Abstract
Background and Aims:
We sought to correlate hepcidin levels in inflammatory bowel disease [IBD] children with disease activity, inflammatory markers, and iron load test [ILT] and to compare IBD patients with coeliac and healthy patients.
Methods:
Between December 2012 and June 2013, 145 subjects [50 IBD patients, 45 coeliac patients and 50 healthy controls] were included in the study. All patients underwent the following examinations: blood count, iron status, erythropoiesis parameters, serum hepcidin, C-reactive protein [CRP], and erythrocyte sedimentation rate [ESR]. In order to evaluate the efficacy of iron absorption, ILT was performed in IBD patients. Disease activity indexes and IBD duration, localisation, and therapy were also evaluated, and a faecal sample for calprotectin collected.
Results:
Serum hepcidin was significantly higher in IBD patients with active disease compared with both coeliac and healthy patients [p = 0.005, p = 0.003 respectively]. In a multivariate logistic regression model, having a Paediatric Crohn’s Disease Activity Index [PCDAI] / Paediatric Ulcerative Colitis Activity Index [PUCAI] ≥ 30 resulted in the only variable independently associated with a positive serum hepcidin (odds ratio [OR] = 6.87; 95% confidence interval [CI] 1.4–33, p = 0.01]]. Patients with iron malabsorption [IM] showed higher values of ESR, CRP, and hepcidin [p = 0.02, p = 0.001, and p = 0.06, respectively]. Eight out of 12 [66.7%] children with IM showed an active disease compared with 6/31 [19.3%] children with normal ILT [p = 0.01]. Hepcidin levels correlated negatively with ILT [r = -0.451, p = 0.002], and positively with ferritin and CRP [r = 0.442, p = 0.0001; r = 0.243, p = 0.009, respectively]
Conclusions:
Our study demonstrates that serum hepcidin is increased in IBD children with active disease and it is responsible for IM.
Keywords: Hepcidin; IBD; iron absorption
1. Introduction
Anaemia is the most frequent extra-intestinal manifestation of inflammatory bowel disease [IBD], with a great impact on the patient’s quality of life.1,2 The prevalence of anaemia in IBD varies between 15% and 75%, depending on the definition and the subgroup of examined patients.3,4 The main types of anaemia in IBD are iron deficiency anaemia [IDA], anaemia of inflammatory aetiology, anaemia of chronic disease [ACD], and combined IDA + ACD.4,5 IDA is mainly the result of the chronic blood loss from the gastrointestinal [GI] tract, due to prolonged inflammation of the small and large intestine epithelium. On the other hand inflammation, through an inflammatory cytokines-mediated mechanism, leads to a decreased iron level in the circulation and thus to a limited availability of iron for erythroid cells.6 Hepcidin, a 25 amino-acid peptide mainly secreted by hepatocytes, controls the amount of iron entering the blood circulation by binding and downregulating ferroportin, a plasma membrane transporter that pumps iron out of phagocytes and duodenal enterocytes. The hepcidin expression is regulated transcriptionally in response to changing serum iron levels. Elevated serum iron promotes hepcidin expression, leading to downregulation of ferroportin and decreased entry of iron into the circulation. Conversely, low serum iron leads to reduced hepcidin expression, elevated ferroportin, and increased movement of iron into the circulation.7,8 In addition to iron status, inflammatory cytokines can also influence transcription of the hepcidin gene. Interleukin-6 [IL-6] has been shown to increase hepcidin expression in vitro and in vivo, and IL-6 induced hepcidin upregulation has been proposed to play an important role in the pathogenesis of ACD.9,10 The role of hepcidin in the mechanisms of anaemia in paediatric IBD is limited and shows conflicting results. Increased urine11 or serum12 hepcidin has been reported in two studies, correlating with the rise of IL-6 levels, ferritin, and disease activity. Conversely, Arnold et al. found significantly decreased hepcidin levels in IBD patients compared with healthy controls.13 Hepcidin precursor, pro-hepcidin, has also been evaluated in three different studies in IBD patients, with variable results.12,14,13,15 Conflicting results may suggest that hepcidin in human IBD is likely to be influenced by various factors such as age, type of disease, and disease activity.
The primary aim of this study was to correlate hepcidin serum levels in patients affected by paediatric IBD with disease activity, inflammatory markers, and iron absorption. The secondary aims were to compare serum hepcidin levels of IBD patients with a group of coeliac and healthy patients, and to establish which iron parameter better correlates with hepcidin.
2. Materials and Methods
2.1. Study population
We conducted a comparative, cross-sectional, single-centre study in paediatric patients with a diagnosis of IBD. Children and adolescents aged from 2 to 18 years with a diagnosis of IBD were prospectively enrolled between December 2012 and June 2013 at the Department of Translational Medical Science, Section of Paediatrics, University of Naples ‘Federico II’, Italy. The diagnosis of IBD was established on the basis of clinical, endoscopic, radiological, and histological criteria according to the Porto criteria.16 During the same study period, we also recruited a group of children who were referred to our centre with suspected coeliac disease due to pathological serum levels of anti-tissue transglutaminase [tTG] [> 7U/ml] and/or positive anti-endomysium [EMA] antibodies. Only patients with a confirmed diagnosis of coeliac disease or potential coeliac disease [positive serology and normal duodenal architecture] were finally included in the study. In addition, we enrolled a group of healthy children referred to our primary care centre for routine well-child visits. Exclusion criteria from the study were: age ≤ 2 years or > 18 years; patients with suspected coeliac disease without confirmation of diagnosis; the presence of other comorbidities; patients having iron supplementation during the month preceding the enrolment; and inability or unwillingness to give informed consent. At the time of the enrolment, all patients underwent the following examinations: full blood count, reticulocytes, serum iron, ferritin, transferrin, soluble transferrin receptor [STfR], total iron-binding capacity [TIBC], transferrin saturation [Tsat], and inflammatory indexes (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR]). In addition, blood samples from all enrolled patients were also obtained for hepcidin 25 isoform analysis. After being centrifuged, the serum of all patients was stored at -80°C in aliquots in order to avoid multiple-frozen thaw. Once collected, all samples were sent to the Section of Internal Medicine, Department of Medicine, University of Verona for the analysis. Hepcidin 25 isoform was measured through a validated mass spectrometry-based assay, as previously described.17 Synthetic hepcidin 25 [Peptides International, Louisville, KY] was used for external calibration and a synthetic hepcidin analogue [Hepcidin 24, Peptides International] as an internal standard. The lower sensitivity limit of the assay was 0.55nM. All samples were measured in duplicate.18 For the IBD group, type of IBD, anatomical distribution of disease, symptoms, disease activity, and treatments, including surgery, were recorded. For the purpose of this manuscript, disease location was described according to the Paris classification.19 Disease activity was scored by the Paediatric Crohn’s Disease Activity Index [PCDAI] or the Paediatric Ulcerative Colitis Activity Index [PUCAI] for CD and UC, respectively.20, 21 In addition, a stool sample for faecal calprotectin determination was also obtained. For the coeliac group, tTG, EMA, and duodenal histology [according to Marsh grading] were also evaluated.
2.2. Differential diagnosis of anaemia
Anaemia was defined on the basis of World Health Organization Criteria 22: in boys aged > 15 years, as Hb < 13g/dl; in non-pregnant girls aged > 15 years, as Hb < 12g/dl; in children aged 5–11 years, as Hb < 11.5g/dl; and in children aged < 5 years, as Hb < 11g/dl. Iron deficiency was defined as a ferritin < 12ng/ml in children aged < 5 years, or ferritin < 15ng/ml in children aged > 5 years, when the corresponding CRP was < 1mg/dl and Tsat < 20%.22 In the presence of biochemical evidence of inflammation, the diagnostic criteria for ACD were a serum ferritin > 100ng/ml and TfS < 20%, whereas if the serum ferritin level was ≤ 100ng /ml, a combination of true iron deficiency and ACD was diagnosed.23
2.3. Iron absorption in IBD patients
In order to evaluate the efficacy of iron absorption, an iron load test [ILT] was performed in children affected by IBD. We used a previously described protocol.24 After an overnight fast and baseline serum iron determination, ferrous sulphate [dosed as 1mg/kg elemental iron with a 60-mg maximum] was administered orally as a liquid preparation, followed by determination of serum iron after 2h. The change in iron levels between the baseline and the 2-h period (Δ[Fe]2hr) was calculated. Iron malabsorption [IM] was defined using the normative data, when the increase of serum iron after 2h from the ILT was lower than the fifth percentile.24
2.4. Ethical approval
All parents or guardians signed a consent form indicating their awareness of the investigative nature of the study and possible risks. When appropriate, we also obtained children’s assent. The study was approved by the Institutional Review Board of University of Naples Federico II.
2.5. Statistical analysis
Variables were screened for their distribution, and appropriate parametric or non-parametric tests were adopted as necessary. Student’s t-test, the ANOVA test, and the Mann-Whitney test for continuous variables, and the chi-square and Fisher’s exact tests for categorical variables, were used where appropriate. Multivariate conditional logistic regression analysis was used to explore the odds associated with a positive serum hepcidin and a pathological ILT. Serum hepcidin and ILT were used as dependent variables, and the effect of all the parameters was analysed by a stepwise procedure. Serum hepcidin was considered positive for values higher than the measurable cut-off [0.55 nM]. Correlations between serum hepcidin and ∆[Fe]2hr with continuous variables were evaluated through linear regression and expressed by Pearson’s correlation coefficient. Variables not normally distributed [serum hepcidin and ferritin] were log-transformed before performing the correlations. Statistical significance was predetermined as p < 0.05. Percentages were rounded to the nearest whole numbers. SPSS version 15 was used for all statistical analyses. The sample size of 50 children in each group was estimated with a 90% power to detect a difference of at least 20% between the three groups, with an alpha of 0.05.
3. Results
Initially, 150 subjects were enrolled between December 2012 and June 2013, comprising 50 IBD patients (UC: 28; CD: 20; IBD-unclassified [IBD-U]: 2; mean age ± standard deviation [SD:] 12.6±3.5; range 4 to 18 years; M/F: 27/23), 50 children with a suspicion of coeliac disease, and 50 healthy controls [mean age ± SD: 11.1±4.2 years; range 3.2 to 18 years; M/F: 25/25]. Among the coeliac disease group, 5 patients [10%] were subsequently excluded from the study for non-confirmed positive coeliac serology and therefore only 45 coeliac patients were included in the study [mean age ± SD: 8±3.2 years; range 2 to 14 years; M/F: 20/25]. Of these 45 children, 38 [84.4%] were affected by coeliac disease and 7 [15.6%] by potential coeliac disease. The baseline and laboratory characteristics of all subjects included in the study are described in Table 1 and Table 2.
Table 1.
Demographic and clinical characteristics of enrolled patients.
| Characteristics [n, %] | IBD patients | Coeliac patients | Healthy controls | p |
|---|---|---|---|---|
| N = 50 | N = 45 | N = 50 | ||
| Age, years [range] | 12.6±3.5 [4–18] | 8±3.2 [2–14] | 11.1±4.2[3.2–18] | 0.01 |
| Gender [n, %] | ||||
| Male | 27 [54] | 20 [44.4] | 25 [50] | 0.6 |
| Coeliac disease diagnosis [n, %] | NA | |||
| Coeliac disease | - | 38 [84.4] | - | |
| Potential coeliac disease | - | 7 [15.6] | - | |
| tTG | - | 110.8±87.6 [3.4–200] | - | |
| EMA positive | - | 41 [91.1] | - | |
| Marsh grading [n, %] | - | - | ||
| T1 | - | 3 [6.6] | - | |
| T2 | - | 0 | ||
| T3a | - | 0 | ||
| T3b | - | 12 [26.6] | - | |
| T3c | - | 21 [46.6] | - | |
| IBD type [n, %] | NA | |||
| UC | 28 [56] | - | - | |
| CD | 20 [40] | - | - | |
| IBD-U | 2 [4] | - | - | |
| IBD disease location [n, %] | - | - | NA | |
| UC | ||||
| Proctosigmoiditis [E1] | 9 [32] | - | - | |
| Left-sided colitis [E2] | 2 [8] | - | - | |
| Extensive colitis [E3] | 6 [21] | - | - | |
| Pancolitis [E4] | 11 [39] | - | - | |
| CD | - | - | ||
| Ileum only [L1] | 1 [5] | - | - | |
| Colon only [L2] | 5 [25] | - | - | |
| Ileum and colon [L3] | 14 [70] | - | - | |
| Upper gastrointestinal tract [L4a] | 2 [10] | - | - | |
| - | - | |||
| IBD disease activity indexes | NA | |||
| PUCAI | 13.8±16.4 [0–47.5] | - | - | |
| PCDAI | 11.8±14.4 [0–60] | - | - | |
| IBD therapy [n, %] | NA | |||
| Steroids | 10 [20] | - | - | |
| Enteral nutrition | 3 [6] | - | - | |
| Azathioprine | 11 [22] | - | - | |
| Methotrexate | 1 [2] | - | - | |
| Biologicals | 2 [4] | - | - | |
| Mesalazine | 31 [62] | - | - |
All continuous variables values are expressed as means ± standard deviation [range].
CD, Crohn’s disease; EMA, anti-endomysium; tTg, anti-tissue transglutaminase; IBD, inflammatory bowel disease; UC, ulcerative colitis; IBD-U, inflammatory bowel disease unclassified; PCDAI, Paediatric Crohn’s Disease Activity Index; PUCAI, Paediatric Ulcerative Colitis Activity Index; NA, not applicable.
Table 2.
Laboratory parameters of enrolled patients.
| Parameters | IBD patients [n = 50] | Coeliac patients [n = 45] | Healthy controls [n = 50] | p* |
|---|---|---|---|---|
| Hb, g/dl | 12.2±1.7 [8.3–14.9] | 12.1±1 [9.1–14.6] | 12.8±1 [11–16] | 0.04 |
| MCV, fl | 78.6±8.1 [60–94] | 78.5±5.3 [60.3–91] | 82.5±4.1 [74–92] | 0.002 |
| Serum iron, µg/dl | 56.6±32.5 [13–148] | 60.4±33.4 [19–176] | 80.7±26.9 [28–138] | 0.001 |
| Ferritin, ng/ml | 45.8±36.8 [6–217] | 33.8±30.5 [6–157] | 56.8±31 [10–179] | 0.0 001 |
| Transferrin, g/l | 2.5±0.6 [1.3–4.3] | 2.7±0.3 [2.1–3.4] | 1.4±0.3 8 [0.7–2.6] | 0.02 |
| STfR, mg/dl | 1.9±1.2 [0.8–8.5] | 1.7±0.4 [1.1–2.8] | 1.4±0.3 [0.4–2.6] | 0.02 |
| Tsat, % | 16.1±9.8 [3–41] | 15±8.6 [5–42] | 21.4±8.3 [6–44] | 0.01 |
| TIBC, µg/dl | 315.5±77.7 [139–460] | 365.1±72.8 [236–576] | 350.8±43.8 [268–450] | 0.3 |
| Reticulocytes, % | 3.8±3.6 [0.6–9] | 4.0±3.3 [1–4] | 3.2±3.4 [0.6–9] | 0.01 |
| Serum hepcidin, nM | 4.3±8.3 [0.55–49.2] | 2.1±3.1 [0.55–15.5] | 2±2.6 [0.55–11.3] | 0.06 |
| ESR, mm | 12.2±11.8 [1–46] | 9.5±6.1 [2–23] | 6.2±3.9 [1–17] | 0.03 |
| CRP, mg/dl | 1.4±3.1 [0.33–18.6] | 0.35±0 .04 [0.33–0.4] | 0.36±0.2 [0.33–2] | 0.0 001 |
All values are expressed as means ± standard deviation [range].
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, haemoglobin; MCV. mean corpuscolate volume; STfR, soluble transferrin receptor; TIBC, total iron binding capacity; TSat, transferrin saturation.
* ANOVA test.
3.1. Prevalence of anaemia among different study groups
The prevalence of anaemia in IBD patients was significantly higher compared with both the coeliac group and the healthy controls (17/50 [34%] versus 5/45 [11.1%]; OR = 4.1; 95% CI 1.3 to 12.3, p = 0.01; 17/50 [34%] versus 0/50 [0%]; p = 0.001; OR 2.5; 95% CI 1.9 to 2.3, respectively]. In detail, 3 out of 17 IBD patients [17.6%] were affected by IDA, 2/17 [11.7%] by ACD, and in 12 out of 17 [70.5%] IBD children a combination of IDA and ACD was identified. All five anaemic coeliac patients were affected by IDA. In addition, an iron deficiency status without anaemia was found in 1/50 [2%] IBD patients, 7/45 [14%] coeliac patients, and 2/50 [4%] healthy controls [p = 0.03].
3.2. Serum hepcidin among different groups
Serum hepcidin was significantly higher in IBD patients with a PCDAI/PUCAI ≥ 30 compared with patients with PCDAI/PUCAI < 30, coeliac patients, and healthy controls [p = 0.02, p = 0.005, p = 0.003, respectively] [Table 3]. In IBD patients with PCDAI/PUCAI < 30, serum hepcidin values were higher than in coeliac or in healthy children, but statistical significance was not reached [p = 0.3, p = 0.2, respectively]. No difference was observed when comparing coeliac patients with healthy controls [p = 0.9] [Table 3]. In addition, serum hepcidin was significantly higher in patients with ACD compared with children with IDA and ACD + IDA [p = 0.001, p = 0.001, respectively]. Patients with ACD + IDA showed higher values of serum hepcidin compared with patients with IDA, with a trend toward statistical significance [p = 0.06] [Table 3]. In detail, none of the patients with pure IDA showed hepcidin values higher than the lower limit of the assay [0.55 nM], whereas 7 out of 12 [58.3%] patients with combined IDA and ACD showed values > 0.55nM. Both of the two patients [100%] with pure ACD showed values higher than the cut-off.
Table 3.
Serum hepcidin levels in different groups of patients.
| Groups [n] | Serum hepcidin* | p |
|---|---|---|
| IBD with PCDAI/PUCAI ≥ 30 [11] | 9.4±15.8 [0.55–49.2] | 0.02 |
| IBD with PCDAI/PUCAI < 30 [39] | 2.8±3.7 [0.55–19.2] | |
| IBD type | 0.1 | |
| UC [28] | 3.3±6.2 [0.55–28.5] | |
| CD [20] | 5.7±10.9 [0.55–49.2] | |
| IBD-U [2] | 1.8±1.7 [0.55–3] | |
| IBD with PCDAI/PUCAI ≥ 30 [11] | 9.4±15.8 [0.55–49.2] | 0.005 |
| Coeliac patients [45] | 2.1±3.1 [0.55–15.5] | |
| IBD with PCDAI/PUCAI ≥ 30 [11] | 9.4±15.8 [0.55–49.2] | 0.003 |
| Healthy controls [50] | 2.1±2.6 [0.55–11.3] | |
| Coeliac patients [45] | 2.1±3.1 [0.55–15.5] | 0.9 |
| Healthy controls [50] | 2.1±2.6 [0.55–11.3] | |
| Coeliac disease group | 0.3 | |
| Coeliac disease [38] | 1.9±3.0 [0.55–15.5] | |
| Potential coeliac disease [7] | 3.1±3.3 [0.55–8.3] | |
| Patients with anaemia | ||
| IDA [8] | 0.55 [0.55] | 0.001 |
| ACD [2] | 25.1±34 [1–49.2] | |
| ACD [2] | 25.1±34 [1–49.2] | 0.001 |
| IDA + ACD [12] | 1.9±4.3 [0.55–15] | |
| IDA [8] | 0.55 [0.55] | 0.06 |
| IDA + ACD [12] | 1.9±4.3 [0.55–15] |
*Values are expressed as means ± standard deviation [range].
ACD, anaemia of chronic disease; CD, Crohn’s disease; IDA, iron deficiency anaemia; IBD, inflammatory bowel disease; IBD-U: inflammatory bowel disease unclassified; PCDAI, Paediatric Crohn’s Disease Activity Index; PUCAI, Pediatric Ulcerative Colitis Activity Index; UC, ulcerative colitis.
3.2.1. Multivariate analysis
In a multivariate logistic regression model, serum hepcidin was considered positive for values > 0.55nM. Having a PCDAI/PUCAI ≥ 30 resulted in the only variable independently associated with a positive serum hepcidin (9/11 [81.8%] patients with a PCDAI/PUCAI ≥ 30 versus 53/134 [39.5%] of the remaining patients; OR = 6.87; 95% CI 1.4–33, p = 0.01). None of the other variables was associated with positive serum hepcidin.
3.3. Iron absorption in IBD patients
Off 50 IBD patients, 43 [86%] performed the ILT. Twelve of the 43 [27.9%] patients showed a pathological ILT. Iron absorption was not associated with patient age or gender [p = 0.6 and p = 0.3, respectively] [Table 4]. Specific type of IBD and duration and extension of disease did not associate with the IM [p = 0.1, p = 0.1, and p = 0.7, respectively]. Patients with IM showed significant higher values of ESR and CRP compared with patients with normal iron absorption [p = 0.02 and p = 0.001, respectively]. Active disease was more frequent in children with a pathological ILT when compared with children with normal iron absorption (8/12 [66.7%] versus 6/31 [19.3%] [p = 0.01]. In particular, a PUCAI/PCDAI ≥ 30 was more common in children with pathological ILT when compared with children with normal ILT (5/12 [41.6%] versus 1/31 [1.3%]; p = 0.004). Mean PUCAI and PCDAI are shown in Table 4. In addition, IBD children with abnormal ILT were significantly more often taking immunosuppressive therapy (9/12 [75%] versus 11/31 [35.5%]; p = 0.03). Baseline serum iron, haemoglobin, transferrin, and Tsat values were significantly lower in patients with IM [p = 0.001, p = 0.005, p = 0.04, and p = 0.003, respectively] [Table 4]. Although baseline ferritin and faecal calprotectin were found to be higher in patients with IM, these differences were not statistically significant [p = 0.2 and p = 0.6, respectively] [Table 4]. Serum hepcidin was higher in patients with pathological ILT, with a trend toward statistical significance [p = 0.06] [Table 4]. In a multivariate logistic regression model, being affected by active IBD resulted in the only variable independently associated with a pathological ILT [OR = 15.4; 95% CI 1.4–160.2, p = 0.007].
Table 4.
Characteristics associated with iron malabsorption in IBD children.
| Characteristics | Pathological ILT [n = 12] | Normal ILT [n = 31] | p |
|---|---|---|---|
| Mean age [years, range] | 12.9±3.6 [2–18] | 12.3±3.5 [2–18] | 0.6 |
| Gender [n, %] | 0.3 | ||
| Male | 4 [33.3] | 17 [54.8] | |
| Duration of disease [months] | 25.5±29.6 [0–82] | 43.8±36.3 [1–113] | 0.1 |
| IBD type [n, %] | 0.1 | ||
| CD | 6 [50] | 10 [32.3] | |
| UC | 5 [41.7] | 20 [64.5] | |
| IBD-U | 1 [8.3] | 1 [3.2] | |
| Active disease [n,%] | 8 [66.7] | 6 [19.4] | 0.01 |
| PCDAI/PUCAI ≥ 30 [n, %] | 5 [41.7] | 1 [3.2] | 0.004 |
| Disease activity indexes | |||
| PCDAI | 17.1±17.4 [0–47.5] | 3.7±4.6 [0–10] | 0.03 |
| PUCAI | 25.8±15.3 [0–40] | 7.5±5.1 [0–35] | 0.002 |
| Immunosuppressants [n,%] | 9 [75] | 11 [35.5] | 0.03 |
| Laboratory parameters | |||
| Haemoglobin, g/dl | 11.3±1.5 [9.1–14.5] | 12.8±1.5 [8.3–14.9] | 0.005 |
| Pre-load sideraemia, µg/dl | 29.7±14.4 [13–52] | 71.6±31 [18–148] | 0.001 |
| Ferritin, ng/ml | 46.8±28 [6–99] | 36.7±23.9 [10–115] | 0.2 |
| Serum hepcidin, nM | 4.8±7.8 [0.55–28.5] | 2.7±3.9 [0.55–19.2] | 0.06 |
| Transferrin, g/l | 2.3±0.8 [1.3–4.3] | 2.7±0.4 [1.6–3.6] | 0.04 |
| Tsat, % | 9.9±5.7 [4–21] | 19.8±9.5 [3–41] | 0.003 |
| STfR, mg/dl | 1.8±0.5 [0.8–2.4] | 1.9±1.5 [0.9–8.5] | 0.8 |
| TIBC, µg/dl | 321±51 [220–370] | 327.6±78.2 [139–460] | 0.8 |
| Reticulocytes,% | 2.6±3 [0.8–9] | 4.6±3.9 [0.6–9] | 0.1 |
| CRP, mg/dl | 1.7 [0.2–5.1] | 0.4 [0–2.6] | 0.001 |
| ESR, mm | 16.7±11.4 [5–35] | 8.4±9.6 [1–46] | 0.02 |
| Calprotectin, µg/g | 316 [25–485] | 234.1 [30–493] | 0.6 |
All continuous variables values are expressed as means ± standard deviation [range].
CD, Crohn’s disease; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IBD, inflammatory bowel disease; IBD-U, inflammatory bowel disease unclassified; PCDAI, Paediatric Crohn’s Disease Activity Index; PUCAI, Paediatric Ulcerative Colitis Activity Index; STfR, soluble transferrin receptor; TIBC, total iron binding capacity; Tsat, transferrin saturation; UC, ulcerative colitis.
3.4. Correlations of serum hepcidin and Δ[Fe]2hr
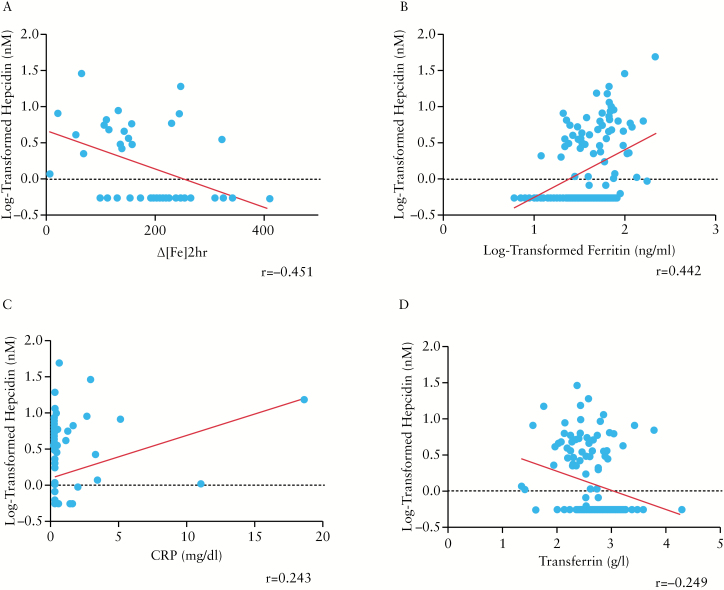
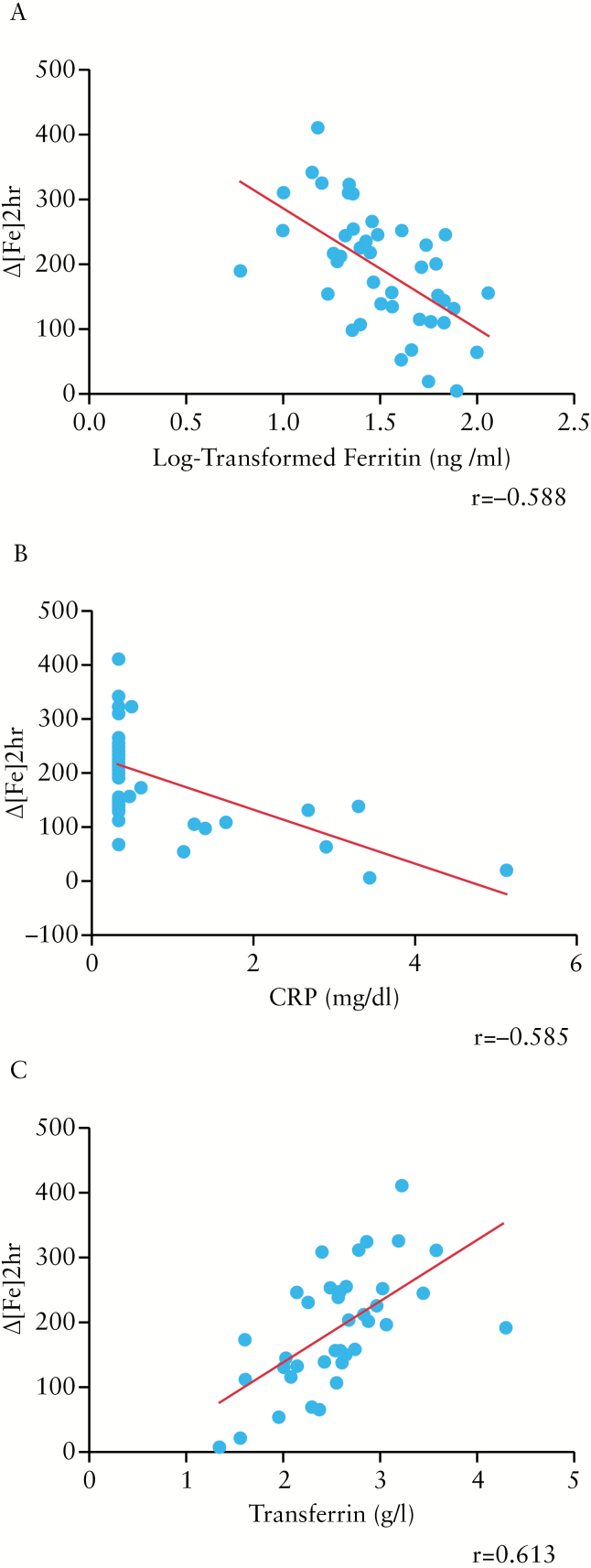
In order to determine correlations, we included all the patients [n = 145] in the analysis, except for Δ[Fe]2hr, PUCAI, PCDAI, calprotectin, and tTg, which were determined only in IBD patients or in coeliac patients. Log-tranformed hepcidin levels correlated negatively with Δ[Fe]2hr [r = -0.451, p = 0.002] [Figure 1A], and positively with log-transformed ferritin and CRP [r = 0.442, p = 0.0 001; r = 0.243, p = 0.009, respectively] [Figure 1B and C]. An inverse relationship was found with serum transferrin [r = -0.249, p = 0.005, respectively] [Figure 1D]. A direct correlation with a trend toward statistical significance was found between log-transformed hepcidin and ESR [r = 0.158, p = 0.09]. No specific correlation was found comparing log-transformed hepcidin with age, duration of IBD, serum iron, STfR, TIBC, or reticulocytes. Among coeliac patients, no significant correlation was identified between log-transformed hepcidin levels and tTG titres [r = -0.201, p = 0.2]. Δ[Fe]2hr was found to be inversely correlated with log-transformed ferritin and CRP [r = -0.588, p = 0.0001; r = -0.585, p = 0.0001, respectively] [Figure 2A and B]. A direct correlation was found between Δ[Fe]2hr and transferrin [r = 0.613, p = 0.0001] [Figure 2C]. An inverse correlation with a trend toward statistical significance was found with ESR, PUCAI, and faecal calprotectin [r = -0.296, p = 0.07; r = -0.292, p = 0.06; r = -0.325, p = 0.08, respectively]. None of the other variables correlated with Δ[Fe]2hr.
Figure 1.
Correlations between log-transformed serum hepcidin and Δ[Fe]2hr [r = -0.451, p = 0.002] in inflammatory bowel disease [IBD patients]. [A] Correlations between log-transformed serum hepcidin and log-transformed ferritin [r = 0.442, p = 0.0 001]. [B] C-reactive protein [CRP] [r = 0.243, p = 0.009] and [C] transferrin [r = -0.249, p = 0.005], [D] in all the enrolled patients.
Figure 2.
Correlations between Δ[Fe]2hr and log-transformed ferritin [r = -0.588, p = 0.0 001]. [A] C-reactive protein [CRP] [r = -0.585, p = 0.0 001] and [B] transferrin [r = 0.613, p = 0.0 001], [C] in inflammatory bowel disease [IBD] patients.
4. Discussion
To the best of our knowledge, this is the first paediatric study evaluating iron absorption and serum hepcidin levels in IBD paediatric patients. Our data show that IBD children with active disease tend to have impaired iron absorption, driven through the hepcidin pathway. Indeed, serum hepcidin levels were significantly higher in IBD patients with moderate to severe activity as compared with all other groups, including patients with mild activity or in remission, coeliac patients, and healthy controls. In addition, a significant inverse correlation was found between hepcidin levels and iron absorption. Our results are in agreement with the paper from Semrin and colleagues, the only paediatric study investigating the relationship between iron absorption and hepcidin.11 The authors, using urinary hepcidin as a proxy for serum hepcidin, enrolled 19 paediatric patients with CD and found that hepcidin was increased in those with active disease and inversely correlated with iron absorption.11
Anaemia is a relevant problem frequently occurring during IBD management. Despite its relative high prevalence, costs, and impact on patient quality of life, it is rarely considered and adequately treated.2,25,26 In our study population, the prevalence of anaemia among IBD patients was 34%. It is well known that the origin of IBD-related anaemia is usually multifactorial.23 Not surprisingly, the majority of the anaemic patients in our study population showed a combined IDA and ACD [70.5%]. This finding once more highlights the difficulties in the therapeutic management of IBD-related anaemia. Indeed, in those cases with a mixed pathogenesis, the clinician usually faces the dilemma whether or not to use oral iron. As previously reported, the treatment with oral iron has significant limitations in IBD, being less efficacious than the intravenous route.27 In addition, absorption of iron from the GI tract is limited, and unabsorbed iron is exposed to the intestinal surface. Iron mucosal harm has been described in IBD.28 Studies in animal models of colitis indicate that luminal iron may exacerbate disease activity.29,30 In a more recent study, iron supplementation affected microbiota and increased faecal calprotectin.31 Taking into account this possible warning, the recently published ECCO guidelines state that the intravenous route should be preferred in those patients with suspected ACD.23 It is therefore important to detect ACD in order to avoid an unnecessary and possibly harmful oral iron therapy.
The development of ACD is influenced by numerous factors, among which hepcidin is now considered the leading actor. Indeed, the decrease in ferroportin expression, which results from elevated hepcidin levels, would block entry of iron into the circulation, with consequent erythropoiesis impairment. Furthermore, since intestinal absorption of iron is inhibited by downregulation of ferroportin on enterocytes, the anaemia would be resistant to oral iron supplementation.7,8 Therefore, serum hepcidin may represent an useful surrogate marker to distinguish patients with impaired iron absorption in whom intravenous iron could be used as first-line option. To date, no paediatric study has evaluated serum hepcidin sensitivity and specificity in IBD-related anaemia. As previously reported in adult populations, the low specificity together with the lack of standardisation should be considered the main limitations of this marker.17,32 Although strongly limited by the small sample of anaemic patients, our data suggest that patients with pure IDA have undetectable hepcidin levels. This finding, if confirmed by larger series, may at least allow hepcidin use in the differential diagnosis of pure IDA from ACD. On the other hand, hepcidin’s role in the diagnosis of combined IDA and ACD is still questionable and needs to be addressed by further studies.
Based on a recent paper published by Wang and colleagues, hepcidin may also act beyond the simple role of iron regulator.33 The authors, inhibiting hepcidin expression in a mouse model of colitis, not only corrected IBD-related anaemia but also reduced colonic inflammatory cytokine expression.33 This finding suggests that hepcidin may be some way involved in perpetuating inflammation, representing a new potential IBD therapeutic target. Our study does not provide information about hepcidin’s role in the inflammatory process. Nevertheless, IBD children with pathological ILT showed higher values of disease activity indexes and acute phase reactants and were more often under immunosuppressive therapy, indicating the more severe phenotype of disease. In addition, the positive correlations of hepcidin with ESR and CRP, confirming that inflammation plays a major role in hepcidin induction, may also indicate that hepcidin could be directly implicated in the IBD inflammatory cascade. Anyhow, targeted studies are still necessary to confirm hepcidin’s role in the pathogenesis of IBD’s inflammatory course.
Our study was also meant to find out which iron parameters better correlate with hepcidin levels, and serum ferritin was found to be the most strictly correlated. This finding is in agreement with previous literature,17,34 and once more demonstrates that ferritin is the primary biochemical marker correlated to hepcidin concentration. However, 58.3% of patients, with a diagnosis of combined IDA + ACD and normal ferritin values, showed an increased hepcidin, demonstrating that the ferritin value is not sufficient in the differential diagnosis of IBD-related anaemia.
This study has some limitations. First of all, intestinal iron absorption was simply determined using the ILT based on the increment of iron level 2h after administrating an iron load, and this constitutes a methodological limitation. However, studies using dual stable iron isotope techniques, which are considered the gold standard for iron absorption determination, have shown a similar inverse correlation between serum hepcidin and iron absorption in healthy controls.35,36 In addition, ILT has already been correlated with urinary hepcidin in IBD.11 Finally, due to the earlier diagnosis, coeliac children were not age-matched with IBD and healthy controls and this may have influenced differences in hepcidin levels.
4.1. Conclusion
In conclusion, this comparative, cross-sectional study demonstrates that serum hepcidin is increased in IBD children with active disease and plays an important role in the process of iron malabsorption. ACD is significantly prevalent in paediatric IBD and should be taken in consideration before starting oral iron therapy. If confirmed by further studies, the negative correlation between hepcidin levels and iron malabsorption during the ILT may have important practical implications for a tailored management of anaemia in children with IBD-associated IDA. Indeed, serum hepcidin may serve as a useful, sensitive, surrogate marker to distinguish patients with impaired iron absorption in whom intravenous iron could be used as first-line option, avoiding the waste of time with a futile and possibly harmful cycle of oral iron. Further studies are needed to better elucidate the role of hepcidin in both iron metabolism and inflammation in IBD, in order to design new therapeutic strategies for ACD in paediatric IBD.
Funding
This work was partially supported by the Italian Ministry of Health Grant RF-2010-2312048 to DG and by the Department of Women, Children and of General and Specialist Surgery at the Second University of Naples [Grant on Normal and Pathological Hematopoiesis] to SP.
Conflict of Interest
The authors declare no conflict of interest to disclose regarding this paper; AS is a speaker for Valeas Angelini, Milte Italia, and Danone, and consultant of D.M.G. Italy and Sucampo.
Author Contributions
MM: substantial contributions to conception and design, analysis and interpretation of data, drafting the article, and final approval of the version to be published. CS: substantial contributions to conception and design, analysis and interpretation of data, drafting the article, and final approval of the version to be published. AA: substantial contributions to conception and design, analysis and interpretation of data, drafting the article, and final approval of the version to be published. FR: substantial contributions to conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be published. RA: substantial contributions to conception and design, analysis and interpretation of data, drafting the article, and final approval of the version to be published. NC:substantial contributions to conception and design, analysis and interpretation of data, drafting the article, and final approval of the version to be published. DG: substantial contributions to conception and design, analysis and interpretation of data, drafting the article, and final approval of the version to be published. BN: revising the article critically for important intellectual content and final approval of the version to be published. AS: revising the article critically for important intellectual content and final approval of the version to be published. SP: revising the article critically for important intellectual content and final approval of the version to be published. EM: substantial contributions to conception and design, interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be published.
References
- 1. Gasche C, Lomer MCE, Cavill I, Weiss G. Iron, anemia, and inflammatory bowel diseases. Gut 2004;53:1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wells CW, Lewis S, Barton JR, Corbett S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis 2006;12:123–30. [DOI] [PubMed] [Google Scholar]
- 3. Goodhand JR, Kamperidis N, Rao A, et al. Prevalence and management of anemia in children, adolescents, and adults with inflammatory bowel disease. Inflamm Bowel Dis 2012;18:513–9. [DOI] [PubMed] [Google Scholar]
- 4. Oustamanolakis P, Koutroubakis IE, Kouroumalis EA. Diagnosing anemia in inflammatory bowel disease: beyond the established markers. J Crohns Colitis 2011;5:381–91. [DOI] [PubMed] [Google Scholar]
- 5. Zhu A, Kaneshiro M, Kaunitz JD. Evaluation and treatment of iron deficiency anemia: a gastroenterological perspective. Dig Dis Sci 2010;55:548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica 2010;95:175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 2001;276:7806–10. [DOI] [PubMed] [Google Scholar]
- 8. Ganz T. Hepcidin. a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003;102:783–8. [DOI] [PubMed] [Google Scholar]
- 9. Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 2004;113:1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A 2005;102:1906–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Semrin G, Fishman DS, Bousvaros A, et al. Impaired intestinal iron absorption in Crohn’s disease correlates with disease activity and markers of inflammation. Inflamm Bowel Dis 2006;12:1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oustamanolakis P, Koutroubakis IE, Messaritakis I, Malliaraki N, Sfiridaki A, Kouroumalis EA. Serum hepcidin and prohepcidin concentrations in inflammatory bowel disease. Eur J Gastroenterol Hepatol 2011;23:262–8. [DOI] [PubMed] [Google Scholar]
- 13. Arnold J, Sangwaiya A, Bhatkal B, Geoghegan F, Busbridge M. Hepcidin and inflammatory bowel disease: dual role in host defence and iron homoeostasis. Eur J Gastroenterol Hepatol 2009;21:425–9. [DOI] [PubMed] [Google Scholar]
- 14. Kaya Z, Yildiz E, Gursel T, et al. Serum prohepcidin levels in children with solid tumors, inflammatory bowel disease and iron deficiency anemia. J Trop Pediatr 2011;57:120–5. [DOI] [PubMed] [Google Scholar]
- 15. Nagy J, Lakner L, Poór VS, et al. Serum prohepcidin levels in chronic inflammatory bowel diseases. J Crohns Colitis 2010;4:649–53. [DOI] [PubMed] [Google Scholar]
- 16. IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis the Porto criteria. J Pediatr Gastroenterol Nutr 2005;41:1–7. [DOI] [PubMed] [Google Scholar]
- 17. Bergamaschi G, Di Sabatino A, Albertini R, et al. Serum hepcidin in inflammatory bowel diseases: biological and clinical significance. Inflamm Bowel Dis 2013;19:2166–72. [DOI] [PubMed] [Google Scholar]
- 18. Campostrini N, Castagna A, Zaninotto F, et al. Evaluation of hepcidin isoforms in hemodialysis patients by a proteomic approach based on SELDI-TOF MS. J Biomed Biotechnol 2010;2010:329646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- 20. Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr 1991;12:439–47. [PubMed] [Google Scholar]
- 21. Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133:423–32. [DOI] [PubMed] [Google Scholar]
- 22. WHO, UNICEF, UNU. Iron Deficiency Anemia: Assessment, Prevention and Control. Report of a joint WHO/UNICEF/UNU consultation. Geneva: World Health Organization, 1998. [Google Scholar]
- 23. Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis 2015;9:211–22. [DOI] [PubMed] [Google Scholar]
- 24. De Vizia B, Poggi V, Conenna R, Fiorillo A, Scippa L. Iron absorption and iron deficiency in infants and children with gastrointestinal diseases. J Pediatr Gastroenterol Nutr 1992;14:21–6. [DOI] [PubMed] [Google Scholar]
- 25. Ott C, Liebold A, Takses A, Strauch UG, Obermeier F. High prevalence but insufficient treatment of iron-deficiency anemia in patients with inflammatory bowel disease: results of a population-based cohort. Gastroenterol Res Pract 2012;2012:595970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lugg S, Beal F, Nightingale P, Bhala N, Iqbal T. Iron treatment and inflammatory bowel disease: What happens in real practice? J Crohn’s Colitis 2014;8:876–80. [DOI] [PubMed] [Google Scholar]
- 27. Lee TW, Kolber MR, Fedorak RN, van Zanten SV. Iron replacement therapy in inflammatory bowel disease patients with iron deficiency anemia: a systematic review and meta-analysis. J Crohns Colitis 2012;6:267–75. [DOI] [PubMed] [Google Scholar]
- 28. De Silva AD, Tsironi E, Feakins RM, Rampton DS. Efficacy and tolerability of oral iron therapy in inflammatory bowel disease: a prospective, comparative trial. Aliment Pharmacol Ther 2005;22:1097–105. [DOI] [PubMed] [Google Scholar]
- 29. Seril DN, Liao J, West AB, Yang GY. High-iron diet: foe or feat in ulcerative colitis and ulcerative colitis-associated carcinogenesis. J Clin Gastroenterol 2006;40:391–7. [DOI] [PubMed] [Google Scholar]
- 30. Oldenburg B, Berge Henegouwen GP, Rennick D, Van Asbeck BS, Konigsberger JC. Iron supplementation affects the production of pro-inflammatory cytokines in IL-10 deficient mice. Eur J Clin Invest 2000;30:505–10. [DOI] [PubMed] [Google Scholar]
- 31. Zimmermann MB, Chassard C, Rohner F, et al. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d’Ivoire. Am J Clin Nutr 2010;92:1406–15. [DOI] [PubMed] [Google Scholar]
- 32. Oustamanolakis P, Koutroubakis IE. Soluble transferrin receptor-ferritin index is the most efficient marker for the diagnosis of iron deficiency anemia in patients with IBD. Inflamm Bowel Dis 2011;17:E158–9. [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Trebicka E, Fu Y, et al. The bone morphogenetic protein-hepcidin axis as a therapeutic target in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mecklenburg I, Reznik D, Fasler-Kan E, Drewe J, Beglinger C, Hruz P. On behalf of the Swiss IBD Cohort Study Group. Serum hepcidin concentrations correlate with ferritin in patients with inflammatory bowel disease. J Crohns Colitis 2014;8:1392–7. [DOI] [PubMed] [Google Scholar]
- 35. Young MF, Glahn RP, Ariza-Nieto M, et al. Serum hepcidin is significantly associated with iron absorption from food and supplemental sources in healthy young women. Am J Clin Nutr 2009;89:533–8. [DOI] [PubMed] [Google Scholar]
- 36. Roe MA, Collings R, Dainty JR, Swinkels DW, Fairweather-Tait SJ. Plasma hepcidin concentrations significantly predict interindividual variation in iron absorption in healthy men. Am J Clin Nutr 2009;89:1088–91. [DOI] [PubMed] [Google Scholar]