Abstract
Background and Aims:
A number of observational studies have reported an association between serum levels of infliximab [IFX] at various thresholds, and clinical outcomes in inflammatory bowel disease [IBD]. This association has not previously been systematically analysed.
Methods:
Systematic review of studies that reported serum infliximab levels according to outcomes in IBD. Primary outcome was clinical remission, and secondary outcomes included endoscopic remission, C-reactive protein [CRP] levels, and colectomy. Meta-analysis of raw data was performed where appropriate. A quality assessment was also undertaken.
Results:
A total of 22 studies met the inclusion criteria, including 3483 patients; 12 studies reported IFX levels in a manner suitable for determining effect estimates. During maintenance therapy, patients in clinical remission had significantly higher mean trough IFX levels than patients not in remission: 3.1 µg/ml versus 0.9 µg/ml. The standardised mean difference in serum IFX levels between groups was 0.6 µg/ml (95% confidence interval [CI] 0.4-0.9, p = 0.0002]. Patients with an IFX level > 2 µg/ml were more likely to be in clinical remission (risk ratio [RR] 2.9, 95% CI 1.8-4.7, p < 0.001], or achieve endoscopic remission [RR 3, 95% CI 1.4-6.5, p = 0.004] than patients with levels < 2 µg/ml.
Conclusions:
There is a significant difference between serum infliximab levels in patients with IBD in remission, compared with those who relapse. A trough threshold during maintenance > 2 µg/ml is associated with a greater probability of clinical remission and mucosal healing.
Keywords: Infliximab, trough, remission, mucosal healing
1. Introduction
‘Loss of response’ to anti-tumour necrosis factor [TNF] agents is a common problem in practice, and has led to many studies of causative factors.1 One common observation is that patients who relapse while on anti-TNF agents often have lower trough serum drug levels and/or higher anti-drug antibody [ADA] levels than those who remain in remission.2,3 Data from clinical trials and observational cohorts have reported a significant difference in trough infliximab levels between groups, leading to an assumption that this is a causative factor in loss of response.4,5 A response to these data in practice has been to increase infliximab doses to raise trough drug levels, with mixed results.5
The relationship between clinical outcomes and serum infliximab [IFX] levels is complex, as prospective studies have also demonstrated that many patients clinically relapse despite adequate serum drug levels and no anti-drug antibodies, and conversely many patients remain in clinical remission despite low levels.3,6 One challenge to the interpretation of these results in practice has been the variations in the assays used and mechanisms of reporting results.7 For example, many studies have used assays in which the presence of infliximab precluded additional data on ADAs in a given sample.2 A recent well-designed study, that compared three different assays, showed close correlation between the results of laboratory and commercial assays that are used in Europe and the USA for the detection of infliximab.3
Given the increasing measurement of IFX levels in practice, and the implications of their use on clinical management and healthcare costs, we sought to perform a systematic review and meta-analysis of the data on this topic. The reported close correlation between the results of different assay types permits pooling of the results from various studies to determine effect estimates.3,8 We previously reported on the association between antibodies to infliximab [ATIs] and clinical outcomes, demonstrating that meaningful summary effect estimates can be determined for clinicians from studies in a variety of healthcare settings and patient populations.8,9 This approach has not been undertaken for clinical outcomes according to serum IFX levels previously.
2. Methods
2.1. Literature search
A literature search was performed to identify all published and unpublished studies in any language for consideration for inclusion studies that had measured serum IFX levels and reported on clinical outcomes according to IFX levels in patients with IBD. A systematic search of the following databases was performed: MEDLINE [Pubmed]—1966 to 2015, Web of Science—2000 to 2015], Allied Health Literature [CINAHL] —1990 to 2015, Scopus—2000 to 2015, and EMBASE—2000 to 2015. The following search strategy was constructed by using a combination of MeSH subject headings and text-words relating to antibodies to IFX: ‘infliximab’, ‘infliximab levels’, ‘ulcerative colitis’, ‘Crohn’s disease’, ‘loss of response’, ‘remission’, ‘mucosal healing’, ‘CRP’. Abstracts from American Digestive Diseases Week and the United European Gastroenterology Week [2002–2015] were searched manually, reference lists of all articles read, and several previously published reviews were scrutinised to disclose additional literature on the topic. Any eligible non-English abstracts identified were translated using Google translate. The corresponding authors of studies published only as abstracts were contacted for additional information. All eligible abstracts were included, given the rapid developments in this field.
2.2. Inclusion and exclusion criteria
We included all studies [controlled trials, observational studies] that reported outcomes [clinical, mucosal, CRP, colectomy] in patients who were treated with IFX for ulcerative colitis [UC] or Crohn’s disease [CD], and grouped these outcomes according to mean/median IFX levels, or according to a cut-off threshold level of IFX. The primary outcome measure was clinical remission defined as absence of clinical symptoms in patients who had responded to IFX. We expected there would be many definitions of ‘remission’ across the studies, so no particular score was pre-specified. We did not require that included studies report the objective confirmation of active inflammation as the cause of symptoms.
We excluded studies if they: a] were review articles; b] examined IFX use in non-IBD patients; c] did not measure IFX levels; d] only reported quartiles of levels, not averages or thresholds; e] only measured TNF-binding capacity, not serum drug levels; f] did not report clinical outcomes of IFX therapy or serum IFX levels; g] were studies using other anti-TNFs only [adalimumab or certolizumab]; or h] only included levels during the induction phase of infliximab.
2.3. Study selection
Two authors [CM and GC] independently scanned the abstract of every trial identified by the search to determine eligibility. The senior author [ACM, clinical investigator] also performed an independent search. Blinding to source was not performed. Full articles were selected for further assessment if the abstract suggested the study included patients with Crohn’s disease or ulcerative colitis, serum IFX levels were measured, and clinical outcomes were reported. If these criteria were unclear from the abstract, the full article was retrieved for clarification. Papers not meeting the inclusion criteria were excluded. Duplicates were screened for by comparing the authors, timelines, and patient populations of selected studies. Any disagreements were resolved by discussion and, if required, by consultation with the senior author [ACM]. All final included and excluded studies were reviewed by the senior investigator [ACM].
2.4. Data extraction and quality assessment
The following data were retrieved [where possible] from published reports using standardised forms, with disagreements resolved by discussion between the reviewers: number of patients in the study; method of selection of cohorts; schedule of anti-TNF administration; assay type used to measure IFX; mean [standard deviation; SD] and median [interquartile range: IQR] IFX trough levels; anti-infliximab antibody levels; methods of measurement of clinical outcomes; and reporting outcomes used. Where details were not available in published results, contact authors were emailed to request additional information. Descriptions of the characteristics and comparisons of outcome groups [eg remission/non-remission] were examined, where provided. Assessment of quality was performed using the Newcastle Ottawa Quality Assessment Scale.10
2.5. Outcomes
The primary outcome of interest was the difference in mean trough IFX levels between patients in remission and those not in remission. The secondary outcomes were relative risk of remission, endoscopic remission, or colectomy, according to a threshold serum IFX level. Mean levels of serum CRP above and below a specified level of serum IFX were also compared. Finally, we examined mean IFX levels in ATI-positive and ATI-negative groups.
2.6. Statistical Analysis
Data were analysed and reported consistent with the consensus guidelines by the MOOSE [for observational studies] and PRISMA [for randomised trials] groups [see Supplementary Tables 1 and 2, available as Supplementary data at ECCO-JCC online].10,11 In studies where the outcome measures were reported as medians, these were accepted as means for the purpose of meta-analysis.12 The interquartile Range [IQR] was converted to an estimated standard deviation [SD] using the formula ‘IQR/1.35’, and the range was converted to an estimated standard deviation using the formula ‘range/4’.13 All authors of studies where SD, IQR or Range where not reported were contacted for additional information. Data were pooled for meta-analysis if the outcomes were sufficiently similar [determined by consensus of authors] and data were homogeneous [determined by the degree of clinical and statistical heterogeneity]. Raw data from included studies [absolute numbers] were used to construct 2×2 contingency tables, and unadjusted risk ratios [RRs] were calculated using Review Manager [RevMan 5.1] for dichotomous outcomes. Standardised mean difference [SMD] was used to report the summary statistic for comparison of outcomes presented as continuous scales, to account for difference in methods of measurement of IFX levels. The random effects model was used to account for variations between studies and give a more conservative pooled estimate.14 The Q test was used to assess for heterogeneity and I2 statistic to quantify the percentage of heterogeneity due to between-study variation; a value of p < 0.10 was considered statistically significant. Sensitivity analyses were performed for all outcomes where 10 or more studies were included. Significance levels were set at p < 0.05.
3. Results
3.1. Literature search
The search of databases yielded 62 articles of potential relevance for full-text review. Of these, 22 met the inclusion criteria; 16 were full papers and 6 were abstracts not published as full papers [Table 1].2,4,6,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33 The timing of samples and methods used are detailed in Table 1. Eleven of these described patients with only Crohn’s disease, four with only ulcerative colitis and seven with any IBD or were unspecified. Five of the 22 reported data from randomised controlled trials; the remainder were observational studies. There was a total of 3483 adult patients in the included studies; 40 studies were excluded for reasons identified in Supplementary Table 3, available as Supplementary data at ECCO-JCC online. The quality assessment and risk of bias score for each study are detailed in Supplementary Table 4 [available as Supplementary data at ECCO-JCC online]. We emailed 18 authors of the included studies to request additional raw data, but only 7 replied with further details, and 4 replied that they were unable to provide this information, despite publishing their results.
Table 1.
Characteristics and outcomes of included studies.
| Author | Year | Type | Disease | Design | N | Method | Timing |
|---|---|---|---|---|---|---|---|
| Adedokun | 2014 | Paper | UC | RCT | 728 | ELISA | Week 8, 30, 54 |
| Ainsworth | 2008 | Paper | CD | Obs | 33 | RIA | Week 8 |
| Baert | 2014 | Paper | IBD | Obs | 128 | HMSA | Week 14 |
| Ben-Bassat | 2013 | Abstract | CD | Obs | 234 | HMSA | Various |
| Bortlik | 2012 | Paper | CD | Obs | 84 | ELISA | Weeks 14, 22 |
| Cornillie | 2014 | Paper | CD | RCT | 144 | ELISA | Week 14 |
| Daperno | 2013 | Abstract | IBD | Obs | 66 | ELISA | Various |
| Drastich | 2011 | Abstract | CD | Obs | 26 | EIA | Various |
| Drobne | 2015 | Paper | CD | Obs | 223 | ELISA | Various |
| Echarri | 2014 | Abstract | CD | Obs | 32 | ELISA | Week 14 |
| Hibi | 2014 | Paper | CD | Obs | 48 | ELISA | Week 14 |
| Maser | 2006 | Paper | CD | Obs | 105 | ELISA | Every 68 weeks |
| Murthy | 2012 | Abstract | UC | Obs | 134 | HMSA | Various |
| Pariente | 2012 | Paper | IBD | Obs | 76 | ELISA | At relapse |
| Paul | 2013 | Paper | IBD | Obs | 120 | ELISA | Week 8 |
| Reinisch | 2015 | Paper | CD | RCT | 203 | ELISA | Weeks 26, 30, 50 |
| Ron | 2012 | Abstract | UC | Obs | 30 | ? | Weeks 12, 52 |
| Seow | 2010 | Paper | UC | Obs | 115 | ELISA | Various |
| Steenholdt | 2011 | Paper | IBD | Obs | 106 | RIA | Various |
| Vande Casteele | 2015 | Paper | IBD | RCT | 275 | ELISA | Various |
| Vande Casteele | 2013 | Paper | IBD | Obs | 90 | HMSA | Week 14 |
| Vande Casteele | 2015 | Paper | CD | Obs & RCTs | 483 | HMSA | Various |
CD, Crohn’s disease; IBD, inflammatory bowel disease; UC, ulcerative colitis; Obs, observational study; RCT, randomised controlled trial; RIA, radio-immunoassay; HMSA, homogeneous mobility shift assay; ELISA, enzyme-linked immunosorbent assay.
3.2. Mean serum IFX level associated with clinical outcomes
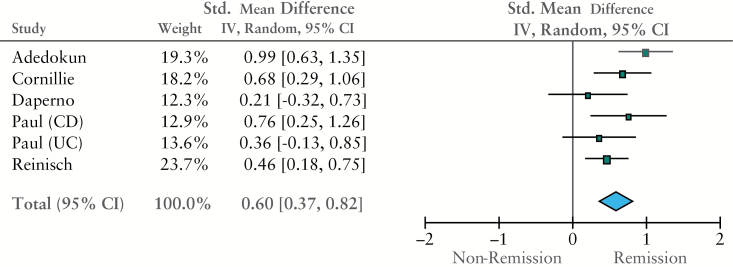
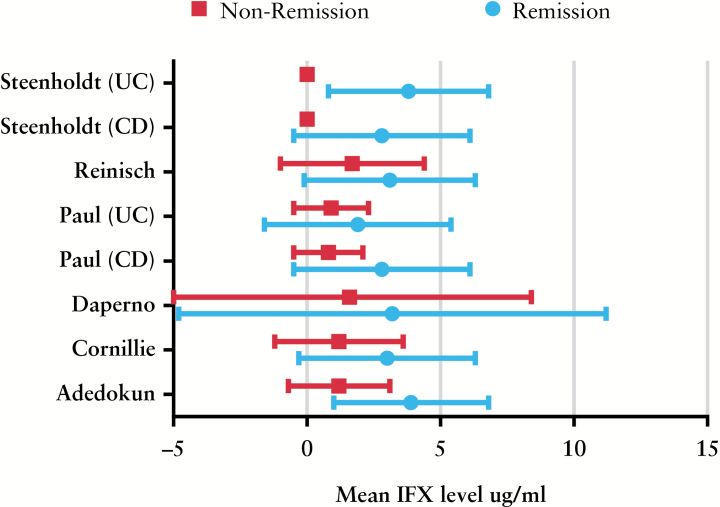
Eight studies reported mean serum IFX levels, grouped according to remission status.4,18,19,20,23,27,28,31 Five used an enzyme-linked immunosorbent assay [ELISA] to measure IFX, and one used a radioimmunoassay [RIA] method; both methods have demonstrated good correlation in independent tests.3 Pooled mean IFX level was 3.1 µg/ml in all remitters, and 0.9 µg/ml in all non-remitters. There was a significant difference in mean serum IFX levels between remission/non-remission patients in five studies suitable for meta-analysis; the standardised mean difference [SMD] in serum IFX levels between groups was 0.6 µg/ml [95% CI 0.4-0.9, p < 0.001 using the random effects model, Figure 1]]. There was moderate statistical heterogeneity for this outcome [I2 = 44%]. There were insufficient included studies [> 10] for sensitivity analysis. When studies that provided separate data for populations with Crohn’s disease were analysed, the difference remained significant; SMD between remitters/non-remitters was 0.7 µg/ml [95% CI 0.3-1.1, p = 0.004] in patients with Crohn’s disease. When the raw mean and standard deviation from each study in remitters/non-remitters are plotted, it can be noted that there is considerable overlap in levels between groups [Figure 2].
Figure 1.
Forest plot of standardized mean difference [SMD] by inverse variance method [random effects model].
Figure 2.
Mean and standard deviation [SD] of serum infliximab [IFX] level in each study grouped by clinical status.
3.3. Remission rates & cut-off serum IFX levels
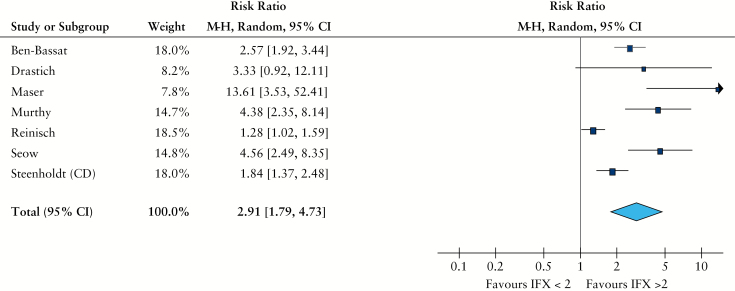
Rather than report mean/median drug levels, many abstracts and studies have reported only remission rates in patients above or below a specific cut-off level of IFX. Seven studies provided remission rates based on IFX thresholds.2,17,21,25,28,30,31 [2, 17, 21, 28, 30, 31]. Four of these used an ELISA assay, two used homogeneous mobility shift assay [HMSA], and one an RIA; all methods show good correlation, as noted above. When remission rates from all seven studies were pooled, comparisons between patients with an IFX level < 2 µg/ml, and those with a level > 2 µg/ml could be made. Crude remission rates were 25% when IFX levels < 2 µg/ml, and 79% when levels > 2 µg/ml. When raw remission rates were analysed to determine risk ratios, patients with an IFX level greater than 2 µg/ml were more likely to be in remission than those with an IFX level < 2 µg/ml [RR 2.9, 95% CI 1.8-4.7, p < 0.001, Figure 3]. There was high statistical heterogeneity for this outcome [I2 = 88%], due to inclusion of the Reinisch and Steenholdt studies; if these are excluded, the effect estimate remains significant but the heterogeneity falls to 59%. There was insufficient number of studies [> 10] to do sensitivity analyses for this outcome. If the studies with ulcerative colitis patients were excluded, the effect remained significant in just Crohn’s disease [RR 2.3, 95% CI 1.4-3.9].
Figure 3.
Risk ratio of clinical remission grouped by serum infliximab [IFX] cut-off [2 µg/ml]. Random effect, Maentel-Haenszel ratio.
3.4. Secondary outcomes & cut-off serum IFX levels
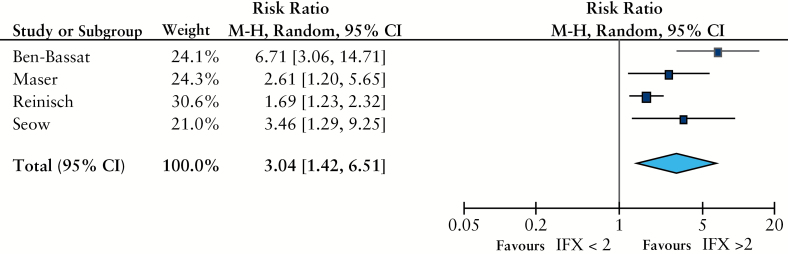
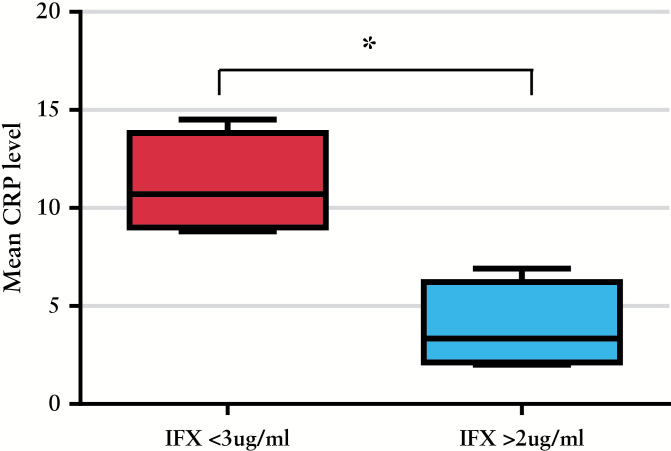
Four studies reported mucosal healing rates according to whether serum IFX was detectable [> 2 µg/ml], or not.2,17,28,30 All studies defined endoscopic remission as ‘no ulcers’ or ‘no mucosal lesions’. In these studies, patients with detectable serum IFX levels during maintenance therapy were more likely to achieve endoscopic remission than patients with undetectable levels [RR 3, 95% CI 1.4-6.5, p = 0.004, Figure 4 ]. Four studies described mean CRP levels at different ranges of serum IFX levels.2,17,18,32 Mean CRP was 11.1g/dl in patients with a serum IFX level < 3 µg/ml, and 3.9g/dl in patients whose serum IFX was > 2 µg/ml [p = 0.005 by unpaired t-test, Figure 5]. We also looked for studies where mean IFX levels in ATI+ and ATI- subjects were compared, but only two studies reported this, and not according to clinical outcome.17,26 Finally, two studies reported colectomy rates based on serum IFX levels in patient with ulcerative colitis.25,30 Patients with undetectable serum IFX levels during maintenance therapy were more likely to require a colectomy than patients with detectable levels [RR 5.4, 95% CI 3.1-9.3, p < 0.001].
Figure 4.
Risk ratio of mucosal healing grouped by serum infliximab [IFX] cut-off [2 µg/ml]. Random effect, Maentel-Haenszel ratio.
Figure 5.
Mean and standard deviation [SD] of serum C-reactive protein [CRP] level in patients with an infliximab [IFX] level < 3 µg/ml, and those with an IFX > 2 µg/ml.
4. Discussion
Many observational studies have linked serum levels of infliximab [IFX] to clinical and objective outcomes in patients with IBD, but the interpretation of these data in clinical practice has been challenging.34 The literature on this topic has used various assays [ELISA, HMSA, RIA] and various descriptors of drug levels [mean, median, quartile, cut-off] according to various outcomes [loss of response, remission] during the course of IFX therapy.7 Most also could not account for the role of ATIs in this relationship between IFX levels and remission, because of the use of drug-sensitive ELISA methodology. This inconsistent reporting provides challenges to the practising clinician when interpreting serum IFX results in an individual patient with commercial assays.34 Methodological analyses of the data from many studies can provide guidance for evidence-based decisions in these situations.
This systematic review and meta-analyses provide a number of conclusions for clinical practice and future research. Patients with IBD who relapse during IFX therapy have consistently lower levels of drug at trough than patients who have remained in remission, regardless of method of testing used. Similarly, absolute differences in many important outcomes [colectomy, mucosal healing] were noted when subjects were grouped by a cut-off level of serum IFX. The assumption is that this is a causative relationship [low levels lead to relapse/colectomy/ mucosal inflammation], and studies that report the impact of dose escalation in patients with low drug levels support this hypothesis.5 Since the cut-off levels of IFX were specific to each study, meta-analysis cannot determine if different cut-offs would provide different results. Cumulatively, these data would imply that our patients with low trough IFX levels experience worse outcomes than patients with higher levels, and thus interventions to identify and address this are warranted. This could be in the form of shorter infusion intervals, higher doses, or the addition of thiopurines.35
However, it should be noted that there is considerable overlap in the range of drug levels in ‘remitters/relapsers’, suggesting that serum IFX levels alone do not explain clinical status in most patients. The recent Steenholdt study reinforced this point; 60–70% of patients lost response to IFX despite ‘high’ serum drug levels and no anti-drug antibody levels.36 This cohort of patients would not benefit from dose/frequency escalation. In the TAXIT trial, 20% of patients who were in clinical remission at baseline had what would be considered sub-therapeutic IFX levels.6 A noted caveat in patients with Crohn’s disease is the limitation of clinical assessment in the absence of objective determination of relapse.37 Getting everyone’s serum IFX levels to a higher range will not improve outcomes for those with overlap IBS or infections, and there could be unintended consequences from sustained supratherapeutic levels.38,39
For research, the reporting standards of these studies have been quite variable. Some have been presented as abstracts only, and lack important details on assay used and timing of levels. For peer reviewers and editors, studies that report outcomes according to quartiles only should be discouraged, as they unrealistically assume homogeneity of risk within quartiles, and the data-driven categories preclude comparisons across studies. Second, the clinical specificity of IFX measurements appears to be comparable across assays. Results from two different groups have suggested that differences between assays are minimal, and that commercially available ELISA assays provide clinically meaningful results similar to the HMSA assays.3,40 However, there may still be inter-assay differences in absolute values for IFX levels, so this needs to be considered when comparing effect estimates. Finally, more data are needed on the predictive value for future relapse of low IFX levels in patients in remission, and how this compares with other biomarkers, such as faecal calprotectin or CRP.
This study includes limitations similar to all meta-analyses. Heterogeneous patient populations, assays, and outcome measures were used in included studies. Of all the included studies [N = 22], few contained raw data suitable for extraction for the determination of mean differences in levels, and requests for additional data from authors were mostly unsuccessful. As noted above, the level of correlation between the different assays used appears sufficient to allow pooling of means/medians for effect estimates. The differences in the lower limit of detection of different assays may have led to varying thresholds for ‘0’ levels between studies. A random effects model was used to conservatively account for the clinical and statistical heterogeneity in pooled studies. Insufficient studies have been published to perform sensitivity analyses for disease [CD or UC] or assay type. Although the correlation between assays is acceptable, absolute concentrations [in the setting of thresholds] might differ from assay to assay. Finally, we have not accounted for confounding factors, eg ATI levels or immunomodulators, that also influence both outcomes and infliximab levels in IBD. Some included studies reported no difference in mean serum IFX according to immunomodulator use,16,23,26,27 whereas others noted higher levels in patients taking immunomodulators.18,19,20,28 Only two studies reported mean IFX levels according to ATI status, without linked clinical outcome data.17,26
In conclusion, this systematic review and meta-analysis provide evidence of an association between serum IFX levels and outcomes in patients with IBD. The existing literature is limited by incomplete publication of results and the deficiencies of primarily retrospective observational studies. Prospective evaluations of the clinical effectiveness of adjustments of IFX dosing to trough levels are required to support the use of routine evaluations of serum IFX levels in practice.
Funding
None.
Conflict of Interest
ACM received consulting fees from Janssen in 2014, unrelated to this study.
Author Contributions
CM, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content; GC, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content; AM, study concept and design, analysis and interpretation of data, statistical analysis, manuscript preparation, study supervision.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
References
- 1. Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol 2009;104:760–7. [DOI] [PubMed] [Google Scholar]
- 2. Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol 2006;4:1248–54. [DOI] [PubMed] [Google Scholar]
- 3. Steenholdt C, Bendtzen K, Brynskov J, et al. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn’s disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol 2014;109:1055–64. [DOI] [PubMed] [Google Scholar]
- 4. Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014;147:1296–307.e5. [DOI] [PubMed] [Google Scholar]
- 5. Afif W, Loftus EV, Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.e3. [DOI] [PubMed] [Google Scholar]
- 7. Moore C, Vaughn B, Moss AC. Analysis of Reporting Standards of Serum Anti-TNF Levels in Patients with IBD. Inflamm Bowel Dis 2013;19:S57. [Google Scholar]
- 8. Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease [IBD]: a meta-analysis. Am J Gastroenterol 2013;108:40–7; quiz 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Meara S, Nanda KS, Moss AC. Antibodies to infliximab and risk of infusion reactions in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014;20:1–6. [DOI] [PubMed] [Google Scholar]
- 10. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale [NOS] for assessing the quality of nonrandomised studies in meta-analyses. 2014. http://www.ohri.ca/Programs/clinical_epidemiology/oxford. Accessed October, 2015. [Google Scholar]
- 11. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology [MOOSE] group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 12. Lee TW, Kolber MR, Fedorak RN, et al. Iron replacement therapy in inflammatory bowel disease patients with iron deficiency anemia: a systematic review and meta-analysis. J Crohns Colitis 2012;6:267–75. [DOI] [PubMed] [Google Scholar]
- 13. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 15. Ainsworth MA, Bendtzen K, Brynskov J. Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn’s disease. Am J Gastroenterol 2008;103:944–8. [DOI] [PubMed] [Google Scholar]
- 16. Baert F, Drobne D, Gils A, et al. Early trough levels and antibodies to infliximab predict safety and success of reinitiation of infliximab therapy. Clin Gastroenterol Hepatol 2014;12:1474–81.e2; quiz e91. [DOI] [PubMed] [Google Scholar]
- 17. Ben-Basset O, Romanova A, Iacono A, et al. Association of Serum Infliximab and Antibodies to Infliximab to Long-Term Clinical Outcome and Mucosal Healing in Crohn’s Disease. Gastroenterology 2013;144:S775. [Google Scholar]
- 18. Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn’s disease. J Crohns Colitis 2013;7:736–43. [DOI] [PubMed] [Google Scholar]
- 19. Cornillie F, Hanauer S, Diamond RH, et al. Can clinical, biological or pharmacological markers predict sustained response to infliximab? A retrospective analysis of ACCENT 1. Gut 2011;60:A296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daperno M, Lavagna A, Fracchia M, et al. Infliximab trough levels [IFX-Tl] are higher in patients with inflammatory bowel disease [IBD]treated with immunosuppressives: clinical correlations of IFX-LT and antibodies to Infliximab [ATI] in IBD. Gastroenterology 2013;144:Tu1173. [Google Scholar]
- 21. Drastich P, Kozeluhova J, Jaresova M, Spicak J. Infliximab serum trough levels and deep remission in patients with IBD. Gastroenterology 2011;140:S292. [Google Scholar]
- 22. Drobne D, Bossuyt P, Breynaert C, et al. Withdrawal of immunomodulators after co-treatment does not reduce trough level of infliximab in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2015;13:514–21.e4. [DOI] [PubMed] [Google Scholar]
- 23. Echarri A, Ferreiro R, Fraga R, et al. Impact of postinduction infliximab trough level and disease activity on primary response in Crohn′s disease. J Crohns Colitis 2015; 9[S1]: P526. [Google Scholar]
- 24. Hibi T, Sakuraba A, Watanabe M, et al. C-reactive protein is an indicator of serum infliximab level in predicting loss of response in patients with Crohn’s disease. J Gastroenterol 2014;49:254–62. [DOI] [PubMed] [Google Scholar]
- 25. Murthy S, Kevans D, Seow CH, et al. Association of serum infliximab and antibodies to infliximab to long-term clinical outcome in acute ulcerative colitis. Gastroenterology 2012;142:S388. [Google Scholar]
- 26. Pariente B, Pineton dC, Krzysiek R, et al. Trough levels and antibodies to infliximab may not predict response to intensification of infliximab therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis 2012;18:1199–206. [DOI] [PubMed] [Google Scholar]
- 27. Paul S, Del Tedesco E, Marotte H, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis 2013;19:2568–76. [DOI] [PubMed] [Google Scholar]
- 28. Reinisch W, Colombel JF, Sandborn WJ, et al. Factors associated with short- and long-term outcomes of therapy for Crohn’s disease. Clin Gastroenterol Hepatol 2015;13:539–47.e2. [DOI] [PubMed] [Google Scholar]
- 29. Ron Y, Yanai H, Ben Yehoyada M. Infliximab for moderate to severe ulcerative colitis: short and long-term effects and predictors of response. Gastroenterology 2012;142:S385. [Google Scholar]
- 30. Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010;59:49–54. [DOI] [PubMed] [Google Scholar]
- 31. Steenholdt C, Bendtzen K, Brynskov J, et al. Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn’s disease. Scand J Gastroenterol 2011;46:310–8. [DOI] [PubMed] [Google Scholar]
- 32. Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut 2015;64:1539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vande CN, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013;108:962–71. [DOI] [PubMed] [Google Scholar]
- 34. Khanna R, Sattin BD, Afif W, et al. Review article: a clinician’s guide for therapeutic drug monitoring of infliximab in inflammatory bowel disease. Aliment Pharmacol Ther 2013;38:447–59. [DOI] [PubMed] [Google Scholar]
- 35. Moss AC, Brinks V, Carpenter JF. Review article: immunogenicity of anti-TNF biologics in IBD the role of patient, product and prescriber factors. Aliment Pharmacol Ther 2013;38:1188–97. [DOI] [PubMed] [Google Scholar]
- 36. Steenholdt C, Brynskov J, Thomsen OO, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27. [DOI] [PubMed] [Google Scholar]
- 37. Lahiff C, Safaie P, Awais A, et al. The Crohn’s disease activity index [CDAI] is similarly elevated in patients with Crohn’s disease and in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2013;37:786–94. [DOI] [PubMed] [Google Scholar]
- 38. Moss AC. Therapeutic drug monitoring, mucosal healing, deep remission: the path to nirvana in Crohn’s disease? Clin Gastroenterol Hepatol 2014;12:432–3. [DOI] [PubMed] [Google Scholar]
- 39. Malkonen T, Wikstrom A, Heiskanen K, et al. Skin reactions during anti-TNF alpha therapy for pediatric inflammatory bowel disease: a 2-year prospective study. Inflammatory Bowel Dis 2014;20:1309–15. [DOI] [PubMed] [Google Scholar]
- 40. Vande CN, Buurman DJ, Sturkenboom MG, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther 2012;36:765–71. [DOI] [PubMed] [Google Scholar]