Abstract
Background. Placental malaria is caused by Plasmodium falciparum–infected erythrocytes (IEs) that surface-express VAR2CSA and bind chondroitin sulfate A. The inflammatory response to placenta-sequestered parasites is associated with poor pregnancy outcomes, and protection may be mediated in part by VAR2CSA antibodies that block placental IE adhesion.
Methods. In this study, we used a new approach to assess VAR2CSA domains for functional epitopes recognized by naturally acquired antibodies. Antigen-specific immunoglobulin (Ig) G targeting Duffy binding–like (DBL) domains from different alleles were sequentially purified from plasma pooled from multigravid women and then characterized using enzyme-linked immunosorbent assay, flow cytometry, and antiadhesion assays.
Results. Different DBL domain-specific IgGs could react to homologous as well as heterologous antigens and parasites, suggesting that conserved epitopes are shared between allelic variants. Homologous blocking of IE binding was observed with ID1-DBL2-ID2a–, DBL4-, and DBL5-specific IgG (range, 42%–75%), whereas partial cross-inhibition activity was observed with purified IgG specific to ID1-DBL2-ID2a and DBL4 antigens. Plasma retained broadly neutralizing activity after complete depletion of these VAR2CSA specificities.
Conclusions. Broadly neutralizing antibodies of multigravidae are not depleted on VAR2CSA recombinant antigens, and hence development of VAR2CSA vaccines based on a single construct and variant might induce antibodies with limited broadly neutralizing activity.
Keywords: malaria, pregnancy, multigravidae, VAR2CSA, DBL domains, functional antibody, vaccine
The risk of Plasmodium falciparum infection is increased during pregnancy despite immunity gradually acquired by malaria-exposed individuals [1]. Pregnant women become highly susceptible to malaria particularly during their first pregnancy [1], with selective accumulation of P. falciparum–infected erythrocytes (IEs) in the intervillous spaces of the placenta [2]. Placental sequestration of IEs leads to an inflammatory infiltrate in the placenta with major consequences for mothers and fetus, such as anemia and low birth weight [3–5].
Chondroitin sulfate A (CSA) expressed by the syncytiotrophoblast has been identified as the major placental receptor involved in the cytoadherence of the parasites in the placenta [6]. Placental parasites predominantly express a member of P. falciparum erythrocyte membrane protein (PfEMP1) family, named VAR2CSA on the IE surface. VAR2CSA comprises an intracytoplasmic region, a transmembrane domain and an extracellular region that contains 6 Duffy binding–like (DBL) domains with additional interdomain (ID) regions. Several lines of evidence support VAR2CSA as the prime vaccine candidate against placental malaria (PM). Placental parasites consistently bind CSA and dominantly express VAR2CSA [7–10]. Knockout of var2csa blocks IE adhesion to CSA [11]. Anti-VAR2CSA antibodies are acquired over successive pregnancies [12–14] and are associated with protection against PM and low birth weight of neonates [15–18]. This protection correlates with the capacity of these antibodies to inhibit adhesion of the parasites to CSA [19]. Women acquire such functional antibody over successive pregnancies [16], resulting in high levels of these antibodies in multigravid women [18].
Despite the promising features of VAR2CSA, its large molecular weight and amino acid sequence polymorphisms are 2 major challenges that need to be overcome to develop an efficient manufacturable vaccine candidate. One approach has been to define smaller domains of VAR2CSA that can generate broadly neutralizing activity similar to that observed in serum samples from multigravid African women. Numerous studies have reported that smaller fragments from different alleles of VAR2CSA can elicit functional antibodies by animal immunization [20–25]. Evidence of shared epitopes between different alleles of VAR2CSA that cross-reacted with animal antisera raised against individual DBL domains has also been demonstrated [26].
Although several reports have addressed the characteristics of naturally acquired functional antibodies against VAR2CSA [27–29], the relative contribution of antibody specific to individual DBL domains in the total functional activity of immunoglobulin (Ig) G in multigravidae has not yet been defined. Whether antibody induced by animal immunization with recombinant protein displays a similar pattern of activity as naturally acquired antibody needs to be investigated. By dissecting protective or more specifically the adhesion-blocking antibody responses acquired by multigravid women, it may be possible to establish the relative importance of functional VAR2CSA antigen-specific antibodies in humans. In this study, we depleted specific antibodies to DBL domains from different alleles of VAR2CSA by affinity-purification from a pool of plasma from multigravid women (MG pool) and assessed the characteristics of the purified antibodies.
MATERIAL AND METHODS
MG Pool and P. falciparum Isolates
Maternal isolates of P. falciparum were collected from pregnant women during a study conducted from 2002–2006 in Muheza, Tanzania. Details of the project have been reported elsewhere [30]. Plasma samples of multigravid women were collected between 2011 and 2013 at the time of delivery in a study conducted in Ouelessebougou (Mali), according to a protocol approved by the institutional review boards of National Institute of Allergy and Infectious Diseases and of Mali. For this work, the MG pool was prepared, and 5 CSA-binding maternal isolates expressing VAR2CSA as well as 2 laboratory strains (FCR3 and CS2) selected to bind CSA were maintained in culture for the assays.
Production of Recombinant Proteins
Composite amino acid sequences of several P. falciparum VAR2CSA DBL domains from different parasite lines, as illustrated in Figure 1A, were used to generate codon-optimized synthetic genes for expression in Escherichia coli (T7 Express cells), essentially as described elsewhere [31]. The production and purification of the recombinant proteins DBL3X-FCR3, DBL4ε-FCR3, DBL5ε-FCR3, and Pichia pastoris–produced AMA1-C1 have been described elsewhere [25, 32, 33]. E. coli clones expressing the other recombinant DBL proteins (ID1-DBL2X-ID2a-1010, ID1-DBL2X-ID2a-711, ID1-DBL2X-ID2a-FCR3, DBL4ε-1010, DBL4ε-711, DBL5ε-1010, and DBL5ε-466) were grown in 5-L bioreactors (BioFlo 310; New Brunswick Scientific) using standard procedures [34]. Cells were grown at 37°C and induced with isopropyl-1-thio-β-galactopyranoside before cell harvesting by centrifugation. Recombinant DBL proteins were found packed in the inclusion bodies, diluted in Tris buffer, and lysed by microfluidization, as described elsewhere [35].
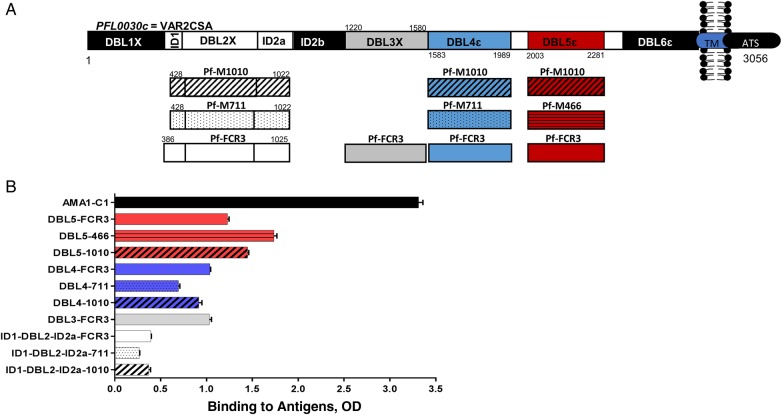
Figure 1.
VAR2CSA antigenic constructs and specific antibodies in the pool of plasma from multigravid women (MG pool). A, Recombinant ID1-DBL2X-ID2a (no color), DBL3X (gray), DBL4ε (blue), and DBL5ε (red) proteins were constructed for VAR2CSA FCR3 variant (no pattern) and maternal isolates M466 ( ), M711 (
), M711 ( ), and M1010 (
), and M1010 ( ). Boundaries of each construct are indicated relative to FCR3 VAR2CSA (GenBank accession No. KU665624). The same residue limit was used for all DBL4ε and DBL5ε constructs [25]. Different boundaries were used for ID1-DBL2X-ID2a-1010 and ID1-DBL2X-ID2a-711 (GenBank for accession No. KU665625), and ID1-DBL2X-ID2a-FCR3 (GenBank for accession No. KU665626) recombinant constructs. B, The MG pool was screened by means of enzyme-linked immunosorbent assay (ELISA) against the VAR2CSA DBL domain recombinant and AMA1 (used as non-VAR2CSA antigen control) antigens. Data are presented as level of antibody binding to the recombinant proteins, measured by optical density (OD). Bars represent means and standard deviations of duplicate wells. Abbreviations: ATS, acidic terminal sequence; TM, transmembrane.
). Boundaries of each construct are indicated relative to FCR3 VAR2CSA (GenBank accession No. KU665624). The same residue limit was used for all DBL4ε and DBL5ε constructs [25]. Different boundaries were used for ID1-DBL2X-ID2a-1010 and ID1-DBL2X-ID2a-711 (GenBank for accession No. KU665625), and ID1-DBL2X-ID2a-FCR3 (GenBank for accession No. KU665626) recombinant constructs. B, The MG pool was screened by means of enzyme-linked immunosorbent assay (ELISA) against the VAR2CSA DBL domain recombinant and AMA1 (used as non-VAR2CSA antigen control) antigens. Data are presented as level of antibody binding to the recombinant proteins, measured by optical density (OD). Bars represent means and standard deviations of duplicate wells. Abbreviations: ATS, acidic terminal sequence; TM, transmembrane.
The identity, purity and integrity of each recombinant ID1-DBL2X-ID2a protein was assessed. Amino-terminal sequencing was performed by the Research Technology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, as described elsewhere [35]. The refold integrity was evaluated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel mobility shifts under reducing and nonreducing conditions. Integrity was also assessed with reversed-phase high-performance liquid chromatography, according to methods reported elsewhere [31, 32, 35], using a Jupiter C4 reversed-phase column (Phenomenex). The theoretical masses were verified by electrospray ionization mass spectrometry [35]. Solution mass and aggregation profile was assessed by means of analytical size exclusion chromatography with multiangle light scattering, as reported elsewhere [31, 35]. The proteins were run on a Zenix-C SEC-150 size exclusion column (Sepax Technologies) with a phosphate-buffered saline (PBS) plus 308-mmol/L sodium chloride mobile phase. The column was in line with a DAWN HELEOS II light scattering detector (Wyatt Technologies) to determine molar mass. Far-UV circular dichroism spectra were collected as described elsewhere [36], but with a signal averaging time of 2.0 seconds.
Surface Plasmon Resonance Analysis
Binding affinity of ID1-DBL2X-ID2a recombinant proteins to chondroitin sulfate proteoglycan (CSPG) was analyzed using a Biacore3000 system (GE Healthcare), as described elsewhere [32]. Briefly, NeutrAvidin protein (Thermo Scientific) was covalently immobilized on a CM5 sensor chip (GE Healthcare) using N-hydroxysuccinimide amine coupling chemistry, followed by the immobilization of biotinylated CSPG (Sigma Aldrich) in flow cell 4, while a biotin-labeled irrelevant peptide was similarly immobilized in reference flow cell 3. Specific protein binding to the immobilized CSPG was obtained by subtracting the response on reference flow cell 3 from that on flow cell 4, after injection of a serial dilution of the ID1-DBL2X-ID2a recombinant proteins. The 1:1 Langmuir binding model was used in the kinetic and affinity analysis in BIAevaluation software (version 4.1.1; GE Healthcare).
Purification of Antigen-Specific IgG
Recombinant proteins were chemically cross-linked to N-hydroxysuccinimide–activated Sepharose beads (GE Healthcare), according to the manufacturer's instruction. VAR2CSA domain-specific and AMA1-specific antibodies were sequentially removed from the MG pool by affinity purification, by applying the flow-through sample from one antigen-specific column to the next column until all specificities were depleted. Bound antibodies were eluted with a low-pH buffer (Invitrogen), neutralized with 2 mol/L Tris buffer (pH 9.0), and dialyzed into PBS (pH 7.4). The concentration of the purified antibodies was measured using a NanoDrop spectrophotometer (ND-2000; Thermo Fisher Scientific). Total IgG was also purified from the MG pool using Protein G Sepharose 4 Fast Flow beads (GE Healthcare Life Sciences), according to the manufacturer's instruction.
Enzyme-Linked Immunosorbent Assay
Antigen recognition of the MG pool and the antigen-specific purified IgG was assessed with enzyme-linked immunosorbent assay (ELISA), as described elsewhere [37]. In brief, recombinant proteins were used to coat flat-bottom 96-well ELISA plates (Immulon 4; Dynex Technology), at 100 ng per well. Plates were incubated at 4°C overnight and blocked with buffer that contained 5% (wt/vol) skim milk powder (Difco) in Tris-buffered saline (BioFluids) for 2 hours at room temperature (RT). Serum samples (dilution, 1:500) or purified specific IgG (final concentration, 1 µg/mL) was added to antigen-coated wells in duplicate and incubated for 2 hours at RT. Plates were washed and incubated with anti-human IgG (heavy and light chains) (KPL) conjugated with alkaline phosphatase for 2 hours at RT, followed by 20 minutes of incubation in the dark at RT with the substrate (0.1 mg of p-nitrophenyl phosphate per well; Sigma 104 substrate; Sigma Aldrich). Absorbance at 405 nm was read using a Spectramax 340PC microplate reader (Molecular Devices). High cross-reactivity was defined as reactivity to heterologous versus homologous of ≥50%, and partial cross-reactivity was defined as reactivity ≥10% but <50%.
Inhibition of Binding Assay
Functional activity of the antibodies was defined as their ability to inhibit adhesion of IEs to CSA and assessed by inhibition of binding assay. Briefly, 20 µg/mL of CSA (Sigma Aldrich) in PBS was coated as spots in a Petri dish (Falcon 351029), incubated overnight at 4°C, and blocked with 3% bovine serum albumin–PBS. Late-stage IEs were gelatin enriched, and parasite density was adjusted to 20% at 0.5% hematocrit. IEs were blocked in 3% bovine serum albumin–Roswell Park Memorial Institute medium for 30 minutes at RT and incubated with serum samples (1:5 dilution) or 0.1 mg/mL of purified IgG for 30 minutes at 37°C. Cells were added to receptor-coated wells in duplicate and incubated for 15 minutes at RT. Nonadherent IEs were washed using a shaker washing system. Bound IEs were immediately fixed with 1.5% glutaraldehyde, stained with Giemsa, and quantified as the mean number of IEs per square millimeter counted in images acquired with an Mi5 microscope (LW Scientific). The percentage of inhibition was determined relative to the well without test sample. Inhibition activity property was attributed to antibody with percentage of inhibition >20%.
Flow Cytometry Analysis
VAR2CSA antigen-specific purified antibodies were analyzed with flow cytometry to assess their capacity to label the native protein expressed on the surface of erythrocytes infected by homologous and in some cases heterologous parasites. Briefly, mature-stage IEs were enriched by 1% gelatin (Sigma Aldrich) flotation and resuspended at 1 × 107 in PBS containing 2% fetal bovine serum. Cells were incubated with purified IgG (final concentration, 20 µg/mL) or a 1:20 dilution of the MG pool samples, and IEs were labeled with 0.1% Sybr Green (Life Technologies). After washing, bound antibodies were detected with phycoerythrin-conjugated goat (Fcγ-specific) anti-human IgG (eBioscience). Then 2 × 105 cells were acquired by an LSRII flow cytometer (BD Bioscience) and analyzed using the FlowJo 9.2 software program (Tree Star).
RESULTS
Strain-transcending Reactivity of Naturally Acquired Antibodies to VAR2CSA DBL Domains
To assess the level of antibodies naturally acquired against VAR2CSA DBL domains, the MG pool was screened with ELISA against the ID1-DBL2-ID2a, DBL3X, DBL4ε, and DBL5ε recombinant protein domains from FCR3, as well as composite sequences for field isolates M1010, M711, and M466 allelic forms of VAR2CSA. Recombinant ID1-DBL2X-ID2a proteins were biochemically and biophysically characterized and were shown to have good identity, integrity, and purity (Supplementary Figure 1). Recombinant ID1-DBL2X-ID2a from FCR3-bound CSPG with nanomolar affinity (36 nmol/L; χ2 = 1.75) (Supplementary Figure 2A), and other ID1-DBL2X-ID2a constructs bound with micromolar affinities (13 µmol/L [χ2 = 11.4] for M1010 and 7.68 µmol/L [χ2 = 11.5] for M711). Interestingly, the secondary structures of these proteins as evaluated by circular dichroism showed similar patterns (Supplementary Figure 2B). ELISA absorbance values were greater for DBL3X, DBL4ε and DBL5ε antigen-constructs than for ID1-DBL2-ID2a (Figure 1B). The sequence identity between the antigenic constructs is provided in Supplementary Table 1. To ensure that antigen-specific antibodies were removed from the MG pool, fractions of the flow-through samples after each antigen-specific antibody purification were also screened with ELISA against the same panel of antigens. Naturally acquired antibodies to VAR2CSA DBL domains and AMA1 antigens used in this study depleted from the MG pool so that the remaining level was similar to that in US naive serum samples (Supplementary Figures 3 and 4).
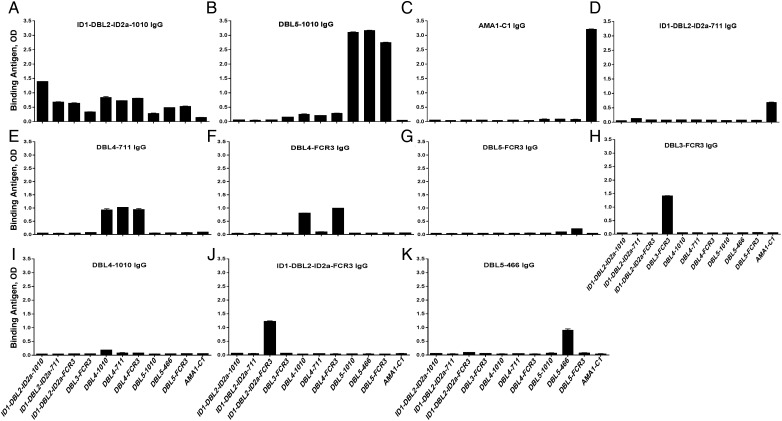
Purified IgG cross-reacted with homologous and heterologous constructs of the antigens, as determined by ELISA (Figure 2). In accordance with the order of the antigen-specific purified antibodies (Table 1), specificity of the purified IgG to the previous antigen was removed in the successive purified IgG targeting the same antigenic constructs from different alleles. For instance, IgG specific to DBL4-711 (first DBL4 antigen used) cross-reacted to DBL4-FCR3 (second DBL4) and DBL4-1010 (third DBL4) antigens, whereas DBL4-FCR3–specific IgG cross-reacted only to DBL4-1010 and not to DBL4-711. IgG specific to DBL4-1010 showed no cross-reactivity to the heterologous DBL4 antigens. Similar pattern of cross-reactivity was observed for ID1-DBL2-ID2a– and DBL5-specific antibodies. Only the ID1-DBL2-ID2a-1010–specific IgG, which was purified first from the MG pool, showed a partial cross-reactivity to other VAR2CSA domains from different allelic forms. As expected, AMA1-specific IgG showed no reactivity to VAR2CSA DBL domain antigens.
Figure 2.
Antigenic reactivity of the purified antigen-specific immunoglobulin (Ig) G. Purified naturally acquired antibody specific to each VAR2CSA Duffy binding–like (DBL) domain and AMA1 recombinant protein were screened by enzyme-linked immunosorbent assay (ELISA) against the panel of antigens to analyze their specificity and cross-reactivity. A–K, Antigen binding profile of each specific purified IgG following the order of depletion. Data are presented as level of antibody binding to the recombinant proteins, measured by optical density (OD). Bars represent means and standard deviations of duplicate wells.
Table 1.
Purified Antigen-Specific Antibody From 4 mL of the MG Pool
| Order of Depletion | Specific Purified Antibodies | Antibody Yield, µg |
|---|---|---|
| 1 | ID1-DBL2-ID2a-1010 | 26 |
| 2 | DBL5-1010 | 22.5 |
| 3 | AMA1 | 298.6 |
| 4 | ID1-DBL2-ID2a-711 | 17.5 |
| 5 | DBL4-711 | 16.9 |
| 6 | DBL4-FCR3 | 15.84 |
| 7 | DBL5-FCR3 | 16.81 |
| 8 | DBL3-FCR3 | 21.84 |
| 9 | DBL4-1010 | 5.51 |
| 10 | ID1-DBL2-ID2a-FCR3 | 7.22 |
| 11 | DBL5-466 | 6.44 |
Abbreviation: MG pool, pool of plasma from multigravid women.
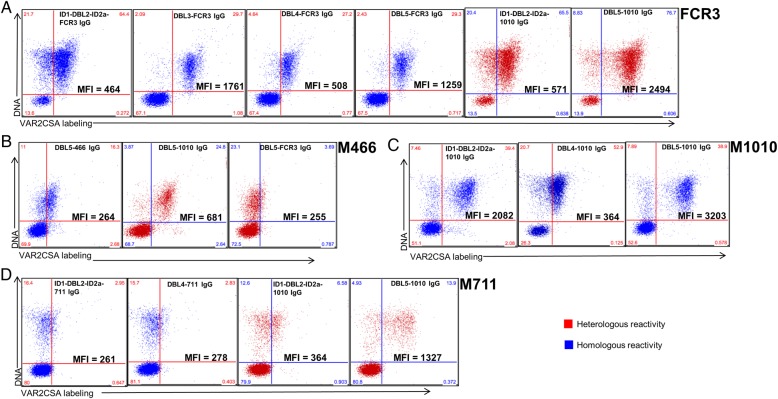
Most importantly, the VAR2CSA DBL domain purified IgG recognized the native protein expressed by homologous parasites at various levels, as shown by flow cytometry (Figure 3). Owing to limited volumes, cross-labeling assay with heterologous parasites could not be performed for all the purified antibodies. However, ID1-DBL2-ID2a-1010–and DBL5-1010–specific IgG (first 2 purified antibodies) cross-labeled heterologous strains, consistent with the cross-reactivity of the purified IgG to immobilized recombinant antigens in ELISA.
Figure 3.
Recognition of the native VAR2CSA by the purified antigen-specific immunoglobulin (Ig) G. In flow cytometry, the ability of the purified VAR2CSA Duffy binding–like (DBL) domain-specific antibody to label the native VAR2CSA expressed on the surface of Plasmodium falciparum–infected erythrocytes by homologous (blue) and heterologous (red) parasites, was assessed on isolates FCR3 (A), M466 (B), M1010 (C), and M711 (D). Median fluorescent intensity (MFI), is shown, as well as the proportion of labeled infected erythrocytes relative to the total cells.
Homologous and Partial Heterologous Blocking Activity of the Purified IgG
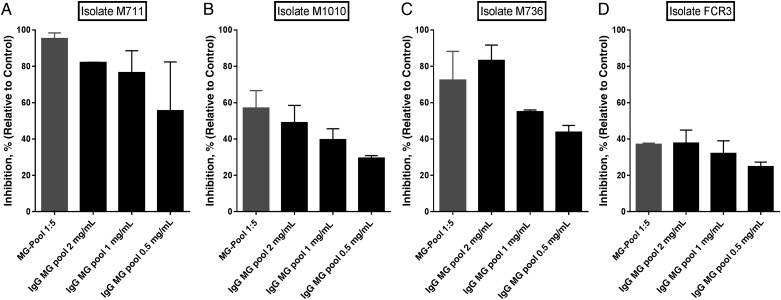
The mean CSA-binding levels of M711, M1010, M736, M918, CS2, and FCR3 isolates determined per square millimeter were 510, 914, 929, 790, 953, and 960, respectively. Inhibition activity of the MG pool was assessed on M711, M1010, M736, and FCR3 isolates. Variable functional activity of the MG pool (dilution 1:5) was observed with inhibition levels that ranged from 37% for FCR3 to 98% for M711 (Figure 4). Isolates M1010 and M736 were similarly inhibited by the MG pool. Notably, adhesion of the maternal isolates to CSA was highly inhibited by the MG pool compared with the laboratory-adapted strain FCR3. However, the degree of FCR3 inhibition varied among different MG pool preparations (data not shown), presumably reflecting variant-specific antibody activity. The total IgG purified from the MG pool showed the similar inhibitory activity against the isolates, supporting that the antiadhesion antibodies are mainly derived from IgG.
Figure 4.
Functional activity of the pool of plasma from multigravid women (MG pool). Inhibitory profile of the MG pool of plasma as well as different concentration of total immunoglobulin (Ig) G purified from the same pool were assessed on isolates M711 (A), M1010 (B), M736 (C) and FCR3 (D). Proportions of inhibited infected erythrocytes relative to the well without test sample are presented. Bars represent means and standard deviations of duplicate wells.
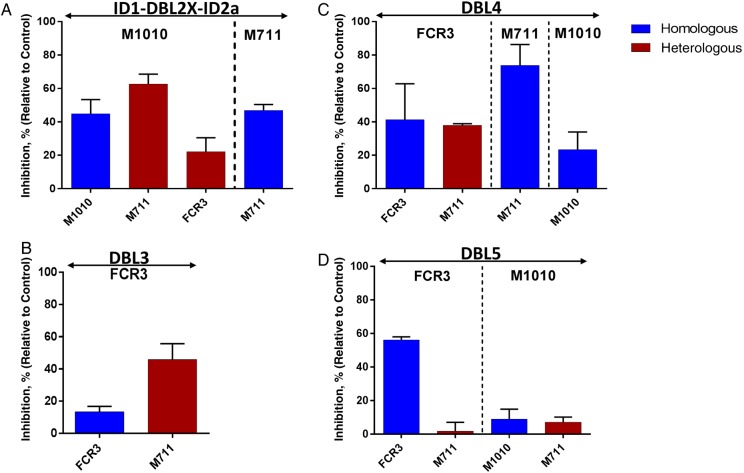
Functional activity of the purified antibodies against VAR2CSA domains was also assessed on homologous and heterologous parasites (Figure 5). All VAR2CSA domain-specific antibodies showed homologous inhibitory activity, except naturally acquired antibodies against DBL3-FCR3 and DBL5-1010. However, of those 2 exceptions, binding of heterologous parasite to CSA was blocked only by DBL3-FCR3 antibodies (Figure 5B). Anti-DBL5-1010 showed no functional activity against either of the tested isolates (Figure 5D), despite the fact that this purified antibody significantly labeled the native VAR2CSA expressed on the surface of several isolates. Interestingly, cross-inhibition activity against different parasite variants was observed with antibodies specific to ID1-DBL2-ID2a-1010 and DBL4-FCR3 (Figure 5A and 5C). These antibodies blocked adhesion to CSA of homologous (45% for ID1-DBL2-ID2a-1010 and 42% for DBL4-FCR3) and heterologous parasites (63% and 38% on M711 isolate for ID1-DBL2-ID2a-1010 and DBL4-FCR3, respectively). Other purified antibodies targeting the same constructs of VAR2CSA (ID1-DBL2-ID2a and DBL4) from different alleles showed modest (25% for DBL4-1010) and moderate (47% for ID1-DBL2-ID2a-711 and 74% for DBL4-711) inhibition activity against homologous parasites but were not tested on heterologous strains due to the limited material. As expected, no inhibitory activity was observed with anti-AMA1 antibodies (Supplementary Figure 5).
Figure 5.
Functional activity of VAR2CSA antigen-specific purified antibodies on different strains of Plasmodium falciparum. Blocking activity of the naturally acquired antibody specific to VAR2CSA Duffy binding–like (DBL) domain against the chondroitin sulfate A (CSA)–binding parasites was assessed. Purified antibodies specific to ID1-DBL2X-ID2a (A), DBL3 (B), DBL4 (C), and DBL5 (D) domain constructs from FCR3, M1010, and M711 variants were tested on the corresponding isolates, to analyze their homologous (blue histogram) and heterologous (red histogram) inhibition activity. Proportion of inhibited P. falciparum–infected erythrocytes relative to the well without test sample are presented. Bars represent means and standard deviations of duplicate wells.
Retention of Broadly Neutralizing Activity by the Depleted MG Pool
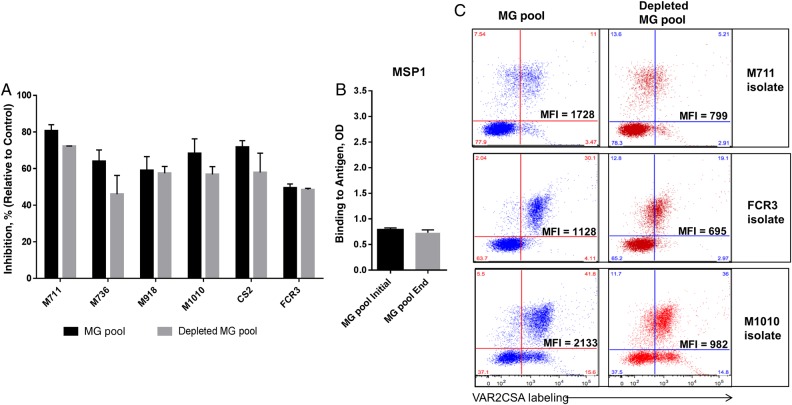
To assess the effect on functional activity of the depletion of purified antibodies against the VAR2CSA domain, we compared the inhibition profile of the MG pool before and after antibody depletion (Figure 6A). Although the level of inhibition had decreased slightly, there was no significant difference before and after the antibody depletions (P > .05 by paired t test for each isolate). Indeed, significant functional activity remained in the MG pool despite the removal of antibodies with CSA-binding inhibition property. Dilution of the MG pool after repeated antibody depletion was assessed by comparing antibody level to merozoite surface protein 1 (MSP1) antigen in both pools (Figure 6B). Level of anti-MSP1 did not significantly decrease in the depleted MG pool (P = .156, by paired t test), suggesting that the slight difference measured in the functional activity of both MG pool is not attributable to the sample dilution. Of note, the level of native VAR2CSA recognition was decreased markedly in the depleted MG pool, suggesting that antibodies targeting immunodominant epitopes in VAR2CSA had been largely removed (Figure 6C).
Figure 6.
Characteristics of the depleted pool of plasma from multigravid women (MG pool). A, The MG pool and depleted MG pool were characterized for their inhibitory activity against laboratory strains (FCR3 and CS2) and 4 maternal isolates (M711, M736, M918, and M1010). B, Levels of antibody specific to merozoite surface protein 1 (MSP1) antigen were compared in both plasma pools. C, Their capacity to label the native VAR2CSA expressed on the surface of Plasmodium falciparum–infected erythrocytes from 3 isolates are reported, shown as median fluorescent intensity (MFI) and the proportion of labeled infected erythrocytes relative to the total cells. Bars represent means and standard deviations of duplicate wells.
DISCUSSION
Naturally acquired immunity that mediates protection of multigravidae against the consequences of PM is the key basis for development of a vaccine to protect women during pregnancy. This specific immunity against placental parasites can recognize geographically diverse CSA-binding isolates [14, 19, 38], suggesting that multigravidae acquire a repertoire of strain-transcending functional antibodies over successive pregnancies [7, 14, 39]. An effective vaccine protecting women against PM should therefore induce antibodies that mimic this broadly neutralizing activity observed in multigravidae. VAR2CSA is currently the leading PM vaccine candidate and efforts to design boundaries that can elicit potent strain-transcending antibody are ongoing [40]. Indeed, despite the available promising data that support a VAR2CSA-based vaccine, particularly its N-terminal fragments [18, 24, 41–43], it remains unclear whether protection is mediated by antibodies targeting conserved VAR2CSA epitopes or by a broad range of antibodies against several alleles of VAR2CSA [28].
In this study, we characterized naturally acquired antibodies to VAR2CSA DBL domains purified from plasma pooled from multigravidae with broadly neutralizing activity. As reported in previous studies, plasma from multigravid women had higher titers of antibodies reacting to DBL3, DBL4, and DBL5 domains compared with ID1-DBL2-ID2a constructs [18, 28], supporting the idea that these DBL domains are more immunogenic. Irrespective of the allelic form of VAR2CSA, this difference in reactivity between DBL domains was maintained.
Specificity and cross-reactivity of the VAR2CSA DBL domain purified antibodies were similar to those generated by immunization of experimental animals against different alleles (3D7 and It4) [26]. This observation is in line with recent reports on antibody responses to VAR2CSA antigens in Cameroonian pregnant women [28], suggesting that naturally acquired antibodies specific to DBL domains target epitopes shared by different alleles of VAR2CSA. Antigenic variation is probably the main factor contributing to differences in antibody recognition. Our data demonstrate that, although multigravid women acquire several VAR2CSA allele-specific antibodies, antibodies against the most conserved shared epitopes were removed by the first antigenic variant. To investigate whether this observation is allele or domain dependent, the antigen order was changed in processing a second MG pool. Purified IgG from the first column (which used different domain and allele) cross-reacted to the same domain from other alleles (Supplementary Figure 4).
Interestingly, all the DBL domain-purified antibody labeled the native VAR2CSA expressed by IEs, and several showed blocking activity against homologous parasites. As reported from animal immunization studies [20, 21, 44], though DBL domain-specific antibodies can recognize recombinant and native epitopes, functional activity is limited to few constructs and variants. For example, anti-DBL5-1010 antibody that cross-reacted with DBL5 antigenic constructs used in this study, and recognized native VAR2CSA from different alleles, showed no blocking activity. Blocking activity of antibody might be mediated by the modification of VAR2CSA conformational epitopes (by binding to the epitopes), by direct inhibition of the key binding-residues involved in the interaction with CSA, or by binding to proximal epitopes, thereby blocking key residues for adhesion to CSA by steric hindrance [45]. In this study, partial blocking activity against homologous and heterologous isolates has been observed with naturally acquired antibody to ID1-DBL2-ID2a and DBL4 constructs, suggesting that these constructs might be of potential interest in the VAR2CSA-based vaccine design [22, 43]. The fact that anti-DBL3 antibody showed no blocking activity against the homologous strain yet disrupted binding of a heterologous strain to CSA was unexpected. An explanation might be that functional antibodies were impeded by nonfunctional variant-specific antibodies that react to homologous but not heterologous parasites. An example of impeding nonfunctional antibody has been observed with IgG against the merozoite antigen MSP1 [46] as well as IgM against VAR2CSA [47].
Of particular interest, we found that the functional antibodies naturally acquired by multigravid women were largely not depleted by VAR2CSA constructs used in this study, including N-terminal constructs, which are currently being evaluated in clinical trials. This may suggest that functional antibodies target multiple conserved epitopes from several alleles of VAR2CSA. Indeed, multigravid women may have developed a repertoire of functional antibodies against several alleles of VAR2CSA that are not all represented among the variants used in this study. It is therefore possible that the functional antibodies in the depleted MG pool might have affinity either to conformational epitopes not displayed by any of the antigenic constructs used in this study or to epitopes from different variants of VAR2CSA. Investigations are needed to identify conformational epitopes in VAR2CSA that react to functional antibodies. Approaches that map functional epitopes throughout the full-length VAR2CSA might help define the residues of interest. The functional antibody in the depleted pool might also be directed to other conserved proteins [48] that could form a complex with VAR2CSA and display epitopes recognized by broadly neutralizing antibody [40]. Studies aiming to identify additional proteins as potential vaccine candidates for use in combination with VAR2CSA are still needed.
Overall, this study yielded some key observations regarding the specificity of functional antibodies naturally acquired by multigravid women. Several functional epitopes targeted by naturally acquired functional antibodies in multigravidae are present throughout the extracellular part of VAR2CSA and appear in different alleles. Antibodies against a subunit construct of VAR2CSA from a single allele might not display the complete repertoire of functional epitopes and might show limited breadth of activity against field isolates. As with vaccines against other malaria antigens, such as AMA1, a multivalent vaccine comprising 3–5 alleles [49, 50], or incorporating additional PM vaccine candidates [40], may be needed to elicit the broadly neutralizing activity seen in serum samples from African multigravidae.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We are thankful to all the women who participated in Mother-Offspring Malaria Study (MOMS) project. We thank Drs Scaria Puthupparampil and Daniel Shaji for useful discussion and advice. Special thanks to Michael Murphy for assistance with surface plasmon resonance analysis and Srinivasan Prakash for providing the biotinylated peptide used in the surface plasmon resonance experiment.
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ 1983; 61:1005. [PMC free article] [PubMed] [Google Scholar]
- 2.Muthusamy A, Achur RN, Bhavanandan VP, Fouda GG, Taylor DW, Gowda DC. Plasmodium falciparum-infected erythrocytes adhere both in the intervillous space and on the villous surface of human placenta by binding to the low-sulfated chondroitin sulfate proteoglycan receptor. Am J Pathol 2004; 164:2013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg 2003; 68:115–9. [PubMed] [Google Scholar]
- 4.Shulman CE, Graham WJ, Jilo H et al. Malaria is an important cause of anaemia in primigravidae: evidence from a district hospital in coastal Kenya. Trans R Soc Trop Med Hyg 1996; 90:535–9. [DOI] [PubMed] [Google Scholar]
- 5.Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg 1996; 55(1 Suppl):33–41. [DOI] [PubMed] [Google Scholar]
- 6.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 1996; 272:1502–4. [DOI] [PubMed] [Google Scholar]
- 7.Tuikue Ndam NG, Fievet N, Bertin G, Cottrell G, Gaye A, Deloron P. Variable adhesion abilities and overlapping antigenic properties in placental Plasmodium falciparum isolates. J Infect Dis 2004; 190:2001–9. [DOI] [PubMed] [Google Scholar]
- 8.Duffy MF, Maier AG, Byrne TJ et al. VAR2CSA is the principal ligand for chondroitin sulfate A in two allogeneic isolates of Plasmodium falciparum. Mol Biochem Parasitol 2006; 148:117–24. [DOI] [PubMed] [Google Scholar]
- 9.Magistrado P, Salanti A, Tuikue Ndam NG et al. VAR2CSA expression on the surface of placenta-derived Plasmodium falciparum-infected erythrocytes. J Infect Dis 2008; 198:1071–4. [DOI] [PubMed] [Google Scholar]
- 10.Doritchamou J, Sossou-Tchatcha S, Cottrell G et al. Dynamics in the cytoadherence phenotypes of Plasmodium falciparum infected erythrocytes isolated during pregnancy. PloS One 2014; 9:e98577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viebig NK, Gamain B, Scheidig C et al. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Rep 2005; 6:775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neil-Dunne I, Achur RN, Agbor-Enoh ST et al. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect Immun 2001; 69:7487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuikue Ndam NG, Salanti A, Le-Hesran JY et al. Dynamics of anti-VAR2CSA immunoglobulin G response in a cohort of Senegalese pregnant women. J Infect Dis 2006; 193:713–20. [DOI] [PubMed] [Google Scholar]
- 14.Ricke CH, Staalsoe T, Koram K et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J Immunol Baltim Md 1950 2000; 165:3309–16. [DOI] [PubMed] [Google Scholar]
- 15.Fried M, Duffy PE. Maternal malaria and parasite adhesion. J Mol Med Berl Ger 1998; 76:162–71. [DOI] [PubMed] [Google Scholar]
- 16.Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun 2003; 71:6620–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 2004; 363:283–9. [DOI] [PubMed] [Google Scholar]
- 18.Ndam NT, Denoeud-Ndam L, Doritchamou J et al. Protective antibodies against placental malaria and poor outcomes during pregnancy, Benin. Emerg Infect Dis J 2015; 21:813-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature 1998; 395:851–2. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen MA, Pinto VV, Resende M et al. Induction of adhesion-inhibitory antibodies against placental Plasmodium falciparum parasites by using single domains of VAR2CSA. Infect Immun 2009; 77:2482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salanti A, Resende M, Ditlev SB et al. Several domains from VAR2CSA can induce Plasmodium falciparum adhesion-blocking antibodies. Malar J 2010; 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magistrado PA, Minja D, Doritchamou J et al. High efficacy of anti DBL4ε-VAR2CSA antibodies in inhibition of CSA-binding Plasmodium falciparum-infected erythrocytes from pregnant women. Vaccine 2011; 29:437–43. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez P, Petres S, Mécheri S, Gysin J, Scherf A. Strain-transcendent immune response to recombinant Var2CSA DBL5-ε domain block P. falciparum adhesion to placenta-derived BeWo cells under flow conditions. PLoS One 2010; 5:e12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doritchamou J, Bigey P, Nielsen MA et al. Differential adhesion-inhibitory patterns of antibodies raised against two major variants of the NTS-DBL2X region of VAR2CSA. Vaccine 2013; 31:4516–22. [DOI] [PubMed] [Google Scholar]
- 25.Fried M, Avril M, Chaturvedi R et al. Multilaboratory approach to preclinical evaluation of vaccine immunogens for placental malaria. Infect Immun 2013; 81:487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avril M, Kulasekara BR, Gose SO et al. Evidence for globally shared, cross-reacting polymorphic epitopes in the pregnancy-associated malaria vaccine candidate VAR2CSA. Infect Immun 2008; 76:1791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ndam NT, Denoeud-Ndam L, Doritchamou J et al. Protective Antibodies against Placental Malaria and Poor Outcomes during Pregnancy, Benin. Emerg Infect Dis 2015; 21:813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babakhanyan A, Fang R, Wey A et al. Comparison of the specificity of antibodies to VAR2CSA in Cameroonian multigravidae with and without placental malaria: a retrospective case-control study. Malar J 2015; 14:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barfod L, Dobrilovic T, Magistrado P et al. Chondroitin sulfate A-adhering Plasmodium falciparum-infected erythrocytes express functionally important antibody epitopes shared by multiple variants. J Immunol Baltim Md 1950 2010; 185:7553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutabingwa TK, Bolla MC, Li JL et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med 2005; 2:e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrera R, Anderson C, Kumar K et al. Reversible conformational change in the Plasmodium falciparum circumsporozoite protein masks its adhesion domains. Infect Immun 2015; 83:3771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obiakor H, Avril M, Macdonald NJ et al. Identification of VAR2CSA domain-specific inhibitory antibodies of the Plasmodium falciparum erythrocyte membrane protein 1 using a novel flow cytometry assay. Clin Vaccine Immunol CVI 2013; 20:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giersing B, Miura K, Shimp R et al. posttranslational modification of recombinant Plasmodium falciparum apical membrane antigen 1: impact on functional immune responses to a malaria vaccine candidate. Infect Immun 2005; 73:3963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimp RL, Martin LB, Zhang Y et al. Production and characterization of clinical grade Escherichia coli derived Plasmodium falciparum 42 kDa merozoite surface protein 1 (MSP1(42)) in the absence of an affinity tag. Protein Expr Purif 2006; 50:58–67. [DOI] [PubMed] [Google Scholar]
- 35.Uchime O, Herrera R, Reiter K et al. Analysis of the conformation and function of the Plasmodium falciparum merozoite proteins MTRAP and PTRAMP. Eukaryot Cell 2012; 11:615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plassmeyer ML, Reiter K, Shimp RL et al. Structure of the Plasmodium falciparum circumsporozoite protein, a leading malaria vaccine candidate. J Biol Chem 2009; 284:26951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine 2008; 26:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maubert B, Fievet N, Tami G, Cot M, Boudin C, Deloron P. Development of antibodies against chondroitin sulfate A-adherent Plasmodium falciparum in pregnant women. Infect Immun 1999; 67:5367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beeson JG, Mann EJ, Elliott SR et al. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes and adhesion inhibitory antibodies are associated with placental malaria and have overlapping and distinct targets. J Infect Dis 2004; 189:540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fried M, Duffy PE. Designing a VAR2CSA-based vaccine to prevent placental malaria. Vaccine 2015; 33:7483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bigey P, Gnidehou S, Doritchamou J et al. The NTS-DBL2X region of VAR2CSA induces cross-reactive antibodies that inhibit adhesion of several Plasmodium falciparum isolates to chondroitin sulfate A. J Infect Dis 2011; 204:1125–33. [DOI] [PubMed] [Google Scholar]
- 42.Clausen TM, Christoffersen S, Dahlback M et al. Structural and functional nsight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J Biol Chem 2012; 287:23332–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen MA, Resende M, de Jongh WA et al. The influence of sub-unit composition and expression system on the functional antibody response in the development of a VAR2CSA based Plasmodium falciparum placental malaria vaccine. PloS One 2015; 10:e0135406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinto VV, Ditlev SB, Jensen KE et al. Differential induction of functional IgG using the Plasmodium falciparum placental malaria vaccine candidate VAR2CSA. PLoS One 2011; 6:e17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nunes-Silva S, Gangnard S, Vidal M et al. Llama immunization with full-length VAR2CSA generates cross-reactive and inhibitory single-domain antibodies against the DBL1X domain. Sci Rep 2014; 4:7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nwuba RI, Sodeinde O, Anumudu CI et al. The human immune response to Plasmodium falciparum includes both antibodies that inhibit merozoite surface protein 1 secondary processing and blocking antibodies. Infect Immun 2002; 70:5328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevenson L, Huda P, Jeppesen A et al. Investigating the function of Fc-specific binding of IgM to Plasmodium falciparum erythrocyte membrane protein 1 mediating erythrocyte rosetting. Cell Microbiol 2015; 17:819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francis SE, Malkov VA, Oleinikov AV et al. Six genes are preferentially transcribed by the circulating and sequestered forms of Plasmodium falciparum parasites that infect pregnant women. Infect Immun 2007; 75:4838–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Remarque EJ, Faber BW, Kocken CHM, Thomas AW. A diversity-covering approach to immunization with Plasmodium falciparum apical membrane antigen 1 induces broader allelic recognition and growth inhibition responses in rabbits. Infect Immun 2008; 76:2660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drew DR, Hodder AN, Wilson DW et al. Defining the antigenic diversity of Plasmodium falciparum apical membrane antigen 1 and the requirements for a multi-allele vaccine against malaria. PloS One 2012; 7:e51023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.