Abstract
Background:
Anti-tumour necrosis factor [TNF] therapy in combination with thiopurine is the most effective strategy for Crohn’s disease, but raises safety concerns.
Methods:
In a retrospective multicentre study, we investigated long-term outcome of patients starting anti-TNF monotherapy for Crohn’s disease and investigated whether introducing an immunomodulator in patients losing response to anti-TNF monotherapy is effective for resetting immunogenicity.
Results:
A total of 350 adult patients with Crohn’s disease received either infliximab [n = 178, 51%] or adalimumab [n = 172, 49%] monotherapy. Mean duration of follow-up was 42 months. An immunomodulator was initiated in 53 patients [15%]. At last follow-up, 73.1% [n = 38] were in clinical remission [one patient with missing data]. Multivariate analysis identified anti-TNF type [higher need for starting immunomodulator for infliximab than for adalimumab; p = 0.0058] and first- vs second-/third-/fourth-line anti-TNF therapy [p = 0.014] as predictors of immunomodulator initiation. Among the 18 patients with available data, introduction of an immunomodulator was able to restore infliximab trough level within the therapeutic range and to induce clinical remission in 10 patients [55%]. Cumulative probability of remaining on anti-TNF therapy was 57.9% at 5 years among the 297 patients not starting an immunomodulator during follow-up.
Conclusion:
An immunomodulator was initiated in 15% of patients with Crohn’s disease starting anti-TNF monotherapy. Independent predictors of immunomodulator initiation were infliximab use and second-/third-/fourth-line anti-TNF therapy. Resetting immunogenicity with an immunomodulator was effective in half of patients in a sub-study. Persistence of anti-TNF treatment at 5 years was observed in half of the 297 patients not starting an immumodulator in a real-life setting.
Key words: Infliximab, adalimumab, Crohn’s disease, anti-TNF monotherapy
1. Introduction
Crohn’s disease [CD] is a chronic disabling and progressive condition.1 Anti-tumour necrosis factor [TNF] therapy is increasingly used to treat inflammatory bowel disease [IBD] refractory to standard medications.2,3 The SONIC trial demonstrated that infliximab in combination therapy with azathioprine is the most effective strategy for CD.4 A SONIC-like trial is not available for other biologicals known to be effective in CD, namely adalimumab, certolizumab pegol, vedolizumab, and natalizumab. A recent French nationwide survey among private gastroenterologists found that the use of combination therapy is very low, representing only 4.5% of IBD patients in a real-life setting.5 This may be partly explained by safety concerns such as lymphoma risk associated with the use of combination therapy.6,7 By contrast, there is no increased risk of malignancy among IBD patients treated with anti-TNF monotherapy except for skin cancers.6,7
Anti-TNF monotherapy may be associated with a better safety profile than combination therapy, especially in some specific patient populations such as young males and elderly people.6,7
Accumulating evidence indicates that combination therapy reduces immunogenicity of anti-TNF therapy and improves trough levels in IBD patients.6,7 Interestingly, a recent pilot study suggested that the addition of an immunomodulator to anti-TNF therapy might be effective in patients who have lost response to anti-TNF agents owing to formation of antidrug antibodies.8 Levels of antibodies to infliximab [ATI] gradually decreased while trough levels of infliximab [TRI] increased; clinical response was restored in all five included patients.8
We therefore evaluated in a large multicentre retrospective study, enrolling all consecutive adult patients who started anti-TNF monotherapy for CD in four referral centres, the short- and long-term outcomes of these patients. We also investigated whether starting an immunomodulator among patients losing response to anti-TNF monotherapy could be used for resetting immunogenicity and improving pharmacokinetics without representing a loss of opportunity for these patients.
2. Methods
2.1. Study population
All consecutive adult patients treated between January 1, 2000 and March 16, 2013 in four referral centres (Nancy, Nice, and Saint-Etienne [France] and Liège [Belgium]) with anti-TNF monotherapy [infliximab or adalimumab] as a first-, second-, third- or fourth-line anti-TNF-therapy were included in this retrospective study. Patients receiving episodic anti-TNF treatment were excluded. Short-term efficacy of anti-TNF monotherapy was evaluated at Weeks 4–12 and was classified as no response, partial response, or complete response. Response was judged by the physician, as no clinical score is routinely used in any of the participating centres and as it was a retrospective study. Long-term efficacy was evaluated by recording anti-TNF persistence as well as clinical benefit at last known follow-up. The need for initiating an immunomodulator during follow-up was evaluated. All CD-related surgeries and hospitalisations were collected. All adverse events occurring during follow-up were recorded.
In the subgroup of patients followed at Saint-Etienne University Hospital, TRI and ATI were measured according to routine practice in all patients losing clinical response to infliximab therapy [ELISA technique from Theradiag, Marne La Vallée, France]. Faecal calprotectin levels and Crohn’s Disease Activity Index [CDAI] scores were also collected in this centre according to routine practice. If patients had undetectable TRI with high ATI [> 200ng/ml]9, azathioprine was then introduced [2.5mg/kg/d] without modification of infliximab dose.
2.2. Statistical analysis
Qualitative variables were given as frequency and percentage. Quantitative variables were calculated as medians with interquartile range [IQR].
The cumulative incidences of optimising anti-TNF therapy, remaining on anti-TNF therapy and on combination therapy, starting an immunomodulator, and experiencing an adverse event related to the immunomodulator were calculated using the Kaplan-Meier estimator. When possible, the median survival time and its 95% confidence interval [95% CI] were computed.
Potential predictive factors of immunomodulator initiation were first tested by bivariate analyses using the Cox proportional hazards model. Parameters with a p-value less than 0.2 in bivariate analysis were introduced in a multivariate Cox proportional hazards model with backward selection. Results were expressed with hazard ratio and its 95% confidence interval [95% CI]. To investigate predictors of clinical remission after immunomodulatory introduction, uncontrolled disease and partial response were pooled and we used bivariate Cox proportional-hazards model. Univariate analysis was performed to identify independent predictors of clinical remission and pharmacokinetics normalisation after introduction of azathioprine in the subgroup of patients with available information.
Data were analysed with SAS software version 9.2 [SAS Inc., Cary, NC 27513]. A p-value less than 0.05 was considered significant.
3. Results
3.1. Baseline characteristics of the 350 patients
The median duration of follow-up since anti-TNF monotherapy initiation for the overall population was 35 months [IQR: 18.3–53.3] [Table 1]. Median age at anti-TNF monotherapy initiation was 32.2 years [23.7–43.9] and 155 patients were males [44.3%]. Median disease duration at time of anti-TNF monotherapy initiation was 4.4 years [1.5–12.1]; 240 patients [68.6%] started anti-TNF monotherapy as first-line anti-TNF therapy; the remaining 110 patients started first-line anti-TNF therapy in association with an immunomodulator and received anti-TNF monotherapy as second-, third-, or fourth-line anti-TNF therapy. Anti-TNF monotherapy was infliximab [n = 178, 51%] or adalimumab [n = 172, 49%]. According to the Montreal classification, 126 [36.7%] patients had ileal disase [L1], 70 [20.3%] colonic disease [L2], and 148 [43.0%] ileocolonic disease. A total of 195 [57.7%] patients had uncomplicated disease, 85 [25.1%] patients had stricturing behaviour, and 58 [17.1%] had penetrating behaviour. Of the 350 patients, 137 [39%] had perianal disease. One-third of patients had previous intestinal resection. About 10% of patients were on an oral 5-amonisalicylate [5-ASA], 30 [8.7%] on budesonide, and 81 [23.3%] on systemic [oral or intravenous] steroids. The main indications of anti-TNF monotherapy were a complicated behaviour for 121 patients [34.6%], steroid-dependent or -refractory disease for 108 patients [31%], immunosuppressive therapy failure for 92 [26.3%], and failure of a first anti-TNF therapy for 44 [12.6%] patients [Table 1].
Table 1.
Demographic and baseline characteristics at anti- tumour necrosis factor [TNF] monotherapy initiation.
| Parameter | N = 350 |
|---|---|
| Age | 35.2 [23.7;43.9] |
| Gender [male] | 44.3% [155] |
| Active smoker | 54.9% [162] |
| Duration of disease [years] | 4.4 [1.5;12.1] |
| Number line of biological therapy | |
| •1st line | 68.6% [240] |
| •2nd line | 29.1% [102] |
| •3rd line | 2.0% [7] |
| •4th line | 0.3% [1] |
| Age at diagnosis [years]a | |
| •< 16 | 13.9% [47] |
| •17–40 | 68.7% [233] |
| •> 40 | 17.4% [59] |
| Localisation of diseasea | |
| •Ileum: L1 | 36.6% [123] |
| •Colon: L2 | 20.3% [70] |
| •Ileocolon: L3 | 43.0% [148] |
| Behavioura | |
| •Pure inflammatory: B1 | 57.7% [195] |
| •Stricturing: B2 | 25.1% [85] |
| •Penetrating: B3 | 17.2% [58] |
| Perianal disease | 39.0% [137] |
| Previous intestinal resection | 33.1% [115] |
| Concomitant medications | |
| •Oral 5-ASA | 9.8% [34] |
| •Budesonide | 8.7% [30] |
| •Systemic [oral or intravenous] steroids | 23.3% [81] |
| •Ciclosporin | 1.1% [4] |
| Indication of anti-TNF monotherapy initiation | |
| •Complicated behaviour | 34.6% [121] |
| •Flare | 13.1% [46] |
| •Fistula | 19.4% [68] |
| •Stenosis | 15.1% [53] |
| •Prophylaxis | 8.3% [29] |
| •Manifestation extra-digestive | 7.7% [27] |
| •Steroid-dependent disease | 16.9% [59] |
| •Steroid-refractory disease | 14.0% [49] |
| •Immunosuppressicve therapy failure | 26.3% [92] |
| •Failure of a first anti-TNF therapy | 12.6% [44] |
Results are expressed as median [interquartile range] for quantitative parameters and as percentage [frequency] for qualitative parameters.
5-ASA, 5-aminosalicylic acid.
aAccording to the Montreal classification
3.2. Short-term clinical efficacy of anti-TNF monotherapy
Clinical efficacy as judged by the physician after anti-TNF induction therapy was observed in 94% of patients [complete response 65.7% and partial response 28.4%], and non-primary response in 6% of patients.
3.3. Long-term outcome of anti-TNF monotherapy
Anti-TNF therapy optimisation [shortening interval and/or increasing the dose] was performed in 163 [47.8%; missing data for 9 out of 350 patients] of 350 patients during follow-up. The cumulative probability of optimising anti-TNF therapy was 26.9% [95% CI: 22.4%; 32.1%] and 58.8% [51.8%; 65.9%] at 1 and 5 years, respectively [Supplementary Figure 1, available as Supplementary data at ECCO-JCC online]. Median time to optimisation was 47 months [28;56]. Of the 163 patients, 80% of patients shortened the interval between infliximab infusions or adalimumab injections, whereas 20% increased the dose of anti-TNF treatment.
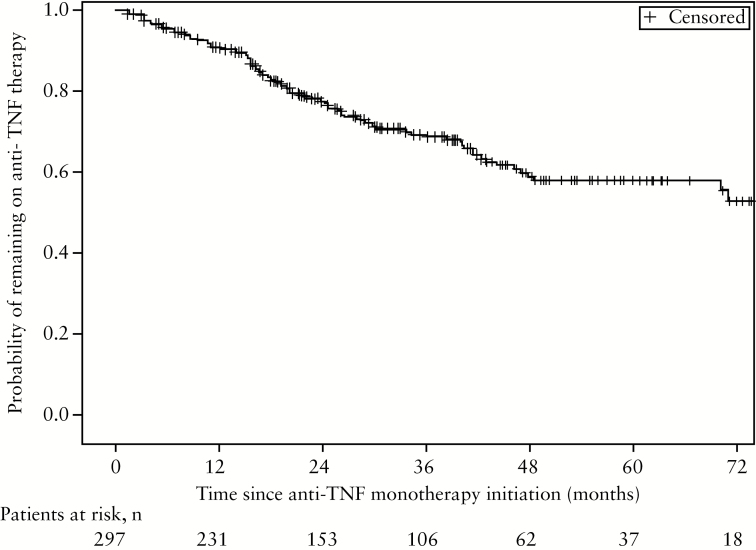
After a median follow-up of 33 months [IQR: 17–52], 297 [85%] out of 350 patients did not require the introduction of an immunomodulator; 92 out of 297 patients stopped anti-TNF therapy during follow-up. Of these 92 patients, 42 [46.7%] were switched to another anti-TNF agent, 10 [11.1%] stopped when they underwent intestinal resection, 8 [8.9%] stopped while being in clinical benefit, and the 32 remaining patients for other reasons. The cumulative probability of remaining on anti-TNF therapy from time of anti-TNF monotherapy initiation for the 297 patients was 90.6% [86.0%;93.2%] and 57.9% [49.9%;64.9%] at 1 and 5 years, respectively [Figure 1 ]. At last follow-up, 75.4% [n = 215] of the 297 patients who did not start an immunomodulator were in clinical remission as judged by the physician, 16.8% [n = 48] had partial clinical response, and only 7.7% [n = 22] had uncontrolled disease.
Figure 1.
Cumulative probability of remaining on anti-tumour necrosis factor [TNF] therapy from time of anti-TNF monotherapy initiation in the 297 patients not needing an immunomodulator during follow-up.
3.4. Long-term outcome of the 53 patients starting an immunomodulator
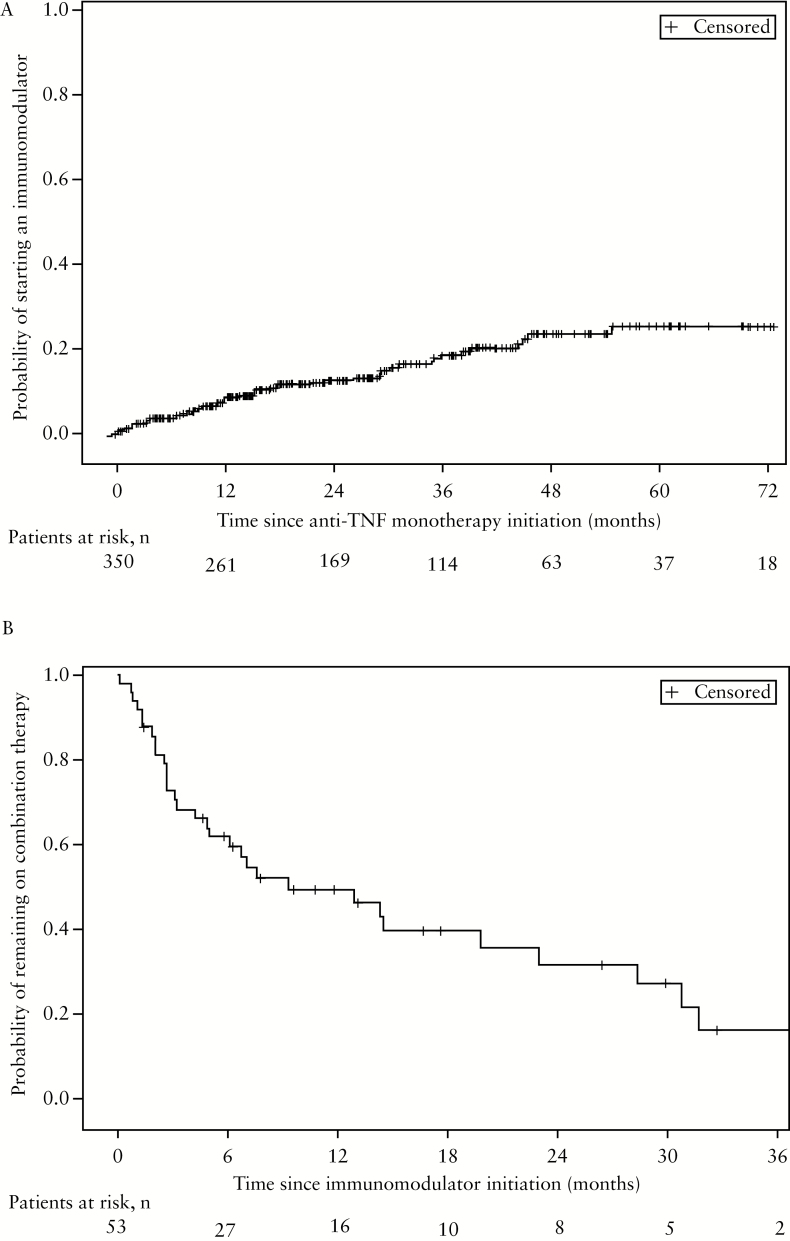
After a median follow-up of 12.8 months [range, 4.5–30.1], an immunomodulator was initiated in 53 out of 350 patients [15%]: 33 [62%] with azathioprine and 20 [38%] with methotrexate. The cumulative probability of starting an immunomodulator was 7.1% [4.8%;10.5%] and 25.9% [19.5%;33.9%] at 1 and 5 years, respectively [Figure 2A]. At last follow-up, 28.9% of the 53 patients were still on combination therapy, 46.1% were on anti-TNF monotherapy [and thus had stopped the immunomodulator], 9.6% were on immunomodulator alone [and thus had stopped the anti-TNF agent], and 13.4% had stopped both anti-TNF therapy and immunomodulator. The probability of remaining on combination therapy [ie patients who stopped neither the anti-TNF agent nor the immunomodulator] from time of immunomodulator initiation for the 53 patients was 61.8% [46.3%; 73.9%] and 31.8% [17.1%; 47.6%] at 6 and 24 months, respectively [Figure 2B]. This probability was 50.6% [30.3%; 74.8%] at 12 months for patients on methotrexate and 48.6% [31.5%; 68.9%] for patients on azathioprine [p = 0.964]. Perianal disease was not predictive of remaining on combination therapy [p = 0.067]: 39.4% [21.0%; 65.4%] at 12 months for patients with perianal disease and 56.2% [38.4%; 79.6%] for patients with no perianal disease. The cumulative probability of experiencing an adverse event related to immumodulator use from time of drug initiation was 17.4% [8.6%;33.3%] and 22% [11.3%;40.4%] at 6 and 12 months, respectively [Supplementary Figure 2, available as Supplementary data at ECCO-JCC online]. This cumulative probability was 5.6% [0.8%;33.4%] at 12 months for patients on methotrexate and 32.8% [16.5%;58.4%] for patients on azathioprine [p = 0.114]. At last follow-up, 13.5% [n = 7] had uncontrolled disease, 13.5% [n = 7] had partial response, and 73.1% [n = 38] were in clinical remission as judged by the physician [one patient with missing data at last follow-up]. In bivariate analysis, predictors of immunomodulator initiation were anti-TNF type [higher need for immunomodulator initiation for infliximab than for adalimumab; p = 0.02] and first- vs second-/third-/fourth-line anti-TNF therapy [p = 0.06] [Table 2]. In multivariate analysis, both variables were statistically significant [p = 0.0058 and 0.014, respectively] [Table 2]. Predictors of clinical remission after immunomodulatory introduction are presented in Table 3. The type of immunomodulator was not a predictive factor of clinical remission after immunomodulator introduction [p = 0.065, Table 3]. Two [10.5%] out of the 19 patients treated with methotrexate had uncontrolled disease (vs 15.1% [n = 5] of the 33 patients on azathioprine); 5.3% [n = 1] had partial response (vs 18.2% [n = 6]); and 84.2% [n = 16] were in clinical remission (vs 66.7% [n = 22]) as judged by the physician.
Figure 2.
Panel A: Cumulative probability of starting an immunomodulator from time of anti- tumour necrosis factor [TNF] monotherapy initiation in the overall population [n = 350]. Panel B: Cumulative probability of remaining on combination therapy from time of immunomodulator initiation in the 53 patients.
Table 2.
Bivariate and multivariate analyses on predictors of immunomodulator initiation.
| Bivariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR [95% CI] | p-Value | HR [95% CI] | p-Value | |
| Gender [male] | 0.79 [0.46;1.37] | 0.416 | ||
| Active smoker | 1.32 [0.72;2.42] | 0.361 | ||
| Localisation of disease * | ||||
| Ileum; L1 | 1 | 0.166 | ||
| Colon: L2 | 1.69 [0.80;3.56] | 0.283 | ||
| Ileocolon: L3 | 1.43 [0.74;2.74] | |||
| Behavioura,b | ||||
| Pure inflammatory: B1 | 1 | |||
| Stricturing: B2 | 0.46 [0.21;1.04] | 0.063 | ||
| Penetrating: B3 | 0.81 [0.37;1.74] | 0.585 | ||
| Age at diagnosis [years]a | ||||
| < 16 | 1 | |||
| 17–40 | 1.03 [0.46;2.32] | 0.938 | ||
| > 40 | 1.24 [0.47;3.25] | 0.665 | ||
| Perianal disease | 0.97 [0.56;1.67] | 0.903 | ||
| Previous intestinal resection | 0.85 [0.47;1.53] | 0.591 | ||
| 2nd/3rd/4th line of anti-TNF therapy vs 1st lineb | 1.67 [0.97;2.87] | 0.0622 | 2.00[1.15;3.49] | 0.014 |
| Anti-TNF type[IFX vs ADA] α | 1.92 [1.10;3.35] | 0.0214 | 2.23[1.26;3.95] | 0.006 |
| Concomitant treatment | ||||
| Oral 5-ASA | 1.08 [0.62;1.86] | 0.786 | ||
| Budesonide | 1.41 [0.64;3.14] | 0.396 | ||
| Systemic [oral or intravenous] | 1.52 [0.68;3.36] | 0303 | ||
| steroids | 1.35 [0.73;2.49] | 0.334 | ||
| Indication of anti-TNF initiation | ||||
| Steroid-dependent disease | 1.21 [0.62;2.34] | 0.580 | ||
| Steroid-refractory disease | 0.73 [0.31;1.71] | 0.474 | ||
| Failure of a first anti-TNF therapy | 1.12 [0.63;2.02] | 0.694 | ||
| Flare | 1.03 [0.44;2.42] | 0.938 | ||
5-ASA, 5-aminosalicylic acid; IFX, infliximab; ADA, adalimumab; HR, hazard ratio. 95% CI, 95% confidence interval; TNF, tumour necrosis factor.
aAccording to the Montreal classification.
bFactors introduced in multivariate analysis.
Table 3.
Bivariate analysis on predictors of clinical remission after immunomodulator introduction.
| Bivariate analysis | ||
|---|---|---|
| HR [95% CI] | p-Value | |
| Gender [male] | 0.87 [0.45;1.68] | 0.690 |
| Active smoker | 1.31 [0.62;2.77] | 0.479 |
| Disease locationa | ||
| Ileum: L1 | 1 | |
| Colon: L2 | 1.55 [0.61;3.95] | 0.359 |
| Ileocolonic: L3 | 0.88 [0.39;1.96] | 0.758 |
| Behavioura,b | ||
| Uncomplicated [B1] | 1 | |
| Complicated [B2 + B3] | 1.21 [0.59;2.48] | 0.593 |
| Age at diagnosis [years]a | ||
| < 16 | 1 | |
| 17–40 | 1.07 [0.43;2.63] | 0.890 |
| > 40 | 1.25 [0.41;3.79] | 0.689 |
| Perianal disease | 1.19 [0.61;2.34] | 0.601 |
| Previous intestinal resection | 0.90 [0.45;1.80] | 0.764 |
| 2nd/3rd/4th line of anti-TNF therapy vs 1st line α | 1.67 [0.83;3.30] | 0.148 |
| Anti-TNF type [IFX vs ADA] α | 0.60 [0.29;1.26] | 0.178 |
| Concomitant treatment | ||
| Oral 5-ASA | 1.39 [0.61;3.22] | 0.413 |
| Budesonide | 1.18 [0.48;2.88] | 0.714 |
| Systemic [oral or intravenous] | 1.53 [0.76;30.7] | 0.234 |
| Steroids | 1.54 [0.79;2.97] | 0.205 |
| IMM type [methotrexate vs azathioprine] | 1.94 [0.96;3.92] | 0.065 |
| Time between anti-TNF therapy and IMM introduction < 12 months vs > = 12 months | 1.11 [0.56;2.34] | 0.756 |
5-ASA, 5-aminosalicylic acid; IFX, infliximab; ADA, adalimumab; IMM, immunomodulatory; TNF, tumour necrosis factor; HR, hazard ratio; CI, confidence interval.
HR, hazard ratio. 95% CI, 95% confidence interval
aAccording to the Montreal classification.
bFactors included in multivariate analysis.
Perianal disease was not a predictor of clinical remission at last follow-up after immunomodulator introduction [p = 0.065; table 3]: 9.5% [n = 2] of the 21 patients with perianal disease and available data at last follow-up had uncontrolled luminal disease at last follow-up (vs 16.1% [n = 5] of the 31 patients with no perianal disease), 14.3% [n = 3] had partial response (vs 12.9% [n = 4] in the no perianal disease group), and 76.2% [n = 16] were in clinical remission (vs 71.0% [n = 22] in the no perianal disease group) as judged by the physician.
3.5. Resetting immunogenicity by adding azathioprine in case of loss of response
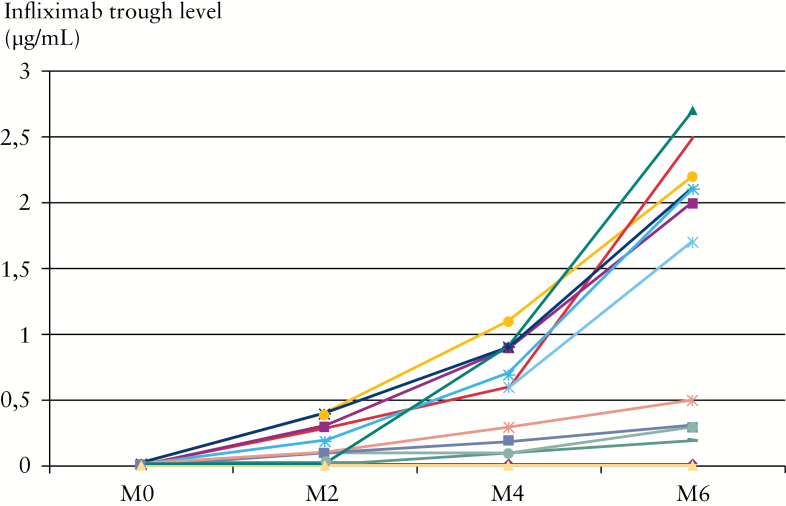
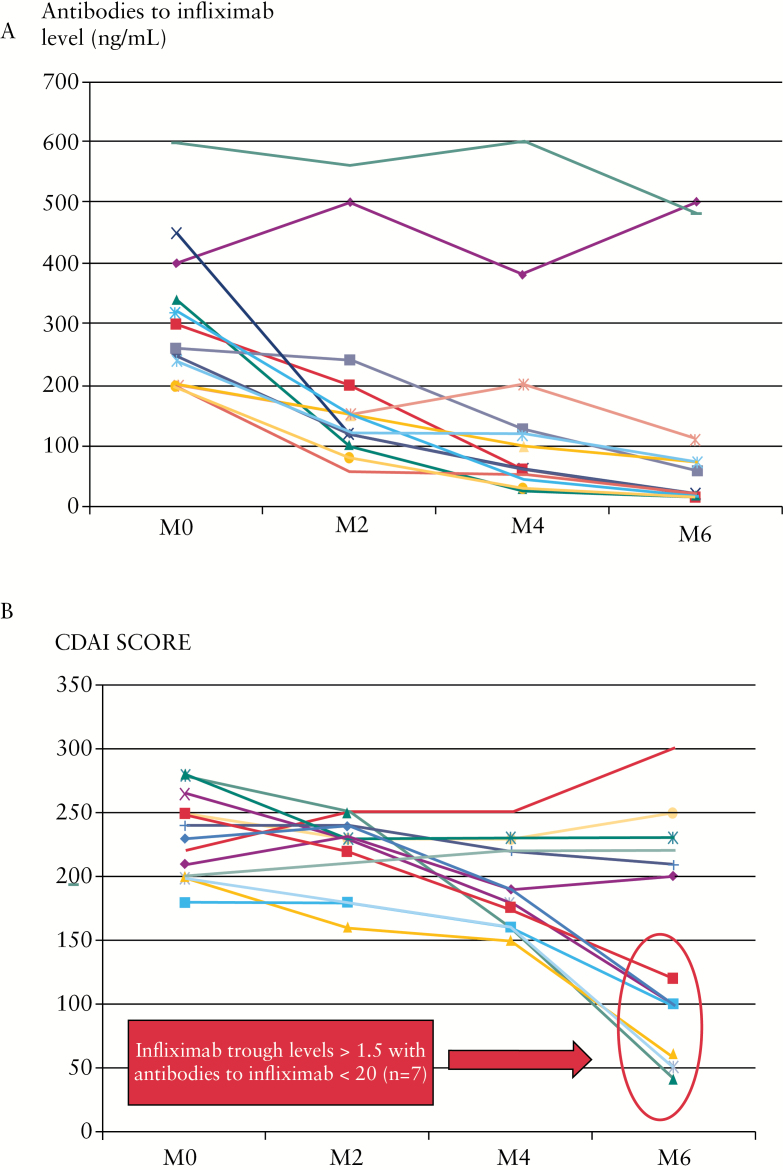
Pharmacokinetics of infliximab after adding azathioprine in IBD patients who lost response and having undetectable TRI with very high ATI [> 200ng/ml] were analysed. A total of 13 CD patients [mean age: 36 years, sex ratio 1] with available data could be analysed. All 13 patients had permanent ATI defined by ATI > 200ng/ml at two consecutive infliximab infusions. CDAI ranged between 150 and 300 and faecal calprotectin level 450 to 800 µg/g stools. The mean TRI increased at 6 months from 0.015 [0.01–0.02] to 0.9 [0.01–2.7] µg/ml [p = 0.01, Figure 3]. Conversely, mean ATI level decreased at 6 months from 320 [200–600] to 60 [10–500] [p = 0.01, Figure 4A]. For 7 out of 13 patients, the trough level of infliximab was within normal range at 6 months following initiation of azathioprine [TRI > 1.5 µg/ml with ATI < 20ng/ml] and all 7 patients were in clinical remission at 6 months [CDAI < 150] [Figure 4B]. A biomarker remission [faecal calprotectin < 250 µg/g stools] was obtained at 4 months in 7 out of 13 patients [Supplementary Figure 3, available as Supplementary data at ECCO-JCC online] and all 7 patients also had trough level of infliximab within normal range. No factor at time of immunomodulator initiation [disease duration, C-reactive protein level, duration of infliximab therapy, previous use of an immunomodulator] was associated with clinical remission or pharmacokinetics normalisation [considered as resetting of immunogenicity] [data not shown]. A TRI ≥ 1 µg/ml at 4 months predicted clinical remission, biomarker remission, and normalisation of both TRI and ATI at 6 months with positive predictive value and negative predictive value of 100%. Results were comparable to adalimumab therapy. Five patients presenting loss of response under adalimumab maintenance therapy [40mg every other week] and with undetectable TRA and positive antibodies to adalimumab were optimised with the addition of azathioprine. Three out of five patients were in clinical remission at 6 months [CDAI < 150], with biomarker remission [faecal calprotectin < 250 µg/g stools] and trough level of adalimumab within therapeutic range [TRA > 4.9 µg/ml].
Figure 3.
Infliximab trough levels [µg/ml] before start of azathioprine treatment at Month [M] 0 (mean: 0.015 [0.01–0.02]) and at 6 months (M6; mean: 0.9 [0.01–2.7]; p = 0.01) in 13 infliximab-treated patients.
Figure 4.
Panel A: Antibodies to infliximab [ng/ml] before start of azathioprine treatment at Month [M] 0 (mean: 320 [200–600]) and at 6 months (M6; mean: 60 [10–500]; p = 0.01) in 13 infliximab-treated patients. Panel B: Crohn’s Disease Activity Index [CDAI] scores before start of azathioprine treatment at Month [M] 0 and at 6 months [M6] in 13 infliximab-treated patients.
3.6. Crohn’s disease-related surgeries and hospitalisations
Among the 350 patients, the cumulative probability of having intestinal resection was 6.2% [4.0%; 9.4%] and 24.9% [19.2%; 32.0%] at 1 and 5 years, respectively. Among the 350 patients, the cumulative probability of having at least one hospitalisation was 7.4% [4.9%; 10.8%] and 19.2% [13.9%; 26.6%] at 1 and 5 years, respectively.
3.7. Safety in the overall population [n = 350]
A total of 157 out of 350 patients [44.8%] experienced adverse events judged as possibly related to anti-TNF therapy during follow-up, with 30 serious adverse events [Table 4]. Of the 127 non-serious adverse events, 43 were infections, 11 allergic reactions, 13 psoriasiform lesions, 28 unspecific skin lesions, and 32 others; of the 30 serious adverse events, 7 were infections, 8 allergic reactions, 4 psoriasiform lesions, 2 unspecific skin lesions, and 9 others. No cancer or lymphoma was detected during follow-up. The cumulative probability of experiencing at least one adverse event among the 350 patients was 25.4% [21.0%; 30.7%] and 43.7% [37.7%; 50.6%] at 1 and 5 years, respectively.
Table 4.
Safety [%, n] in the overall population [n = 350].
| Adverse events | 44.8% [157] |
|---|---|
| Serious adverse events | 8.6% [30] |
| -infections | 23.3% [7] |
| -allergic reactions | 26.7% [8] |
| -psoriasiform lesions | 13.3% [4] |
| -unspecific skin lesions | 6.7% [2] |
| -others | 30.0% [9] |
| Non-serious adverse events | 36.3% [127] |
| -infections | 33.9% [43] |
| -allergic reactions | 8.7% [11] |
| -psoriasiform lesions | 10.2% [13] |
| -unspecific skin lesions | 22.0% [28] |
| -others | 25.2% [32] |
4. Discussion
This is the first study investigating the long-term efficacy and safety profile of anti-TNF monotherapy for CD. The SONIC trial clearly demonstrated that combination therapy is superior to anti-TNF monotherapy for maintaining steroid-free clinical remission at 6 months.4 However, several studies showed that combination therapy is associated with an increased risk of malignancies, particularly an increased risk of lymphoma.6,7 Importantly, this risk is known to be mainly driven by concomitant thiopurine use.6,7 By contrast, all available studies failed to identify an increased risk of malignancy among IBD patients treated with anti-TNF monotherapy.6,7 In this context, some physicians use anti-TNF as monotherapy in IBD patients, as demonstrated in a recent survey conducted among private gastroenterologists in France.5 Whether starting anti-TNF monotherapy should be considered as an option in clinical practice for CD patients is still a matter of debate.
We found that only 6% of patients were primary non-responders. This is consistent with data from a large observational study assessing the long-term clinical benefit of infliximab in 614 consecutive patients with CD from a single centre during a median follow-up of 55 months and showing that 10.9% of patients were primary non-responders to infliximab.10 Our data indicate that in the short term, monotherapy with anti-TNF therapy is highly efficacious in CD in a real-life setting.
However, long-term data are needed to further evaluate whether some patients can be treated with anti-TNF therapy without needing a concomitant immunomodulator. We showed that only 15% of patients needed initiation of an immunomodulator during follow-up. Importantly, three-quarters of patients on anti-TNF monotherapy were in clinical remission as judged by the physician at last report, showing that most patients not needing an immunomodulator have long-term clinical benefit. In the overall population, the cumulative probability of having intestinal resection was 24.9% at 5 years and the cumulative probability of having at least one hospitalisation was 19.2% at 5 years. In a recent French observational study of a referral-centre cohort, the cumulative probability of the first CD-related major abdominal surgery was 25.9% at 5 years.2 In CD, the annual incidence of hospitalisations was 20%.11 Loss of response is frequent with anti-TNF therapy in IBD.12,13 Consistently, we found that the cumulative probability of optimising anti-TNF therapy was 58.8% at 5 years. Overall, these findings indicate that starting with anti-TNF monotherapy does not seem to dramatically worsen disease outcomes.
Combination therapy reduces immunogenicity and increases anti-TNF trough levels.6,7 This likely contributes to the greater efficacy profile of combination therapy over anti-TNF monotherapy. Starting a concomitant immunomodulator while being on anti-TNF monotherapy is often perceived as a missed opportunity for these patients, as it might be too late to prevent immunogenicity. Preliminary findings suggest that this approach may be used for resetting immunogenicity in IBD patients on anti-TNF monotherapy losing drug response.8 However, this awaits confirmation in a larger series of patients. In our study, starting azathioprine treatment in case of loss of response to anti-TNF in 13 infliximab- and 5 adalimumab-treated patients induced clinical remission, biomarker remission, and normalisation of anti-TNF trough levels in 55% of patients at 6 months. It is noteworthy that one-quarter of patients starting an immunomodulator after initiation of anti-TNF monotherapy were still on combination therapy and three-quarters of these were in clinical remission at last follow-up. The safety profile of azathioprine, when introduced after start of anti-TNF monotherapy, is consistent with previous reports on thiopurine in IBD. Overall, these results indicate that introduction of an immunomodulator could be considered in clinical practice among patients who lose response to anti-TNF monotherapy, as it may be efficacious in terms of clinical remission and improvement of pharmacokinetics profile.
Interestingly, independent predictors of immunomodulator initiation were anti-TNF type [higher need for immunomodulator initiation for infliximab than for adalimumab] and first- vs second-/third-/fourth-line anti-TNF therapy in multivariate analysis. Recently, two meta-analyses showed that clinical remission rates at 6 months or 1 year were similar with adalimumab monotherapy and with combination therapy in CD.14,15 However, all monoclonal antibodies have the potential for immunogenicity. Furthermore, whereas combination therapy with infliximab is known to be more effective than infliximab monotherapy, no SONIC-like trial is available for adalimumab. The fact that first-line anti-TNF therapy was associated with a lower need for combination therapy may only reflect more refractory disease.
Limitations of our study include the retrospective study design and the lack of evaluation of objective signs of inflammation [C-reactive protein and endoscopy] due to missing data. We did not include a control group of patients receiving combination therapy as by definition this would lead to major bias due to uncontrolled study. However, there are several strengths. This is a large cohort of patients with CD [n = 350] treated over a 13-year period; large pharmacokinetics data after introduction of an immunomodulator were available in 18 patients; the mean duration of follow-up was 42 months; and it was a multicentre cohort study. Our findings were confirmed by other groups.16,17
In conclusion, an immunomodulator was initiated in 15% of patients with Crohn’s disease starting anti-TNF monotherapy. Independent predictors of immunomodulator initiation were infliximab use and second-/third-/fourth-line anti-TNF therapy. Resetting immunogenicity with an immunomodulator was effective in half of patients in a sub-study. Persistence of anti-TNF treatment at 5 years was observed in half of the 297 patients not starting an immumodulator during follow-up. Taken together, our findings indicate that some CD patients could remain on anti-TNF monotherapy in the long term, without representing a missed opportunity for these patients. This might improve the risk-benefit profile of current strategies based on a widespread and earlier use of biologicals in CD patients. Indeed, this may be a way to improve the long-term safety profile of anti-TNF agents by limiting the number of patients being exposed to combination therapy.
Conflict of Interest
LPB: consulting fees from Merck, Abbott, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Shire, Therakos, Pharmacosmos, Pilège, BMS, UCB-pharma, Hospira, Celltrion, Takeda, Boerhinger-Ingelheim, Lilly, Pfizer; lecture fees from Merck, Abbott, Janssen, Ferring, Norgine, Tillots, Vifor, Therakos, HAC-pharma. XR: consulting and lecture fees from MSD, Abbvie, Hospira, Janssen, Pfizer, Takeda, Norgine, Ferring, HAC Pharma, Theradiag. JF: consulting fees from Abbvie, Astellas pharma, Janssen, Takeda; lecture fees from Abbvie, Chugaï pharma, Ferring, MSD, Norgine. XH: consulting fees from Abbvie, Baxter, Fresenius Kabi, Nestlé Health Sciences, Takeda; lecture fees from Abbvie, Mayoli-Spindler, MSD, Nestlé Health Sciences, Norgine, Nutricia, Takeda, Vifor. CR: consulting fees from Abbvie, MSD, Janssen; lectures fees from Abbvie, MSD, Roche, Takeda, Falk. EL; consulting and lecture fees from Abbvie and MSD.
Author Contributions
LPB, study design and concept, data interpretation, drafting of the manuscript; JS, data analysis and interpretation, critical review of the manuscript; JF, CR, OA, VF, EL, XH, data collection and critical review of the manuscript; XR, data interpretation, drafting of the manuscript.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
References
- 1. Peyrin-Biroulet L, Cieza A, Sandborn WJ, et al. ; International Programme to Develop New Indexes for Crohn’s Disease [IPNIC] group Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut 2012;61:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peyrin-Biroulet L, Oussalah A, Williet N, Pillot C, Bresler L, Bigard MA. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn’s disease. Gut 2011;60:930–6. [DOI] [PubMed] [Google Scholar]
- 3. Williet N, Pillot C, Oussalah A, et al. Incidence of and impact of medications on colectomy in newly diagnosed ulcerative colitis in the era of biologics. Inflamm Bowel Dis 2012;18:1641–6. [DOI] [PubMed] [Google Scholar]
- 4. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 5. Duchesne C, Faure P, Kohler F, et al. ; CREGG [Club de Reflexion des cabinets et Groupes d’Hépato-Gastroentérologie] Management of inflammatory bowel disease in France: a nationwide survey among private gastroenterologists. Dig Liver Dis 2014;46:675–81. [DOI] [PubMed] [Google Scholar]
- 6. Dulai PS, Siegel CA, Peyrin-Biroulet L. Anti-tumor necrosis factor-α monotherapy vs combination therapy with an immunomodulator in IBD. Gastroenterol Clin North Am 2014;43:441–56. [DOI] [PubMed] [Google Scholar]
- 7. Dulai PS, Siegel CA, Colombel JF, Sandborn WJ, Peyrin-Biroulet L. Systematic review: monotherapy with antitumour necrosis factor α agents vs combination therapy with an immunosuppressive for IBD. Gut 2014;63:1843–53. [DOI] [PubMed] [Google Scholar]
- 8. Ben-Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013;11:444–7. [DOI] [PubMed] [Google Scholar]
- 9. Paul S, Del Tedesco E, Marotte H, Rinaudo-Gaujous M, Moreau A, Phelip JM, Genin C, Peyrin-Biroulet L, Roblin X. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis 2013;19:2568–76. [DOI] [PubMed] [Google Scholar]
- . Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut 2009;58:492–500. [DOI] [PubMed] [Google Scholar]
- 11. Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- 12. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol 2009;104:760–7. [DOI] [PubMed] [Google Scholar]
- 13. Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol 2011;106:674–84. [DOI] [PubMed] [Google Scholar]
- 14. Kopylov U, Al-Taweel T, Yaghoobi M, et al. Adalimumab monotherapy vs combination therapy with immunomodulators in patients with Crohn’s disease: A systematic review and meta-analysis. J Crohns Colitis 2014;8:1632 41. [DOI] [PubMed] [Google Scholar]
- 15. Jones J, Kaplan GG, Peyrin-Biroulet L, et al. Impact of Concomitant Immunomodulator Treatment on Efficacy and Safety of Anti-TNF Therapy in Crohn’s Disease: A Meta-Analysis of Placebo Controlled Trials With Individual Patient-Level Data. Gastroenterology 2013;144:S179. [Google Scholar]
- 16. Ong DE, Kamm MA, Hartono JL, Lust M. Addition of thiopurines can recapture response in patients with Crohn’s disease who have lost response to anti-tumor necrosis factor monotherapy. J Gastroenterol Hepatol 2013;28:1595–9. [DOI] [PubMed] [Google Scholar]
- 17. Papamichael K, Karatzas P, Mantzaris GJ. Addition of an immunomodulator as a rescue therapy for loss of response to adalimumab dose escalation in patients with Crohn’s disease. J Crohns Colitis 2015;9:589–90. [DOI] [PubMed] [Google Scholar]