Abstract
Background:
The Notch signalling pathway plays an essential role in mucosal regeneration, which constitutes a key goal of Crohn’s disease (CD) treatment. Macrophages coordinate tissue repair and several phenotypes have been reported which differ in the expression of surface proteins, cytokines and hypoxia-inducible factors (HIFs). We analysed the role of HIFs in the expression of Notch ligands in macrophages and the relevance of this pathway in mucosal regeneration.
Methods:
Human monocytes and U937-derived macrophages were polarized towards the M1 and M2 phenotypes and the expression levels of HIF-1α, HIF-2α, Jagged 1 (Jag1) and delta-like 4 (Dll4) were evaluated. The effects of macrophages on the expression of hairy and enhancer of split-1 (HES1, the main target of Notch signalling) and intestinal alkaline phosphatase (IAP, enterocyte marker) in epithelial cells in co-culture were also analysed. Phenotype macrophage markers and Notch signalling were evaluated in the mucosa of CD patients.
Results:
M1 macrophages were associated with HIF-1-dependent induction of Jag1 and Dll4, which increased HES1 protein levels and IAP activity in co-cultured epithelial cells. In the mucosa of CD patients a high percentage of M1 macrophages expressed both HIF-1α and Jag1 while M2 macrophages mainly expressed HIF-2α and we detected a good correlation between the ratio of M1/M2 macrophages and both HES1 and IAP protein levels.
Conclusion:
M1, but not M2, macrophages are associated with HIF-1-dependent induction of Notch ligands and activation of epithelial Notch signalling pathway. In the mucosa of chronic CD patients, the prevalence of M2 macrophages is associated with diminution of Notch signalling and impaired enterocyte differentiation.
Key Words: Macrophages, Crohn’s disease, mucosal healing, Notch signalling
1. Introduction
Crohn’s disease (CD) is a chronic relapsing inflammatory disorder of the gastrointestinal tract characterized by transmural inflammation, architectural distortion and thickening of all the layers of the bowel wall, which leads to intestinal fibrosis and stricture development.1 The aim of current clinical management is to prolong periods of remission and halt the destructive and progressive course of the disease. In recent years mucosal healing has been established as a key treatment goal in CD that predicts sustained clinical remission and resection-free survival of patients.2,3 This process is highly dependent on the adequate reconstruction of the intestinal epithelium, which depends on proliferation and differentiation of the progenitor cells located at the base of the crypts. The coordination of several signalling pathways, including Wnt and Notch, plays an essential role in epithelial regeneration.4–10
The Notch signalling pathway is mediated by Notch proteins, which act as receptors for the transmembrane ligands Jagged (Jag) and Delta-like (Dll) proteins. Upon binding to their ligands, Notch receptors are cleaved by secretase and the Notch intracellular domain translocates to the nucleus, where it up-regulates the expression of specific target genes, such as hairy and enhancer split-1 (HES1). This gene, in turn, represses the expression of Math1, a master regulator of secretory cell lineage differentiation.6,7 It was initially reported that deletion of the HES1 gene resulted in the generation of an excessive number of secretory cells.11 Later studies demonstrated that inactivation of Notch signalling results in conversion of proliferating progenitors into post-mitotic goblet cells,12 which led to the assumption that Notch signalling plays an essential role in regulating cell-fate decisions in intestinal homeostasis. However, there is still controversy regarding the regulation of Notch signalling in CD. An increase in Math1 mRNA expression has been reported in the damaged mucosa of CD patients13 while increased cleavage of Notch-1, which is the upstream signal regulating HES1 expression, has also been described.14
Macrophages constitute one of the central components of the inflamed mucosa, where local hypoxia and inflammatory mediators modulate their gene expression through the activity of hypoxia-inducible factors (HIFs).15,16 Several macrophage phenotypes have been characterized, and differ in the expression of surface proteins and the production of cytokines.17 The M1 or pro-inflammatory phenotype mediates the defence of the host from microorganisms and contributes to inflammatory injury. There is evidence in the literature of a role for the transcription factor HIF-1 in M1 polarization,18 and several studies report the up-regulation of Notch receptors and Notch signalling in classical macrophage differentiation.19–21 The M2 macrophage phenotype expresses high levels of anti-inflammatory cytokines and coordinates tissue repair.22,23 It has recently been reported that inhibition of Notch signalling enhances M2 polarization.20 In the present study we analysed the role of HIF in the expression of Notch ligands in macrophages. In addition, taking into account the strategic position of macrophages in maintaining communication with epithelial cells, we explored the relevance of macrophages in the regulation of Notch signalling and regeneration of the mucosa of CD patients.
2. Materials and methods
2.1. Intestinal mucosal samples
Colonic surgical resections were obtained from the damaged mucosa of CD patients and from the healthy mucosa of patients with colorectal cancer (as controls) (Table 1). The study was approved by the Institutional Review Board of The Hospital of Manises (Valencia). Written informed consent was obtained from all participating patients.
Table 1.
Patient characteristics.
| Patient group and characteristic | n |
|---|---|
| Crohn’s disease | 16 |
| Age (y) | |
| 17–40 | 6 |
| >40 | 10 |
| Gender | |
| Male | 9 |
| Female | 7 |
| Concomitant medication | |
| Azathioprine | 3 |
| Anti-tumour necrosis factor | 16 |
| Mesalazine | 2 |
| Control | 11 |
| Age (y) | |
| 17–40 | 0 |
| >40 | 11 |
| Gender | |
| Male | 7 |
| Female | 4 |
2.2. Isolation of colonic crypts
Human intestinal crypts were isolated from the mucosa of surgical resections obtained from control and CD patients, as described previously.24
2.3. Isolation of macrophages from human intestine
Macrophages were isolated from the mucosa of surgical resections obtained from control and CD patients as described previously.24
2.4. Cell culture
Caco-2 cells (American Type Culture Collection, VA) were cultured in MEM medium (Sigma-Aldrich) supplemented with 20% inactivated foetal bovine serum (FBS) with 100 Um/L penicillin, 100mg/mL streptomycin, 2mM l-glutamine (Lonza), 100mM sodium pyruvate (Lonza) and 1% non-essential amino acids (Lonza).
HT29 cells (American Type Culture Collection, VA, USA) were cultured in McCoy’s Medium Modified (Sigma-Aldrich) supplemented with 10% inactivated FBS, 100U/mL penicillin, 100 μg/mL streptomycin and 2mM l-glutamine.
Human monocytes (U937, European Collection of Cell Culture, Salisbury, UK) were cultured in RPMI medium with 10% inactivated FBS with 100U/mL penicillin and 100 μg/mL streptomycin. Monocytes were differentiated into macrophages by culturing them in the presence of phorbol myristate acetate (PMA) for 48h.25 U937-derived macrophages were stimulated with lipopolysaccharide (LPS; 0.1 µg/mL; E. coli 0111:B4) and interferon γ (IFN-γ; 20ng/mL) or with interleukin 4 (IL-4; 20ng/mL) in order to polarize them towards M1 or M2 phenotypes, respectively, as previously reported.26
Hypoxia (3% O2) was established by incubating macrophages in a CO2/O2 incubator (Invivo2 400, Ruskinn Technology Ltd, Pencoed, UK) with a blend of 5% CO2 and the appropriate percentages of O2 and N2 to a total of 100%. Normoxic controls were obtained by incubating the cells at 21% O2.
2.5. Isolation of mononuclear cells
Human peripheral blood mononuclear cells were isolated from both healthy donors and CD patients by Ficoll density-gradient centrifugation at 400g for 40 minutes. Monocyte-derived macrophages were obtained from monocytes seeded in 12-well tissue culture plates and differentiated into macrophages by culture in X-Vivo 15 medium (Lonza) supplemented with 1% human serum, 100U/mL penicillin, 100 μg/mL streptomycin and 20ng/mL recombinant human macrophage colony-stimulating factor (M-CSF, Peprotech, London, UK) at 37ºC in 5% CO2 for 6 days.
2.6. Co-culture
U937-derived macrophages were seeded and differentiated as above. Afterwards the epithelial cells were placed in the same wells at a ratio 1:1 and were maintained in co-culture for 24 hours.
2.7. Alkaline phosphatase activity
Following 24 hours of co-culture with macrophages, cells were washed with cold phosphate-buffered saline (PBS) and lysed in 150 μL of 0.5% Triton X-100, 10mM Tris–HCl (pH 8) and 150mM NaCl. Each sample was mixed with a p-nitrophenyl phosphate solution (Sigma-Aldrich). Thirty minutes later, absorbance at 405nm was measured. Protein content was quantified using the Bradford assay (Bio-Rad Laboratories, Madrid, Spain). Alkaline phosphatase activity was also determined in macrophages cultured alone.
2.8. Immunohistochemistry, immunofluorescence and goblet cell count
Immunohistochemistry for HES1, CD68, CD86 and CD206 cells was performed in 5 µm sections of paraffin-embedded tissues (Table 2). A horse anti-mouse/rabbit biotinylated antibody (Vector Laboratories, CA, USA, 1:200) was used as a secondary antibody as previously described.26 An area of 0.3mm2 was selected for quantitative analysis.
Table 2.
Specific antibodies used for immunohistochemistry, immunofluorescence studies and Western blot analysis.
| Antibody | Immunofluorescence | Immunohistochemistry | Western blot |
|---|---|---|---|
| Antibody dilution | Antigen retrieval | Antibody dilution | |
| IAP (Santa Cruz Biotechnology) | 1:1000 | ||
| HES1 (Santa Cruz Biotechnology) | 1:100 | Sodium citrate buffer pH 6°, 20 min, 1:200 | 1:500 |
| Jag1 (Santa Cruz Biotechnology) | 1:100 | Sodium citrate buffer pH 9°, 20 min, 1:200 | |
| CD18 (BD, Barcelona Spain) | 1:100 | ||
| CD68 (Biolegend, Madrid, Spain) | 1:100 | α-Chymotrypsin 37°C, 20 min, 1:100 | |
| CD86 (Epitomics, Burlingame, CA, USA) | 1:100 | α-Chymotrypsin 37°C, 20 min, 1:200 | |
| CD206 (Novus Biologicals, Cambridge, UK) | 1:100 | Sodium citrate buffer pH 9°, 20 min, 1:200 | |
| Arginase I (Santa Cruz Biotechnology) | 1:100 | ||
| iNOs (Santa Cruz Biotechnology) | 1:100 | ||
| HIF-1α (Novus Biologicals) | 1:100 | 1:500 | |
| HIF-2α (Santa Cruz Biotechnology) | 1:100 | ||
| Muc2 (Santa Cruz Biotechnology) | 1:100 | ||
| β-actin (Sigma-Aldrich) | 1:10000 |
Goblet cells were counted following standard periodic acid–Schiff staining of the sections adjacent to those used for immunostaining. We counted the number of goblet cells (by counting the vacuoles) in at least three crypts per sample and results were normalized to the total number of epithelial cells (by counting the nuclei) in the same crypt.
2.9. Static cytometry
Macrophages isolated from the mucosa of surgical resections obtained from control and CD patients were fixed with 2% paraformaldehyde, permeabilized with 0.1% Triton-X100 and double-stained (Jag1/CD86, Jag1/CD206, HIF1α/CD86, HIF1α/CD206, HIF2α/CD86, HIF2α/CD206, CD86/CD68, CD206/CD68, arginase I/CD68, iNOs/CD68) with specific monoclonal antibodies (Table 2) as previously described.24 Fluorescence-labelled (Texas red [TR] and Fluorescein isothiocyanate [FITC]) goat anti-mouse or goat anti-rabbit (1:100, Santa Cruz Biotechnology) was used as the secondary antibody, and Hoechst 33342 was added to stain the nuclei.
U937 macrophages co-cultured with epithelial cells were incubated overnight at 4ºC with monoclonal antibodies against HES1 or Muc2 combined with an antibody against CD18 to identify and exclude macrophages from the cytometric analysis (Table 2). Fluorescence-labelled (TR and FITC) goat anti-mouse or goat anti-rabbit (1:100, Santa Cruz Biotechnology) were used as the secondary antibodies, and Hoechst 33342 was added to stain the nuclei. In all cases the fluorescent signal (16 images per well) was quantified using the static cytometer software Scan® version 2.03.2 (Olympus, Barcelona, Spain).
2.10. RNA interference and cellular transfection
U937 cells were transfected with a vector targeting human HIF-1α (miHIF-1α, described previously27) or a non-targeting control vector (mock), as described previously.27 In addition, we have now designed vectors targeting human Jag1 (miJag1; 28.82±15.70% of reduction vs mock, based on the targeting sequence 5ʹ-CCTAAGCATGGGTCTTGCAAA-3ʹ; GenBank accession number NM_000214.2) and Dll4 (miDll4; 27.74±13.39% of reduction vs mock, based on the targeting sequence 5ʹ-TCCAACTGCCCTTCAATTTCA-3ʹ; GenBank accession number NM_019074.3). Lipofectamine-2000 (Invitrogen Life Technologies, Carlsbad, CA) was employed as a transfection reagent according to the manufacturer’s instructions. Twenty-four hours post-transfection, cells were incubated for 8h in normoxic or hypoxic conditions, as described above. M1 macrophages were transfected with miHIF-1α, miJag1, miDll4 or a mock vector before M1 polarization.
2.11. Protein extraction and Western blot analysis
Equal amounts of protein from macrophages, HT29 cells, Caco-2 cells or colonic tissue28 were loaded onto sodium dodecyl sulphate/polyacrylamide gel electrophoresis gels and analysed by Western blot as described previously (Table 2). Protein expression was quantified by means of densitometry using Image Gauge Version 4.0 software (Fujifilm). Data were normalized to β-actin.
2.12. RNA extraction and quantitative reverse transcription–polymerase chain reaction (qRT-PCR) analysis
Total RNA and cDNA from macrophages or colonic tissue was obtained as described previously.25 Real-time PCR was performed with the PrimeScript Reagent Kit Perfect Real Time (Takara) in a thermocycler (LightCycler, Roche Diagnostics). Specific oligonucleotides were designed according to the sequences shown in Table 3.
Table 3.
Primer sequences of specific PCR products for each gene analysed.
| Human gene | Sense | Antisense | Length (bp) |
|---|---|---|---|
| Jag1 | 5ʹ-gaacacgggcgttgcccact-3ʹ | 5ʹ-gtggacgcatcccgggtgtg-3ʹ | 304 |
| Dll4 | 5ʹ-gtgcagcgtacaccggcact-3ʹ | 5ʹ- tctgttcgcgacgccgcttt-3ʹ | 223 |
| HES1 | 5ʹ-aaaattcctcgtccccggtg-3ʹ | 5ʹ-tttgt tatccgttcg-3ʹ | 64 |
| Muc2 | 5ʹ-gctggccgccggctattacc-3ʹ | 5ʹ-accccggccgtcatccatca-3ʹ | 79 |
| Math1 | 5ʹ-ccgcccagtatttgctacat-3ʹ | 5ʹ-cattcacctgtttgctggaa-3ʹ | 234 |
| β-actin | 5ʹ-ggacttcgagcaagagatgg-3ʹ | 5ʹ-agcactgtgttggcgtacag-3ʹ | 67 |
2.13. Chromatin immunoprecipitation (ChIP) assay
A ChIP assay was carried out in U937-derived macrophages, incubated under hypoxia or normoxia for 5h, as previously described.25 Immunoprecipitation was performed with anti-HIF1α antibody (BD, Madrid, Spain) or control IgG antibody. After reverse crosslinking, DNA fragments were purified with a Montage PCR Kit (Millipore, Germany). PCR was performed using PCR Master (Roche Diagnostics, Mannheim, Germany) with the primers 5ʹ-TGTCCACCCTTCAAAGGAAGTC-3ʹ and 5ʹ-CAAATCCGAGTCTGCGGAGC-3ʹ, detecting the region −1646 to −1166 in the Jag1 promoter, or 5ʹ-CCCTGAGCATCCCGCTG-3ʹ and 5ʹ-CCGGCTCTAATATACTCCGCC-3ʹ, detecting the region −638 to −106 in the Jag1 promoter, as shown in Figure 1c. The PCR products were separated by electrophoresis in 2% agarose gel.
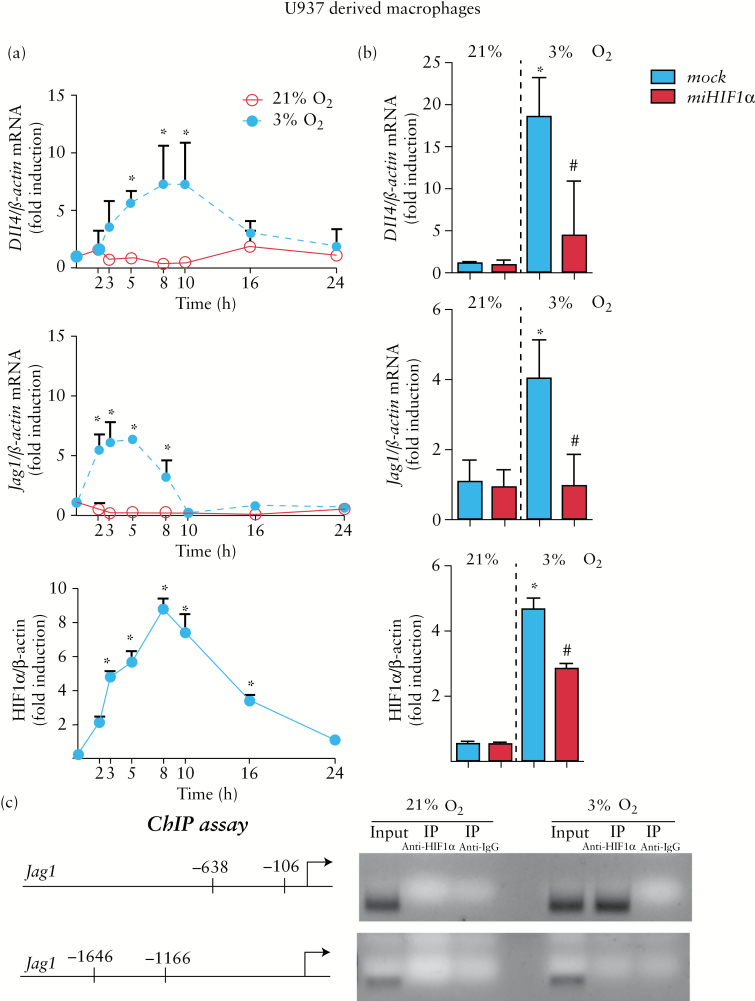
Figure 1.
HIF-1 mediates the hypoxic up-regulation of Dll4 and Jag1 in macrophages. (a) Graphs showing time-course analysis of the effects of hypoxia on HIF-1α protein levels and mRNA expression of Jag1 and Dll4 in U937 macrophages. In all cases, points in the graphs represent mean ± SEM (n > 3). *p < 0.05 vs time 0h or vs macrophages in normoxia at the same time point. (b) Graphs showing mRNA expression of Dll4 and Jag1 and protein levels of HIF-1α in mock-transfected U937 cells and cells treated with miHIF-1α under normoxic or hypoxic conditions (8h). In all cases, bars in the graphs represent mean ± SEM (n > 3). *p < 0.05 vs the same group in normoxia; # p < 0.05 vs mock-transfected cells in hypoxia. (c) Representative chromatin immunoprecipitation (ChIP) experiment performed in samples from U937-derived macrophages in normoxia or hypoxia. Chromatin was immunoprecipitated with anti-HIF-1α antibody or a non-related antibody anti-IgG as a control. An aliquot of the input chromatin is also shown. Primers specific to the promoter region of the Jag1 gene were used to amplify the DNA isolated from the ChIP assay.
2.14. Statistical analysis
Data were expressed as mean ± SEM and compared by one-way analysis of variance (ANOVA) with Newman–Keuls post hoc correction for multiple comparisons or a t-test when appropriate. A p value <0.05 was considered to be statistically significant. Clinical correlations were analysed in the human samples using Pearson’s correlation coefficient.
3. Results
3.1. HIF-1 mediates the expression of Notch ligands in M1 macrophages
Hypoxia induced a time-dependent increase in HIF-1α stabilization in macrophages, which peaked at 8 hours and then progressively decreased. In parallel, hypoxia induced a time-dependent increase in the mRNA expression of Jag1 and Dll4 compared with the expression detected in cells in normoxia (Figure 1a). To evaluate the role of HIF-1 in gene expression, we used an micro RNA (miRNA) approach to selectively knockdown this transcription factor in U937-derived macrophages. As shown in Figure 1b, up-regulation of the mRNA expression of both Dll4 and Jag1 induced by hypoxia was significantly reduced in cells transfected with miHIF1α, showing that HIF-1 is involved in the induction of these ligands in hypoxia.
Analysis of the Jag1 gene promoter identified potential HIF-1 binding sites (HRE sequence). To examine the binding of HIF-1α to the promoter region of Jag1, we performed ChIP assays using an affinity-purified antibody directed against HIF-1α and primers specific for two Jag1 promoter regions containing HIF-1 binding sites (Figure 1c). Our data revealed HIF-1α binding to the proximal promoter region of the Jag1 gene in hypoxia through the HRE sequences located between positions −106 and −638 (Figure 1c) from the start codon.
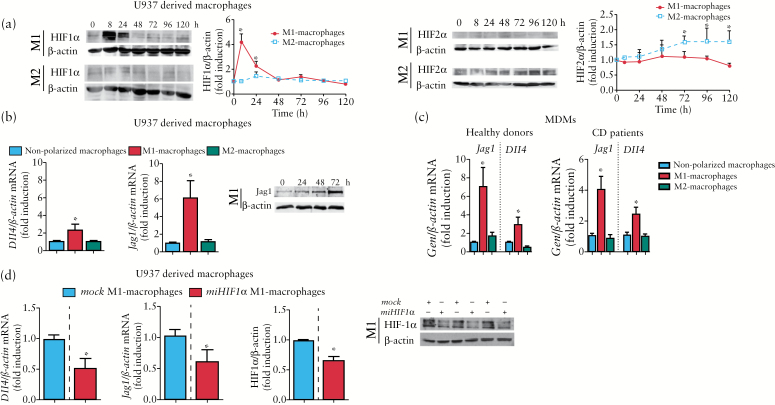
Polarization of U937 macrophages towards an M1 phenotype26 following treatment with LPS + IFN induced HIF-1α stabilization within the first 24 hours and failed to induce HIF-2α stabilization significantly at any time analysed (Figure 2a). In contrast, polarization towards an M2 phenotype26 as a result of treatment with IL-4 induced HIF-2α stabilization but not HIF-1α stabilization (Figure 2a). Analysis of the expression of HIF-1 target genes revealed a significant increase (fold induction) in the mRNA expression of LDHA (2.2±0.4) and iNOS (6.0±1.7) by M1 macrophages compared with both non-polarized (1.0±0.2 and 0.9±0.1, respectively) and M2 (1.2±0.3 and 2.4±1.3, respectively) macrophages. In addition, the expression of an HIF-2 target gene, ArgI, was increased in M2 macrophages (2.7±0.7) compared with non-polarized (1.0±0.1) and M1 (0.8±0.1) cells. Analysis of the expression of Notch ligands revealed a significant increase in mRNA expression of Dll4 and mRNA expression and protein levels of Jag1 in M1 macrophages but not in M2 cells (Figure 2b). These effects were also observed in macrophages derived from primary monocytes obtained from both healthy donors and CD patients (Figure 2c). The up-regulation of the mRNA expression of both Dll4 and Jag1 that was detected in U-937 macrophages polarized towards an M1 phenotype was significantly reduced in cells transfected with miHIF1α (Figure 2d), demonstrating that HIF-1 is involved in the induction of Notch ligands in M1 macrophages.
Figure 2.
HIF-1 mediates the expression of Notch ligands associated with M1 macrophages. U937-derived macrophages (n = 6) were either treated with lipopolysaccharide (LPS) and interferon-γ (IFN-γ) and polarized towards M1 macrophages or treated with interleukin 4 (IL-4) and polarized towards M2 macrophages; some cells were treated with the vehicle (non-polarized macrophages). (a) Representative Western blots and graph showing HIF-1α or HIF-2α protein levels at different time points. Points in the graphs represent mean ± SEM (n > 3). *p < 0.05 vs time 0h. (b) Graphs show relative mRNA expression levels of Dll4 and Jag1 in macrophages and protein levels of Jag1 in M1 macrophages at different time points. Bars represent mean ± SEM. *p < 0.05 vs the respective value in non-polarized macrophages and M2 macrophages. (c). Graphs showing relative mRNA expression levels of Dll4 and Jag1 in macrophages derived from primary monocytes (healthy donors, n = 6; Crohn’s disease patients, n = 6). Bars represent mean ± SEM. *p < 0.05 vs the respective value in non-polarized macrophages and M2 macrophages. (d) Representative Western blots and graphs showing protein levels of HIF-1α and mRNA expression of Dll4 and Jag1 in U937-derived macrophages transfected with mock or miHIF-1α and polarized towards M1. Bars represent mean ± SEM. *p < 0.05 vs mock M1 macrophages.
3.2. HIF-1-dependent induction of Notch ligands mediates the increase in HES1 expression and IAP activity induced by M1 macrophages
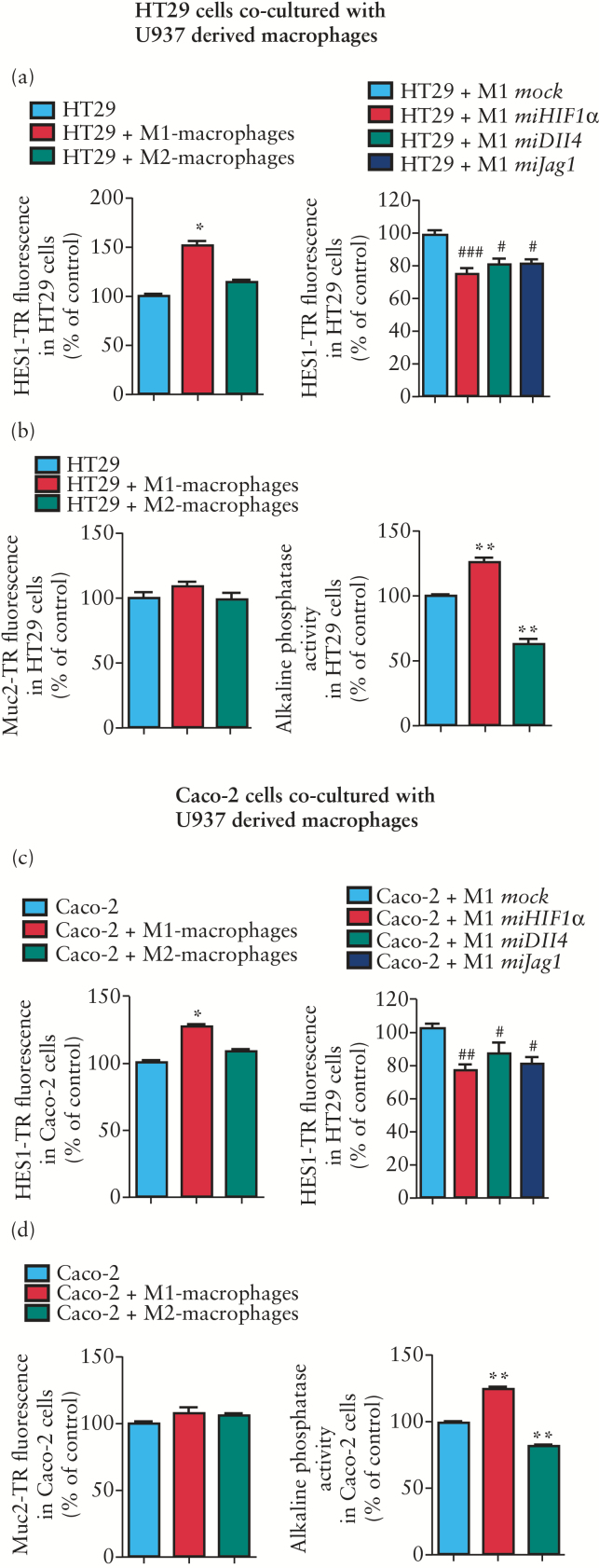
Next we analysed whether macrophages modulate the Notch signalling pathway and markers of differentiation in co-cultured epithelial cells. First, we determined the expression of HES1 and IAP (a marker of enterocyte differentiation) protein levels in two epithelial cell lines, HT29 and Caco-2, at sub-confluence and at different times after reaching cell confluence. Our data show a time-dependent increase in protein levels of both HES1 and IAP as well as IAP enzymatic activity in both HT29 and Caco-2 cells by culturing post-confluence (Supplementary Figure 1).
In the co-culture experiments, M1 macrophages increased protein levels of HES1 and IAP enzymatic activity with no effect on Muc2 expression in either HT29 or Caco-2 cells (Figure 3a–d). The effects induced by M1 macrophages on epithelial protein levels of HES1 were significantly reduced in macrophages treated with miHIF1, miDll4 and miJag1, suggesting that the HIF-1-dependent induction of Notch ligands mediates the activation of Notch signalling in epithelial cells (Figure 3a, c). In contrast, M2 macrophages did not significantly modify HES1 protein levels but induced a significant reduction in IAP activity in both HT29 and Caco-2 cells (Figure 3a–d). No IAP activity was detected in macrophages.
Figure 3.
M1 macrophages activate HES1 expression and markers of differentiation in epithelial cells. HT29 cells (a) or Caco-2 cells (b) at pre-confluence were co-cultured (24h) with M1 or M2 macrophages (stained with CD18 and fluorescein isothiocyanate). In some cases macrophages were transfected with mock, miHIF1α, miDll4 or miJag1 vectors before M1 polarization. Levels of HES1 staining (TR) or Muc2 staining (TR) in epithelial cells were determined by static cytometry (n = 6). Graphs show a significant increase in the expression of epithelial HES1 induced by M1 but not M2 macrophages compared with that detected in epithelial cells cultured alone. M1-miHIF1α, M1-miDll4 and M1-miJag1 macrophages significantly reduced HES1 expression in either HT29 or Caco-2 cells. M1 or M2 macrophages failed to significantly modify the expression of Muc2 in either HT29 or Caco-2 cells. IAP enzymatic activity in epithelial cells was significantly increased by M1 macrophages and significantly reduced by M2 macrophages. In all cases bars represent mean ± SEM. *p < 0.05 and **p < 0.01 vs epithelial cells; # p < 0.05 and ### p < 0.001 vs epithelial cells co-cultured with M1-mock macrophages.
3.3. M1 macrophages express HIF-1α and Notch ligands while M2 macrophages express HIF-2α in the mucosa of CD patients
Next we performed a comparative study of control and CD patients to characterize the macrophage phenotype present in the mucosa of CD patients and the expression of HIF and Notch ligands in these cells.
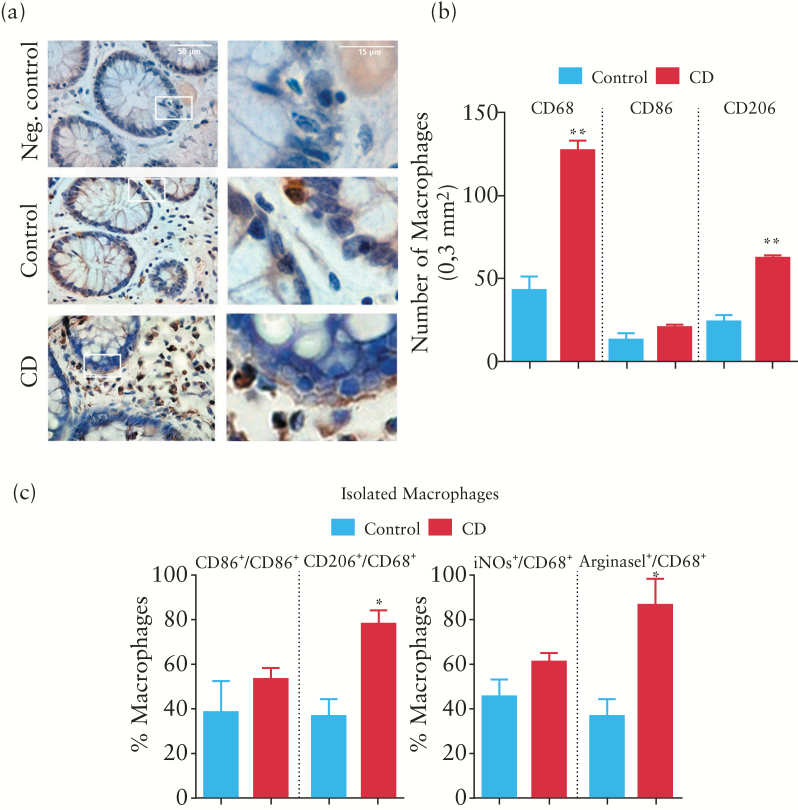
Immunohistochemical experiments revealed macrophages in an adjacent position to epithelial cells (Figure 4a) and a quantitative analysis showed that the numbers of CD68+ cells and CD206+ cells were significantly higher in the mucosa of chronic CD patients than in that of control patients. In contrast, no significant differences were observed in the number of CD86+ cells (Figure 4b). Double immunofluorescence experiments in macrophages isolated from the mucosa revealed that the percentage of CD68-positive cells that expressed M1 markers CD86 and iNOS was similar in CD and control patients. In contrast, the percentage of CD68-positive cells that expressed M2 markers CD206 and ArgI was significantly higher in macrophages isolated from the mucosa of CD patients (Figure 4c).
Figure 4.
Characterization of the macrophage phenotype in the mucosa of Crohn’s disease (CD) patients. (a) Representative images showing CD68 immunostaining in lamina propria and in close proximity to epithelial cells in the mucosa of control and CD patients. (b) Graph showing quantitative analysis of CD68+, CD86+ and CD206+ cells in a total area of 0.3mm2. Bars represent mean ± SEM. **p < 0.01 vs control mucosa. (c) Graphs showing the percentages of CD86+/CD68+ cells, CD206+/CD68+ cells, iNOS+/CD68+ cells and arginase I (ArgI)+/CD68+ cells in isolated macrophages obtained from the mucosa (static cytometry). Bars represent mean ± SEM. *p < 0.01 vs macrophages from control mucosa.
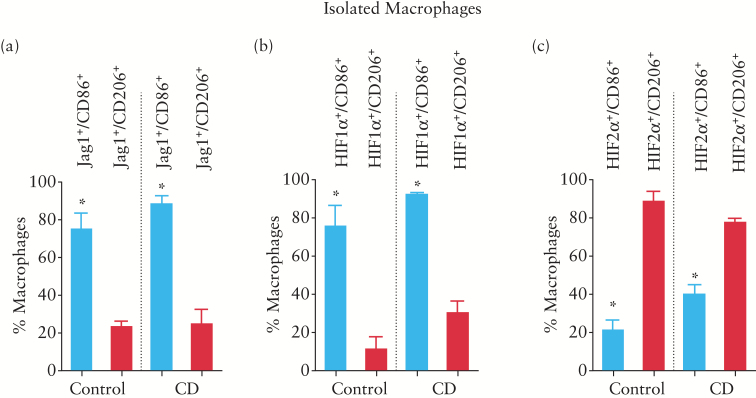
We also detected a high percentage of CD86+ cells expressing HIF-1α in macrophages isolated from both control and CD patients, while a very low percentage of CD206+ cells expressed HIF-1α (Figure 5b). In contrast, a large percentage of CD206+ cells from the mucosa of both control and CD patients expressed HIF-2α while a low percentage of CD86+ cells expressed HIF-2α (Figure 5c). Finally, the percentage of cells expressing the Notch ligand Jag1 in the population of CD86+ cells was higher than that recorded in cells expressing CD206 (Figure 5a) and these values were similar between macrophages obtained from control and CD patients (Figure 5a).
Figure 5.
CD86+ macrophages from human mucosa express HIF1α and Jag1. Quantitative analysis (static cytometry) was performed in macrophages isolated from the mucosa and the graph shows the percentages of CD86+ and CD206+ cells that expressed Jag1 (a), HIF-1α (b) or HIF-2α (c). Bars represent mean ± SEM. *p < 0.05 vs CD206+ cells expressing the respective marker in the same group of patients.
3.4. Macrophages modulate Notch signalling and markers of differentiation in human intestine in a phenotype-dependent manner
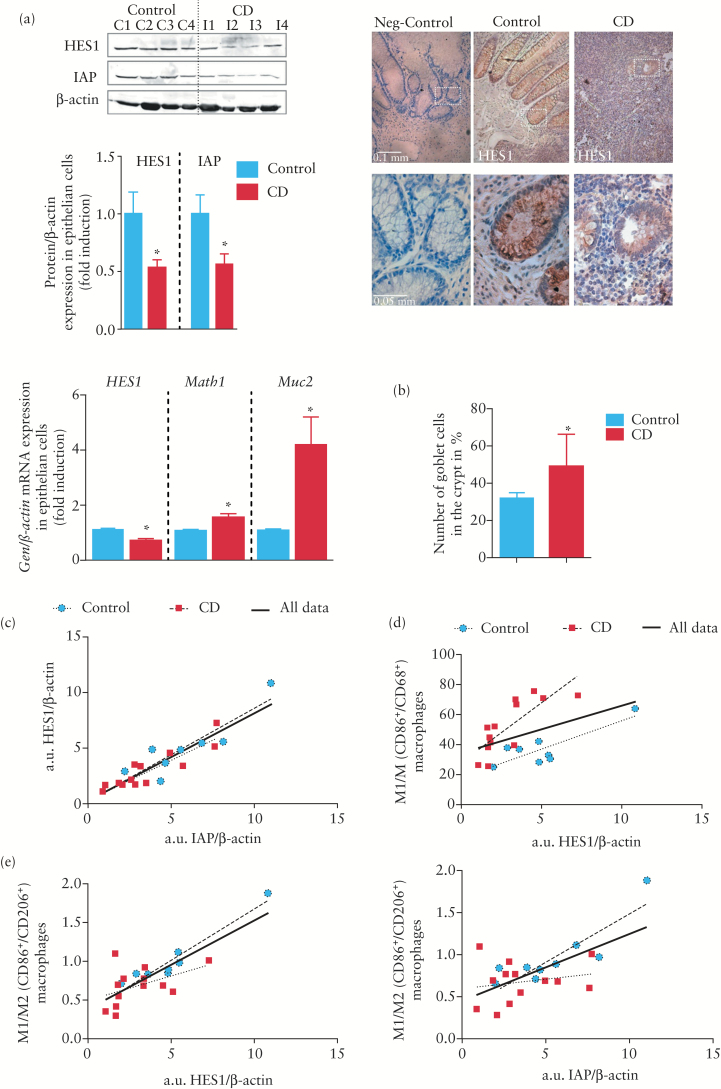
To determine whether macrophages in the mucosa modulate Notch signalling in epithelial cells, we analysed this pathway specifically in crypts isolated from the mucosa of control and CD patients. Results revealed low HES1 immunostaining and decreased mRNA and protein HES1 expression in the mucosa of CD patients compared with controls (Figure 6a). In addition, we also detected enhanced Math1 mRNA expression, increased Muc2 mRNA expression, a higher percentage of goblet cells per crypt and decreased IAP protein levels in the mucosa of chronic CD patients compared with control mucosa (Figure 6a, b), suggesting a diminution of the Notch signalling pathway and impaired differentiation associated with CD. To study a possible regulatory link between M1 macrophages and Notch signalling, the relationship between HES1 protein levels detected by western blot and the proportion of CD86+/CD68+ macrophages was analysed and a positive correlation coefficient (r = 0.4631, p = 0.045) (Figure 6d) was obtained. A detailed analysis revealed a different distribution of points marked by the presence of CD, and when data were analysed separately the correlation coefficient was closer for both controls (r = 0.89, p = 0.001) and CD patients (r = 0.79, p = 0.001). This suggests that other factors were regulating HES1 in the mucosa. Interestingly, a better correlation coefficient (r = 0.804, p < 0.001) was obtained between HES1 protein levels and the ratio of CD86+ (M1) to CD206+ (M2) macrophages (Figure 6e). The M1/M2 ratio also exhibited a positive and significant correlation with IAP protein levels (r = 0.66, p = 0.002) (Figure 6e), which suggests that both M1 and M2 macrophages regulate Notch signalling in the mucosa.
Figure 6.
The M1/M2 ratio correlates with HES1 and IAP protein levels in human intestinal mucosa. (a) Representative Western blots showing HES1 and IAP protein levels in the mucosa of Crohn’s disease (CD) and control patients and photographs showing HES1 immunostaining. Graphs show quantification of HES1 and IAP protein levels and the mRNA expression of HES1, Math1, and Muc2 in intestinal crypts isolated from the mucosa of control and CD patients. (b) Quantitative analysis showing the percentage of goblet cell vs nuclei per crypt in the mucosa (three crypts were analysed per sample). In all cases bars in graphs represent mean ± SEM. Significant difference from control mucosa is shown by *p < 0.05. (c) A positive and significant correlation was observed between HES1 and IAP protein levels in the mucosa of CD and control patients. (d) A positive and significant correlation was observed between CD86+/CD68+ cells and HES1 protein levels in the mucosa of CD and control patients. (e) A closer correlation was established between the CD86+/CD206+ ratio and HES1 protein levels as well as the CD86+/CD206+ ratio and IAP protein levels.
4. Discussion
The present study demonstrates that M1 macrophages, but not M2 macrophages, are associated with HIF-1-dependent induction of Jag1 and Dll4, which increases HES1 protein levels and IAP activity in co-cultured epithelial cells. In the mucosa of chronic CD patients, the M1/M2 macrophage ratio closely correlates with Notch signalling and markers of enterocyte differentiation, suggesting that macrophages play a role in the diminished Notch signalling and impaired enterocyte differentiation observed.
Our data show that HIF-1, a transcription factor induced by hypoxia and inflammatory conditions, mediates the expression of Dll4 and Jag1 in hypoxic macrophages. A previous study reported activation of the Dll4 promoter by HIF-1 in endothelial cells.29 We demonstrate for the first time the activation of the Jag1 promoter by HIF-1 and provide further evidence that HIF-1 regulates the expression of Notch ligands. Emerging evidence suggests that the functional phenotype of macrophages is regulated by transcription factors that define alternative activation.18 We found HIF-1α stabilization in human macrophages polarized towards an M1 phenotype and HIF-2α stabilization in those that had been polarized towards an M2 phenotype. Of interest, our data associate for the first time M1, but not M2, macrophages with HIF-1-dependent increases in the mRNA expression of Dll4 and Jag1, which suggests that this transcription factor mediates the selective Notch ligand expression that characterizes the macrophage phenotype. These effects have also been observed in polarized macrophages derived from primary monocytes obtained from both healthy subjects and CD patients, supporting the idea of preferential expression of Notch ligands by the M1 phenotype. The pattern of Notch ligand expression is functionally relevant since M1, and not M2, macrophages increased the expression of the main target gene of the canonical Notch signalling, HES1, in epithelial cells in co-culture through an action mediated by the HIF-1-dependent induction of Jag1 and Dll4. This was observed in two epithelial cell lines capable of expressing differentiation features characteristic of mature intestinal cells, such as enterocytes or mucus cells.30,31 In line with this, the increase in HES1 induced by M1 macrophages was paralleled by an increase in IAP activity, a well-known marker of enterocyte differentiation,32 with no changes in Muc2 expression. Considering that our results show that spontaneous differentiation of these cells is associated with a time-dependent increase in both IAP activity and HES1 protein level, our results strongly suggest that M1 macrophages promote enterocyte differentiation in epithelial cells. Previous studies have shown that Jag1 up-regulates alkaline phosphatase in stem cells,33 which leads us to propose that M1 macrophages activate the Notch signalling pathway and enterocyte differentiation in epithelial cells through the expression of Dll4 and Jag1.
We analysed the pathophysiological relevance of these observations in the mucosa of CD patients, in which we found an increased number of macrophages compared with that of control patients. The expression of both M1 and M2 markers was detected but, in a similar manner to that previously reported in the mucosa of ulcerative colitis patients,26 the number of M2 macrophages was higher than the number of M1 macrophages. Of interest, a high percentage of M1 macrophages were positive for HIF-1α and Jag1, reinforcing the observations reported in vitro and suggesting that the expression of Notch ligands by M1 macrophages in human intestine is also associated with HIF-1. Of particular interest, macrophages were frequently detected in an adjacent position to epithelial cells and we observed a positive and significant correlation between CD86+ cells and HES1 protein levels in crypts isolated from the mucosa, which strongly supports the idea that M1 macrophages activate Notch signalling pathways in epithelial cells. A detailed analysis of this correlation revealed differences in the distribution of data marked by the presence or absence of CD; a higher number of macrophages and lower protein levels of epithelial HES1 were detected in the mucosa of CD patients compared with control patients. These observations led us to suggest that, in addition to M1 macrophages, HES1 expression was modulated by other factors present in the inflamed mucosa. Considering our data showing that M2 macrophages prevail in the mucosa of CD patients and that most of them express HIF-2α, which has been related to Notch signalling inhibition,34 the results suggest that M2 macrophages may also be modulating the Notch pathway. Reinforcing this observation, we have previously demonstrated that M2 macrophages activate Wnt signalling in epithelial cells,26 and this pathway has been widely associated with inhibition of Notch signalling.35–37 In line with this, our data show a very good correlation between HES1 protein levels and the ratio of M1/M2 macrophages, and we propose that M2 macrophages act in an opposite manner to M1 cells in the modulation of Notch signalling.
The Notch pathway governs the intestinal binary cell-fate decision between the secretory and absorptive cell lineages38. Our results reveal diminished HES1 expression in crypts isolated from the mucosa of CD patients in parallel with enhanced expression of Math1, a transcription factor that is repressed by HES1, strongly suggesting that the Notch signalling pathway was impaired.13 It has been reported that the up-regulation of Math 1 directs epithelial cell fate towards secretory lineage cells, including goblet cells.7,39 Our data demonstrate increased mRNA expression of Muc2, a marker of goblet cells, and a higher number of goblet cells per crypt, in parallel with decreased IAP protein levels in the mucosa of CD patients compared with controls, suggesting that enterocyte differentiation is specifically impaired. Previous studies have reported diminished IAP mRNA and protein expression40,41 in the intestinal mucosa of adults and children with CD. We extend these observations and show that these diminished IAP protein levels correlate with diminished HES1 protein levels, which leads us to propose that enterocyte differentiation is impaired in CD as a consequence of an undermined Notch signalling pathway. This hypothesis is backed by the fact that macrophages, which were closely correlated with HES1 protein levels, were also correlated with the expression of the enterocyte marker IAP.
As a whole, our results provide evidence of HIF-1 dependent induction of Notch ligands associated with M1 macrophages. In contrast to M2 macrophages, M1 cells activate the Notch signalling pathway in epithelial cells. The prevalence of M2 over M1 macrophages in the mucosa of chronic CD patients may mediate the diminished enterocyte differentiation and impaired mucosal regeneration observed in these patients. A better understanding of the reciprocal regulation of macrophage phenotype and mucosal repair following intestinal damage will help to establish new approaches to CD therapy.
Funding
This work was supported by Ministerio de Ciencia e Innovación (grant number SAF2013-43441P), CIBERehd (grant number CB06/04/0071) and Generalitat Valenciana (grant number PROMETEOII/2014/0635). Jesús Cosín-Roger is supported by FPU fellowships from Ministerio de Educación, Cultura y Deporte. Carlos Hernández acknowledges support from the ‘Ramon y Cajal’ programme of the Spanish Government.
Conflict of Interest
None declared.
Author Contributions
All authors have made substantial contributions to the article. DO, JC, SC, CH and MDB: conception and design of the study, acquisition of data and/or analysis and interpretation of data. JH, RA, JVE and MDB: drafting the article or revising it critically for important intellectual content. All authors: final approval of the version to be submitted.
Supplementary Data
Supplementary data to this article can be found online at ECCO-JCC online.
Acknowledgments
We thank Brian Normanly for his English language editing.
References
- 1. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- 2. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 2012;61:1619–35. [DOI] [PubMed] [Google Scholar]
- 3. Walsh A, Palmer R, Travis S. Mucosal healing as a target of therapy for colonic inflammatory bowel disease and methods to score disease activity. Gastrointest Endosc Clin N Am 2014;24:367–78. [DOI] [PubMed] [Google Scholar]
- 4. Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011;474:298–306. [DOI] [PubMed] [Google Scholar]
- 5. Henderson P, van Limbergen JE, Schwarze J, Wilson DC. Function of the intestinal epithelium and its dysregulation in inflammatory bowel disease. Inflamm Bowel Dis 2011;17:382–95. [DOI] [PubMed] [Google Scholar]
- 6. Obata Y, Takahashi D, Ebisawa M, et al. Epithelial cell-intrinsic Notch signaling plays an essential role in the maintenance of gut immune homeostasis. J Immunol 2012;188:2427–36. [DOI] [PubMed] [Google Scholar]
- 7. Nakamura T, Tsuchiya K, Watanabe M. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J Gastroenterol 2007;42:705–10. [DOI] [PubMed] [Google Scholar]
- 8. Liu L, Rao JN, Zou T, et al. Activation of Wnt3a signaling stimulates intestinal epithelial repair by promoting c-Myc-regulated gene expression. Am J Physiol Cell Physiol 2012;302:C277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koch S, Nava P, Addis C, et al. The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology 2011;141:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taniguchi K, Wu LW, Grivennikov SI, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 2015;519:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jensen J, Pedersen EE, Galante P, et al. Control of endodermal endocrine development by Hes-1. Nat Genet 2000;24:36–44. [DOI] [PubMed] [Google Scholar]
- 12. Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature 2005;435:964–8. [DOI] [PubMed] [Google Scholar]
- 13. Gersemann M, Becker S, Kubler I, et al. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation 2009;77:84–94. [DOI] [PubMed] [Google Scholar]
- 14. Dahan S, Rabinowitz KM, Martin AP, Berin MC, Unkeless JC, Mayer L. Notch-1 signaling regulates intestinal epithelial barrier function, through interaction with CD4+ T cells, in mice and humans. Gastroenterology 2011;140:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glover LE, Colgan SP. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology 2011;140:1748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013;496:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takeda N, O’Dea EL, Doedens A, et al. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev 2010;24:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang YC, He F, Feng F, et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res 2010;70:4840–9. [DOI] [PubMed] [Google Scholar]
- 20. Singla RD, Wang J, Singla DK. Regulation of Notch 1 signaling in THP-1 cells enhances M2 macrophage differentiation. Am J Physiol Heart Circ Physiol 2014;307:H1634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu H, Zhu J, Smith S, Foldi J, et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol 2012;13:642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol 2013;183:1352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol 2013;93:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ortiz-Masia D, Cosin-Roger J, et al. Hypoxic macrophages impair autophagy in epithelial cells through Wnt1: relevance in IBD. Mucosal Immunol 2013;7:929–38. [DOI] [PubMed] [Google Scholar]
- 25. Ortiz-Masia D, Diez I, Calatayud S, et al. Induction of CD36 and thrombospondin-1 in macrophages by hypoxia-inducible factor 1 and its relevance in the inflammatory process. PLoS One 2012;7:e48535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cosin-Roger J, Ortiz-Masia D, Calatayud S, et al. M2 macrophages activate WNT signaling pathway in epithelial cells: relevance in ulcerative colitis. PLoS One 2013;8:e78128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ortiz-Masia D, Hernandez C, Quintana E, et al. iNOS-derived nitric oxide mediates the increase in TFF2 expression associated with gastric damage: role of HIF-1. FASEB J 2010;24:136–45. [DOI] [PubMed] [Google Scholar]
- 28. Riano A, Ortiz-Masia D, Velazquez M, Calatayud S, Esplugues JV, Barrachina MD. Nitric oxide induces HIF-1alpha stabilization and expression of intestinal trefoil factor in the damaged rat jejunum and modulates ulcer healing. J Gastroenterol 2011;46:565–76. [DOI] [PubMed] [Google Scholar]
- 29. Diez H, Fischer A, Winkler A, et al. Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp Cell Res 2007;313:1–9. [DOI] [PubMed] [Google Scholar]
- 30. Cohen E, Ophir I, Shaul YB. Induced differentiation in HT29, a human colon adenocarcinoma cell line. J Cell Sci 1999;112:2657–66. [DOI] [PubMed] [Google Scholar]
- 31. Hodin RA, Meng S, Archer S, Tang R. Cellular growth state differentially regulates enterocyte gene expression in butyrate-treated HT-29 cells. Cell Growth Differ 1996;7:647–53. [PubMed] [Google Scholar]
- 32. Sussman NL, Eliakim R, Rubin D, Perlmutter DH, DeSchryver-Kecskemeti K, Alpers DH. Intestinal alkaline phosphatase is secreted bidirectionally from villous enterocytes. Am J Physiol 1989;257:G14–23. [DOI] [PubMed] [Google Scholar]
- 33. Osathanon T, Nowwarote N, Manokawinchoke J, Pavasant P. bFGF and JAGGED1 regulate alkaline phosphatase expression and mineralization in dental tissue-derived mesenchymal stem cells. J Cell Biochem 2013;114:2551–61. [DOI] [PubMed] [Google Scholar]
- 34. Hu YY, Fu LA, Li SZ, et al. Hif-1alpha and Hif-2alpha differentially regulate Notch signaling through competitive interaction with the intracellular domain of Notch receptors in glioma stem cells. Cancer Lett 2014;349:67–76. [DOI] [PubMed] [Google Scholar]
- 35. Boulter L, Govaere O, Bird TG, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 2012;18:572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fukunaga-Kalabis M, Hristova DM, Wang JX, et al. UV-induced Wnt7a in the human skin microenvironment specifies the fate of neural crest-like cells via suppression of Notch. J Invest Dermatol 2015;135:1521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang M, Chang A, Choi M, Zhou D, Anania FA, Shin CH. Antagonistic interaction between Wnt and Notch activity modulates the regenerative capacity of a zebrafish fibrotic liver model. Hepatology 2014;60:1753–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noah TK, Shroyer NF. Notch in the intestine: regulation of homeostasis and pathogenesis. Annu Rev Physiol 2013;75:263–88. [DOI] [PubMed] [Google Scholar]
- 39. Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 2001;294:2155–8. [DOI] [PubMed] [Google Scholar]
- 40. Tuin A, Poelstra K, de Jager-Krikken A, et al. Role of alkaline phosphatase in colitis in man and rats. Gut 2009;58:379–87. [DOI] [PubMed] [Google Scholar]
- 41. Molnar K, Vannay A, Szebeni B, et al. Intestinal alkaline phosphatase in the colonic mucosa of children with inflammatory bowel disease. World J Gastroenterol 2012;18:3254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]