Abstract
Background and aims:
The classification of Crohn’s disease (CD) is usually determined at initial diagnosis and is frequently based on ileocolonoscopic and cross-sectional imaging data. Advanced endoscopic and imaging techniques such as small-bowel video capsule endoscopy (VCE) and magnetic resonance enterography (MRE) may provide additional data regarding disease extent and phenotype. Our aim was to examine whether VCE or MRE performed after the initial diagnosis may alter the original disease classification.
Methods:
Consecutive patients with known small-bowel CD in clinical remission or mild disease were prospectively recruited and underwent MRE and VCE (if small-bowel patency was confirmed by a patency capsule (PC). Montreal classifications before and after evaluation were compared.
Results:
Seventy-nine patients underwent MRE and VCE was performed in 56. Previously unrecognized disease locations were detected with VCE and MRE in 51 and 25%, respectively (p < 0.01) and by both modalities combined in 44 patients (55%). Twenty-two patients (27%) were reclassified as having an advanced phenotype (B2/B3). MRE and VCE reclassified the phenotype in 26 and 11% of cases, respectively (p < 0.05). Overall, both modalities combined altered the original Montreal classification in 49/76 patients (64%).
Conclusion:
VCE and MRE may lead to reclassification of the original phenotype in a significant percentage of CD patients in remission. VCE was more sensitive for detection of previously unrecognized locations, while MRE was superior for detection of phenotype shift. The described changes in the disease classification may have an important impact on both clinical management and long-term prognosis in these patients.
Key Words: Capsule endoscopy, Crohn’s disease, magnetic resonance enterography, Montreal classification, phenotype
1. Introduction
The Montreal classification was introduced in 2005 and is currently regarded as a standard for classifying Crohn’s disease (CD) in adults.1,2 This classification is usually determined during the initial diagnosis and is usually based on ileocolonoscopy.3 However, small-bowel involvement in CD is frequently proximal to the distal ileum and is not within the reach of standard ileo-colonoscopy.4
Advanced endoscopic and imaging techniques such as small-bowel video capsule endoscopy (VCE) and magnetic resonance enterography (MRE) provide additional data regarding the location, extent of luminal disease and disease phenotype.5–7 MRE is accurate for the detection of strictures and extra-luminal complications.8 VCE is highly sensitive for the diagnosis of mucosal lesions, particularly for the detection of superficial mucosal lesions, as well as proximal lesions.5,9 Recently, a paediatric modification, named the Paris classification,10 was proposed. The Paris classification uses a more accurate definition of proximal small-bowel disease, along with some additional alterations. However, it has not yet been incorporated into adult clinical practice.
After the initial diagnosis, many patients remain clinically asymptomatic on therapy. This, however, does not ensure sustained control of mucosal inflammation and structural intestinal damage. It is well known there is often no correlation between symptoms and progression of anatomic damage.11 Persistent low-grade inflammation and ongoing bowel damage may potentially lead to deviation from the original classification regarding disease location and phenotype.
Close monitoring of CD patients in remission or with mild disease activity might help identify patients at risk of subsequent disease relapse and/or complications.12 Colonoscopy is inconvenient and often unacceptable by patients, and has low accessibility to the proximal small bowel.
The classification of CD is important in prediction of the disease course and selection of management strategy. Data from several cohorts suggest that ileocolonic location and/or the presence of penetrating disease is associated with a high risk of disabling disease within the first years after diagnosis.13–15 Other studies have shown that the presence of proximal small-bowel disease is strongly associated with an increased risk of relapse and complicated disease.16–18
Therefore, our aim was to examine whether VCE and/or MRE performed in CD patients in remission or with mild disease activity can identify changes that will modify the original disease classification.
2. Methods
2.1. Patient population
The study population included adult (>18 years) consecutive CD patients with known small-bowel disease in remission or experiencing mild disease symptoms, as determined by the validated Crohn’s disease activity index (CDAI) of <150 or 150–220, respectively. In order to be included, patients had to be in steroid-free remission for 3–24 months and to have been treated with a stable medication dose (60 days for thiopurines and methotrexate, 60 days for infliximab, 30 days for adalimumab and for 5-aminosalicylic acid [5-ASA] agents).
Patients were excluded if they were unable to understand or provide informed consent; had severe comorbidities such as liver, kidney neurological, metabolic or cardio-respiratory disorders not controlled at the time of enrolment; difficulty in swallowing; a history of aspiration or dysphagia; claustrophobia or implanted metal objects or cardiac pacemaker precluding performance of MRI; or a known or suspected intestinal obstruction or severe stricture.
2.2. MRE studies
All patients underwent MRE upon enrolment. All MRE examinations were performed using a 1.5T GE Optima MR450w scanner with GEM Suite (GE Healthcare) with oral and intravenous contrast. Distension of the small bowel was obtained by using oral contrast: 360ml of Osmitrol 20% diluted in 1.5L of water. Patients were instructed to drink 4 doses of 465ml every 15 minutes for an hour before undergoing the MRE examination. During the last 15 minutes, patients received via infusion 150mL of saline containing 0.5mg of glucagon in a slow drip. Magnetic resonance image acquisition was performed using a previously described protocol.19 A board-certified abdominal radiologist (MMA) with 10 years of experience in reading MRE reviewed all MRE examinations.
2.3. Capsule endoscopy studies
A patency capsule (PC) test was performed in all patients with active small-bowel disease detected on MRE. If no active small-bowel disease was detected by MRE, a PC study was not performed. If a PC was not eliminated from the small bowel within 30 hours, the patient was withdrawn from the study. In patients with isolated small-bowel CD, small bowel-III capsule (Given Imaging, Yokneam, Israel) was used. In patients with established ileo-colonic CD, a colonic capsule (PillCam2 colon capsule, Given Imaging, Yokneam, Israel) was administered.
The preparation for VCE included ingestion of clear fluids only for 24 hours prior to the procedure and a 12-hour overnight fast. For a colonic capsule study, a split dose of 4L of polyethylene glycol preparation was used. An additional fluid bolus was given after 2 hours from ingestion of the capsule in order to facilitate small-bowel transit. All images were reviewed using the RAPID 8 software (Given Imaging, Yokneam, Israel). Mucosal inflammation was quantified using the Lewis score (LS).20 Active inflammation was defined as a segmental LS ≥135. A board-certified gastroenterologist with over 10 years of experience in capsule endoscopy read the capsule videos. We defined capsule retention in accordance with the international consensus on capsule endoscopy consensus definitions.21
2.4. Montreal classification determination
For each patient recruited to our study, a pre-study Montreal classification22 was determined according to data extracted from the electronic health records and extensive chart review up to the last clinic visit before entering the study. The distribution of the disease (L) was determined by different diagnostic modalities (imaging, endoscopy and VCE). Proximal disease (L4) was determined by MRE as the presence of the active disease in bowel segments located in the upper left quadrant. Proximal disease (L4 disease) was determined in VCE studies as LS>135 in the first or second tertile (excluding the distal/terminal ileum segments).
The phenotype (B) was established based on prior imaging or medical history, including surgery. A new Montreal classification was determined according to the first MRE and VCE studies performed at recruitment. When the PC was retained in patients with documented strictures on MRE, the phenotype was defined as B2.
2.5. Statistical analysis
Descriptive statistics are presented as means ± standard deviations for continuous variables and percentages for categorical variables. Categorical variables were analysed using χ2 and Fisher’s exact tests and continuous variables by the t-test and the Mann–Whitney test. A two-tailed p value <0.05 was considered statistically significant. The analysis was performed using IBM SPSS (Version 20.0) (Armonk, NY, USA).
3. Results
3.1. Patient population
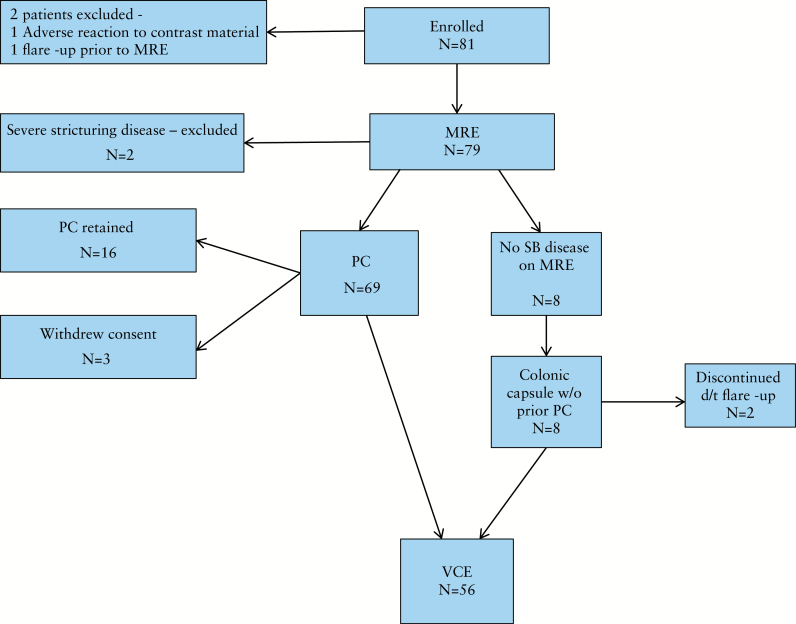
Eight-one patients were enrolled. Two were excluded before undergoing MRE (1 due to an adverse reaction to contrast material and 1 due to a flare-up). Seven of the 79 patients who underwent MRE were excluded before performing a VCE for variable causes (flare-up, severe stricturing disease, withdrawal of consent). Sixteen patients were not eligible for a VCE due to failure to excrete the PC. Subsequently, VCE was performed in 56 patients (Figure 1).
Figure 1.
Study inclusion flowchart. MRE, magnetic resonance enterography; PC, patency capsule; VCE, small-bowel video capsule endoscopy; SB, small bowel.
Both tests were performed 5.7 years (range 19-0.3) after original diagnosis. Results of previous ileocolonoscopy, cross-imaging studies CTE, MRE and VCE, before recruitment, were available in 100, 75 and 6% of the cases, respectively. Five patients had a VCE at the initial diagnostic stage, in addition to other examinations. Baseline demographic characteristics of all 79 patients (43% females) with CD are shown in Table 1.
Table 1.
Clinical and demographic characteristics of the patients included in the study
| Male/female, n (%) | 45/34 (57/43) |
| Age at diagnosis, y | 26±11 |
| Disease duration, y | 5.7±5.5 |
| Smoking status, n (%) | |
| Current | 14 (17.7) |
| Never smoked | 53 (67) |
| Former smoker | 11 (13.9) |
| Previous surgery, n (%) | 13 (16.4) |
| Perianal disease, n (%) | 17 (21.5) |
| Current treatment, n (%) | 18 (22.7) |
| None | 23 (29.1) |
| 5-ASA | 5 (6.3) |
| Thiopurines | 20 (25) |
| Anti-TNF | 24 (31.6) |
| Combined anti-TNF + thiopurines | 6 (7.5) |
5-ASA, 5-aminosalicylic acid; TNF, tumour necrosis factor.
3.2. Change in disease classification
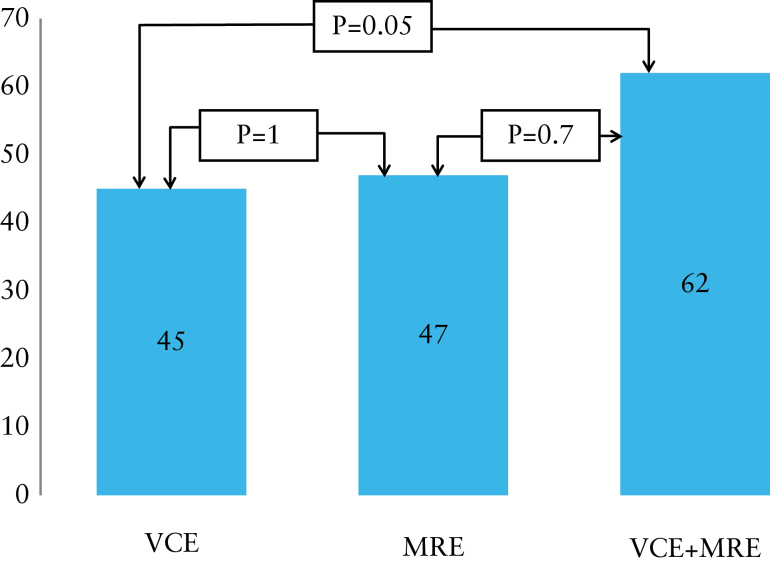
The pre-study and study Montreal classifications are described in Figure 2. Overall, according to the findings of both modalities (VCE and MRE) the original Montreal classification was reclassified in 49/79 patients (62%). VCE and MRE altered the original classification in 36/79 (45.5%) and 37/79 (47%) of cases, respectively (p = 1).
Figure 2.
Change in Montreal classification assessed by MRE, VCE or both. MRE, magnetic resonance enterography; VCE, small-bowel video capsule endoscopy.
3.3. Change in disease location
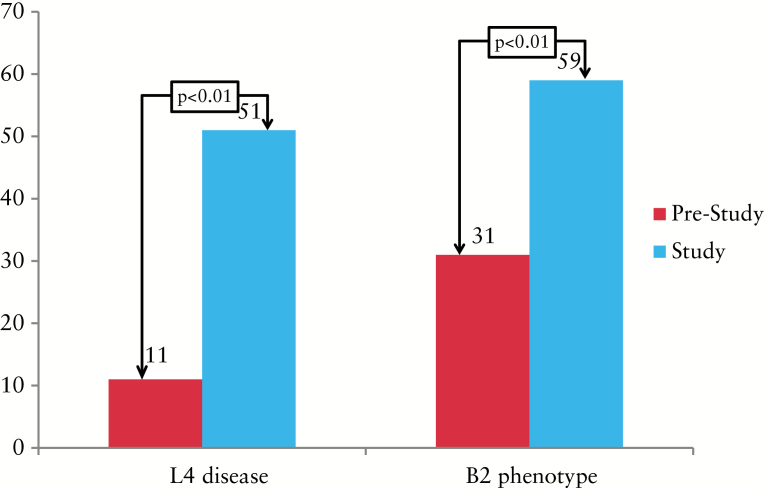
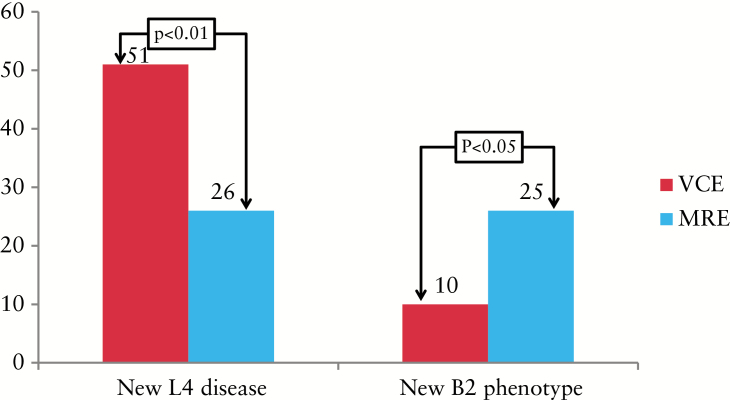
Previously unrecognized disease location was detected using both modalities in 44/79 (55%) patients; in 40 (91%) of these, new proximal disease (L4) was identified. Pre- and post-study proximal disease location is shown in Figure 3. Eleven (14%) patients had a known pre-study proximal disease location vs 51 patients (64%) post-study (p < 0.01). VCE and MRE independently detected previously unrecognized proximal disease location in 29/56 (51%) and 20/79 (26%) of patients (p < 0.01) (Figure 4). Evidence of active disease in the 1st and 2nd tertiles was determined in 29 (51.7%) and 20 (35%) of the patients that underwent VCE, respectively. Severe disease (LS >790) in any segment was detected in 12 (21%) patients; 5/12 had severe proximal disease. Nine (16%) patients had isolated proximal disease on VCE; 3/9 had severe disease (LS <790).
Figure 3.
Pre-study and study proximal disease and fibrostenotic phenotype. L4, proximal disease; B2, fibrostenotic phenotype.
Figure 4.
Detection of previously unrecognized proximal and fibrostenotic disease with each modality. L4, proximal disease; B2, fibrostenotic phenotype; MRE, magnetic resonance enterography; VCE, small-bowel video capsule endoscopy.
A sub-analysis was carried out comparing patients whose initial diagnosis was performed up to 2 years prior to recruitment compared with those diagnosed earlier. In the more recently diagnosed patients, the initial classification was modified in 66% in comparison to 55.5% of the cases in the group of patients diagnosed up to 2 years before recruitment (p = 0.6). In addition, no significant difference was detected between the 2 groups concerning previously unrecognized proximal disease (51.8 and 44.2% respectively, p = 0.46). An additional comparison was made between patients diagnosed by ileocolonoscopy alone (19/79) with those diagnosed by cross-imaging studies (60/74) and/or VCE (5/79) and an ileocolonoscopy. The number of patients reclassified according to their original Montreal classification was similar in the 2 groups (57.7 and 53.5% respectively; p = 0.6). Additionally, new proximal disease was detected in similar proportions of the patients (52 vs 45%, p = 0.4)
3.4. Change in disease behaviour
Twenty-two patients (27%) originally diagnosed with an inflammatory phenotype (B1) were reclassified as having an advanced phenotype. Pre- and post-study prevalences of B2 phenotype are presented in Figure 3. Twenty-five (31%) and 47 (59%) patients had pre-study and study stricturing (B2) phenotypes, respectively (p < 0.01). MRE and VCE reclassified the phenotype in 26 and 11% of cases, respectively (p < 0.05) (Figure 4). Out of the 22 patients reclassified as having an advanced phenotype, all had B2 disease. Three patients (3/22) also had penetrating features (B3) that included sinus tracts and fistulae but without an evident abscess. A sub-analysis for change in phenotype according to date and modality of diagnosis was performed. Patients diagnosed up to 2 years prior to recruitment showed similar detection rates of complicated disease (26 vs 27.7%, p = 1.0) compared with those diagnosed earlier. This was also true in relation to modality (diagnosis with and without imaging) (23 and 26.3%, respectively, p = 0.76). Similarly, a history of smoking, perianal disease and/or inflammatory bowel disease (IBD)-related surgery was not found to be associated with a higher prevalence of phenotype change (21.5 vs 20%, p = 1.0; 23.7 vs 18.1%, p = 0.9; 26 vs 14.2%, p = 0.47, respectively).
3.5. Safety
No cases of VCE retention occurred in this study. One patient had a symptomatic temporary PC retention manifested by abdominal pain and vomiting, and another patient had an episode of severe abdominal pain following ingestion of oral contrast material for MRE, resulting in exclusion from the study.
4. Discussion
The present study examined the ability of VCE and MRE to detect changes in disease classification that occur over time in regard to the location and behaviour of the disease in patients with quiescent CD, or stemming from the limitations of the original modalities employed in the initial diagnosis.
Our most important observation is that previously unrecognized proximal small-bowel disease was detected by VCE in half of the patients. MRE was less sensitive in detection of proximal small-bowel disease (only 25%), but was superior for detection of phenotype shift. Our results are in line with several previous reports showing similar detection rates of active proximal disease using VCE.18,23,24 However, the patients in our study differed in that they had quiescent or only mildly active disease.
The clinical relevance of our observations merits further evaluation. Based on previous data, it is agreed that proximal disease in CD patients is generally associated with more severe and complicated disease.16–18,25 However, the question of whether the detection of proximal disease in a quiescent patient should influence the therapeutic management remains unanswered and will require additional trials. It should also be mentioned that the impact of low-grade inflammation (detected in two-thirds of our patients) on the natural history of the disease remains to be established.
A number of studies have revealed the relatively low sensitivity of radiological imaging in the detection of proximal small-bowel lesions.26–28 In our study, the accuracy of MRE in detecting proximal disease coincides with this earlier data.
According to data from several clinical cohorts, CD location remains relatively stable over time after diagnosis. However, behaviour phenotype progresses over time, even in patients in clinical remission, with an increasing number of patients progressing from an inflammatory phenotype to stricturing or penetrating disease.29,30 Up to 70% of CD patients develop either penetrating or stricturing disease during the course of the disease.23 Similar results were published by the IBSEN group, in which 36% had stenosing or penetrating disease at diagnosis, rising to 49 and 53% after 5 and 10 years, respectively.30 A Belgian group reported similar results, with 46% of the patients having a change in disease behaviour within 10 years of follow-up.31 Monitoring of patients in clinical remission usually includes review of their symptoms with or without calculation of disease activity scores, and measurement of inflammatory markers (C-reactive protein [CRP] and/or calprotectin). Small-bowel anatomical damage may well go undetected under these routine follow-ups.32 Previous studies have documented the poor correlation between CRP and small-bowel endoscopic damage, with faecal calprotectin performing somewhat better.32–35 The present study shows that the risk of developing stricturing and/or penetrating complications exists even when the disease is clinically quiescent. Intensive monitoring with advanced imaging and endoscopic modalities may potentially lead to detection of disease features associated with a more severe prognosis. The recently published STRIDE (Selecting Therapeutic Targets in Inflammatory Bowel Disease) initiative suggests targeting the treatment by endoscopic remission and cross-sectional imaging in patients with disease location not amenable to endoscopic access.36 In most of these patients, capsule endoscopy will indeed provide an accurate, safe and quantitative assessment of the proximal small-bowel mucosa.32 The Lewis score, recently validated in established Crohn’s disease,24 provides an accurate quantification of inflammatory activity, along with a definition of mucosal healing. We believe that in patients with documented patency of the small bowel, VCE will provide a more accurate assessment of the inflammatory lesions, especially in proximal small-bowel disease37 or when the inflammation is relatively mild.38
Capsule retention is a concern for both providers and patients and is dependent upon the clinical indication for use. A recent meta-analysis reported VCE retention in 2.8% of patients with suspected or determined CD.39 In a recent real-life study, the rate of small-bowel retention in patients with known CD approximated 2.5%.40 In our study, there were no cases of capsule retention according to the definitions of the ICCE consensus.21 Moreover, all capsules reached the caecum before the recording time elapsed. This is probably due to our very cautious strategy, with all patients screened by MRE and subsequently undergoing a PC study, unless a very severe stricture was demonstrated by MRE (leading to withdrawal from the study) or, alternatively, no active SB was demonstrated.41 In one patient the PC was retained, necessitating corticosteroid therapy, with resolution.
One of the drawbacks of our study is related to the retrospective nature of the data concerning the initial assessment of patients. Our study cohort comprised patients that in most cases were diagnosed several years before entering the study. Not all the patients had imaging during their initial evaluation and only a few patients had an earlier VCE. In many of these patients, the diagnosis was obtained using different equipment (a CT scanner or a different MR scanner) in another institution and the studies were interpreted by a different reader. In some cases, the location and behaviour characteristics may have been changed after revision of the original studies by a different viewer, and may not represent the true progression of the disease. However, this limitation accurately represents the real-life nature of the present study and the clinical challenge addressed by it, especially for referral IBD centres that care for patients that frequently have been diagnosed elsewhere. Although the Paris classification uses a more specific definition of proximal small-bowel disease, we chose to use the Montreal classification mainly because the Paris classification has not been validated in adult patients. Furthermore, the presence of mild gastric and proximal duodenal involvement could have over-diagnosed proximal CD.
In conclusion, monitoring of CD patients in remission or with mild disease activity using VCE and MRE can safely identify unknown new proximal involvement and progression to stricturing/penetrating disease. Further prospective studies are required in order to evaluate the impact of CD reclassification on long-term outcome.
Funding
The study was partially supported by a generous grant from the Leona M. and Harry B. Helmsley Charitable Trust.
Conflict of Interest
Fred Saibil: advisory boards/fellow support from Abbvie, Janssen, Ferring and Takeda. Iris Dotan: advisory boards/lectures/research support from Janssen, AbbVie, MSD, Takeda, Ferring, Falk Pharma, Rafa, Genentech, Pfizer, Given Imaging, Teva and Protalix. Shomron Ben-Horin: research support and/or consultancy fees from MSD, AbbVie, Janssen, CellTrion and Takeda. Rami Eliakim: advisory board/lecture fees from Given Imaging, AbbVie, Janssen, Rafa, Falk Pharma and Takeda. All other authors: none to declare.
Author Contributions
Tomer Greener: study design, data collection and analysis, and manuscript drafting. Uri Kopylov: study design, data collection and analysis and manuscript drafting. Doron Yablecovitch, Adi Lahat, Sandra Neuman, Nina Levhar, Henit Yanai, Batya Weiss, Benjamin Avidan, Iris Dotan, Yehuda Chowers, Michal Amitai, Fred Saibil: data collection and reviewing the manuscript for important scientific content. Shomron Ben- Horin, Rami Eliakim: study initiation and design, and reviewing the manuscript for important scientific content. All authors reviewed and approved the final version of the manuscript
References
- 1. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19(Suppl A):5–36. [DOI] [PubMed] [Google Scholar]
- 2. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis 2010;4:7–27. [DOI] [PubMed] [Google Scholar]
- 4. Petruziello C, Onali S, Calabrese E, et al. Wireless capsule endoscopy and proximal small bowel lesions in Crohn’s disease. World J Gastroenterol 2010;16:3299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voderholzer WA, Beinhoelzl J, Rogalla P, et al. Small bowel involvement in Crohn’s disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut 2005;54:369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koh DM, Miao Y, Chinn RJ, et al. MR imaging evaluation of the activity of Crohn’s disease. Am J Roentgenol 2001;177:1325–32. [DOI] [PubMed] [Google Scholar]
- 7. Horsthuis K, Bipat S, Bennink RJ, Stoker J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: metaanalysis of prospective studies. Radiology 2008;247:6479. [DOI] [PubMed] [Google Scholar]
- 8. Rimola J, Rodriguez S, Garcia-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in Crohn disease. Gut 2009;58:1113–20. [DOI] [PubMed] [Google Scholar]
- 9. Eliakim R, Fischer D, Suissa A, et al. Wireless capsule video endoscopy is a superior diagnostic tool in comparison to barium follow-through and computerized tomography in patients with suspected Crohn’s disease. Eur J Gastroenterol Hepatol 2003;15: 363–7. [DOI] [PubMed] [Google Scholar]
- 10. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- 11. Kopylov U, Yablecovitch D, Lahat A, et al. Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn’s disease using biomarkers, capsule endoscopy, and imaging. Am J Gastrenterol 2015;110:1316–23. [DOI] [PubMed] [Google Scholar]
- 12. Sauter B, Beglinger C, Girardin M, et al. Monitoring disease activity and progression in Crohn’s disease. A Swiss perspective on the IBD Ahead ‘Optimised Monitoring’ recommendations. Digestion 2014;89:299–309. [DOI] [PubMed] [Google Scholar]
- 13. Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn’s disease. Gastroenterology 2006;130:650–6. [DOI] [PubMed] [Google Scholar]
- 14. Loly C, Belaiche J, Louis E. Predictors of severe Crohn’s disease. Scand J Gastroenterol 2008;43:948–54. [DOI] [PubMed] [Google Scholar]
- 15. Seksik P, Loftus EV, Jr, Beaugerie L, et al. Validation of predictors of 5-year disabling CD in a population-based cohort from Olmsted County, Minnesota, 1983–1996. Gastroenterology 2007;132:A17. [Google Scholar]
- 16. Wolters FL, Russel MG, Sijbrandij J, et al. Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut 2006;55:1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow DK, Sung J, Yu JC, et al. Upper gastrointestinal tract phenotype of Crohn’s disease is associated with early surgery and further hospitalization. Inflamm Bowel Dis 2009;15:551–7. [DOI] [PubMed] [Google Scholar]
- 18. Flamant M, Trang C, Maillard O, et al. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn’s disease. Inflamm Bowel Dis 2013;19:1390–6. [DOI] [PubMed] [Google Scholar]
- 19. Shrot S, Konen E, Hertz M, Amitai MM. Magnetic resonance enterography: 4 years experience in a tertiary medical center. Isr Med Assoc J 2011;13:172–7. [PubMed] [Google Scholar]
- 20. Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther 2008;27:146–54. [DOI] [PubMed] [Google Scholar]
- 21. Cave D, Legnanu P., de FR, Lewis BS. ICCE consensus for capsule retention. Endoscopy 2005;37:1065–67. [DOI] [PubMed] [Google Scholar]
- 22. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petruziello C, Onali S, Calabrese E, et al. Wireless capsule endoscopy and proximal small bowel lesions in Crohn’s disease. World J Gastroenterol 2010;16:3299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cotter J, Dias de Castro F, Moreira MJ, Rosa B. Tailoring Crohn’s disease treatment: the impact of small bowel capsule endoscopy. J Crohns Colitis 2014;8:1610–5. [DOI] [PubMed] [Google Scholar]
- 25. Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 2002;8:244–50. [DOI] [PubMed] [Google Scholar]
- 26. Triester SL, Leighton JA, Leontiadis GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn’s disease. Am J Gastroenterol 2006;101:954–64. [DOI] [PubMed] [Google Scholar]
- 27. Panés J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther 2011;34:125–45. [DOI] [PubMed] [Google Scholar]
- 28. Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol 2011;9:124–9. [DOI] [PubMed] [Google Scholar]
- 29. Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn’s disease phenotype-results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol 2008;103:3082–3093. [DOI] [PubMed] [Google Scholar]
- 30. Henriksen M, Jahnsen J, Lygren I, et al. Clinical course in Crohn’s disease: results of a five-year population-based follow-up study (the IBSEN study). Scand Gastroenterol 2007;42:602–610. [DOI] [PubMed] [Google Scholar]
- 31. Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut 2001;49:777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kopylov U RG, Bressler B, Seidman EG. Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory boweldisease. Inflamm Bowel Dis 2014;20:742–56. [DOI] [PubMed] [Google Scholar]
- 33. Jones J, Loftus EV, Jr, Panaccione R, et al. Relationship between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gatroenterol Hepatol 2008;6:1218–24. [DOI] [PubMed] [Google Scholar]
- 34. Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 2011;140:1817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 36. Dionisio PM, Gurudu SR, Leighton JA, et al. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol 105:1240–8. [DOI] [PubMed] [Google Scholar]
- 37. Kopylov U, Klang E, Yablecovitch D, et al. Diffusion-weighted and contrast-enhanced magnetic resonance enterography versus capsule endoscopy activity indices for quantification of small bowel inflammation in patients with quiescent Crohn’s disease. In: UEGW; 2015, October, Barcelona, Spain. [Google Scholar]
- 38. Liao Z, Gao R, Xu C, et al. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc 2010;71:280–6. [DOI] [PubMed] [Google Scholar]
- . Kopylov U, Nemeth A, Koulaouzidis A, et al. Small bowel capsule endoscopy in the management of established Crohn’s disease: clinical impact, safety and correlation with inflammatory biomarkers. Inflamm Bowel Dis 2015;21:93–100. [DOI] [PubMed] [Google Scholar]
- 40. Rozendorn N, Klang E, Lahat A, et al. Prediction of patency capsule retention in known Crohn’s disease patients by using magnetic resonance imaging. Gastrointest Endosc 2016;83:182–7. [DOI] [PubMed] [Google Scholar]