as described for Fig. 5) of zebrafish embryos (n = 66/group) grown at two different temperatures (22 °C, top of panel and 28.5 °C, bottom of panel). A panel of embryos grown for 24 h starting at 24 h post-fertilization (hpf) at 22 °C and 28.5 °C were analyzed for fiber lengths and lengths. Images were captured at 48 hpf. Data were calculated from the archived images and are presented as means ± SDs for fiber length (B) and length (C). *P = <0.05.
Discussion
Growth retardation during development is often observed in teratogen-exposed zebrafish embryos (van den Brandhof and Montforts, 2010). Although developmental delay is usually considered a reversible and non-specific effect, these early developmental delays might persist later and cause anomalies (Weis and Weis, 1987). Some previous studies have attempted to distinguish between growth retardation and other specific developmental effects in zebrafish embryo assays. It has been reported that ‘growth-retardation’ at 24/48 hpf is a ‘teratogenic endpoint’, and defects of development of somites, eyes, or blood circulation are ‘development endpoints’ (Nagel, 2002). In another report, an extensive scoring system included various morphological effects but eliminated endpoints of growth retardation as they were non-discriminating and not cost-effective (Brannen et al., 2010). However, others have considered ‘growth retardation’ as a teratogenic endpoint (Hermsen et al., 2011a, b). In addition, it has been shown that longer exposures to 2 and 3 dpf result in better consistency with observations seen in adults (Khetan and Collins, 2007). It was, therefore, compelling to investigate treatment effects specifically during this period.
Fetal exposure to alcohol is associated with a number of adverse long-term outcomes (Jones et al., 2013). Birth defects such as multiple clefts, as well as an increased risk for Pierre–Robin Disease (Romitti et al., 2007) and congenital heart defects have been associated with mothers’ alcohol intake (Grewal et al., 2008). Our data show that higher concentrations of ethanol (>2%) can induce growth retardation. These data are consistent with previous studies where zebrafish embryos were exposed to ethanol from 8 to 24 hpf; additionally, abnormal axonogenesis was noticeable in 3-day-old embryos and the embryos treated with ethanol (>1.5% v/v) were smaller than the controls (Sylvain et al., 2010). Again, the measurements for fiberlength are double those of length with the latter being those predicted for embryos of this age. These results indicate that, with respect to length, this high content assay is reproducible and predictive.
In in vitro assays, nicotine concentrations greater than 1 mM are toxic to primary normal human oral keratinocytes (Gemenetzidis et al., 2009). A marginally toxic dose of nicotine is 10 mM and it has been shown that salivary nicotine concentration can reach these kinds of concentrations in tobacco chewers (Homsy et al., 1997). Smoking during pregnancy is a well-established detriment to fetal growth and increases the risk of a low birth weight (Kramer, 1987a, b). High-content analysis of zebrafish embryos exposed to nicotine shows significant growth retardation suggesting that these embryos may be predictive of nicotine’s effects on humans.
Ketamine has been found to induce acute pathomorphological changes in specific brain neurons in a variety of animal models. It also induces neuronal brain apoptosis and disrupts spontaneous activities and learning processes in animals (Olney et al., 1989). Although chronic in vitro exposure to low and subanesthetic doses of ketamine does not affect survival of neural cells, it can impair neuronal morphology and lead to neural dysfunction (Vutskits et al., 2007). It has been recognized as a developmental neurotoxicant [(reviewed in Wang and Slikker, 2008; Dong and Anand, 2013)]. Although ketamine has not been described as an environmental agent causing neonatal malformations like alcohol, one case study presented data suggesting that intrauterine growth retardation, remarkable hypotonia, and poor reflex responses were associated with ketamine exposure (Su et al., 2010). In chicks, ketamine at concentrations of 2 mM or higher were embryo lethal, whereas 100–200 μg ml–1 (0.1–0.8 mM) preferentially inhibited elevation of neural folds. The latter effect was detectable within 3 h of treatment and was readily reversible (Lee and Nagele, 1985). Ketamine treatment of very early stage zebrafish embryos suggests a teratogenic effect of the anesthetic in a NMDA receptor-independent manner (Felix et al., 2013). Our data derived from the high content analysis portray ketamine as a developmental toxicant.
Maternal plasma caffeine levels less than 60 μg ml–1 or lower have not been reported to produce congenital malformations or growth retardation in infants [reviewed in (Brent et al., 2011)]. Consumption of caffeinated beverages during pregnancy by mothers has been associated with anencephalic offspring and children with various malformations such as absence of, or cleft palate (Johansen et al., 2009). Increased cleft palate and digital defects in the offspring of mice exposed to caffeine at a dose of 250 mg kg–1 has been reported (Nishimura and Nakai, 1960). In rats, a reduction in fetal weight was found after gestational exposure to caffeine (Aeschbacher et al., 1980). In zebrafish, kinked tails have been reported upon acute exposure to caffeine in 2- to 3-day-old embryos (Rana et al., 2010). Caffeine also induced shorter tails at 0.25 mM and dorsal curvature at 0.5 mM in all surviving (96.8%) larvae. At 3 mM, edema and hemorrhages were also observed (about 30% of surviving larvae presented both), whereas at higher concentrations all survivors presented edema (Pruvot et al., 2012). Developmental defects could occur in the 2–3 dpf zebrafish at a caffeine dose of 286 μM. Treatment with 0.5 mM caffeine reportedly caused them to suffer from shorter tails and body curvature at 3 dpf; however, an increased incidence of edema and hemorrhages with concentrations higher than 3 mM were observed (Pruvot et al., 2012). In the present study, using a fast high content assay, we show that caffeine at doses over 0.5 mM can induce developmental toxicity in the zebrafish embryos. In humans, the LD50 for caffeine ranges from 50–500 mg kg–1 body weight, which is equivalent to the 0.26–2.5 mM range [reviewed in (Dews, 1982)]. Our results indicate that the doses of caffeine that can evoke a response in zebrafish are equivalent to those in humans.
Although the temperature of the zebrafish habitat ranges from 6 °C in the winter up to 38 °C in the summer (Spence et al., 2008), there are still contradictions concerning the temperature range in which reproduction and early development take place (Sfakianakis et al., 2011). The variance in fish body shape can be explained by the general morphological plasticity that fish exhibit in different environments, owing to alterations in muscle and bone developmental patterns (Wimberger, 1992). It was first reported that body shape differentiation in a fish species, the sea bass (Dicentrarchus labrax), can be induced by the environmental temperature during early life stages (Georgakopoulou et al., 2007). The authors used two different temperatures (15 and 20 °C) during larval rearing (from the egg stage until metamorphosis) of the species and concluded that developmental temperature strongly affects body shape at the subsequent juvenile stage. In particular, they reported that body shape at 15 °C tended to be more slender than at 20 °C. Consistent with these reported data based on manual measurements, our results, based on the rapid high content analysis, show that zebrafish embryos grown at a lower temperature are significantly smaller than those grown in an ambient temperature.
Both environmental and human hazard assessments, based on morphology-based teratogenicity using zebrafish embryos, have been reported (Selderslaghs et al., 2009; Gustafson et al., 2012). While mammalian models are time and cost-intensive and can be fraught with ethical issues relating to animal use, cell-based in vitro assays do not exhibit the complexity of animal models and, hence, often have limited predictive capability. In this context, the zebrafish embryo is a superior model system for safety assessment. Utilization of data from zebrafish embryos in the human risk assessment process relies upon dose-response data obtained using the embryos and how those data compare with those in mammalian models. The pharmacokinetics of chemicals in cultured cells in vitro, as well as in fish embryos, are different from those of typical mammalian models; however, drug metabolizing systems in embryos have been shown to be highly conserved compared with those of mammals (Hung et al., 2012). Numerous previous studies that have explored the utility of zebrafish assays for assessing the teratogenic potential (death, malformation, and growth retardation) of chemicals have shown good concordance with results obtained in vivo using mammals (Nagel, 2002; Selderslaghs et al., 2009; Brannen et al., 2010; Hermsen et al., 2011a, b; Van den Bulck et al., 2011). However, no universally accepted standard protocol with specified endpoints exposure durations, or embryo ages has been adopted.
The current study serves to begin the discourse as to whether a morphological parameter such as body length, presumably refiecting growth rate of zebrafish embryos, can serve as a reliable endpoint for teratogenicity and/or developmental toxicity testing. In the present study, we explored the effects of a select few representative compounds known to have deleterious biological effects on zebrafish larvae. In this first attempt, we sought to demonstrate a method that is both fast and cost-effective using a state-of-the-art procedure. Although many previous reports have described the effects of compounds on early zebrafish development, those efforts have been based on manual measurements and do not lend themselves to conduct experiments in a high throughput fashion, free from operator bias. Other concerns with the earlier approaches relate to the accuracy and reproducibility of results. While differences in body length of only a few micrometers are typically not detectable using manual measurements, the automated method described herein can reproducibly quantitate these subtle differences. In order to further demonstrate the utility of this HCS approach and assess its predictive potential, a larger number of compounds need to be tested.
Acknowledgments
We thank Paula Gedraitis, Sylvia de Bruin and Tim Baranowski of Molecular Devices for their technical support and the NCTR zebrafish facility support staff for their help in animal maintenance.
Footnotes
Disclaimer
This document has been reviewed in accordance with United States Food and Drug Administration (FDA) policy and approved for publication. Approval does not signify that the contents necessarily reflect the position or opinions of the FDA, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the FDA.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Aeschbacher HU, Milon H, Poot A, Wurzner HP. Effect of caffeine on rat offspring from treated dams. Toxicol Lett. 1980;7:71–77. doi: 10.1016/0378-4274(80)90087-9. [DOI] [PubMed] [Google Scholar]
- Anderson C, Bartlett SJ, Gansner JM, Wilson D, He L, Gitlin JD, Kelsh RN, Dowden J. Chemical genetics suggests a critical role for lysyl oxidase in zebrafish notochord morphogenesis. Mol Biosyst. 2007;3:51–59. doi: 10.1039/b613673g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenzana FJ, Carvan MJ, 3rd, Aijon J, Sanchez-Gonzalez R, Arevalo R, Porteros A. Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicol Teratol. 2006;28:342–348. doi: 10.1016/j.ntt.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Biala G, Kruk M. Effects of co-administration of bupropion and nicotine or D-amphetamine on the elevated plus maze test in mice. J Pharm Pharmacol. 2009;61:493–502. doi: 10.1211/jpp/61.04.0012. [DOI] [PubMed] [Google Scholar]
- Bilotta J, Barnett JA, Hancock L, Saszik S. Ethanol exposure alters zebrafish development: a novel model of fetal alcohol syndrome. Neurotoxicol Teratol. 2004;26:737–743. doi: 10.1016/j.ntt.2004.06.011. [DOI] [PubMed] [Google Scholar]
- van den Brandhof EJ, Montforts M. Fish embryo toxicity of carbamazepine, diclofenac and metoprolol. Ecotoxicol Environ Saf. 2010;73:1862–1866. doi: 10.1016/j.ecoenv.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Brannen KC, Panzica-Kelly JM, Danberry TL, Augustine-Rauch KA. Development of a zebrafish embryo teratogenicity assay and quantitative prediction model. Birth Defects Res B Dev Reprod Toxicol. 2010;89:66–77. doi: 10.1002/bdrb.20223. [DOI] [PubMed] [Google Scholar]
- Brent RL, Christian MS, Diener RM. Evaluation of the reproductive and developmental risks of caffeine. Birth Defects Res B Dev Reprod Toxicol. 2011;92:152–187. doi: 10.1002/bdrb.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittijn SA, Duivesteijn SJ, Belmamoune M, Bertens LF, Bitter W, de Bruijn JD, Champagne DL, Cuppen E, Flik G, Vandenbroucke-Grauls CM, Janssen RA, de Jong IM, de Kloet ER, Kros A, Meijer AH, Metz JR, van der Sar AM, Schaaf MJ, Schulte-Merker S, Spaink HP, Tak PP, Verbeek FJ, Vervoordeldonk MJ, Vonk FJ, Witte F, Yuan H, Richardson MK. Zebrafish development and regeneration: new tools for biomedical research. Int J Dev Biol. 2009;53:835–850. doi: 10.1387/ijdb.082615sb. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J Neurosci. 2007;27:4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvan MJ, 3rd, Loucks E, Weber DN, Williams FE. Ethanol effects on the developing zebrafish: neurobehavior and skeletal morphogenesis. Neurotoxicol Teratol. 2004;26:757–768. doi: 10.1016/j.ntt.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Cheesman SE, Layden MJ, Von Ohlen T, Doe CQ, Eisen JS. Zebrafish and fly Nkx6 proteins have similar CNS expression patterns and regulate motoneuron formation. Development. 2004;131:5221–5232. doi: 10.1242/dev.01397. [DOI] [PubMed] [Google Scholar]
- Chen YH, Huang YH, Wen CC, Wang YH, Chen WL, Chen LC, Tsay HJ. Movement disorder and neuromuscular change in zebrafish embryos after exposure to caffeine. Neurotoxicol Teratol. 2008;30:440–447. doi: 10.1016/j.ntt.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Choi TY, Kim JH, Ko DH, Kim CH, Hwang JS, Ahn S, Kim SY, Kim CD, Lee JH, Yoon TJ. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007;20:120–127. doi: 10.1111/j.1600-0749.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 2005;230:389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- Cuevas E, Trickler WJ, Guo X, Ali SF, Paule MG, Kanungo J. Acetyl l-carnitine protects motor neurons and Rohon-Beard sensory neurons against ketamine-induced neurotoxicity in zebrafish embryos. Neurotoxicol Teratol. 2013;39:69–76. doi: 10.1016/j.ntt.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Welch SP, Martin BR. Nicotine-induced antinociception in mice: role of G-proteins and adenylate cyclase. Pharmacol Biochem Behav. 1994;48:37–42. doi: 10.1016/0091-3057(94)90494-4. [DOI] [PubMed] [Google Scholar]
- Dews PB. Caffeine. Annu Rev Nutr. 1982;2:323–341. doi: 10.1146/annurev.nu.02.070182.001543. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. J Fam Pract. 1995;40:385–394. [PubMed] [Google Scholar]
- Dingledine R. Excitatory amino acids: modes of action on hippocampal pyramidal cells. Fed Proc. 1983;42:2881–2885. [PubMed] [Google Scholar]
- Dlugos CA, Rabin RA. Ocular deficits associated with alcohol exposure during zebrafish development. J Comp Neurol. 2007;502:497–506. doi: 10.1002/cne.21320. [DOI] [PubMed] [Google Scholar]
- Dong C, Anand KJ. Developmental neurotoxicity of ketamine in pediatric clinical use. Toxicol Lett. 2013;220:53–60. doi: 10.1016/j.toxlet.2013.03.030. [DOI] [PubMed] [Google Scholar]
- Driever W, Fishman MC. The zebrafish: heritable disorders in transparent embryos. J Clin Invest. 1996;97:1788–1794. doi: 10.1172/JCI118608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotoxicol Teratol. 1990;12:231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Felix LM, Antunes LM, Coimbra AM. Ketamine NMDA receptor-independent toxicity during zebrafish (Danio rerio) embryonic development. Neurotoxicol Teratol. 2013;41C:27–34. doi: 10.1016/j.ntt.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Flanagan-Steet H, Fox MA, Meyer D, Sanes JR. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynap-tic specializations. Development. 2005;132:4471–4481. doi: 10.1242/dev.02044. [DOI] [PubMed] [Google Scholar]
- Gemenetzidis E, Bose A, Riaz AM, Chaplin T, Young BD, Ali M, Sugden D, Thurlow JK, Cheong SC, Teo SH, Wan H, Waseem A, Parkinson EK, Fortune F, Teh MT. FOXM1 upregulation is an early event in human squamous cell carcinoma and it is enhanced by nicotine during malignant transformation. PLoS One. 2009;4:e4849. doi: 10.1371/journal.pone.0004849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulou E, Sfakianakis DG, Kouttouki S, Divanach P, Kentouri M, Koumoundouros G. The influence of temperature during early life on phenotypic expression at later ontogenetic stages in sea bass. J Fish Biol. 2007;70:278–291. [Google Scholar]
- Goldsmith P. Zebrafish as a pharmacological tool: the how, why and when. Curr Opin Pharmacol. 2004;4:504–512. doi: 10.1016/j.coph.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Gorge G, Nagel R. Toxicity of lindane, atrazine, and deltamethrin to early life stages of zebrafish (Brachydanio rerio) Ecotoxicol Environ Saf. 1990;20:246–255. doi: 10.1016/0147-6513(90)90004-o. [DOI] [PubMed] [Google Scholar]
- Granato M, Nusslein-Volhard C. Fishing for genes controlling development. Curr Opin Genet Dev. 1996;6:461–468. doi: 10.1016/s0959-437x(96)80068-2. [DOI] [PubMed] [Google Scholar]
- Grewal J, Carmichael SL, Ma C, Lammer EJ, Shaw GM. Maternal periconceptional smoking and alcohol consumption and risk for select congenital anomalies. Birth Defects Res A Clin Mol Teratol. 2008;82:519–526. doi: 10.1002/bdra.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson AL, Stedman DB, Ball J, Hillegass JM, Flood A, Zhang CX, Panzica-Kelly J, Cao J, Coburn A, Enright BP, Tornesi MB, Hetheridge M, Augustine-Rauch KA. Inter-laboratory assessment of a harmonized zebrafish developmental toxicology assay - progress report on phase I. Reprod Toxicol. 2012;33:155–164. doi: 10.1016/j.reprotox.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP, Kelsh RN, Furutani-Seiki M, Vogelsang E, Beuchle D, Schach U, Fabian C, Nusslein-Volhard C. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- Hermsen SA, Pronk TE, van den Brandhof EJ, van der Ven LT, Piersma AH. Chemical class-specific gene expression changes in the zebrafish embryo after exposure to glycol ether alkoxy acids and 1,2,4-triazole antifungals. Reprod Toxicol. 2011a;32:245–252. doi: 10.1016/j.reprotox.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Hermsen SA, van den Brandhof EJ, van der Ven LT, Piersma AH. Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo potencies. Toxicol In Vitro. 2011b;25:745–753. doi: 10.1016/j.tiv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Homsy W, Yan K, Houle JM, Besner JG, Gossard D, Pierce CH, Caille G. Plasma levels of nicotine and safety of smokers wearing transdermal delivery systems during multiple simultaneous intake of nicotine and during exercise. J Clin Pharmacol. 1997;37:728–736. doi: 10.1002/j.1552-4604.1997.tb04360.x. [DOI] [PubMed] [Google Scholar]
- Hultman KA, Scott AW, Johnson SL. Small molecule modifier screen for kit-dependent functions in zebrafish embryonic melanocytes. Zebrafish. 2008;5:279–287. doi: 10.1089/zeb.2008.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung MW, Zhang ZJ, Li S, Lei B, Yuan S, Cui GZ, Man Hoi P, Chan K, Lee SM. From omics to drug metabolism and high content screen of natural product in zebrafish: a new model for discovery of neuroactive compound. Evid Based Complement Alternat Med. 2012;2012:605303. doi: 10.1155/2012/605303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janhunen M, Piironen J, Peuhkuri N. Parental effects on embryonic viability and growth in Arctic charr Salvelinus alpinus at two incubation temperatures. J Fish Biol. 2010;76:2558–2570. doi: 10.1111/j.1095-8649.2010.02648.x. [DOI] [PubMed] [Google Scholar]
- Johansen AM, Wilcox AJ, Lie RT, Andersen LF, Drevon CA. Maternal consumption of coffee and caffeine-containing beverages and oral clefts: a population-based case-control study in Norway. Am J Epidemiol. 2009;169:1216–1222. doi: 10.1093/aje/kwp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Jones TB, Bailey BA, Sokol RJ. Alcohol use in pregnancy: insights in screening and intervention for the clinician. Clin Obstet Gynecol. 2013;56:114–123. doi: 10.1097/GRF.0b013e31827957c0. [DOI] [PubMed] [Google Scholar]
- Jung DW, Williams D, Khersonsky SM, Kang TW, Heidary N, Chang YT, Orlow SJ. Identification of the F1F0 mitochondrial ATPase as a target for modulating skin pigmentation by screening a tagged triazine library in zebrafish. Mol Biosyst. 2005;1:85–92. doi: 10.1039/b417765g. [DOI] [PubMed] [Google Scholar]
- Kanungo J, Lantz S, Paule MG. In vivo imaging and quantitative analysis of changes in axon length using transgenic zebrafish embryos. Neurotoxicol Teratol. 2011;33:618–623. doi: 10.1016/j.ntt.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanungo J, Cuevas E, Ali SF, Paule MG. l-Carnitine rescues ketamine-induced attenuated heart rate and MAPK (ERK) activity in zebrafish embryos. Reprod Toxicol. 2012;33:205–212. doi: 10.1016/j.reprotox.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanungo J, Cuevas E, Ali SF, Paule MG. Zebrafish Model in Drug Safety Assessment. Curr Pharm Des. 2014 doi: 10.2174/138161282-0666140205145658. [DOI] [PubMed] [Google Scholar]
- Kari G, Rodeck U, Dicker AP. Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharmacol Ther. 2007;82:70–80. doi: 10.1038/sj.clpt.6100223. [DOI] [PubMed] [Google Scholar]
- Khersonsky SM, Jung DW, Kang TW, Walsh DP, Moon HS, Jo H, Jacobson EM, Shetty V, Neubert TA, Chang YT. Facilitated forward chemical genetics using a tagged triazine library and zebrafish embryo screening. J Am Chem Soc. 2003;125:11804–11805. doi: 10.1021/ja035334d. [DOI] [PubMed] [Google Scholar]
- Khetan SK, Collins TJ. Human pharmaceuticals in the aquatic environment: a challenge to Green Chemistry. Chem Rev. 2007;107:2319–2364. doi: 10.1021/cr020441w. [DOI] [PubMed] [Google Scholar]
- Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- Koumoundouros G, Ashton C, Sfakianakis DG, Divanach P, Kentouri M, Anthwal N, Stickland NC. Thermally induced phenotypic plasticity of swimming performance in European sea bass Dicentrarchus labrax juveniles. J Fish Biol. 2009;74:1309–1322. doi: 10.1111/j.1095-8649.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987a;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- Kramer MS. Intrauterine growth and gestational duration determinants. Pediatrics. 1987b;80:502–511. [PubMed] [Google Scholar]
- Lammer E, Kamp HG, Hisgen V, Koch M, Reinhard D, Salinas ER, Wendler K, Zok S, Braunbeck T. Development of a flow-through system for the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) Toxicol In Vitro. 2009;23:1436–1442. doi: 10.1016/j.tiv.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Lee H, Nagele RG. Neural tube defects caused by local anesthetics in early chick embryos. Teratology. 1985;31:119–127. doi: 10.1002/tera.1420310114. [DOI] [PubMed] [Google Scholar]
- Lockwood B, Bjerke S, Kobayashi K, Guo S. Acute effects of alcohol on larval zebrafish: a genetic system for large-scale screening. Pharmacol Biochem Behav. 2004;77:647–654. doi: 10.1016/j.pbb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- MacRae CA, Peterson RT. Zebrafish-based small molecule discovery. Chem Biol. 2003;10:901–908. doi: 10.1016/j.chembiol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Matsui JI, Egana AL, Sponholtz TR, Adolph AR, Dowling JE. Effects of ethanol on photoreceptors and visual function in developing zebrafish. Invest Ophthalmol Vis Sci. 2006;47:4589–4597. doi: 10.1167/iovs.05-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley GA, Kenmuir CL, McMullen CA, Snyder A, Yocom AM, Likins-Fowler D, Valentine EL, Weber B, Biada JM. Long-term age-dependent behavioral changes following a single episode of fetal N-methyl-D-Aspartate (NMDA) receptor blockade. BMC Pharmacol. 2004;4:28. doi: 10.1186/1471-2210-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey RD, Zon LI. Small molecule screening in the zebrafish. Methods. 2006;39:255–261. doi: 10.1016/j.ymeth.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Nagel R. DarT: The embryo test with the Zebrafish Danio rerio--a general model in ecotoxicology and toxicology. ALTEX. 2002;19(Suppl 1):38–48. [PubMed] [Google Scholar]
- Nishimura H, Nakai K. Congenital malformations in offspring of mice treated with caffeine. Proc Soc Exp Biol Med. 1960;104:140–142. doi: 10.3181/00379727-104-25757. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Kita T, Okuda H, Tanaka T, Nakashima T. Effects of aging on acute toxicity of nicotine in rats. Pharmacol Toxicol. 1994;75:1–6. doi: 10.1111/j.1600-0773.1994.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Olds DL, Henderson CR, Jr, Tatelbaum R. Intellectual impairment in children of women who smoke cigarettes during pregnancy. Pediatrics. 1994;93:221–227. [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- Pakkasmaa S, Penttinen OP, Piironen J. Metabolic rate of Arctic charr eggs depends on their parentage. J Comp Physiol B. 2006;176:387–391. doi: 10.1007/s00360-005-0057-4. [DOI] [PubMed] [Google Scholar]
- Park HC, Shin J, Appel B. Spatial and temporal regulation of ventral spinal cord precursor specification by Hedgehog signaling. Development. 2004;131:5959–5969. doi: 10.1242/dev.01456. [DOI] [PubMed] [Google Scholar]
- Parng C, Roy NM, Ton C, Lin Y, McGrath P. Neurotoxicity assessment using zebrafish. J Pharmacol Toxicol Methods. 2007;55:103–112. doi: 10.1016/j.vascn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Woods IG, Ngo-Hazelett P, Yan YL, Kelly PD, Chu F, Huang H, Hill-Force A, Talbot WS. Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res. 2000;10:1890–1902. doi: 10.1101/gr.164800. [DOI] [PubMed] [Google Scholar]
- Pruvot B, Quiroz Y, Voncken A, Jeanray N, Piot A, Martial JA, Muller M. A panel of biological tests reveals developmental effects of pharmaceutical pollutants on late stage zebrafish embryos. Reprod Toxicol. 2012;34:568–583. doi: 10.1016/j.reprotox.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Raftery TD, Isales GM, Yozzo KL, Volz DC. High-content screening assay for identification of chemicals impacting spontaneous activity in zebrafish embryos. Environ Sci Technol. 2014;48:804–810. doi: 10.1021/es404322p. [DOI] [PubMed] [Google Scholar]
- Rana N, Moond M, Marthi A, Bapatla S, Sarvepalli T, Chatti K, Challa AK. Caffeine-induced effects on heart rate in zebrafish embryos and possible mechanisms of action: an effective system for experiments in chemical biology. Zebrafish. 2010;7:69–81. doi: 10.1089/zeb.2009.0631. [DOI] [PubMed] [Google Scholar]
- Reimers MJ, La Du JK, Periera CB, Giovanini J, Tanguay RL. Ethanol-dependent toxicity in zebrafish is partially attenuated by antioxidants. Neurotoxicol Teratol. 2006;28:497–508. doi: 10.1016/j.ntt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Romitti PA, Sun L, Honein MA, Reefhuis J, Correa A, Rasmussen SA. Maternal periconceptional alcohol consumption and risk of orofacial clefts. Am J Epidemiol. 2007;166:775–785. doi: 10.1093/aje/kwm146. [DOI] [PubMed] [Google Scholar]
- Selderslaghs IW, Van Rompay AR, De Coen W, Witters HE. Development of a screening assay to identify teratogenic and embryotoxic chemicals using the zebrafish embryo. Reprod Toxicol. 2009;28:308–320. doi: 10.1016/j.reprotox.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Sengpiel V, Elind E, Bacelis J, Nilsson S, Grove J, Myhre R, Haugen M, Meltzer HM, Alexander J, Jacobsson B, Brantsaeter AL. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results from a large prospective observational cohort study. BMC Med. 2013;11:42. doi: 10.1186/1741-7015-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M, Fox NL, Hebel JR. Prenatal exposure to tobacco: II. Effects on cognitive functioning at age three. Int J Epidemiol. 1990;19:72–77. doi: 10.1093/ije/19.1.72. [DOI] [PubMed] [Google Scholar]
- Sfakianakis DG, Kentouri M. Effect of temperature on muscle lactate metabolic recovery in sea bass (Dicentrarchus labrax, L.) juveniles exposed to exhaustive exercise. Fish Physiol Biochem. 2010;36:387–390. doi: 10.1007/s10695-009-9305-y. [DOI] [PubMed] [Google Scholar]
- Sfakianakis DG, Leris I, Laggis A, Kentouri M. The effect of rearing temperature on body shape and meristic characters in zebrafish (Danio rerio) juveniles. Environ Biol Fishes. 2011;92:197–205. [Google Scholar]
- Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008;182:313–333. doi: 10.1007/978-3-540-74806-9_15. [DOI] [PubMed] [Google Scholar]
- Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev Camb Philos Soc. 2008;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research--advantages and current limitations. Toxicol Pathol. 2003;31(Suppl):62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DY, Fishman MC. The zebrafish as a model system to study cardiovascular development. Trends Cardiovasc Med. 1994;4:207–212. doi: 10.1016/1050-1738(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Staisey NL, Fried PA. Relationships between moderate maternal alcohol consumption during pregnancy and infant neurological development. J Stud Alcohol. 1983;44:262–270. doi: 10.15288/jsa.1983.44.262. [DOI] [PubMed] [Google Scholar]
- Stewart A, Wong K, Cachat J, Gaikwad S, Kyzar E, Wu N, Hart P, Piet V, Utterback E, Elegante M, Tien D, Kalueff AV. Zebrafish models to study drug abuse-related phenotypes. Rev Neurosci. 2011;22:95–105. doi: 10.1515/RNS.2011.011. [DOI] [PubMed] [Google Scholar]
- Su PH, Chang YZ, Chen JY. Infant with in utero ketamine exposure: quantitative measurement of residual dosage in hair. Pediatr Neonatol. 2010;51:279–284. doi: 10.1016/S1875-9572(10)60054-X. [DOI] [PubMed] [Google Scholar]
- Sylvain NJ, Brewster DL, Ali DW. Zebrafish embryos exposed to alcohol undergo abnormal development of motor neurons and muscle fibers. Neurotoxicol Teratol. 2010;32:472–480. doi: 10.1016/j.ntt.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem Biophys Res Commun. 2003;304:223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- Trickler WJ, Guo X, Cuevas E, Ali SF, Paule MG, Kanungo J. Ketamine attenuates cytochrome p450 aromatase gene expression and estradiol-17beta levels in zebrafish early life stages. J Appl Toxicol. 2013;39:69–76. doi: 10.1002/jat.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Belt K, Van Puymbroeck S, Witters H. Toxicity of cadmium-contaminated clay to the zebrafish Danio rerio. Arch Environ Contam Toxicol. 2000;38:191–196. doi: 10.1007/s002449910025. [DOI] [PubMed] [Google Scholar]
- Van den Bulck K, Hill A, Mesens N, Diekman H, De Schaepdrijver L, Lammens L. Zebrafish developmental toxicity assay: A fishy solution to reproductive toxicity screening, or just a red herring? Reprod Toxicol. 2011;32:213–219. doi: 10.1016/j.reprotox.2011.06.119. [DOI] [PubMed] [Google Scholar]
- Vogt A, Cholewinski A, Shen X, Nelson SG, Lazo JS, Tsang M, Hukriede NA. Automated image-based phenotypic analysis in zebrafish embryos. Dev Dyn. 2009;238:656–663. doi: 10.1002/dvdy.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vutskits L, Gascon E, Potter G, Tassonyi E, Kiss JZ. Low concentrations of ketamine initiate dendritic atrophy of differentiated GABAergic neurons in culture. Toxicology. 2007;234:216–226. doi: 10.1016/j.tox.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Wang C, Slikker W., Jr Strategies and experimental models for evaluating anesthetics: effects on the developing nervous system. Anesth Analg. 2008;106:1643–1658. doi: 10.1213/ane.ob013e3181732c01. [DOI] [PubMed] [Google Scholar]
- Wang LH, Tsai CL. Effects of temperature on the deformity and sex differentiation of tilapia, Oreochromis mossambicus. J Exp Zool. 2000;286:534–537. [PubMed] [Google Scholar]
- Weis JS, Weis P. Pollutants as developmental toxicants in aquatic organisms. Environ Health Perspect. 1987;71:77–85. doi: 10.1289/ehp.877177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William CM, Tanabe Y, Jessell TM. Regulation of motor neuron subtype identity by repressor activity of Mnx class homeodomain proteins. Development. 2003;130:1523–1536. doi: 10.1242/dev.00358. [DOI] [PubMed] [Google Scholar]
- Wimberger PH. Plasticity of fish body shape - the effects of diet, development, family and age in 2 species of Geophagus(Pisces, Cichlidae) Biol J Linn Soc Lond. 1992;45:197–218. [Google Scholar]
- Yozzo KL, Isales GM, Raftery TD, Volz DC. High-content screening assay for identification of chemicals impacting cardiovascular function in zebrafish embryos. Environ Sci Technol Lett. 2013;47:11302–11310. doi: 10.1021/es403360y. [DOI] [PubMed] [Google Scholar]
- Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4:35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]