Abstract
Background and Aims:
Golimumab has been approved recently to treat refractory moderate-to-severe ulcerative colitis [UC]. To date it is not clear why a considerable fraction of patients do not respond, or lose initial response, to golimumab therapy. Our aim was to investigate whether a low golimumab serum concentration and/or a positive anti-golimumab antibody status reduces the efficacy of this drug in patients with UC.
Methods:
Serum samples of 21 patients with moderate-to-severe UC were collected during the first 14 weeks of golimumab therapy. For measurement of golimumab serum concentrations, both a tumour necrosis factor [TNF]-coated enzyme-linked immunosorbent assay [ELISA] and a sandwich-type ELISA were developed. Anti-golimumab antibodies were measured using a bridging ELISA and a newly-developed drug-tolerant immunoassay. Clinical response and mucosal healing were assessed 14 weeks after start of treatment.
Results
Out of 21 patients, 10 [48%] reached partial clinical response at Week 14. Median [interquartile range] serum golimumab concentration was significantly higher in partial clinical responders than in non-responders: 10.0 [7.8–10.5] µg/ml versus 7.4 [4.8–8.3] µg/ml at Week 2 [p = 0.035] and 5.1 [4.0–7.9] µg/ml versus 2.1 [1.8–4.2] µg/ml at week 6 [p = 0.037]. Four out of 21 UC patients developed anti-golimumab antibodies, detectable only using a drug-tolerant immunoassay, and three had a partial clinical response at that time. Clinical non-responders had a significantly more severe colitis, indicated by a higher endoscopic Mayo score at baseline compared with partial clinical responders [p = 0.048].
Conclusion:
Adequate exposure to golimumab drives clinical response. A worse disease at baseline influences clinical response rate negatively.
Keywords: Therapeutic drug monitoring, golimumab, ulcerative colitis
1. Introduction
Ulcerative colitis [UC] is a chronic relapsing intestinal inflammatory disorder characterised by mucosal ulceration of the colon resulting in bloody diarrhoea, urgency, and pain. The onset of the disease mainly occurs in young adulthood and can therefore significantly erode patients’ productivity and quality of life.1 Until recently, the main goal in treatment of UC was to bring a patient to full clinical remission. The launching of the anti-tumour necrosis factor [TNF] agents, infliximab [Remicade®, Remsima®, and Inflectra®] and adalimumab [Humira®] radically changed the concept of treating refractory UC.2 These biologicals have indeed proven to induce endoscopic remission [mucosal healing] and to decrease hospitalisation and colectomy rates in patients with UC; however, loss of response remains a major concern in clinical practice.3,4
Golimumab [Simponi®] is a human IgG1 kappa monoclonal antibody [MA], derived from TNF-immunised transgenic mice engineered to express human IgGs.5 Golimumab binds to both the soluble and transmembrane bioactive forms of human TNF, giving rise to stable high-affinity complexes and thereby preventing the binding of TNF to its receptors.6 Golimumab is the first subcutaneously administered, once monthly dosed, anti-TNF biological that has been approved for the treatment of UC.7
The PURSUIT-SC induction study demonstrated a positive exposure-effect relation between serum golimumab concentration and clinical outcomes at Week 6.8 In this trial, 51.0% of the patients in the golimumab 200/100mg group responded clinically upon induction therapy. The PURSUIT maintenance trial provided additional evidence that higher golimumab serum concentrations were associated with greater rates of clinical response and remission.9 However, at this moment, the optimal therapeutic window for golimumab in UC is unknown. As previously reported for both infliximab and adalimumab, it can be hypothesised that golimumab also evokes an immune response resulting in the formation of anti-drug antibodies [ADAb] that may or may not be neutralising and may or may not be persistent. To date, little is known about anti-golimumab antibody development and its relation to clinical response in patients with UC. In addition, many questions concerning immunogenicity have not yet been answered and different methods of detection are being used, which makes the results difficult to compare.
In this study, we generated monoclonal antibodies towards golimumab and developed assays to determine golimumab and anti-golimumab concentrations in a cohort of patients with UC who started golimumab therapy.
2. Materials and Methods
2.1. Patients
At Week 14, 21 consecutive patients with moderate-to-severely active UC [endoscopic Mayo score 2/3] who were started on 200mg and 100mg subcutaneous golimumab at Weeks 0 and 2, respectively, were evaluated and included in the study. All 21 patients had symptoms of diarrhoea and blood at baseline. Serum samples were taken before the first injection and at Weeks 1, 2, 4, 6, and 14. Both golimumab and anti-golimumab concentrations were retrospectively analysed. Clinical response to golimumab and mucosal healing were assessed at Week 14 by the treating physician who was unaware of the serum drug concentrations. Clinical response was defined as complete if there was absence of diarrhoea and blood, and partial if there was marked clinical improvement but still persistent rectal blood loss.10 Mucosal healing was defined as a Mayo endoscopic subscore of 0 or 1 at Week 10–14.10 Laboratory tests included albumin and C-reactive protein [CRP] determination. This study was approved by the Ethical Committee UZ Leuven in the framework of the Flemish inheritance study for Crohn’s and colitis [B322201213950/S53684]. Informed consent was provided by all patients.
2.2. Generation and characterisation of monoclonal antibodies towards golimumab
Through hybridoma technology, three mouse monoclonal antibodies were generated towards golimumab [ie MA-GOM131E3, MA-GOM159B8, and MA-GOM171D8]. Production, purification, and conjugation of the MA-GOM were carried out as described previously.11 The antibodies were characterised with respect to: 1] their differential cross-reactivity towards infliximab, adalimumab, and a human IgG-mixture [in ELISA setting]11; 2] their ability to inhibit the TNF-golimumab interaction [in a functional cell-based assay]12; and 3] their affinity for golimumab [using surface plasmon resonance analysis].11 The isotypes of the MA-GOM were determined by the IsoStripTM Mouse Monoclonal Antibody Isotyping kit [Roche Diagnostics®, Belgium].
2.3. Measurement of serum golimumab concentrations
Serum concentrations of golimumab were determined using a TNF-coated enzyme-linked immunosorbent assay [ELISA] set-up, which is in principle similar to the test that is routinely carried out for quantification of infliximab concentrations.11 A conjugated anti-golimumab monoclonal antibody [MA-GOM] was used for the detection of TNF-bound golimumab. In addition, serum concentrations of golimumab were also quantified using a sandwich-type ELISA, based on the principle that golimumab is captured between an immobilised MA-GOM-1 and an added horseradish peroxidase [HRP]-labelled MA-GOM-2 that targets a different epitope on the golimumab molecule. Analytical validation including the performance characteristics of the assay [calibration standards, sensitivity, accuracy, and imprecision] was performed in a similar way to that described for the infliximab ELISA [Van Stappen et al.].11 External analytical validation was performed by Sanquin Diagnostic Services [Amsterdam, The Netherlands] using an in-house developed ELISA. In this assay, golimumab levels were measured using TNF for capture and rabbit anti-golimumab for detection [lower limit of quantification 5ng/ml; accuracy 103%, imprecision 12%].13
2.4. Measurement of serum anti-golimumab antibody concentrations
Anti-golimumab antibodies were determined in a bridging ELISA, using a set-up as described for the detection of anti-infliximab antibodies [Van Stappen et al.].14 This drug-sensitive assay measures serum concentrations of ‘free’ or excess ADAb and is not able to detect ADAb in the presence of drug. In addition, the assay lacks sensitivity towards IgG4-type of antibodies, since only bivalent IgG will be detected. Recently, a novel assay for anti-infliximab antibodies that enables measurement in the presence of infliximab, was developed [Van Stappen et al., manuscript in preparation]. In this study, a similar approach was adopted for the measurement of anti-golimumab antibodies in the presence of golimumab.
2.5. Statistical analysis
Quantitative data were summarised by median and interquartile range [IQR]. A ShapiroWilk test was used to assess the normality of continuous variables. Differences between responders and non-responders were analysed with the MannWhitney test. The Pearson correlation test and Spearman rank test were used for correlation analysis. To quantify the agreement between serum golimumab concentrations, the intraclass correlation coefficient [ICC] was calculated using the two-way mixed single measures test [absolute agreement]. The area under the curve for serum golimumab concentrations versus time was calculated by using the trapezoidal rule. Diagnostic performance was assessed with receiver operating characteristics [ROC] curve analysis. A two-sided p-value less than 0.05 was considered statistically significant. The software packages SPSS version 22.0 [IBM, New York, NY, USA] and GraphPad Prism version 5.03 [Graphpad Software, San Diego, CA, USA] were used for all statistical analyses.
3. Results
3.1. Demographic characteristics
At study entry, 11 out of 21 patients [52%] were anti-TNF naïve, whereas 10/21 patients [48%] had previously received infliximab and/or adalimumab. The demographic characteristics of the patients are shown in Table 1. The reason for anti-TNF discontinuation for the anti-TNF exposed patients is depicted in Table 2. In all, 18 patients received golimumab monotherapy, whereas 3 patients received combination therapy with thiopurines [Table 1].
Table 1.
Baseline characteristics of the 21 patients with UC.
| N = 21 | |
|---|---|
| Gender: male, n [%] | 10/21 [48%] |
| Age [years] | 45 [29–53] |
| Disease duration [years] | 9 [6–14] |
| BMI [kg/m2] | 24 [22.5–27] |
| Smoker, n [%] | 0/21 [0%] |
| Extent of disease | |
| Proctitis, n [%] | 1/21 [5%] |
| Left-sided colitis, n [%] | 13/21 [62%] |
| Extensive colitis, n [%] | 7/21 [33%] |
| Endoscopic Mayo score 2, n [%] | 6/21 [29%] |
| Endoscopic Mayo score 3, n [%] | 15/21 [71%] |
| Previous anti-TNF use, n [%] | 10/21 [48%] |
| Concomitant thiopurine, n [%] | 3/21 [14%] |
| Serum albumin [g/l] | 41.9 [39.7–44.5] |
| C-reactive protein [mg/l] | 5.7 [1.3–25.6] |
Values are expressed as median [interquartile range], unless stated otherwise. BMI, body mass Index; TNF, tumour necrosis factor; UC, ulcerative colitis.
Table 2.
Reason for infliximab/adalimumab discontinuation for the anti-TNF exposed patients.
| Discontinuation of infliximab | Discontinuation of adalimumab | |
|---|---|---|
| Patient 1 | Reason: anti-drug antibody status | - |
| Patient 2 | Reason: failure [loss of response] | - |
| Patient 3 | Reason: failure [loss of response] | - |
| Patient 4 | Reason: failure [loss of response] | - |
| Patient 5 | Reason: intolerance [delayed hypersensitivity] | - |
| Patient 6 | Reason: intolerance [infusion reaction] | Reason: failure [loss of response] |
| Patient 7 | Reason: failure [loss of response] | Reason: failure [loss of response] |
| Patient 8 | Reason: anti-drug antibody status | Reason: failure [primary non-response] |
| Patient 9 | Reason: failure [loss of response] | Reason: failure [primary non-response] |
| Patient 10 | Reason: failure [loss of response] | - |
3.2. Golimumab treatment
All patients received golimumab 200mg and 100mg at Weeks 0 and 2, respectively; 18 out of 21 patients used 50mg golimumab every 4 weeks as maintenance treatment, whereas 3 patients [with body weight more than 80 kg] started on a 4-week dosing interval of golimumab 100mg. Among the 18 patients who received 50mg golimumab, 13 patients continued receiving 50mg and 5 patients increased their dose to golimumab 100mg when reaching Week 14.
3.3. Response rates
Overall, 10 [48%] out of 21 UC patients showed partial clinical response at Week 14; of these, 3 had a complete clinical response at Week 14. Mucosal healing was present in 4 of 10 partial clinical responders and 2 of 3 complete clinical responders. Finally, 5 out of 11 clinical non-responders needed a colectomy within 1 year after the first golimumab injection.
3.4. Golimumab serum concentration
3.4.1. Development of golimumab serum concentration assays
Two different types of assay were developed to determine golimumab serum concentrations. In the TNF-coated ELISA, MA-GOM131E3, a high-affinity non-neutralising mouse monoclonal antibody, was selected for detection of TNF-bound golimumab. The golimumab calibration curve was linear between 0.6ng/ml and 37.5ng/ml. The cut-off determined using anti-TNF naïve UC patient samples was set at 0.5 µg/ml, taking into account a serum dilution of 1:300. Diluting serum 1:300 to 1:600 allows measurement of golimumab serum concentrations between 0.5 µg/ml and 22.5 µg/ml. To develop a sandwich-type ELISA, a compatible pair of high-affinity antibodies [MA-GOM171D8/MA-GOM159B8-HRP] was selected to capture and detect golimumab. The golimumab calibration curve was linear between 0.04ng/ml and 1.25ng/ml. The cut-off determined using anti-TNF naïve UC patient samples was set at 0.1 µg/ml, taking into account a serum dilution of 1:2000 revealing a higher sensitivity compared with the TNF-coated ELISA. Diluting serum 1:2000 to 1:16000 allows golimumab serum concentration quantification between 0.1 µg/ml and 20 µg/ml.
3.4.2. Determination of golimumab serum concentrations
Golimumab concentrations were determined in 88 samples [average of five samples/patient] using both the TNF-coated and sandwich-type ELISA, revealing an excellent correlation [Spearman’s rho 0.982, p < 0.0 001] and agreement [ICC of 0.974] between both assays. Nevertheless, the TNF-coated ELISA detected golimumab in one sample [concentration < 1 µg/mll] in which no golimumab was detected using the sandwich-type ELISA. This sample was a golimumab-naïve sample. Because of its higher sensitivity and specificity as compared with the TNF-coated ELISA, the sandwich-type ELISA was therefore ultimately selected for quantification of golimumab serum concentrations. Accuracy and imprecision of the sandwich-type ELISA were calculated to be 100% and 5%, respectively. All baseline samples taken before the initiation of golimumab treatment were below the cut-off of the assay [< 0.1 µg/ml]. Re-analysis of 20 randomly selected samples by Sanquin revealed a very good Pearson’s r [0.969, p < 0.0 001] and ICC [0.926].
3.4.3. Relationship between serum golimumab concentrations and treatment outcome
After 2 and 6 weeks of golimumab therapy, median [IQR] serum golimumab concentrations were 8.0 [5.3–10.3] µg/ml and 4.3 [2.0–6.9] µg/ml, respectively, measured by the sandwich-type ELISA. Median [IQR] serum golimumab concentrations were 10.0 [7.8–10.5] µg/ml versus 7.4 [4.8–8.3] µg/ml at Week 2 [p = 0.035] and 5.1 [4.0–7.9] µg/ml versus 2.1 [1.8–4.2] µg/ml at Week 6 [p = 0.037] in partial clinical responders versus non-responders, respectively. Drug exposure as defined by median [IQR] area under the curve (AUC [Week 0–6]) of golimumab was significantly greater for partial clinical responders (7354 [5803–9469] µg.h/ml] than non-responders (4990 [3830–6317] µg.h/ml) [p = 0.034] [Figure 1]. ROC curve analysis revealed a cut-off of 2.6 µg/ml at Week 6 (90% specificity, 56% sensitivity, AUROC 0.79 [95% CI], p = 0.034) for the association with a partial clinical response at Week 14. Clinical non-responders had a significantly more severe colitis, indicated by a higher endoscopic Mayo score at baseline compared with partial clinical responders (median [IQR] endoscopic Mayo score 3.0 [3.0-3.0] versus 2.5 [2.0–3.0], p = 0.048). In addition, baseline serum albumin concentrations were relatively lower (40.2 [39.4–42.5] g/l versus 43.6 [40.3–46.2] g/l [p = 0.082]), and baseline CRP concentrations relatively higher (8.0 [1.1–33.2] mg/l versus 4.7 [1.3–15.6] mg/l [p = 0.481]), in non-responders versus partial clinical responders, respectively.
Figure 1.
Drug exposure as defined by area under the curve (AUC [Weeks 0–6]) of golimumab for partial clinical responders [solid black line] and non-responders [dashed black line].
Median [IQR] serum golimumab concentrations in complete clinical responders [n = 3] versus partial clinical responders and clinical non-responders [n = 18] were 10.4 [7.8–10.6] versus 8.0 [5.2–9.8] [p = 0.263] at Week 2 and 7.7 [4.3–8.4] versus 3.7 [2.0–5.4] at Week 6 [p = 0.130], respectively.
Median [IQR] serum golimumab concentrations in patients who had achieved mucosal healing [n = 4] versus patients who did not [n = 17] were 10.2 [8.4–10.6] versus 8.0 [5.2–9.0] [p = 0.121] at Week 2 and 6.3 [4.7–8.0] versus 3.3 [2.0–4.5] [p = 0.098] at Week 6, respectively. In addition, baseline serum albumin concentrations were significantly higher (45.9 [43.2–47.9] g/l versus 40.5 [39.3–43.2] g/l [p = 0.012]), baseline CRP concentrations were relatively lower (4.6 [0.4–9.8] mg/l versus 5.7 [1.6–32.6] mg/l [p = 0.244]), and baseline endoscopic Mayo score was relatively lower (2.0 [2.0–2.8] versus 3.0 [2.8–3.0] [p = 0.051]) in patients who had achieved mucosal healing versus patients who did not.
Finally, patients with low serum CRP [≤ 10mg/l] and/or high serum albumin [≥ 40g/l] at baseline had a significant higher golimumab exposure [w0-w6] compared with patients with high serum CRP [> 10mg/l] and/or lower serum albumin [< 40g/l], whereas endoscopic Mayo score was not associated with golimumab exposure [w0-w6].
3.5. Anti-golimumab antibody concentration
3.5.1. Development of anti-golimumab assay
MA-GOM159B8 is a mouse monoclonal antibody, with a high specificity and high affinity for golimumab, that is able to inhibit binding of TNF to golimumab [data not shown]. Based upon its ability to cross-link coated golimumab with biotin-labelled golimumab, MA-GOM159B8 was selected as calibrator in the developed golimumab bridging ELISA and all values obtained were expressed as ng/ml MA-GOM159B8 equivalents. The MA-GOM159B8 calibration curve was linear in the range of 0.16ng/ml to 10.0ng/ml. The cut-off determined using anti-TNF naïve UC patient samples was set at 3.2ng/ml, taking into account a serum dilution of 1:20. Accuracy and imprecision were calculated to be 98% and 7%, respectively. In order to be able to detect anti-golimumab antibodies in the presence of golimumab, a drug-tolerant immunoassay was developed using the same MA-GOM159B8 calibrator. The MA-GOM159B8 calibration curve was non-linear in the range of 0.2ng/ml to 180ng/ml. The cut-off determined using anti-TNF naïve UC patient samples was set at 25ng/ml, taking into account a serum dilution of 1:18. As shown in Figure 2, a 20-fold excess of golimumab over MA-GOM159B8 still gave a signal well above the cut-off of this drug-tolerant assay.
Figure 2.
Detection of anti-golimumab antibody in the presence of various concentrations of golimumab using the drug-tolerant immunoassay. Determination of 500ng/ml MA-GOM159B8, spiked with golimumab concentrations between 0 and 10 µg/ml [molar excess up to 20-fold].
3.5.2. Determination of anti-golimumab serum concentrations
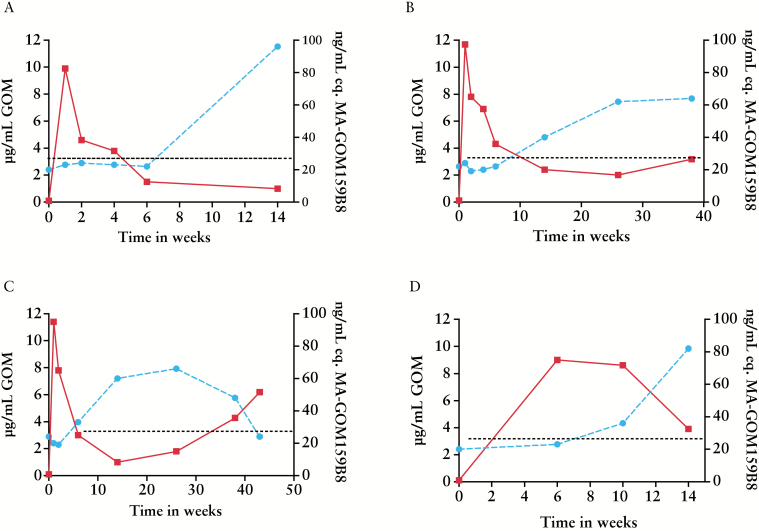
All patient samples were tested for presence of anti-golimumab antibodies using bridging ELISA. In the drug-sensitive bridging assay set-up, no antibodies towards golimumab were found in any of the patient samples analysed between Week 0 and Week 14. Subsequently, samples were re-analysed in the drug-tolerant anti-golimumab immunoassay. Baseline samples of all patients were negative for anti-golimumab antibodies, but 4 out of 21 UC patients tested positive for anti-golimumab antibodies within 14 weeks of treatment and on at least one time point [Figure 3].
Figure 3.
The course of golimumab concentrations [solid line, left y-axis] and anti-golimumab antibody concentrations as measured using drug-tolerant immunoassay [dashed line, right y-axis] in four individual patients. The dotted line represents the cut-off [25ng/ml MA-GOM159B8 equivalents] of the drug-tolerant immunoassay. [A] Non-responder. [B, C, and D] Partial clinical responders.
3.5.3. Relationship between serum anti-golimumab antibody concentrations and treatment outcome
Using the drug-tolerant immunoassay, anti-golimumab antibodies were detectable within 14 weeks of therapy. Of the four patients in whom anti-golimumab antibodies were detected, two were in partial clinical response at time of detection, one patient had complete clinical response and mucosal healing, and one patient lacked clinical response. Figure 3 shows the course of golimumab concentrations and anti-golimumab antibody concentrations in these four patients. Because the clinical condition of patient A [Figure 3A] deteriorated, therapy was switched to vedolizumab [Entyvio®]. In this patient, anti-golimumab antibodies were detectable [96ng/ml] at Week 14 using the drug-tolerant immunoassay, but undetectable using the drug-sensitive bridging ELISA. However, when an additional serum sample of the patient was taken 6 months after the final golimumab injection, the drug was no longer present in the sample and, even in the drug-sensitive bridging ELISA, the titre of anti-golimumab antibodies was borderline positive [5ng/mL]. In two other patients [Figure 3B and C], who completed a minimal follow-up of 38 weeks, samples measured using the bridging ELISA were always negative. Using the drug-tolerant immunoassay, the antibody titre of patient B remained stable between Week 26 and Week 38 as well as the serum golimumab concentration [Figure 3B]. By contrast, the antibody titre of patient C decreased from Week 26 onwards, and this phenomenon was accompanied by an increase of drug concentrations after intensification of the golimumab dose. In a last patient [Figure 3D], anti-golimumab antibodies were detectable in the drug-tolerant immunoassay when the serum golimumab concentration still reached 8.6 µg/ml. This patient was in partial clinical response but no further samples are yet available.
There was no association in patients who developed ADAb [measured using a drug-tolerant ADAb assay] with CRP, albumin or Mayo score at baseline.
4. Discussion
Golimumab has recently been approved to treat refractory moderate-to-severe UC. The pivotal PURSUIT trial demonstrated that many patients do not reach clinical remission within 6 weeks.8 We sought to establish whether differences in golimumab exposure could form a basis for clinical effectiveness of golimumab therapy.
Based on the results of the PURSUIT-SC induction study,8 golimumab is administered to patients with UC as an induction dose of 200mg at Week 0 followed by 100mg 2 weeks later and, every4weeks, injections of 50mg or 100mg [when patient weight is more than 80 kg]. In the study of Sandborn et al., an exposure-response relationship was observed, with patients in the highest serum golimumab concentration quartiles having greater rates of clinical response when compared with those in the lower quartiles, at Week 6.8 In our cohort, we could confirm that 80% of the partial clinical responders had a serum golimumab concentration located in the highest quartiles at Week 6. However, in contrast to the study of Sandborn et al., which only included anti-TNF naïve patients, 10 out of 21 patients in our study had previously received anti-TNF treatment. Only 3 of these patients [30%] responded to golimumab therapy, compared with 7 out of 11 [64%] anti-TNF naïve patients. Moreover, of all 21 patients studied here, complete clinical response and mucosal healing at Week 14 were observed in only 3 and 4 patients, respectively, which is much lower than those established in a clinical trial setting.8 The fact that some patients needed a dose increase to maintain response after induction therapy is an interesting occurrence. Data from the PURSUIT-M study showed that administration of golimumab 100mg 4-weekly in patients who lost response to initial treatment, did not result in significantly higher response rates [34.6% versus 28.0%] compared with patients who continued the 50-mg dose,9 although drug concentrations were not taken into account. In our study, four out of five patients with an intensification of golimumab therapy had a partial clinical response at Week 14; however, in all five patients serum golimumab concentration at Week 6 was above our clinical cut-off. As golimumab is expensive, it might be more efficient to adjust the golimumab dosing schedule based on the measured serum golimumab concentration.
In most studies, trough concentrations [TC; concentration measured just before the next injection] are the marker for drug exposure, and doses are adjusted based on these troughs. In this paper, only samples at Weeks 2, 6, and 14 were taken just before the next injection and only these can be referred to as trough concentrations. The problem with subcutaneously administered drugs is that the exact moments when the lowest [usually referred to as trough] and the highest [peak] concentrations are reached are much harder to predict, due to the difference in absorption/degradation/distribution rates of the drug. Typically, peak concentration is reached 3–8 days after injection. Moreover, a higher TC does not necessarily imply a larger area under the concentration-time curve [AUC]. The combination of a higher peak concentration and a higher clearance can result in the same trough concentration as the combination of a lower peak concentration and a lower clearance. Therefore, therapeutic drug monitoring ideally takes into account the individual’s whole pharmacokinetics [PK] profile of the biological administered.
A common objection that limits implementation of measurements in clinical practice is the claim that the magnitude of results varies systematically between different assays and technologies, and assay standardisation is lacking.9 We have developed two enzyme-linked immunosorbent assays to measure golimumab serum concentrations. Comparison between both assays revealed that the sandwich-type ELISA was more specific and more sensitive compared with the TNF-coated ELISA. However, an excellent agreement between both assays was observed. Subsequently, we performed a small comparative test with Sanquin Diagnostic Services. Despite different analytical performances and different dilution steps, no significant differences in golimumab concentrations were observed, suggesting that both methods will result in similar correlations with clinical outcomes and comparable therapeutic actions.
An important cause of non-response to adalimumab or infliximab treatment is subclinical drug concentrations due to ADAb formation.15 As reported in the PURSUIT-M study,9 the incidence of antibodies to golimumab is low, reaching only 2.9%; however, the presence of drug in the serum might have confounded the evaluation. Here we show that anti-golimumab antibodies were detected in 19% [n = 4] of patients using a newly-developed drug-tolerant immunoassay; three of these patients maintained response and a fourth patient had to be switched to another biological agent. All four patients tested negative in the drug-sensitive bridging ELISA at time of first detection. A recent study of van Schouwenburg et al.,16 in which 99 rheumatoid arthritis patients were treated with adalimumab, showed that the clinical relevance of measuring ADAb in complex with drug is limited. This study also indicates that immunogenicity does not play an important role in explaining the rather high ‘failure’ rate to golimumab, as observed in our cohort. First of all, the presence of anti-golimumab antibodies [detected using a drug-tolerant immunoassay] did not lead to undetectable golimumab serum concentrations. Moreover, according to Figure 1, the difference in concentration versus time lines is already established before the second infusion [at Week 1], indicating that the B-cell response might not be the cause of this difference in exposure. The clinical relevance of these antibodies can therefore be disputed. However, the lack of association may not be conclusive because of the low rate of anti-golimumab positivity and the short duration of follow-up. Dose escalation in one patient confirmed previous findings that development of ADAb might be transient and can be overcome.17 Future work should focus on the relationship between anti-golimumab antibodies and golimumab concentrations and explore inter-individual differences for their clinical significance.
Besides immunogenicity, identification of other patient-, drug-, or disease-related covariates that influence drug pharmacokinetics may help to explain the inter-individual variability in drug exposure, observed after standard dosing of golimumab. In this cohort, patients with more severe disease at baseline were less likely to respond to treatment with golimumab. Predictive PK modelling integrating this [and other] variable[s], together with early drug monitoring, may allow for refinement of the dosing regimen, which in turn may enhance the therapeutic success of golimumab therapy.
This study has some limitations. Since golimumab is a self-administered agent, there might have been some variation in the timing of dosing. Patient number was small, and no hard clinical endpoint could be used to assess a therapeutic response. Moreover, patients have been treated in a non-controlled fashion, including dose escalation based on clinical and endoscopic evaluation.
In conclusion, despite treatment with golimumab subcutaneous injections, many patients with UC still have persistent disease activity. The response to golimumab treatment is related to serum golimumab concentrations and shows a large variation between patients. Further investigation into the mechanisms that determine golimumab exposure may help to optimise this treatment.
Funding
This work was supported by the Fund for Scientific Research Flanders [grant number G.0617.12]; and the Agency for the Promotion and Innovation through Science and Technology in Flanders to ID and TVS.
Conflict of Interest
GVA received financial support for research from Abbott and Ferring Pharmaceuticals, lecture fees from Janssen, MSD and Abbott, and consultancy fees from PDL BioPharma, UCB Pharma, Sanofi-Aventis, Abbott, Abbvie, Ferring, Novartis, Biogen Idec, Janssen Biologics, NovoNordisk, Zealand Pharma A/S, Millenium/Takeda, Shire, Novartis, and Bristol Mayer Squibb. SV received financial support for research from MSD, Abbvie, and Takeda, lecture fees from Abbvie, MSD, Ferring Pharmaceuticals, Pfizer, Hospira, and Takeda, and consultancy fees from Pfizer, Ferring Pharmaceuticals, Shire Pharmaceuticals Group, MSD, Abbvie, Hospira, Mundipharma, and Takeda. MF received financial support for research from Takeda, lecture fees from MSD, Janssen, Abbvie, Boehringer-Ingelheim, Ferring, Chiesi, Tillotts, Zeria, and Mitsubishi Tanabe, and consultancy fees from MSD, Janssen, Abbvie, Boehringer-Ingelheim, and Ferring.
AG received financial support for research from Pfizer, speaker fees for Pfizer, Abbvie, Janssen Biologicals, and MSD, and consultant fees from UCB, and has licensed infliximab ELISA to apDia. ID, ED, TVS, AdV, and EB declared no conflict of interest.
Author Contributions
ID performed research, interpreted the data, and implemented statistical analysis. ED helped with the acquisition of data and contributed to manuscript review. TVS helped with part of the research and contributed to manuscript review. AdV and EB performed part of the research. GVA and SV provided the serum samples and contributed to manuscript review. MF provided the serum samples, helped with the acquisition and interpretation of data, and reviewed the manuscript. AG designed research, coordinated the experiments, and reviewed the manuscript. All authors read and approved the final manuscript.
References
- 1. Danese S, Fiocchi C. Ulcerative Colitis. N Engl J Med 2011;365:1713–25. [DOI] [PubMed] [Google Scholar]
- 2. Danese S, Colombel JF, Peyrin-Biroulet L, et al. Review article: the role of anti-TNF in the management of ulcerative colitis – past, present and future. Aliment Pharmacol Ther 2013;37:855–66. [DOI] [PubMed] [Google Scholar]
- 3. Ferrante M, Vermeire S, Fidder H, et al. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis 2008;2:219–25. [DOI] [PubMed] [Google Scholar]
- 4. Armuzzi A, Biancone L, Daperno M, et al. Adalimumab in active ulcerative colitis: A ‘real-life’ observational study. Dig Liver Dis 2013;45:738–43. [DOI] [PubMed] [Google Scholar]
- 5. Hutas G. Golimumab, a fully human monoclonal antibody against TNFα. Curr Opin Mol Ther 2008;10:393–406. [PubMed] [Google Scholar]
- 6. Shealy D, Cai A, Staquet K, et al. Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor alpha. MAbs 2010;2:428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Löwenberg M, De Boer NKH, Hoentjen F. Golimumab for the treatment of ulcerative colitis. Clin Exp Gastroenterol 2014;7:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous Golimumab Induces Clinical Response and Remission in Patients With Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2014;146:85–95. [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous Golimumab Maintains Clinical Response in Patients With Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2014;146:96–109. [DOI] [PubMed] [Google Scholar]
- 10. Ferrante M, Vermeire S, Katsanos KH, et al. Predictors of Early Response to Infliximab in Patients With Ulcerative Colitis. Inflamm Bowel Dis 2007;13:123–8. [DOI] [PubMed] [Google Scholar]
- 11. Van Stappen T, Brouwers E, Tops S, et al. Generation of a highly specific monoclonal anti-infliximab antibody for harmonization of TNF-coated infliximab assays. Ther Drug Monit 2015;37:479–85. [DOI] [PubMed] [Google Scholar]
- 12. Gils A, Vande Casteele N, Poppe R, et al. Development of a Universal Anti-Adalimumab Antibody Standard for Interlaboratory Harmonization. Ther Drug Monit 2014;36:669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Schie KA, Hart MH, de Groot ER, et al. The antibody response against human and chimeric anti-TNF therapeutic antibodies primarily targets the TNF binding region. Ann Rheum Dis 2015;74:311–4. [DOI] [PubMed] [Google Scholar]
- 14. Van Stappen T, Billiet T, Vande Casteele N, et al. An optimized anti-infliximab bridging ELISA for harmonization of anti-infliximab antibody titers in patients with inflammatory bowel diseases. Inflamm Bowel Dis 2015;21:2172–7. [DOI] [PubMed] [Google Scholar]
- 15. Cohena LB, Nanauc RM, Delzord F, et al. Biologic therapies in inflammatory bowel disease. Transl Res 2014;163:533–56. [DOI] [PubMed] [Google Scholar]
- 16. Van Schouwenburg PA, Krieckaert CL, Rispens T, et al. Long-term measurement of anti-adalimumab using pH-shift-anti-idiotype antigen binding test shows predictive value and transient antibody formation. Ann Rheum Dis 2013;72:1680–6. [DOI] [PubMed] [Google Scholar]
- 17. Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013;108:962–71. [DOI] [PubMed] [Google Scholar]