Abstract
Background and Aims:
Non-adherence to anti-tumour necrosis factor [TNF] agents in patients with inflammatory bowel disease [IBD] is a serious problem. In this study, we assessed risk factors for non-adherence and examined the association between adherence to anti-TNF agents and loss of response [LOR].
Methods:
In this multicentre, 12-month observational study, outpatients with IBD were included. Demographic and clinical characteristics were recorded. Adherence was measured with the Modified Morisky Adherence Scale-8 [MMAS-8] and 12-month pharmacy refills [medication possession ratio, MPR]. Risk factors included demographic and clinical characteristics, medication beliefs, and illness perceptions. Cox regression analysis was performed to determine the association between MPR and LOR to anti-TNF, IBD-related surgery or hospitalisation, dose intensification, or discontinuation of anti-TNF.
Results:
In total, 128 patients were included [67 infliximab, 61 adalimumab], mean age 37 ( ± standard deviation [SD] 14) years, 71 [56%] female. Median disease duration was 8 (interquartile range [IQR] 4–14) years. Clinical disease activity was present in 41/128 [32%] patients, 36/127 [28%] patients had an MMAS-8 < 6 [‘low adherence’], and 25/99 [25%] patients had an MPR < 80% [non-adherence]. Risk factors for non-adherence included adalimumab use (odds ratio [OR] 10.1, 95% confidence interval [CI] 2.62–40.00), stronger emotional response [OR 1.16, 95% CI 1.02–1.31], and shorter timeline perception, i.e. short perceived illness duration [OR 0.60, 95% CI 0.38–0.96]. Adherence is linearly and negatively [OR 0.14, 95% CI 0.03–0.63] associated with LOR.
Conclusion:
Non-adherence to anti-TNF agents is strongly associated with LOR to anti-TNF agents, adalimumab use, and illness perceptions. The latter may provide an important target for interventions aimed at improving adherence and health outcomes.
Keywords: Inflammatory bowel disease, adherence, predictors, illness perceptions, medication beliefs
1. Introduction
The introduction of anti-tumour necrosis factor [TNF] agents, including infliximab and adalimumab, has greatly improved the treatment options in patients with Crohn’s disease [CD] and ulcerative colitis [UC]. Both agents are highly efficacious for induction and maintenance of remission1 and have been shown to reduce rates of hospitalisation and surgery.2,3,4
In clinical practice, non-adherence to anti-TNF agents in patients with inflammatory bowel disease [IBD] ranges from 17% to 45%,5,6 and is associated with higher rate of hospitalisation and increased healthcare costs.7,8 Several factors have been associated with non-adherence to anti-TNF agents in IBD, including female gender, smoking, maintenance dosing, constraints related to treatment, anxiety, and moodiness.5,6
Despite our increased understanding of adherence, several controversies still exist. Non-adherence is generally defined as taking less than 80% of medication, but this definition may not be applicable to anti-TNF agents.9,10 Additionally, no prospective studies to date have evaluated the association between adherence and loss of response to anti-TNF agents. Finally, no consistent association between the above-mentioned risk factors and non-adherence to anti-TNF agents has been found and few of them can be modified.11,12
Treatment beliefs and illness perceptions are modifiable factors and have been consistently associated with non-adherence to medication across a range of chronic illnesses, including IBD.13,14,15,16 Treatment beliefs constitute patients’ beliefs about the necessity of their medication for controlling their illness and their concerns about potential side effects.17 Illness perceptions constitute patients’ views, cognitive or emotional, about several components of the illness such as the causes, clinical course, daily consequences, curability, and emotional impact.18
Therefore, in this multicentre prospective study we assessed: 1] risk factors, including treatment beliefs and illness perceptions, for non-adherence to anti-TNF agents; and 2] the association between the level of adherence and loss of response, leading to hospitalisation, surgery, and dose intensification or discontinuation of anti-TNF agents in outpatients with IBD.
2. Patient and Methods
2.1. Patient population and design
Between May 2012 and May 2013, adult [age ≥ 18 years] ambulatory patients with an established diagnosis of IBD19,20 receiving infliximab [intravenous] or adalimumab [subcutaneous] as induction or maintenance treatment were invited to participate in the study. Patients were recruited from three university hospitals [University Medical Centre Utrecht, Leiden University Medical Centre, Maastricht University Medical Centre] and three general hospitals [Jeroen Bosch Hospital, ‘s Hertogenbosch; Diakonessenhuis, Utrecht; Onze Lieve Vrouwe Gasthuis, Amsterdam] in The Netherlands. At baseline, patients completed a baseline questionnaire, which included demographic data, clinical disease activity, treatment beliefs, illness perceptions, and adherence behavior, as described below. Clinical data were collected from electronic medical records. Patients were then prospectively followed for 12 months. Clinical outcomes were also collected from electronic medical records. Local patient pharmacies were contacted for medication refills during follow-up. The study was centrally approved by the Ethics Committee of the University Medical Centre Utrecht. All patients signed informed consent.
2.2. Risk factors for non-adherence to anti-TNF agents
Potential risk factors for non-adherence to anti-TNF agents were derived from two systematic reviews.5,6 Demographic risk factors included gender, age, smoking status [current smoker, ex-smoker, non-smoker], and education level [low versus high]. Low education included no education, primary education, secondary education, and technical or professional school, whereas high education included higher vocational education and university.
Clinical risk factors included age at diagnosis, disease duration, disease localisation, perianal disease, concomitant medication [mesalazine, corticosteroids, immunomodulators], anti-TNF agent, and duration of anti-TNF treatment at enrolment. Clinical disease activity was measured with the validated patient-based Harvey-Bradshaw Index [11 items, excluding question about abdominal mass]21,22 and the patient-based Simple Clinical Colitis Activity Index [9 items]23,24 for patients with CD and UC, respectively. A score more than 4 indicated clinically active disease. The patient-based Harvey-Bradshaw Index consists of 11 items addressing 4 domains, including general well-being [1 item], abdominal pain [1 item], number of liquid stools per day [1 item], and extraintestinal manifestations [8 items: arthralgia, uveitis, erythema nodosum, aphthous ulcer, pyoderma gangrenosum, anal fissure, new fistula, and abscess]. As the third item of the clinician-based Harvey-Bradshaw Index [abdominal mass] requires a physical examination, this item was excluded.22
Behavioural risk factors included illness perceptions and treatment beliefs. Illness perceptions were assessed with the Brief Illness Perception Questionnaire.25 This 9-item questionnaire explores the cognitive and emotional representations of illness across 8 dimensions: Consequences, Timeline, Personal Control, Treatment Control, Identity, Concerns, Understanding, and Emotional Response. Items are assessed on an 11-point Likert scale, for example ‘How much does your illness affect your life?’: 0 [‘not at all’] – 10 [‘severely affects my life’]. Treatment beliefs were assessed with the Beliefs about Medicines Questionnaire specific [BMQ-specific].26,27 This questionnaire consists of two subscales assessing patients’ beliefs about the necessity of the prescribed medication for controlling their illness, and their concerns about the potential adverse consequences of taking it. For example: ‘My health at present depends on this medicine’ or ‘I sometimes worry about becoming too dependent on this medicine’. Patients indicate their degree of agreement with each statement on a 5-point Likert scale, ranging from strongly disagree [1] to strongly agree [5]. A mean score for each subscale is calculated by dividing total scores by the number of items in the scale, resulting in a mean score range of 1–5 for both necessity [8 items] and concerns [9 items] scales.
2.3 Adherence measures
Adherence to anti-TNF agents was measured with an 8-item self-reported measure [Morisky Medication Adherence Scale-8, MMAS-8]28 and pharmacy refills. At baseline, patients were asked to fill out the MMAS-8. This tool addresses several factors of medication- taking behaviour, including failure to remember and problems with the complexity of the anti-TNF regimen It has been reported to correlate well with patients’ adherence as measured with pharmacy refills in IBD.30 A score less than 6 points is interpreted as low adherence, 6–7 points as medium adherence, and 8 points as high adherence.
Medication refills were assessed over 12 months. By tracking refills over a 12-month period, patients’ refill activity was averaged and accounted for ‘medication hoarding’ and premature refills. Pharmacy refill adherence was calculated by dividing the number of days supplied within the refill interval [based on the dosing regimen] by the number of days in the actual refill interval over 12 months, and expressed as percentage. Pharmacy refills are considered a suitable ‘gold standard’ to measure patient adherence.30
2.4. Outcome measures
The main outcome measure included the association between adherence to anti-TNF agents and loss of response. No exact definition of loss of response exist.31 In the present study, loss of response was defined by clinical worsening leading to IBD-related surgery [bowel resection, strictureplasty or ostomy, or perianal surgery30], IBD-related hospitalisation, or dose intensification or discontinuation of anti-TNF agent. Dose intensification was defined as either an increase in dose (from 5mg to 10mg/kg for infliximab [IFX]; from 40mg to 80mg for adalimumab [ADA]) or as a decrease of the interval between infusions [ranging from 4 to 8 weeks] or injections [from every other week to every week]. At the physicians’ discretion, trough serum concentrations of IFX or ADA were measured in patients with loss of response. Trough levels were measured by Sanquin, Amsterdam, The Netherlands, using an enzyme-linked immunosorbent assay [ELISA] as previously described.33 Trough levels of IFX <3 µg/ml and of ADA < 5 µg/ml were considered subtherapeutic.34,35
2.5. Statistical analysis
Data analyses were performed using SPSS 20.0 and SAS 9.2. Descriptive statistics were used to characterise IBD patients. Means and medians were reported with a standard deviation [SD] and interquartile range [IQR], respectively. Comparisons between adherent and non-adherent IBD patients according to MMAS-8 and pharmacy refills [MPR] were analysed with Student’s-t test for continuous variables and χ2 test for dichotomous variables. Association between MMAS-8 and MPR percentage was assessed by means of Spearman’s correlation analysis. In order to determine factors associated with non-adherence, we performed univariate logistic regression analysis with demographic, disease, and behavioural characteristics. Characteristics that were associated [p < 0.10] with non-adherence following univariate analysis were included in the multivariate logistic regression analyses to identify independent risk factors for adherence. Cox proportional hazards regression analysis was performed to determine the association between adherence and time to loss of response. Tests for trends in loss of response across categories of MPR percentage [0–40, 40–60, 60–80, 80–100%] were conducted using the median value in each category as a continuous variable in the linear regression models. We estimated that after 12 months, 70% of all anti-TNF users will have maintained clinical response.36,37 With an alpha of 0.05 and a power of 90%, we were able to have an area under the receiver operating curve of 0.75 [with standard error of 0.05] if we included 32 anti-TNF users without clinical response. Based on 70% maintained clinical response, the inclusion of 107 anti-TNF users was required. After adjustment for missing values and loss to follow-up, we decided to include a total of 130 anti-TNF users.
3. Results
3.1. Patient population
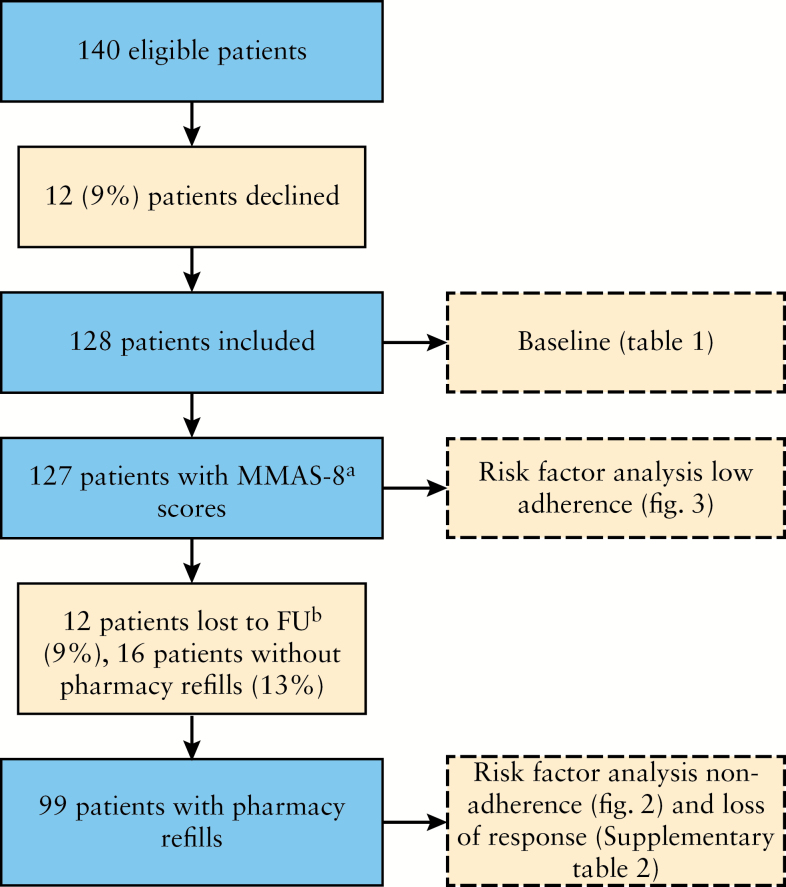
Between May 2012 and May 2013, 128 patients were prospectively enrolled and 12 patients declined to participate [Figure 1]. Patients who declined to participate had a lower mean age [27.3±7.7 versus 36.6±13.6 years, p = 0.02] as compared with patients who did participate [Table 1]. There were no statistically significant differences between groups with regard to relevant demographic and clinical characteristics. Obviously, data on clinical disease activity, illness perceptions, and treatment beliefs of non-responders could not be obtained. Of the 128 patients, 104 [81%] had CD and 24 [19%] had either UC or IBD-unclassified, with a mean age of 36.6 [± SD 13.6] years, and a median disease duration of 8 [IQR 4–14] years; 66 [46%] and 62 [54%] patients received infliximab and adalimumab, respectively; 62 [47%] patients were concomitantly treated with immunosuppressive agents. Overall, 27/61 [44%] adalimumab users and 33/67 [49%] infliximab users were concomitantly treated with immunosuppressive agents [p = 0.60]; 41 [32%] patients had clinically active disease as measured with the clinical index score. During follow-up, 12 patients [9.4%] were lost to follow-up because of no response to induction treatment [4], death [1], transfer [3], or unknown [4]; 12-month pharmacy refills were available for 99 patients.
Figure 1.
Flow-chart. [a] Modified Morisky Adherence Scale, 8 items; [b] follow-up.
Table 1.
Patient characteristics [responders: n = 128; non-responders: n = 12].
| Characteristic: | Responders: | Nonresponders: | p value |
|---|---|---|---|
| Age, mean [± SD] | 36.6 [13.6] | 27.3 [7.7] | 0.02 |
| Female gender, n [%] | 71 [55] | 4 [33] | 0.23 |
| Smoking, n [%] | 25 [20] | ||
| Low education, n [%] | 80 [63] | ||
| Diagnosis, n [%] | |||
| Crohn’s disease | 104 [81] | 11 [92] | 0.47 |
| Ulcerative colitis, IBD-u | 24 [19] | 1 [8] | 0.47 |
| Disease duration, median [IQR] | 8 [4–14] | ||
| IBD localisationa | |||
| Large bowel | 29 [28] | 3 [27] | 0.55 |
| Small bowel | 20 [19] | 3 [27] | 0.42 |
| Both large and small bowel | 55 [53] | 5 [45] | 0.76 |
| Clinical active disease, n [%] | 41 [32] | ||
| Perianal disease, n [%] | 74 [71] | ||
| Anti-TNF agent, n [%] | |||
| Infliximab | 66 [52] | 8 [67] | 0.38 |
| Adalimumab | 62 [48] | 4 [33] | 0.38 |
| Concomitant medication, n [%] | |||
| 5-ASA | 22 [17] | 2 [17] | 1.00 |
| Corticosteroid | 21 [16] | 1 [8] | 0.69 |
| Immunosuppressive agent | 62 [48] | 4 [33] | 0.38 |
| MMAS-8b score, median [IQR]c | 7.00 [5.75–7.75] | ||
| <6 [‘low adherer’], n [%] | 36 [28] | ||
| 6–8 [‘medium adherer’], n [%] | 61 [48] | ||
| 8 [‘high adherer’], n [%] | 30 [24] | ||
SD, standard deviation; IBD-u, inflammatory bowel disease unclassified; IQR, interquartile range; TNF, tumour necrosis factor; 5-ASA, 5-aminosalicylates.
aCrohn’s disease patients.
bModified Morisky Adherence Scale-8.
c1 missing value.
3.2. Adherence
Of 128 patients, 127 filled-out the MMAS-8. The median MMAS-8 score was 7.00 [IQR 5.75–7.75] [Table 1]. Patients who were lost to follow-up did not have a significantly different median MMAS-8 score [7.75, IQR 6.00–8.00 versus 7.00, IQR 5.75–7.75; p = 0.15]; 36 [28%] patients were ‘low adherer’, 61 [48%] ‘medium adherer’, and 30 [24%] ‘high adherer’. Low adherence to infliximab and adalimumab was reported in, respectively, 16/66 [24%] and 20/61 [33%] of patients [p =0 .33]; 12-month pharmacy refills were available for 99 patients; 25 [25%] patients were non-adherent to anti-TNF agents. Non-adherence to infliximab and adalimumab was reported in, respectively, 4/50 [8%] and 21/49 [43%] of patients [p < 0.01]. The correlation coefficient between the MMAS-8 and MPR percentage was 0.28 [p = 0.014].
3.3. Risk factors of non-adherence [MPR < 80%] and low adherence according MMAS-8
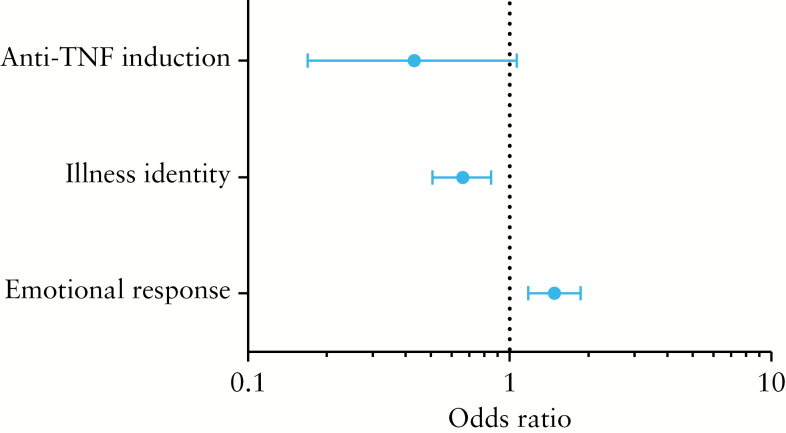
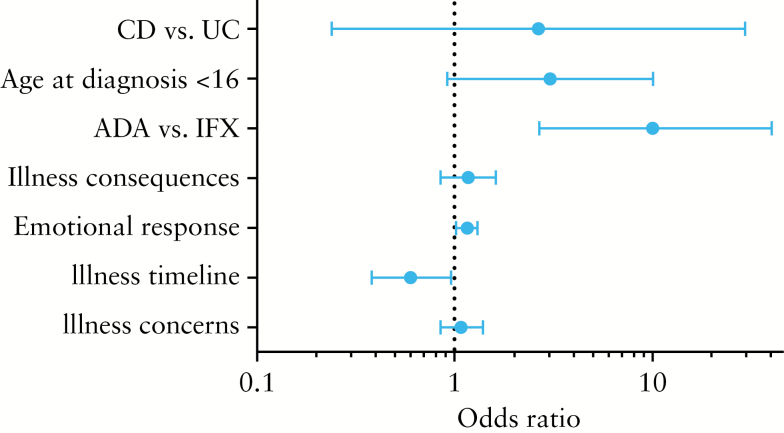
The results of the univariate analyses are shown in Supplementary Tables 1 and 2, available as Supplementary data at ECCO-JCC online. Independent risk factors for non-adherence according to pharmacy refills were adalimumab use [OR 10.1, 95% CI 2.62–40.00], shorter timeline perception, i.e. perceptions of IBD as an acute episodic disease [OR 0.60, 95% CI 0.38–0.96], and stronger emotional response, i.e. negative emotions resulting from IBD [OR 1.16, 95% CI 1.02–1.31] [Figure 2]. Although CD patients were more likely to be non-adherent in univariate analysis [OR 6.10, 95% CI 0.76–50.00], this association disappeared in the multivariate analysis [OR 2.68, 95% CI 0.24–29.42]. Independent risk factors for low adherence according to the MMAS-8 were a weaker illness identity, i.e. less symptoms associated with IBD [OR 0.66, 95% CI 0.51–0.85], and a stronger emotional response [OR 1.48, 95% CI 1.18–1.87]. There was a trend towards a statistically significant and negative association between induction therapy and low adherence [OR 0.43, 95% CI 0.17–1.07] [Figure 3].
Figure 2.
Independent risk factors of low adherence (Modified Morisky Adherence Scale [MMAS]-8 score < 6].
Figure 3.
Independent risk factors of non-adherence (medication possession ratio [MPR] < 80%).
3.4. Association between adherence and loss of response
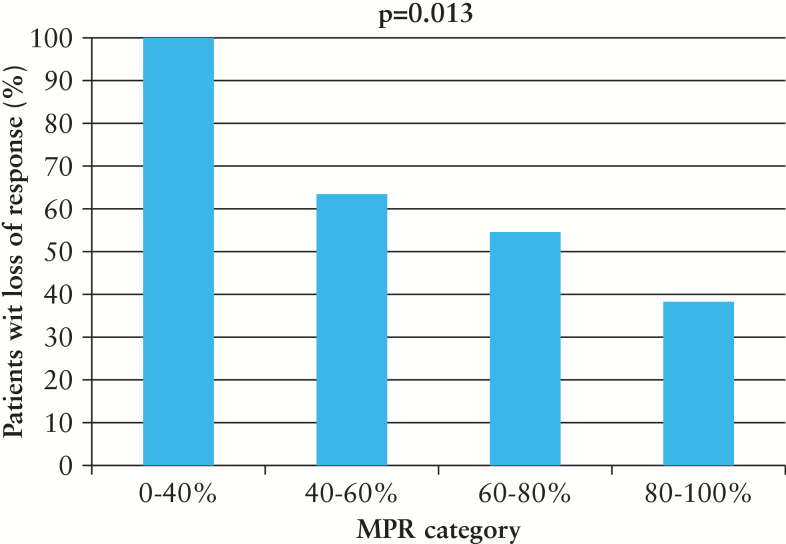
During a median follow-up of 1.4 years [IQR 0.8–2.2] 56 of 116 [48%] IBD patients lost response to an anti-TNF agent [46/100 patient-years], which resulted in IBD-related surgery in 11 patients, hospitalisation in 3 patients, and dose intensification and discontinuation in 42 patients. Trough levels were measured in 36 of 56 [64%] IBD patients, including 19 patients on IFX and 17 patients on ADA; 11 of 19 [58%] IFX users and 8 of 17 [47%] ADA users had a subtherapeutic trough level with a median of, respectively, 2.0 µg/ml [IQR 0.9–9.0] and 4.8 µg/ml [IQR 2.0–8.5]. In univariate analysis, risk factors for loss of response were Crohn’s disease [OR 2.58, 95% CI 1.37–4.87], clinically active disease at baseline [OR 2.12, 95% CI 1.20–3.77], co-treatment with corticosteroids [OR 3.41, 95% CI 1.63–7.12], and low MPR percentage [OR 0.14, 95% CI 0.03–0.63]. Co-treatment with an immunosuppressive agent was not associated with loss of response [OR 0.88, 95% CI 0.48–1.61]. An increase in MPR percentage was parallelled by a significant [p = 0.013] decrease in the proportion loss of response [Figure 4]. Factors that were independently associated with loss of response included co-treatment with corticosteroids [OR 2.93, 95% CI 1.16–7.41] and low MPR percentage [OR 0.08, 95% CI 0.02–0.42].
Figure 4.
Association between medication possession ratio [MPR] category and proportion of patients with loss of response [%].
4. Discussion
In this 12-month observational study, non-adherence rates were significantly higher in patients treated with adalimumab than with infliximab. Non-adherence to anti-TNF agents was strongly determined by adalimumab use and—to a minor extent—by negative beliefs about how IBD affects one’s emotional well-being [i.e. a stronger emotional response]. Additionally, we found that non-adherence was significantly associated with loss of response to anti-TNF agents.
To our knowledge, this is the first study exploring the influence of illness perceptions on adherence to anti-TNF agents and prospectively examining the association between adherence to anti-TNF agents and loss of response. The overall non-adherence rate for anti-TNF agents was 25% [defined as a medication possession ratio < 80%], which is similar to the rates reported by two large systematic reviews [17% to 45%].10,11 Non-adherence rates were higher for adalimumab than for infliximab according to both the Modified Morisky Adherence Scale-8 [MMAS-8] [adalimumab 33% vs infliximab 24%] and the pharmacy refills [adalimumab 43% vs infliximab 8%]. The significantly higher non-adherence rate for adalimumab may be explained by the fact that adalimumab is self-administered, whereas infliximab is administered intravenously by a healthcare professional in a controlled setting. Although one might presume that adalimumab users are more likely to have a non-adherent behaviour, this was not found with the MMAS-8 tool. Additionally, there were no significant differences between adalimumab and infliximab users with regard to illness perceptions and treatment beliefs, which could have explained the difference in non-adherence rates. Notwithstanding, confounding by indication may still have occurred, as physicians are more likely to prescribe infliximab to patients with possibly unfavourable medication-taking behaviour.
We found that the MMAS-8 tool correlated significantly with patients’ refill behaviour. Yet, with a correlation coefficient of only 0.28, this correlation was weak, limiting the use of the MMAS-8 as a useful screening tool to predict patients’ medication adherence behaviour. However, the MMAS-8 may be a useful tool to assess patients’ reasons for non-adherence and differentiate between intentional and unintentional non-adherence.38
This finding is in line with a study that examined the correlation between the MMAS-8 tool and pharmacy refills among 150 IBD patients receiving different drug classes, including 5-aminosalicylates [ASA], budesonide, immunomodulators, and anti-TNF agents.30 A significant association between the MMAS-8 tool and pharmacy refills was found with regard to immunomodulators [r = 0.26, p = 0.02], but not to anti-TNF agents [r = -0.04, p = 0.85]. In contrast, other studies which excluded patients receiving anti-TNF agents, observed a significant association between the MMAS-8 tool and pharmacy refills.29,39 These differences may be explained by the fact that the MMAS-8 tool was originally developed in patients on daily medication. Additionally, several questions on the MMAS-8 simply do not apply for intravenous medication [infliximab], for example: ‘Have you cut back your medication without telling your doctor?’
In order to improve medication adherence, researchers and physicians increasingly focus on modifiable factors such as frequency of dosing, route of administration, measures to reduce anxiety and depression, and beliefs about medication. Beliefs about medication may play a key role in determining non-adherence. A cross-sectional study of 1871 IBD patients reported more doubts about the personal need for maintenance treatment and more concerns about potential adverse effects in non-adherent patients [according to the four-item Medication Adherence Report Scale].28 These findings were confirmed by a cross-sectional study of 356 IBD patients in which adherent patients had a higher belief of necessity to take medication and a trend towards lower concerns about medication.17 There is no clear explanation for the non-significant association between beliefs about medication and non-adherence in the present study. As non-adherent patients in our cohort had a trend towards more concerns about potential adverse effects, our study might have been underpowered to detect significant differences between adherent and non-adherent patients with regard to beliefs about medication. Interestingly, we demonstrated in our study that illness perceptions, in particular negative perceptions about how IBD affects a patient’s emotional well-being [i.e. emotional response] and perceptions of IBD as an acute episodic disease [i.e. timeline perception], were significantly associated with non-adherence. This concurs with previous studies showing a negative impact of emotional responses [feelings of anxiety, anger, and depression] on medication adherence in patients with hypertension.40,41 Additionally, a prospective study of 198 adult patients with allergic asthma reported a significantly lower adherence in patients who perceived their asthma as only present when they experienced symptoms.42 This finding corroborates the Common Sense Model of self-regulation, in which it is stated that individual’s personal beliefs about an illness [here: IBD] play a major role in the adjustment to the illness, i.e. taking the medication as prescribed.19,43
Finally, we demonstrated that adherence was linearly associated with loss of response, i.e. every 10% increase in MPR corresponded with a 24% decreased risk of losing response to an anti-TNF agent. In contrast to previous studies in patients with dyslipidaemia and HIV/AIDS, we have not identified a threshold adherence level above which the risk of losing response significantly decreased.8,9 This may be partly due to the complex pharmacological metabolism of anti-TNF agents [e.g. production of anti-TNF antibodies and routes of administration] in respect to the more ‘simple’ oral lipid-lowering and antiretroviral drugs. Additionally, in the present study adherence was linked to clinical outcomes, whereas previous studies used ‘intermediate’ outcomes such as virological failure and lipid reduction.
This study has several strengths. First, this study was powered to detect a statistically significant association between adherence and loss of response. Second, two methods of adherence measurement, i.e. the MMAS-8 tool and pharmacy refills, were combined in order to maximise accuracy. Third, patients were prospectively followed for 12 months, which enabled assessment of predictors of non-adherence and loss of response. Fourth, patients were included from both university and general hospitals, thereby, in our view, reliably representing the average IBD patient in The Netherlands.
Some limitations of this study need to be addressed as well. First, although the MMAS-8 tool was modified in order to measure adherence to anti-TNF agents, some questions may not be applicable to non-oral medication. Second, as trough levels were not systematically measured in patients with loss of response, we could not reliably assess the association between trough levels and loss of response. Third, although the non-adherence rate found in our study is in line with previous studies, the Hawthorne effect [in which patients improve their medication-taking behaviour in response to their awareness of being observed]44 could not be completely excluded. Consequently, the non-adherence to TNF-inhibitors in clinical practice might even be higher.
In conclusion, our results show a linear association between adherence to anti-TNF agents and loss of response. Additionally, adherence is substantially lower in patients treated with adalimumab, in patients with negative beliefs about how IBD affects one’s emotional well-being, and with a shorter timeline perception. As illness perceptions and beliefs about medication are potentially modifiable factors, they may provide a relevant target for interventions aimed at improving adherence and other health outcomes. These interventions might have a far greater impact on health outcomes than any treatment itself.45 Future studies should focus on the use of trough levels as a reliable measure of adherence and on the development of adherence-enhancing interventions targeted at modifiable factors such as treatment and illness beliefs.
Funding
No specific funding has been received.
Conflict of Interest
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. HHF has acted as a consultant for AbbVie. AAK has received payments for lectures from Ferring and acted as a consultant for AbbVie and Shire. AEvdMJ has acted as consultant for AbbVie. MP has acted as a consultant for MSD and received payments for lectures from MSD, Falk Pharma, Abbvie, and Ferring. BO has received payments for lectures from Ferring and acted as a consultant for AbbVie and MSD. PDS has received a research grant from Falk Pharma.
Author Contributions
The concept and design of the study, or acquisition of data, or analysis and interpretation of data: MvdH, BOld, HHF, AAK, MGHvO; drafting the article or revising it critically for important intellectual content: MvdH, BO, HhF, AAK, MGHvO, JMJ, RCHS, BAvT, AEvdMJ, MP, PDS; final approval of the version to be submitted: all authors.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
Acknowledgments
We thank Janette Gaarenstroom, Toos Schakel, Mia Cillissen, and Andy Peters for assistance with patient enrolment. Use of the MMAS is protected by US copyright laws. Use of the MMAS-8 was made under a licence agreement from Professor Donald E. Morisky, Department of Community Health Sciences, UCLA School of Public Health, Los Angeles, CA, USA.
References
- 1. Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011;106:644–59. [DOI] [PubMed] [Google Scholar]
- 2. Costa J, Magro F, Caldeira D, Alarcão J, Sousa R, Vaz-Carneiro A. Infliximab reduces hospitalisations and surgery interventions in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2013;19:2098–110. [DOI] [PubMed] [Google Scholar]
- 3. Feagan BG, Panaccione R, Sandborn WJ, et al. Effects of adalimumab therapy on incidence of hospitalisation and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology 2008;135:1493–9. [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Rutgeerts P, Feagan BG, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology 2009;137:1250–60. [DOI] [PubMed] [Google Scholar]
- 5. Fidder HH, Singendonk MM, van der Have M, Oldenburg B, van Oijen MG. Low rates of adherence for tumor necrosis factor-α inhibitors in Crohn’s disease and rheumatoid arthritis: results of a systematic review. World J Gastroenterol 2013;19:4344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopez A, Billioud V, Peyrin-Biroulet C, Peyrin-Biroulet L. Adherence to anti-TNF therapy in inflammatory bowel diseases: a systematic review. Inflamm Bowel Dis 2013;19:1528–33. [DOI] [PubMed] [Google Scholar]
- 7. Carter CT, Waters HC, Smith DB. Impact of infliximab adherence on Crohn’s disease-related healthcare utilization and inpatient costs. Adv Ther 2011;28:671–83. [DOI] [PubMed] [Google Scholar]
- 8. Kane SV, Chao J, Mulani PM. Adherence to infliximab maintenance therapy and health care utilization and costs by Crohn’s disease patients. Adv Ther 2009;26:936–46. [DOI] [PubMed] [Google Scholar]
- 9. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. [DOI] [PubMed] [Google Scholar]
- 10. Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133:21–30. [DOI] [PubMed] [Google Scholar]
- 11. Haynes RB, McDonald H, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev 2002:CD000011. [DOI] [PubMed] [Google Scholar]
- 12. Greenley RN, Kunz JH, Walter J, Hommel KA. Practical strategies for enhancing adherence to treatment regimen in inflammatory bowel disease. Inflamm Bowel Dis 2013;19:1534–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One 2013;8:e80633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kucukarslan SN. A review of published studies of patients’ illness perceptions and medication adherence: lessons learned and future directions. Res Social Adm Pharm 2012;8:371–82. [DOI] [PubMed] [Google Scholar]
- 15. Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated with non-adherence to oral medication for inflammatory bowel disease: a systematic review. Am J Gastroenterol 2010;105:525–39. [DOI] [PubMed] [Google Scholar]
- 16. Selinger CP, Eaden J, Jones DB, et al. Modifiable factors associated with nonadherence to maintenance medication for inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2199–206. [DOI] [PubMed] [Google Scholar]
- 17. Horne R. Representations of medication and treatment: advances in theory and measurement In: Petrie KJ, Weinman JA. (eds). Perceptions of Health and Illness: Current Research and Applications. London: Harwood Academic Press, 1997. [Google Scholar]
- 18. Leventhal H, Brissette I, Leventhal EA. The Common-Sense Model of self-regulation of health and illness. In: Cameron LD, Leventhal H. (eds). The Self-Regulation of Health and Illness Behaviour. London: Routledge, 2003. [Google Scholar]
- 19. Stange EF, Travis SP, Vermeire S, et al. ; European Crohn’s and Colitis Organisation. . European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. Gut 2006;55[Suppl 1]:i1–i15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stange EF, Travis SP, Vermeire S, et al. ; European Crohn’s and Colitis Organisation [ECCO] European evidence-based consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohns Colitis 2008;2:1–23. [DOI] [PubMed] [Google Scholar]
- 21. Harvey RF, Bradshaw JM. A simple index of Crohn’s disease activity. Lancet 1980;1:876. [DOI] [PubMed] [Google Scholar]
- 22. Evertsz’ FB, Hoeks CC, Nieuwkerk PT, et al. Development of the patient Harvey Bradshaw index and a comparison with a clinician-based Harvey Bradshaw index assessment of Crohn’s disease activity. J Clin Gastroenterol 2013;47:850–6. [DOI] [PubMed] [Google Scholar]
- 23. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bennebroek Evertsz’ F, Nieuwkerk PT, Stokkers PC, et al. The patient simple clinical colitis activity index [P-SCCAI] can detect ulcerative colitis [UC] disease activity in remission: a comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis 2013;7:890–900. [DOI] [PubMed] [Google Scholar]
- 25. Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631–7. [DOI] [PubMed] [Google Scholar]
- 26. Horne R, Weinman J, Hankins M. The Beliefs about Medicines Questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1–24. [Google Scholar]
- 27. Horne R, Parham R, Driscoll R, Driscoll R, Robinson A. Patients’ attitudes to medicines and adherence to maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis 2009;15:837–44. [DOI] [PubMed] [Google Scholar]
- 28. Trindade AJ, Ehrlich A, Kornbluth A, Ullman TA. Are your patients taking their medicine? Validation of a new adherence scale in patients with inflammatory bowel disease and comparison with physician perception of adherence. Inflamm Bowel Dis.2011;17:599–604. [DOI] [PubMed] [Google Scholar]
- 29. Kane S, Becker B, Harmsen WS, Kurian A, Morisky DE, Zinsmeister AR. Use of a screening tool to determine nonadherent behavior in inflammatory bowel disease. Am J Gastroenterol 2012;107:154–60. [DOI] [PubMed] [Google Scholar]
- 30. Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 2006;40:1280–8. [DOI] [PubMed] [Google Scholar]
- 31. Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther 2011;33:987–95. [DOI] [PubMed] [Google Scholar]
- 32. Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut 2009;58:492–500. [DOI] [PubMed] [Google Scholar]
- 33. Pouw MF, Krieckaert CL, Nurmohamed MT, et al. Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis 2015;74:513–8. [DOI] [PubMed] [Google Scholar]
- 34. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148: 132–09. [DOI] [PubMed] [Google Scholar]
- 35. Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015;13:522–30.e2. [DOI] [PubMed] [Google Scholar]
- 36. Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol 2011;106:674–84. [DOI] [PubMed] [Google Scholar]
- 37. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol 2009;104:760–7. [DOI] [PubMed] [Google Scholar]
- 38. Kane SV, Robinson A. Review article: understanding adherence to medication in ulcerative colitis innovative thinking and evolving concepts. Aliment Pharmacol Ther 2010;32:1051–8. [DOI] [PubMed] [Google Scholar]
- 39. Long MD, Kappelman MD, Martin CF, et al. Development of an internet based cohort of patients with inflammatory bowel disease [CCFA partners]: methodology and initial results. Inflamm Bowel Dis 2012;18:2099–106. [DOI] [PubMed] [Google Scholar]
- 40. Zugelj U, Zupancic M, Komidar L, Kenda R, Varda NM, Gregoric A. Self-reported adherence behavior in adolescent hypertensive patients: the role of illness representations and personality. J Pediatr Psychol 2010;35:1049–60. [DOI] [PubMed] [Google Scholar]
- 41. Ross S, Walker A, MacLeod M. Patient compliance in hypertension: role of illness perceptions and treatment beliefs. J Hum Hypertens 2004;18:607–13. [DOI] [PubMed] [Google Scholar]
- 42. Halm EA, Mora P, Leventhal H. No symptoms, no asthma: the acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthma. Chest 2006;129:573–80. [DOI] [PubMed] [Google Scholar]
- 43. Hagger MS, Orbell S. A meta-analytic review of the common sense model of illness representations. Psychol Health 2003;18:141–84. [Google Scholar]
- 44. McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev.2008;16:CD000011. [DOI] [PubMed] [Google Scholar]