Abstract
Background and Aims:
Minimisation of the placebo responses in randomised controlled trials [RCTs] is essential for efficient evaluation of new interventions. Placebo rates have been high in ulcerative colitis [UC] clinical trials, and factors influencing this are poorly understood. We quantify placebo response and remission rates in UC RCTs and identify trial design factors influencing them.
Methods:
MEDLINE, EMBASE, and the Cochrane Library were searched from inception through April 2014 for placebo-controlled trials in adult patients with UC of a biological agent, corticosteroid, immunosuppressant, or aminosalicylate. Data were independently doubly extracted. Quality was assessed using the Cochrane risk of bias tool.
Results:
In all, 51 trials [48 induction and 10 maintenance phases] were identified. Placebo response and remission rates were pooled according to random-effects models, and mixed-effects meta-regression models were used to evaluate effects of study-level characteristics on these rates. Pooled estimates of placebo remission and response rates for induction trials were 10% (95% confidence interval [CI] 7-13%) and 33% [95% CI 29-37%], respectively. Corresponding values for maintenance trials were 19% [95% CI 11-30%] and 22% [95% CI 17-28%]. Trials enrolling patients with more active disease confirmed by endoscopy [endoscopy subscore ≥ 2] were associated with lower placebo rates. Conversely, placebo rates increased with increasing trial duration and number of study visits.
Conclusions:
Objective assessment of greater disease activity at trial entry by endoscopy lowered placebo rates, whereas increasing trial duration and more interactions with healthcare providers increased placebo rates. These findings have important implications for design and conduct of clinical trials.
Keywords: RCT, randomised controlled trial, DAI, Disease Activity Index, UC, ulcerative colitis, MCS, Mayo Clinic Score
1. Introduction
Over the past 60 years, the randomised controlled trial [RCT] has undergone continuous evolution and refinement as a gold standard for evaluating the efficacy of new treatments. An increased understanding of the placebo response has been an integral part of this process. Determinants of the placebo response include natural variation in underlying disease, regression towards the mean, and the ‘true’ placebo effect 1 which is likely attributable to interrelated environmental and psychosocial factors.2 In clinical trials, these factors include patient expectations of treatment benefits, the response to observation and assessment [Hawthorne effect], the response to administration of a therapeutic ritual, the patient-physician relationship, and intrinsic features of trial design. 3,4 In the context of drug development, minimising the placebo response optimises the capacity to detect differences between active drug and placebo [assay sensitivity] and allows for the conduct of more efficient trials. Thus, identification of determinants of the placebo response is critical to trial design.
Randomised controlled trials in ulcerative colitis [UC] have shown heterogeneous and often large placebo response rates that may have prevented identification of effective therapies. A previous meta-analysis conducted by Su and colleagues identified several design features that influenced the placebo response.5 However, this study was conducted over a decade ago, before the modern-day era of biological therapies for inflammatory bowel disease, and included many trials that used outcome measures that are no longer considered appropriate. Identifying trial design criteria capable of consistently yielding predictable and low placebo rates increases the potential for more efficient trials. Based on these considerations, we conducted a meta-analysis to estimate placebo response and remission rates in both induction and maintenance phases of UC trials that included endoscopic evaluation of disease activity, using either the Disease Activity Index 6 or the nearly identical Mayo Score 7 for enrolment and outcome assessment. A meta-regression was conducted to identify trial design features that affected the placebo response.
2. Methods
2.1. Data sources
We searched MEDLINE [1948-April 2014], EMBASE [1947 May 2014], the Cochrane Central Register of Controlled Trials [2014], and the Cochrane Inflammatory Bowel Disease/Functional Bowel Disorders review group specialised trials register, without language restriction from inception to May 2014. The search strategies are reported in Table S1, available as Supplementary data at ECCO-JCC online. Citations and abstracts for potentially relevant studies were selected and screened, and complete manuscripts were retrieved for assessment of eligibility. Abstracts from conference proceedings [Digestive Disease Week and United European Gastroenterology Week; 2012–2014] and bibliographies of relevant studies, review articles, and meta-analyses were hand-searched to identify additional studies.
2.2. Study selection
Eligible studies were RCTs fulfilling the following criteria: [1] a placebo-controlled trial in adult patients with UC of either a biological agent, corticosteroid, immunosuppressant, or aminosalicylate; [2] use of the Disease Activity Index 6 or the nearly identical Mayo [or modified Mayo] Clinic Score 7,8 as enrolment criteria and for the assessment of clinical response and/or remission; [3] duration of at least 2 weeks for induction, and 4 months for maintenance of remission trials. Trials of probiotics, antibiotics, complementary therapies, or devices were excluded as were trials of hospitalised patients with severe UC.
The Mayo Clinic score 7 and the Disease Activity Index 6 are 12-point scales incorporating four components of disease activity: stool frequency, rectal bleeding, mucosal appearance of the sigmoid colon on sigmoidoscopy, and physician’s global assessment. Although slight differences exist in the definitions used in these two instruments, they are sufficiently similar to be considered equivalent. Trials using modifications of the Mayo score [eg Modified Mayo Disease Activity Index] were also eligible for inclusion. We refer to either score collectively as the Ulcerative Colitis Disease Activity Index [UCDAI] in this paper.
2.3. Data extraction and quality assessment
Articles were independently assessed by pairs of investigators using pre-defined eligibility criteria. Disagreement between investigators was resolved by consensus. All data were extracted independently in duplicate into a Microsoft Excel spreadsheet [XP professional edition; Microsoft Corp., Redmond, WA, USA]. Outcomes extracted included the proportion of patients with clinical response and remission, corticosteroid- free remission, mucosal healing, and histological remission, when available.
Additional features extracted were: [a] trial design and participant characteristics (number of treatment arms, trial development phase, year of publication, study location[s], first author nationality, number of participants, study duration, number of follow-up visits, frequency of follow-up visits, number of participants analysed, percentage of post- randomisation drop-outs, age, gender ratio); [b] type of intervention [drug class, concomitant therapy, dose, route of administration, frequency of administration, ratio of active drug to placebo]; [c] criteria for enrolment and outcome assessment [minimum UCDAI score for inclusion; UCDAI-based definitions of response and remission]; [d] disease severity and duration [baseline C-reactive protein [CRP] and calprotectin, disease distribution, and disease duration].
The Cochrane Collaboration risk of bias tool was used to assess the methodological quality of the studies included.18 Two independent investigators assessed the risk of bias and disagreements were resolved by discussion.
2.4. Data synthesis and analysis
Placebo response and remission rates were pooled, overall and separately, for induction and maintenance phases of trials, using a random-effects model for rates on the logit scale. These were pooled separately to reflect the two common methods of trial design. A random-effects model was chosen to account for both between- and within-study variability. Point estimates and associated 95% confidence intervals [CIs] were converted back to the original scale. Mixed-effects meta-regression analyses with logits of event rates as outcome variables were used to assess the effect of each study-level characteristic on placebo rates.9 Clinical judgement and previously identified predictors of placebo response were used to establish factors for inclusion in the meta-regression.10 A multivariable meta-regression analysis was not performed due to the limited number of studies and co-linearity between study-level characteristics.
Potential sources of clinical heterogeneity were evaluated by constructing stratum-specific rates of placebo response and remission for various patient and trial-level covariates. Statistical heterogeneity within these strata was quantified using I2 and Q-test statistics.5,11 A value below 50% represents lower levels of heterogeneity.12 Potential publication bias related to placebo rates9,13,14 was examined using funnel plots constructed by plotting the log odds ratio against the standard error, to emphasise differences between studies of smaller size within which biases are most likely to operate.
Cumulative meta-analysis was also conducted by date of publication in order to provide an assessment of pooled placebo rates over time.15 Cumulative meta-analysis and its graphical representation were calculated and interpreted using a Bayesian approach whereby the next study continually updates the previous distribution, such that the last posterior distribution becomes the next prior distribution. All analyses were performed using Metafor package16 for R version 3.1.1.17
3. Results
3.1. Search results
The search yielded 7588 citations of which 3605 were duplicates and removed [Figure S1, available as Supplementary data at ECCO-JCC online]. Of the remaining 3983 records screened, 227 full-text articles were selected and reviewed for eligibility. Of these, 77 articles reported 51 trials eligible for data extraction, including 48 induction and 9 maintenance phase studies. For the 48 induction phase6 ,78, 19–60 trials containing 3043 participants randomised to placebo, response rates were evaluable in 43 trials and remission rates in 40 trials. For the 9 maintenance phase trials 8,20,26,50,55,61,62,63 containing 1019 participants randomised to placebo [range 34–260], response rates were evaluable in 5 trials and remission rates in 8 trials. Given the small number of maintenance trials, meta-regression to identify factors modulating the placebo response was only feasible for induction trials.
3.2. Description of included studies
Study characteristics and definitions used for response or remission are shown in Table S2, available as Supplementary data at ECCO-JCC online. For induction trials, the mean age of participants enrolled was 40.8 years [range 30–50 years], mean placebo sample size was 66 [range 5–331], and the number of centres ranged from 1 to 217. Length of follow-up ranged from 2 to 24 weeks. The majority of induction studies were designed as stand-alone [70%], phase 3 [63%], multicentre/multinational [65%], biological therapy [47%] trials with a first author from a North American centre [56%].
For maintenance trials, the mean age of participants was 40.6 years [range 37.3–46 years], the mean placebo sample size was 113 [range 34–260], the number of centres ranged from 9 to 251 and the majority investigated biological therapies [6/10; 60%]. Length of follow-up ranged from 12 to 96 weeks.
3.3. Pooled placebo remission and response rates for induction and maintenance trials
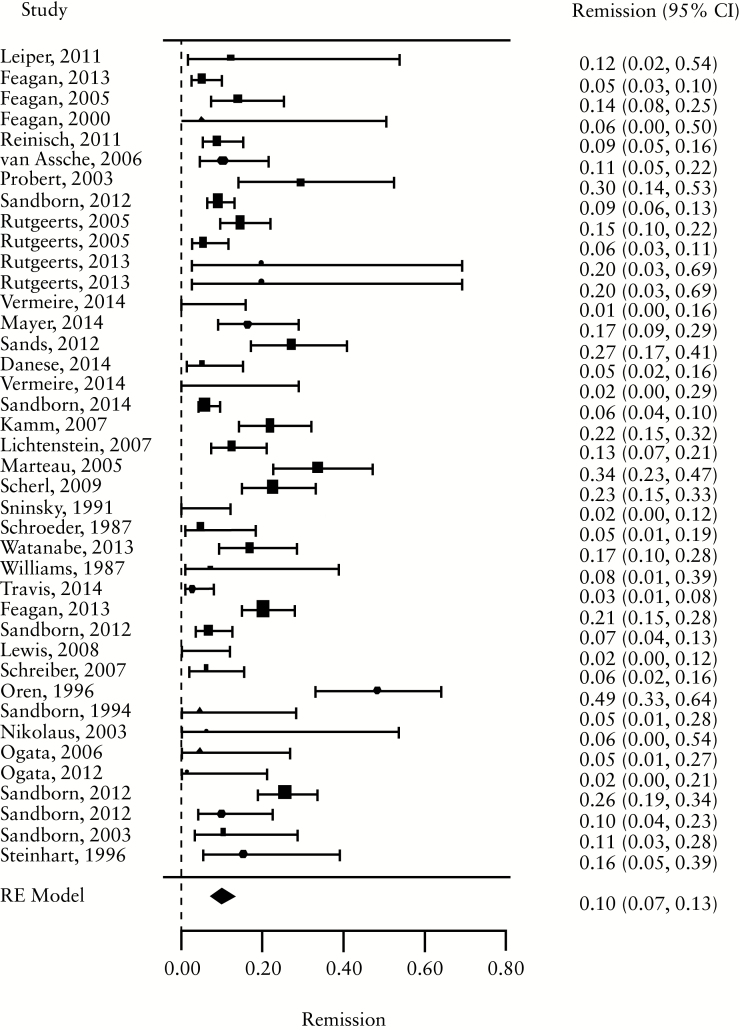
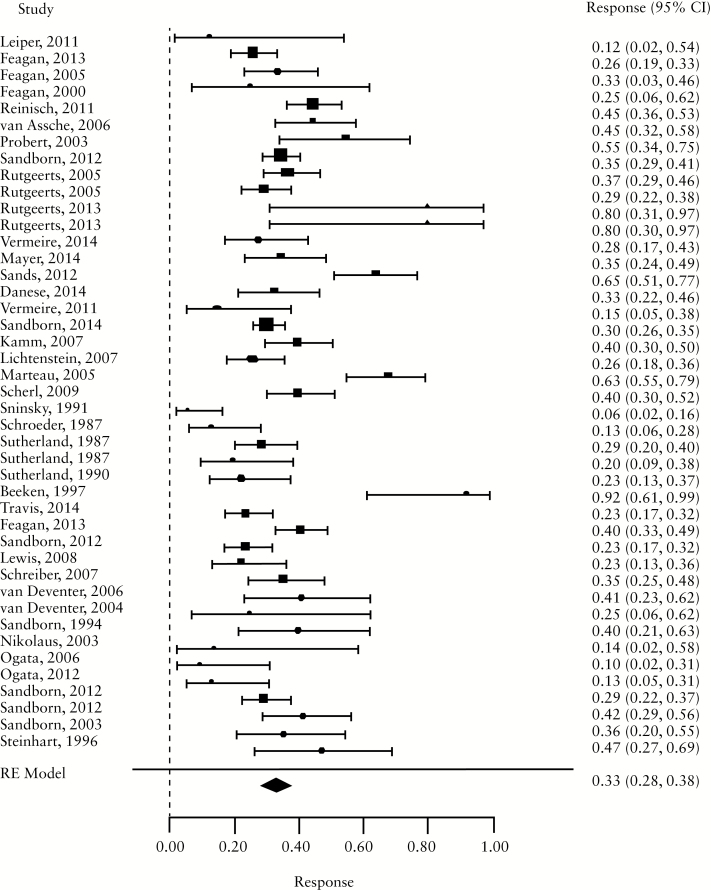
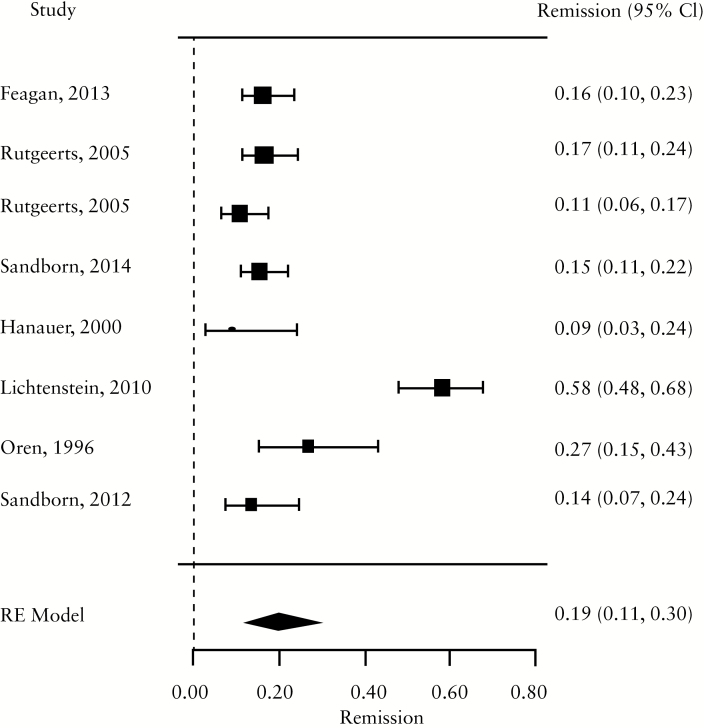
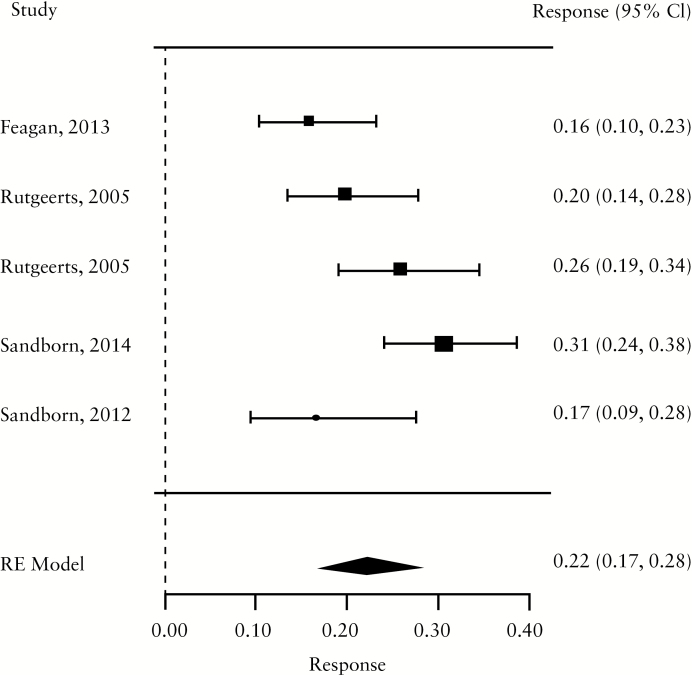
For induction trials, the pooled placebo remission rate was 10% [95% CI 7-13%; range 1–49%] and the pooled placebo response rate was 33% [95% CI 28-38%; range 6–92%]. Significant heterogeneity was observed among trials [I2 = 78.3%, p < 0.001] [Figure 1a, b; Table 1]. For maintenance trials, the pooled placebo remission rate was 19% [95% CI 11-30%; range 9–58%] and the pooled placebo response rate was 22% [95% CI 17-28%; range 16–31%] [Figure 1c, d]; some heterogeneity was also demonstrated amongs these trials [I2 = 64.6%, p = 0.02].
Figure 1.
a. Forest plot of pooled placebo remission rates for induction trials. B. Forest plot of pooled placebo response rates for induction trials. c. Forest plot of pooled placebo remission rates for maintenance trials. d. Forest plot of pooled placebo response rates for maintenance trials.
Table 1.
Stratum-specific placebo rates in induction trials.
| Response | Remission | |||||
|---|---|---|---|---|---|---|
| Trials N | Pooled rate % [95% CI] | I2 p-value | Trials N | Pooled rate [95% CI] | I2 p-value | |
| All trials | 43 | 33 [29-37] | < 0.001 | 40 | 12 [9-15] | < 0.001 |
| Setting | ||||||
| Multicentre, single country | 14 | 26 [18-35] | < 0.001 | 13 | 11 [6-19] | < 0.001 |
| Multicentre, multinational | 29 | 36 [31-41] | < 0.001 | 24 | 12 [9-16] | < 0.001 |
| Single-centre | 3 | 22 [8-45] | 0.06 | 3 | 6 [2-16] | 0.7 |
| Design | ||||||
| Stand-alone induction | 35 | 35 [29-40] | < 0.001 | 30 | 11 [8-15] | < 0.001 |
| Induction and maintenance | 10 | 30 [26-35] | 0.021 | 10 | 13 [8-22] | < 0.001 |
| First author country | ||||||
| North America | 25 | 32 [27-37] | < 0.001 | 21 | 11 [8-15] | < 0.001 |
| Europe | 17 | 38 [31-46] | < 0.001 | 15 | 12 [7-18] | < 0.001 |
| Other | 2 | 12 [5-24] | 0.67 | 4 | 14 [3-45] | < 0.001 |
| Drug class | ||||||
| Corticosteroid | 2 | 23 [19-29] | 1.00 | 2 | 5 [2-11] | 0.166 |
| Aminosalicylate | 13 | 32 [20-47] | < 0.001 | 9 | 18 [12-24] | 0.005 |
| Immunosuppressant | 4 | 19 [7-43] | 0.037 | 4 | 10 [2-41] | < 0.001 |
| Biological | 22 | 36 [31-40] | < 0.001 | 22 | 12 [9-15] | < 0.001 |
| Other | 5 | 34 [25-44] |
0.264 | 3 | 7 [3-18] |
0.142 |
| Route of administration | ||||||
| Topical | 9 | 39 [27-53] | < 0.001 | 5 | 18 [9-31] | 0.044 |
| Oral | 17 | 28 [22-34] | < 0.001 | 15 | 11 [6-18] | < 0.001 |
| Intravenous | 13 | 37 [29-45] | < 0.001 | 13 | 13 [09-18] | < 0.003 |
| Subcutaneous | 7 | 35 [29-41] | 0.032 | 7 | 8 [6-10] | 0.49 |
| Disease severity on entry | ||||||
| Mild-moderate | 21 | 32 [25-39] | < 0.001 | 16 | 10 [7-17] | < 0.001 |
| Moderate-severe | 24 | 34 [29-40] | < 0.001 | 24 | 0.12 [9-17] | < 0.001 |
| Disease duration on entry | ||||||
| ≤ 5 years | 6 | 47 [37-57] | 0.05 | 8 | 20 [16-25] | 0.300 |
| > 5 years | 29 | 33 [28-38] | < 0.001 | 28 | 11 [8-15] | < 0.001 |
| Inclusion criteria | ||||||
| Minimum total score ≥ 6 | 17 | 35 [29-40] | < 0.001 | 18 | 13 [9-19] | < 0.001 |
| Minimum total score < 6 | 22 | 35 [29-41] | < 0.001 | 18 | 12 [9-17] | < 0.001 |
| Endoscopy subscore for inclusion | ||||||
| ≥ 2 | 21 | 34 [30-39] | < 0.001 | 22 | 12 [9-16] | < 0.001 |
| ≥ 1 | 5 | 46 [31-61] | 0.02 | 4 | 25 [11-48] | < 0.001 |
| Not stated | 17 | 29 [21-39] | < 0.001 | 13 | 7 [5-10] | 0.370 |
| Bleeding subscore for inclusion | ||||||
| Yes | 8 | 38 [30-47] | < 0.001 | 8 | 18 [12-25] | 0.002 |
| No/not stated | 35 | 32 [27-36] | < 0.001 | 32 | 10 [8-14] | < 0.001 |
| Duration of follow-up | ||||||
| ≤ 8 weeks | 36 | 33 [28-39] | < 0.001 | 32 | 11 [9-14] | < 0.001 |
| > 8weeks | 7 | 33 [27-40] | 0.064 | 8 | 13 [9-16] | < 0.001 |
| Number of follow-up visits | ||||||
| ≤ 3 | 17 | 32 [23-45] | < 0.001 | 12 | 11 [7-19] | < 0.001 |
| > 3 | 25 | 34 [30-38] | < 0.001 | 24 | 12 [9-16] | < 0.001 |
| Publication date | ||||||
| Before 2005 | 15 | 33 [23-44] | < 0.001 | 12 | 14 [7-23] | < 0.001 |
| After 2005 | 28 | 33 [29-37] | < 0.001 | 28 | 11 [9-14] | < 0.001 |
| Time point to measure remission | ||||||
| ≤ 6 weeks | 16 | 31 [22-42] | < 0.001 | 18 | 11 [7-17] | < 0.001 |
| > 6weeks | 20 | 35 [30-39] | < 0.001 | 20 | 11 [8-15] | < 0.001 |
| Improvement in endoscopy subscore required for definition | ||||||
| Yes | 19 | 32 [27-37] | < 0.001 | 20 | 0.10 [7-14] | < 0.001 |
| No | 24 | 35 [28-43] | < 0.001 | 20 | 0.14 [9-21] | < 0.001 |
| Improvement in bleeding subscore required for definition | ||||||
| Yes | 9 | 30 [21-39] | < 0.001 | 9 | 14 [10-20] | < 0.001 |
| No | 34 | 34 [29-39] | < 0.001 | 31 | 11 [8-15] | < 0.001 |
CI, confidence interval.
Table 2.
Univariable meta-regression analysis of factors contributing to placebo response and remission rates in induction trials.
| Study characteristic | Response | Remission | ||
|---|---|---|---|---|
| Odds ratio [95% CI] | p-Value | Odds ratio [95% CI] | p-Value | |
| Trial setting | ||||
| Multicentre single-country | 1.0 | - | 1.0 | - |
| Multicentre, multinational | 1.59 [1.01-2.51] | 0.047 | 1.17 [0.58-2.34] | 0.664 |
| Single centre | 0.80 [0.30-2.12] | 0.667 | 0.57 [0.12-2.76] | 0.488 |
| Trial design | ||||
| Stand-alone induction vs induction and maintenance trial |
0.75 [0.48-1.16] | 0.193 | 1.26 [0.67-2.38] | 0.475 |
| First author country | ||||
| North America | 1.0 | - | 1.0 | - |
| Europe | 1.30 [0.89-1.90] | 0.165 | 1.07 [0.58-1.98] | 0.83 |
| Other |
0.28 [0.09-0.86] | 0.026 | 1.90 [0.68-5.31] | 0.22 |
| Class of drug | ||||
| Corticosteroid | 1.0 | - | 1.0 | - |
| Aminosalicylate | 1.57 [0.66-3.70] | 0.306 | 3.83 [1.16-12.62] | 0.027 |
| Immunosuppressant | 0.84 [0.26-2.70] | 0.766 | 3.94 [0.89-17.38] | 0.071 |
| Biological | 1.83 [0.81-4.16] | 0.147 | 2.49 [0.80-7.72] | 0.115 |
| Other |
1.69 [0.64-4.48] | 0.292 | 1.47 [0.32-6.69] | 0.618 |
|
Route of administration |
|
|
|
|
| Topical | 1.0 | 1.0 | - | |
| Oral | 0.66 [0.33-1.03] | 0.065 | 0.66 [0.26-1.68] | 0.382 |
| Intravenous | 0.89 [0.49-1.62] | 0.710 | 0.70 [0.27-1.85] | 0.477 |
| Subcutaneous |
0.81 [0.42-1.59] | 0.550 | 0.39 [0.13-1.14] | 0.086 |
| Disease severity at inclusion | ||||
| Moderate-severe vs mild-moderate | 1.10 [0.75-1.62] | 0.624 | 1.06 [0.59-1.92] | 0.847 |
| Disease duration before enrolment | ||||
| > 5 years vs ≤ 5 years | 0.54 [0.32–0.92] | 0.02 | 0.60 [0.30-1.23] | 0.17 |
|
Inclusion criteria |
|
|
|
|
| Minimum entry score < 6 vs minimum entry score ≥ 6 |
1.02 [0.71-1.47] | 0.888 | 0.89 [0.49-1.62] | 0.712 |
| Endoscopy subscore for entry | ||||
| ≥ 2 | 1.0 | - | 1.0 | - |
| ≥ 1 | 1.65 [0.93-2.94] | 0.086 | 2.44 [1.16-5.15] | 0.019 |
| Not stated |
0.77 [0.52-1.13] | 0.178 | 0.53 [0.29-0.98] | 0.042 |
| Bleeding subscore for entry | ||||
| [No/not stated vs Yes] | 0.74 [0.47-1.15] | 0.181 | 0.58 [0.30-1.11] | 0.102 |
|
Duration of follow-up |
|
|
|
|
| > 8 weeks vs ≤8 weeks |
0.93 [0.56-1.55] | 0.79 | 1.43 [0.70-2.92] | 0.321 |
|
Number of follow-up visits |
|
|
|
|
| > 3 vs ≤ 3 |
1.04 [0.69-1.57] | 0.8310 | 1.08 [0.55-2.12] | 0.8236 |
| Publication date After 2005 vs before 2005 |
0.99 [0.65-1.50] |
0.9468 |
0.72 [0.38-1.35] |
0.3047 |
| Improvement in endoscopy subscore required for definition | ||||
| No vs Yes |
1.19 [0.80-1.75] | 0.3870 | 1.54 [0.87-2.70] | 0.1332 |
| Time point to measure response | ||||
| > 6 weeks vs ≤ 6 weeks |
1.11 [0.72-1.69] | 0.6338 | - | - |
| Improvement in bleeding subscore required for definition |
|
|
|
|
| No vs Yes |
1.21 [0.76-1.92] | 0.4123 | 0.86 [0.44-1.67] | 0.655 |
| Number of follow-up visits | ||||
| [per 1-visit increment] |
1.05 [0.94-1.17] | 0.412 | 1.13 [0.99-1.30] | 0.071 |
| Duration of follow-up [per 1-week increment] |
1.02 [0.97-1.06] |
0.528 |
1.05 [1.00-1.09] |
0.039 |
| Screening visits Yes vs No |
1.12 [0.75-0.66] |
0.6 |
0.95 [0.53-1.72] |
0.9 |
| Number of trial centres per 1-centre increment |
1.00 [1.00-1.03] |
0.728 |
1.00 [0.99-1.00] |
0.304 |
|
Publication year per 1=year increment |
1.02 [0.99-1.04] |
0.172 |
0.99 [0.95-1.04] |
0.778 |
| Extensive disease/pancolitis ≥ 30% vs < 30% |
1.01 [0.69-1.47] |
0.969 |
1.23 [0.64-2.36] |
0.532 |
| Concurrent steroid Yes vs No |
0.88 [0.59-1.32] |
0.539 |
1.13 [0.63-2.05] |
0.683 |
| Concurrent immunomodulator Yes vs No |
0.79 [0.53-1.16] |
0.222 |
1.18 [0.66-2.10] |
0.575 |
| Ratio of active drug Placebo > 1 vs ≤ 1 |
1.01 [0.68-1.50] |
0.972 |
0.91 [0.49-1.67] |
0.757 |
| Primary time point to measure endpoint [per 1-week increment] |
1.00 [0.91-1.10] |
0.955 |
1.01 [0.89-1.16] |
0.844 |
CI, confidence interval.
3.4. Determinants of the placebo remission rate in induction trials
3.4.1. Participant- and disease-related characteristics
Studies using an endoscopy subscore ≥ 1 for study entry were associated with a higher pooled placebo remission rate compared with studies using a more stringent criterion of endoscopy subscore ≥ 2 (25% vs 12%; odds ratio [OR] 2.44, 95% CI 1.16-5.15, p = 0.019] [Tables 1 and 2]. Inclusion of an improvement in the endoscopy subscore in the definition of remission was associated with a non-significant reduction in the placebo remission rate [10% vs 14%; OR 0.65, 95% CI 0.37-1.15; p = 0.133]. No significant differences were observed for the pooled placebo remission rates according to the requirement for a minimum rectal bleeding subscore for study entry [required vs not required], study-defined disease severity [mild-moderate vs moderate-severe], composite UCDAI score for trial eligibility [≥ 6 vs < 6], duration of follow-up [≤ 8 weeks vs > 8 weeks] or date of publication [before 2005 vs after 2005].
3.4.2. Trial design
An increasing length of follow-up was associated with a significant increase in the pooled placebo remission rate in induction trials [OR 1.05, 95% CI 1.00-1.09, p = 0.039; per 1-week increment]. A greater number of follow-up visits was also associated with an increase in the placebo remission rate [OR 1.13, 95% CI 0.9-1.30, p = 0.07]. Year of publication had no impact on the placebo remission rate [before 2005 vs after 2005]. There was no difference in the placebo remission rate according to continent of origin [OR 1.07, 95% CI 0.58-1.98, p = 0.83 for trials from Europe; OR 1.90, 95% CI 0.68-5.31, p = 0.22 for trials from other continent; baseline comparator for both trials originating from North America]
3.4.3. Class of drug
Pooled remission rates according to class of drug class ranged from 5% to 18% [Table 1]. The lowest placebo remission rate [5%; 95% CI 2-11%; I2 = 0.166] was observed for trials of corticosteroids whereas the highest placebo remission rate [18%; 95% CI 12-24%; I2 = 0.005] was observed for trials of aminosalicylates [18%; 95% CI 12-24%; I2 = 0.005]. Aminosalicylate trials were associated with an increase in the placebo remission rate [OR 3.83, 95% CI 1.16-12.62, p = 0.027; baseline comparator corticosteroids] as were immunosuppressant trials [OR 3.94, 95% CI 0.89-17.38, p = 0.071; baseline comparator corticosteroids]. Randomisation ratio had no impact on the placebo remission rate.
3.5. Determinants of placebo response rate in induction trials
3.5.1. Participant and disease-related characteristics
Disease duration > 5 years before enrolment was associated with a significantly lower placebo response rate compared with disease duration < 5 years [33% vs 47%, respectively; OR 0.54, 95% CI 0.32-0.92, p = 0.020] [Tables 1 and 2]. Studies using an endoscopy subscore ≥ 1 for study entry were associated with a higher placebo response rate compared with studies using a more stringent entry criterion of endoscopy subscore ≥ 2 [46% vs 34%; OR 1.65, 95% CI 0.93-2.94, p = 0.086]. No differences were observed for the pooled placebo response rates according to requirement for a minimum rectal bleeding subscore for study entry [required vs not required], study-defined clinical disease severity [mild-moderate vs moderate-severe], duration of follow-up [≤ 8 weeks vs > 8 weeks], date of publication [before 2005 vs after 2005], composite UCDAI score for trial eligibility [≥ 6 vs < 6] or the time point for the outcome measure of response [> 6 weeks vs < 6 weeks] [Tables 1 and 2].
3.5.2. Trial design and setting
A higher pooled placebo response rate was observed in multicentre multinational induction trials compared with multicentre single-country induction trials [35% vs 26%, respectively; OR 1.59, 95% CI 1.01-2.51, p = 0.047] [Tables 1 and 2]. No significant differences in placebo response rates were observed between integrated induction/maintenance trials compared with stand-alone induction trials [30% vs 35%; OR 0.75, 95% CI 0.48-1.16, p = 0.193], induction trials published before or after 2005 [33% for both time periods; OR 0.99, 95% CI 0.65-1.50, p = 0.947], or according to number of follow-up visits [OR 1.05, 95% CI 0.94-1.17 per visit increment], or duration of follow-up [OR 1.02, 95% CI 0.97-1.06 per 1-week increment].
3.5.3. Class of drug
Pooled placebo response rates according to class of drug ranged from 19% to 36% [Table 1]. The lowest placebo response rate [19%; 95% CI 7-43%; I2 = 0.037] was observed for trials of immunosuppressants, whereas the highest placebo response rate [36%; 95% CI 31-40%; I2 < 0.001] was observed for trials of biological drugs. Trials of orally administered agents had the lowest placebo response rate [28%; OR 0.66, 95% CI 0.33-1.03] compared with trials of topically administered agents which had the highest placebo response rate [39%; 95% CI 27-53%; p = 0.065 for the comparison]. A randomisation ratio of active drug to placebo of > 1 had no impact on the placebo response rate [OR 1.01, 95% CI 0.68-1.50, p = 0.97] although the limited number of studies using asymmetrical allocation limited our ability to detect such an effect.
3.6. Time trends in the placebo rates
Cumulative meta-analysis demonstrated a consistent increase in the placebo remission rate from 1987 to 2007 [from 5% to 14%] and remained constant [12% to 14%] through 2014 [Supplementary Figure 2, available as Supplementary data at ECCO-JCC online ]. Placebo response rates increased from 1987 to 2007 [from 13% to 33%], after which these rates remained constant [32% to 34% through 2014] [Supplementary Figure 3, available as Supplementary data at ECCO-JCC online, ].
3.7. Sources of heterogeneity and risk of bias
Heterogeneity among strata of relevant study features was slightly less evident in studies that were single-centre, used an integrated induction and maintenance trial design, evaluated an immunosuppressant or subcutaneously administered drug, enrolled patients with disease duration ≤ 5 years, and followed patients for > 8 weeks [Table 1]. Risk of bias for induction studies was assessed as low or unclear for most parameters studied [Supplementary Table 3, available as Supplementary data at ECCO-JCC online].
3.8 Publication bias
The regression test for funnel plot asymmetry demonstrated that the risk of publication bias was significant [z = -3.40, p < 0.001] for induction trials reporting remission [Supplementary Figure 4, available as Supplementary data at ECCO-JCC online] and low [z = -0.31, p = 0.76] for those reporting response [Supplementary Figure 5, available as Supplementary data at ECCO-JCC online]. The asymmetry in the former plot suggests that smaller trials with no difference in treatment effect between intervention and placebo for remission may have been unpublished.
4. Discussion
The response to placebo in clinical trials is a complex phenomenon that is influenced by multiple factors including the type of intervention, the method and frequency of treatment, response expectancy, patient-provider interactions, behavioural conditioning, and the clinical context.64 Understanding the magnitude and modifiers of the placebo response in UC trials is important to the design of efficient clinical trials.
Our most notable finding was that trials which enrolled patients with more active disease confirmed objectively by endoscopy were associated with significantly lower placebo remission rates than trials enrolling patients with less active disease [OR 2.44, 95% CI 1.16-5.15, p = 0.019 for UCDAI endoscopy subscore ≥ 1 vs ≥ 2]. These findings underscore the critical importance of confirming that patients enrolled into clinical trials not only have the required disease, but also objective confirmation of the degree of disease severity. Whereas clinical trials pay greater attention to the adjudication of trial endpoints, little attention is paid to adjudicating patients enrolled into trials to ensure that they not only have the disease under study, but also the necessary degree of severity. The importance of this concept for drug development in UC was highlighted in a post hoc analysis of a placebo-controlled trial of mesalamine, where restricting the analysis of the primary outcome to patients deemed to have sufficiently active disease at trial entry by an independent central reader [Mayo subscore of ≥ 2 at entry] led to a reduction in placebo remission rates from 20.6% to 13.8%.45 Alternatively, no such relationship was identified for symptom-based criteria such as rectal bleeding. These findings indicate that endoscopy at study entry is a more valid tool to ensure active disease and to improve assay sensitivity in RCTs than symptom-based trial entry criteria. Surprisingly, we did not find a similar relationship for use of endoscopy as an outcome measure, suggesting that definition of the patient population by endoscopy is a dominant factor in influencing placebo rates. Consistent with these findings, the trial reporting a placebo remission rate of zero in this study used centralised reading of endoscopy to confirm UCDAI endoscopy subscores of ≥ 2 at trial entry, and to assess response to therapy.28 Similarly, two preliminary trial reports using the same methodology have reported remarkably low placebo remission rates; 6.2% in a phase II trial of an oral selective sphingosine 1-phosphate [S1P] 1 and 5 receptor modulator 65 and 2.7% in a phase II trial of an anti-MadCam antibody [PF-00547659] for moderately-severe UC.66 These results have led to the widespread use of central reading of video endoscopies to confirm eligibility and to evaluate treatment response in RCTs of new therapies for UC.
We also found that placebo response and remission rates varied according to whether trials were designed as induction or maintenance studies. This distinction is important for sample size calculations, since UC trials are commonly designed as either separate induction or maintenance studies, or as integrated trials with re-randomisation after an induction period. In the latter design, patients who respond to placebo usually continue to receive placebo in the maintenance phase of the trial—‘sham randomisation’—and are excluded from the intent-to-treat analysis. Trials must be designed with a sufficient number of patients entering a maintenance phase to account for this design feature. Longer trial duration may account for the higher placebo remission rate observed in maintenance studies [19% vs 12% for induction studies], and be related to an increased likelihood of spontaneous improvement of underlying disease and regression towards the mean. When we examined the impact of increasing trial duration as a continuous variable in the meta-regression, a 5% increase in remission rate per week of follow-up was observed [p = 0.04]. Similarly, a 13% increase in the remission rate was observed per extra follow-up visit [p = 0.07], which may also be related to the positive effects of increasing patient assessment. A study in irritable bowel syndrome [IBS] investigated which components of the clinical encounter moderated the placebo effect and found that positive interaction with the practitioner during follow-up visits was the strongest predictor.3 Standardising trial assessments is thus an important aspect of managing treatment expectations in clinical trials and potentially reducing the response to placebo.
The influence of non-specific moderators of the placebo effect3 may be even greater when using subjective endpoints, such as patient-reported outcomes [PROs] solely, as is the case in IBS trials. Emerging evidence from other therapeutic areas indicate that placebo responses are more pronounced in trials in which outcomes are measured by PROs compared with those in which they are assessed by physicians.2,67 This is an important observation, as regulatory agencies are in the process of re-defining endpoints for IBD trials, and are likely to incorporate subjective PROs. Our data here, demonstrating low placebo rates associated with the use of central reading at trial entry and for the assessment of outcome,45,65,66 combined with evidence from other therapeutic areas such as IBS, suggest that use of PROs alone may be associated with a risk for high placebo responses. It may ultimately be possible to circumvent this risk with the use of endoscopy as an objective co-primary endpoint.
Our cumulative meta-analyses demonstrate that placebo response and remission rates have largely remained stable between 2005 and 2014. One potential use of these new data could be to inform previous probability distributions for placebo treatment effects in Bayesian approaches to early trial design,68 which has the potential to increase efficiency by reducing the number of trial participants required. Further reductions in these rates will likely require alternative approaches to trial design and endpoint evaluation. Use of an active rather than placebo comparator in later phase trials is one consideration. However, research in other therapeutic areas has shown that comparative effectiveness trials paradoxically increase the placebo effect without being able to control for it. This phenomenon may be due to increased expectancy responses, since all patients know they will receive an active treatment.69
Clinical and methodological differences among studies included in a meta-analysis contribute to statistical heterogeneity.70 We observed a high degree of heterogeneity when trials were pooled for both response and remission [I2 p-value for both outcomes < 0.001]. This heterogeneity remained statistically significant after stratification across various categories of variables, although it was less evident in trials that were single-centre, used an integrated trial design, evaluated an immunosuppressive, evaluated a subcutaneously administered drug, enrolled patients with disease duration of < 5 years, and followed up patients for > 8 weeks. The degree of heterogeneity is perhaps surprising given that we refined our eligibility criteria to only include trials that used the UCDAI score, and the outcome definitions for response and remission were broadly similar in the majority of studies. This serves to highlight that many other factors contribute to heterogeneity including demographics, patterns of disease, timing of outcome evaluation, methods of outcome evaluation, and inter-observer variation in outcome evaluation to which endoscopic evaluation is particularly prone.45
Our findings can be compared with those of Su and colleagues who evaluated trials performed before 2005. The current study included 30 RCTs that were published after 2005 and only evaluated trials that used the UCDAI as an outcome measure [51 trials vs 12 in Su et al.].5 Our rationale for using the latter criterion was to focus on trials that used an outcome measure relevant to modern drug development in distinction from those that included indices that are no longer used. Second, we investigated the pooled remission and response rates for induction and maintenance trials separately, which provides new data to inform these separate phases of trial design. Third, we formally assessed the methodological quality of the included trials as well as the possibility of publication bias. Despite this, the findings of the two reviews are broadly similar.
Our review has some limitations. First, there were insufficient trials available to evaluate the effect of study-level characteristics of placebo rates for maintenance studies. Second, only one of the trials included utilised central reading of endoscopy,45 so we were unable to evaluate the independent impact of this methodology on placebo rates. Third, there was evidence of statistically significant heterogeneity when data were pooled. Finally, it is important to recognise that despite the detailed analyses we performed using pooled data, the optimum method to investigate the influence of specific patient characteristics on placebo rates is through use of patient-level data.
In conclusion, objective assessment of greater disease activity at trial entry by endoscopy lowered placebo rates, whereas increasing trial duration and more interactions with healthcare providers through study follow-up visits increased placebo rates. These findings have important implications for design and conduct of future clinical trials in UC, in order to maximise assay sensitivity and improve trial efficiency.
Funding
No external funding for this work was received. VJ: salary is part funded by the UK National Institute for Health Research. PSD is supported by the National Institute of Diabetes and Digestive and Kidney Diseases [Grant 5T32DK007202].
Conflict of Interest
VJ: has received scientific advisory board fees from AbbVie. GYZ is an employee of Robarts Clinical Trials which was the research organisation that conducted this study. MHM is an employee of Robarts Clinical Trials which was the research organisation that conducted this study. RK has received honoraria from Takeda Pharma and consulting fees from AbbVie. LMS is an employee of Robarts Clinical Trials which was the research organisation that conducted this study. MKV is an employee of Robarts Clinical Trials which was the research organisation that conducted this study. ST has received grant support from AbbVie, IOIBD, Lilly, Norman Collison Foundation [all to Institution]; advisory board fees [all suspended Q1 2012-14 as President of ECCO] from AbbVie, Asahi, AstraZeneca, Boehringer Ingelheim, Celgene, Cosmo, ChemoCentryx, Ferring, FPRT Bio, Genentech/Roche, GSK, Lilly, Novartis, Novo Nordisk, Pfizer, Proximagen, Receptos, SigmoidPharma, Synthon,Takeda, Topivert, Trino Therapeutics with Wellcome Trust, UCB Pharma, Vertex, VHSquared, Vifor, Warner Chilcott. GDH has received consulting fees from Abbott/Abbvie, ActoGeniX NV, Amgen, AM-Pharma BV, Boehringer-Ingelheim, ChemoCentryx, Centocor/Jansen Biologics, Cosmo Technologies, Elan/Biogen, EnGene Inc, Ferring Pharmaceuticals, Gilead Sciences, Given Imaging, GSK, Merck Research Laboratories, Merck Serono, Millenium Pharmaceuticals, Novo Nordisk, NPS Pharmaceuticals, PDL Biopharma, Pfizer, Receptos, Salix Pharmaceuticals, Schering Plough, Shire Pharmaceuticals, Sigmoid Pharma Ltd, Teva Pharmaceuticals, Tillotts Pharma AG, UCB Pharma; research grants from Abbvie, GSK, Falk, Janssen, Merck, Given Imaging; payments for lectures/speakers’ bureaux from Abbvie, Jansen, Merck, Takeda, UCB, Shire. BGL is a consultant for Prometheus Laboratories and Santarus; has participated on speakers’ bureau for Salix; and is an employee of Robarts Clinical Trials which was the research organisation that conducted this study. WJS has received consulting fees from Abbott, ActoGeniX NV, AGI Therapeutics Inc., Alba Therapeutics Corp., Albireo, Alfa Wasserman, Amgen, AM-Pharma BV, Anaphore, Astellas, Athersys Inc., Atlantic Healthcare Ltd, Aptalis, BioBalance Corp., Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Celek Pharmaceuticals, Cellerix SL, Cerimon Pharmaceuticals, ChemoCentryx, CoMentis, Cosmo Technologies, Coronado Biosciences, Cytokine Pharmasciences, Eagle Pharmaceuticals, EnGene Inc, Eli Lilly, Enteromedics, Exagen Diagnostics Inc., Ferring Pharmaceuticals, Flexio Therapeutics Inc., Funxional Therapeutics Ltd, Genzyme Corp., Gilead Sciences, Given Imaging, GSK, Human Genome Sciences, Ironwood Pharmaceuticals, KaloBios Pharmaceuticals, Lexicon Pharmaceuticals, Lycera Corp., Meda Pharmaceuticals, Merck Research Laboratories, Merck Serono, Millenium Pharmaceuticals, Nisshin Kyorin Pharmaceuticals, Novo Nordisk, NPS Pharmaceuticals, Optimer Pharmaceuticals, Orexigen Therapeutics Inc., PDL Biopharma, Pfizer, Procter and Gamble, Prometheus Laboratories, ProtAb Ltd, Purgenesis Technologies Inc., Relypsa Inc., Roche, Salient Pharmaceuticals, Salix Pharmaceuticals, Santarus, Schering Plough, Shire Pharmaceuticals, Sigmoid Pharma Ltd, Sirtris Pharmaceuticals, SLA Pharma UK Ltd, Targacept, Teva Pharmaceuticals, Therakos, Tillotts Pharma AG, TxCell SA, UCB Pharma, Viamet Pharmaceuticals, Vascular Biogenics Ltd, Warner Chilcott UK Ltd, and Wyeth; research grants from Abbott, Bristol-Myers Squibb, Genentech, GSK, Janssen, Milennium Pharmaceuticals, Novartis, Pfizer, Procter and Gamble, Shire Pharmaceuticals, and UCB Pharma; payments for lectures/speakers’ bureaux from Abbott, Bristol-Myers Squibb, and Janssen; and holds stock/stock options in Enteromedics. BGF has received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma AG, Abbott Labs, Novartis Pharmaceuticals, Centocor Inc., Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix, Wyeth Pharmaceuticals Inc.; consulting fees from Millennium Pharmaceuticals, Merck, Centocor Inc., Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, Abbott Labs, Astra Zeneca, Serono, Genentech, Tillotts Pharma AG, Unity Pharmaceuticals, Albireo Pharma, Given Imaging Inc., Salix Pharmaceuticals, Novonordisk, GSK, Actogenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, GiCare Pharma Inc., Sigmoid Pharma; speakers’ bureau fees from UCB, Abbott, J&J/Janssen.
Author Contributions
Guarantors of the article: VJ, BGF. Development of study concept and design: VJ, BGL, WJS, BGF. Study supervision: VJ, GYZ, WJS, BGF, CP, JKM. Acquisition, analysis, and interpretation of the data: VJ, TA, MA, MA, TA, NA, SB, TC, PSD, MG, DH, AK, EM, ST, GD, BGL, WJS, BGF. Statistical analysis: GYZ. Drafting of the manuscript: VJ, GYZ, CP, JKM, BGF. Critical revision of the manuscript for important intellectual content: VJ, GYZ, CP, JKM, RK, MHM, MKV, TA, MA, MA, TA, NA, SB, TC, PSD, MG, DH, AK, EM, ST, GD, BGL, WJS, BGF. All authors approved the final version of the manuscript including the authorship list.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
References
- 1. Sedgwick P. Treatment effects and placebo effects. BMJ 2015;350:h267. [DOI] [PubMed] [Google Scholar]
- 2. Enck P, Bingel U, Schedlowski M, et al. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov 2013;12:191–204. [DOI] [PubMed] [Google Scholar]
- 3. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 2008;336:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Enck P, Klosterhalfen S, Weimer K, et al. The placebo response in clinical trials: more questions than answers. Philos Trans R Soc Lond B Biol Sci 2011;366:1889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Su C, Lewis JD, Goldberg B, et al. A meta-analysis of the placebo rates of remission and response in clinical trials of active ulcerative colitis. Gastroenterology 2007;132:516–26. [DOI] [PubMed] [Google Scholar]
- 6. Sutherland LR, Martin F, Greer S. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology 1987;92:1894–8. [DOI] [PubMed] [Google Scholar]
- 7. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomised study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- 8. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- 9. Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559–73. [DOI] [PubMed] [Google Scholar]
- 10. Ilnyckyj A, Shanahan F, Anton PA, et al. Quantification of the placebo response in ulcerative colitis. Gastroenterology 1997;112:1854–8. [DOI] [PubMed] [Google Scholar]
- 11. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 14. Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med 2001;20:641–54. [DOI] [PubMed] [Google Scholar]
- 15. Lau J, Schmid CH, Chalmers TC. Cumulative meta-analysis of clinical trials builds evidence for exemplary medical care. J Clin Epidemiol 1995;48:45–57; discussion 59–60. [DOI] [PubMed] [Google Scholar]
- 16. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software 2010;36:1–48. [Google Scholar]
- 17. Team RC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2012. Open access available at: http://cran. r-project. org 2014. [Google Scholar]
- 18. Higgins J, Altman D, Sterne J. Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions: Version 5.1. 0. [Updated March 2011]. Hoboken, NJ: Wiley-Blackwell, 2011. [Google Scholar]
- 19. Leiper K, Martin K, Ellis A, et al. Randomised placebo-controlled trial of rituximab [anti-CD20] in active ulcerative colitis. Gut 2011;60:1520–6. [DOI] [PubMed] [Google Scholar]
- 20. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 21. Feagan BG, Greenberg GR, Wild G, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med 2005;352:2499–507. [DOI] [PubMed] [Google Scholar]
- 22. Feagan BG, McDonald J, Greenberg G, et al. An ascending dose trial of a humanized A4 B7 antibody in ulcerative colitis [UC]. Gastroenterology 2000;118:A874. [Google Scholar]
- 23. Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011;60:780–7. [DOI] [PubMed] [Google Scholar]
- 24. Van Assche G, Sandborn WJ, Feagan BG, et al. Daclizumab, a humanised monoclonal antibody to the interleukin 2 receptor [CD25], for the treatment of moderately to severely active ulcerative colitis: a randomised, double blind, placebo controlled, dose ranging trial. Gut 2006;55:1568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Probert CS, Hearing SD, Schreiber S, et al. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut 2003;52:998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65 e1-3. [DOI] [PubMed] [Google Scholar]
- 27. Rutgeerts PJ, Fedorak RN, Hommes DW, et al. A randomised phase I study of etrolizumab [rhuMAb beta7] in moderate to severe ulcerative colitis. Gut 2013;62:1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vermeire S, O’Byrne S, Keir M, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet 2014;384:309–18. [DOI] [PubMed] [Google Scholar]
- 29. Mayer L, Sandborn WJ, Stepanov Y, et al. Anti-IP-10 antibody [BMS-936557] for ulcerative colitis: a phase II randomised study. Gut 2014;63:442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sands BE, Sandborn WJ, Creed TJ, et al. Basiliximab does not increase efficacy of corticosteroids in patients with steroid-refractory ulcerative colitis. Gastroenterology 2012;143:356–64 e1. [DOI] [PubMed] [Google Scholar]
- 31. Danese S, Rudzinski J, Brandt W, et al. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut 2015;64:243–9. [DOI] [PubMed] [Google Scholar]
- 32. Vermeire S, Ghosh S, Panes J, et al. The mucosal address in cell adhesion molecule antibody PF-00547,659 in ulcerative colitis: a randomised study. Gut 2011;60:1068–75. [DOI] [PubMed] [Google Scholar]
- 33. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:85–95; quiz e14-5. [DOI] [PubMed] [Google Scholar]
- 34. Kamm MA, Sandborn WJ, Gassull M, et al. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology 2007;132:66–75; quiz 432–3. [DOI] [PubMed] [Google Scholar]
- 35. Lichtenstein GR, Kamm MA, Boddu P, et al. Effect of once- or twice-daily MMX mesalamine [SPD476] for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol 2007;5:95–102. [DOI] [PubMed] [Google Scholar]
- 36. Marteau P, Probert CS, Lindgren S, et al. Combined oral and enema treatment with Pentasa [mesalazine] is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut 2005;54:960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scherl EJ, Pruitt R, Gordon GL, et al. Safety and efficacy of a new 3.3g b.i.d. tablet formulation in patients with mild-to-moderately-active ulcerative colitis: a multicenter, randomised, double-blind, placebo-controlled study. Am J Gastroenterol 2009;104:1452–9. [DOI] [PubMed] [Google Scholar]
- 38. Sninsky CA, Cort DH, Shanahan F, et al. Oral mesalamine [Asacol] for mildly to moderately active ulcerative colitis. A multicenter study. Ann Intern Med 1991;115:350–5. [DOI] [PubMed] [Google Scholar]
- 39. Sutherland LR, Martin F. 5-Aminosalicylic acid enemas in treatment of distal ulcerative colitis and proctitis in Canada. Dig Dis Sci 1987;32:64S–66S. [DOI] [PubMed] [Google Scholar]
- 40. Sutherland LR, Onstad M, Peppercorn G, Greenberger M, N. Goodman N M. A double-blind placebo controlled, multicentre study of the efficacy and safety of 5-aminosalicylic acid tables in the treatment of ulcerative colitis. Can J Gastroenterol 1990;4:463–7. [Google Scholar]
- 41. Watanabe M, Nishino H, Sameshima Y, et al. Randomised clinical trial: Evaluation of the efficacy of mesalazine [mesalamine] suppositories in patients with ulcerative colitis and active rectal inflammation - A placebo-controlled study. Aliment Pharmacol Ther 2013;38:264–73. [DOI] [PubMed] [Google Scholar]
- 42. Beeken W, Howard D, Bigelow J, et al. Controlled trial of 4-ASA in ulcerative colitis. Dig Dis Sci 1997;42:354–8. [DOI] [PubMed] [Google Scholar]
- 43. Williams CN, Haber G, Aquino JA. Double-blind, placebo-controlled evaluation of 5-ASA suppositories in active distal proctitis and measurement of extent of spread using 99mTc-labeled 5-ASA suppositories. Dig Dis Sci 1987;32:71S–75S. [DOI] [PubMed] [Google Scholar]
- 44. Travis SP, Danese S, Kupcinskas L, et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: results from the randomised CORE II study. Gut 2014;63:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feagan BG, Sandborn WJ, D’Haens G, et al. The role of centralized reading of endoscopy in a randomised controlled trial of mesalamine for ulcerative colitis. Gastroenterology 2013;145:149–57 e2. [DOI] [PubMed] [Google Scholar]
- 46. Sandborn WJ, Travis S, Moro L, et al. Once-daily budesonide MMX[R] extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology 2012;143:1218–26 e1-2. [DOI] [PubMed] [Google Scholar]
- 47. Lewis JD, Lichtenstein GR, Deren JJ, et al. Rosiglitazone for active ulcerative colitis: a randomised placebo-controlled trial. Gastroenterology 2008;134:688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schreiber S, Keshavarzian A, Isaacs KL, et al. A randomised, placebo-controlled, phase II study of tetomilast in active ulcerative colitis. Gastroenterology 2007;132:76–86. [DOI] [PubMed] [Google Scholar]
- 49. van Deventer SJ, Tami JA, Wedel MK. A randomised, controlled, double blind, escalating dose study of alicaforsen enema in active ulcerative colitis. Gut 2004;53:1646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oren R, Arber N, Odes S, et al. Methotrexate in chronic active ulcerative colitis: a double-blind, randomised, Israeli multicenter trial. Gastroenterology 1996;110:1416–21. [DOI] [PubMed] [Google Scholar]
- 51. Sandborn WJ, Tremaine WJ, Schroeder KW, et al. A placebo-controlled trial of cyclosporine enemas for mildly to moderately active left-sided ulcerative colitis. Gastroenterology 1994;106:1429–35. [DOI] [PubMed] [Google Scholar]
- 52. Nikolaus S, Rutgeerts P, Fedorak R, et al. Interferon beta-1a in ulcerative colitis: a placebo controlled, randomised, dose escalating study. Gut 2003;52:1286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ogata H, Matsui T, Nakamura M, et al. A randomised dose finding study of oral tacrolimus [FK506] therapy in refractory ulcerative colitis. Gut 2006;55:1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ogata H, Kato J, Hirai F, et al. Double-blind, placebo-controlled trial of oral tacrolimus [FK506] in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm Bowel Dis 2012;18:803–8. [DOI] [PubMed] [Google Scholar]
- 55. Sandborn WJ, Colombel JF, Sands BE, et al. Abatacept for Crohn’s disease and ulcerative colitis. Gastroenterology 2012;143:62–9 e4. [DOI] [PubMed] [Google Scholar]
- 56. Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012;367:616–24. [DOI] [PubMed] [Google Scholar]
- 57. Sandborn WJ, Sands BE, Wolf DC, et al. Repifermin [keratinocyte growth factor-2] for the treatment of active ulcerative colitis: a randomised, double-blind, placebo-controlled, dose-escalation trial. Aliment Pharmacol Ther 2003;17:1355–64. [DOI] [PubMed] [Google Scholar]
- 58. Steinhart AH, Hiruki T, Brzezinski A, et al. Treatment of left-sided ulcerative colitis with butyrate enemas: a controlled trial. Aliment Pharmacol Ther 1996;10:729–36. [DOI] [PubMed] [Google Scholar]
- 59. Sandborn W, Bosworth B, Zakko S, et al. Efficacy and safety of budesonide foam for inducing remission in mildly to moderately active ulcerative proctitis or ulcerative proctosigmoiditis. Inflamm Bowel Dis 2013;19:S83. [Google Scholar]
- 60. Sandborn W, Bosworth B, Zakko S, et al. Budesonide foam for inducing remission in active mild-to-moderate ulcerative proctitis or ulcerative proctosigmoiditis: Results of two randomised, placebo-controlled trials. Am J Gastroenterol 2013;108:S542. [Google Scholar]
- 61. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:96–109 e1. [DOI] [PubMed] [Google Scholar]
- 62. Hanauer S, Good LI, Goodman MW, et al. Long-term use of mesalamine [Rowasa] suppositories in remission maintenance of ulcerative proctitis. Am J Gastroenterol 2000;95:1749–54. [DOI] [PubMed] [Google Scholar]
- 63. Lichtenstein GR, Gordon GL, Zakko S, et al. Clinical trial: once-daily mesalamine granules for maintenance of remission of ulcerative colitis-a 6-month placebo-controlled trial. Aliment Pharmacol Ther 2010;32:990–9. [DOI] [PubMed] [Google Scholar]
- 64. Dieppe P. Trial designs and exploration of the placebo response. Complement Ther Med 2013;21:105–8. [DOI] [PubMed] [Google Scholar]
- 65. Sandborn W, Feagan BG, Wolf DC, et al. The TOUCHSTONE Study: a Randomised, Double-Blind, Placebo-Controlled Induction Trial of an Oral S1P Receptor Modulator [RPC1063] in Moderate to Severe Ulcerative Colitis. Gastrointest Endosc 2015;5:AB147. [Google Scholar]
- 66. Vermeire S, Sandborn W, Danese S, et al. TURANDOT: a randomised, multicentre double-blind, placebo-controlled study of the safety and efficacy of anti-MAdCAM antibody PF-00547659 [PF] in patients with moderate to severe ulcerative colitis [UC]. J Crohns Colitis 2015;9:S13. [Google Scholar]
- 67. Rief W, Nestoriuc Y, Weiss S, et al. Meta-analysis of the placebo response in antidepressant trials. J Affect Disord 2009;118:1–8. [DOI] [PubMed] [Google Scholar]
- 68. Schmid CH, Cappelleri JC, Lau J. Bayesian methods to improve sample size approximations. Methods Enzymol 2004;383:406–27. [DOI] [PubMed] [Google Scholar]
- 69. Rutherford BR, Sneed JR, Roose SP. Does study design influence outcome? The effects of placebo control and treatment duration in antidepressant trials. Psychother Psychosom 2009;78:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. BMJ 1994;309:1351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. van Deventer SJ, Wedel MK, Baker BF, et al. A phase II dose ranging, double-blind, placebo-controlled study of alicaforsen enema in subjects with acute exacerbation of mild to moderate left-sided ulcerative colitis. Aliment Pharmacol Ther 2006;23:1415–25. [DOI] [PubMed] [Google Scholar]