Abstract
Background and Aims: To investigate the macro- and microstructural changes of bone in patients with inflammatory bowel disease [IBD] and to define the factors associated with bone loss in IBD.
Methods: A total of 148 subjects, 59 with Crohn’s disease [CD], 39 with ulcerative colitis [UC], and 50 healthy controls were assessed for the geometric, volumetric and microstructural properties of bone using high-resolution peripheral quantitative computed tomography. In addition, demographic and disease-specific characteristics of IBD patients were recorded.
Results: IBD patients and controls were comparable in age, sex, and body mass index. Total [p = 0.001], cortical [p < 0.001], and trabecular volumetric bone mineral density [BMD] [p = 0.03] were significantly reduced in IBD patients compared with healthy controls. Geometric and microstructural analysis revealed significantly lower cortical area [p = 0.001] and cortical thickness [p < 0.001] without differences in cortical porosity, pore volume, or pore diameter. CD showed a more severe bone phenotype than UC: cortical bone loss was observed in both diseases, but CD additionally showed profound trabecular bone loss with reduced trabecular BMD [p = 0.008], bone volume [p = 0.008], and trabecular thickness [p = 0.009]. Multivariate regression models identified the diagnosis of CD, female sex, lower body mass index, and the lack of remission as factors independently associated with bone loss in IBD.
Conclusion: IBD patients develop significant cortical bone loss, impairing bone strength. Trabecular bone loss is limited to CD patients, who exhibit a more severe bone phenotype compared with UC patients.
Keywords: Inflammatory bowel disease; bone, osteoporosis, computed tomography
1. Introduction
Inflammatory bowel disease [IBD] is associated with substantial comorbidity. Bone loss appears to be one of the most frequent comorbidities in IBD. Its clinical importance is underscored by increased fracture risk in IBD patients.1,2,3 Bone microstructure in IBD has not been investigated to date. Current knowledge on bone changes in IBD is exclusively based on studies using dual X-ray absorptiometry [DXA], which measures overall bone mineral density [BMD] but not bone structure. Hence, despite its high prevalence and clinical importance, the nature of bone loss in IBD is incompletely defined.
DXA is widely used to measure BMD. This technique is based on a planar measurement of X- ray extinction, which is then expressed as BMD [g/cm2] or as respective T-score [in relation to healthy peak bone mass]. DXA studies suggested a rather high prevalence [22-77%] of low bone mass [osteopenia] in patients with IBD, and osteoporosis was found in 17-41% of the patients.4,5,6 DXA however is not able to assess bone microstructure or bone geometry and also fails to selectively measure bone changes in different compartments, such as the trabecular network or the cortical shell. Furthermore, DXA results are prone to bias; for instance due to bone deposition in the spine or the joints in the context of degenerative or inflammatory rheumatic disease. Hence, it is not surprising that the majority of fragility fractures occur in patients who are osteopenic rather than osteoporotic in the DXA measurements.7,8
It is important to mention that bone is composed of two entirely different compartments, the cancellous trabecular network and the cortical bone shell. Bone strength depends not only on BMD but also on the respective microarchitecture of the cancellous and the cortical bone 9,10. Different non-invasive techniques for three-dimensional assessment of bone have been developed in recentyears in order to reliably assess bone microstructure in humans.11 In this context, high-resolution peripheral quantitative computed tomography [HR-pQCT] is the gold standard, allowing standardised and accurate measurement of bone microstructure at the micrometer level, resembling a virtual bone biopsy.12,13 In addition to the assessment of bone microstructure, HR-pQCT allows defined regional BMD measurements, which have shown to correlate with the BMD results obtained by DXA.14 The value of HR-pQCT in bone analysis is further supported by the fact that HR-pQCT results correlate with incident fracture risk in the radius, the hip, and the spine in postmenopausal women.10,15
To better investigate the nature of bone loss in IBD patients, we applied HR-pQCT bone imaging in a prospectively collected cohort of IBD patients. We characterised cancellous and cortical bone changes as well as bone microstructure and geometry and compared them with findings in a healthy control group, which was analysed in parallel. Furthermore, bone changes were related to demographic and disease-specific characteristics of IBD patients.
2. Methods
2.1. Patients and study design
A total number of 101 patients with IBD [CD and UC] were recruited at the tertiary care outpatient clinics of the Department of Internal Medicine 1 and 3 of the University of Erlangen-Nuremberg. The diagnosis of IBD was histologically verified previously and available in the medical history. The study was approved by the local ethics committee and the national radiation safety agency [BundesamtfurStrahlenschutz]. Subjects were enrolled into the study after agreeing to participate and signing informed consent. The study was performed in accordance with the Declaration of Helsinki.
Demographic characteristics, disease duration, serum C- reactive protein and 25[OH]-vitamin D levels were determined in all patients. Oral immunosuppressive drug therapy [azathioprine, mesalazine, 6-mercaptopurine, cyclosporine A, methotrexate, tacrolimus], treatment with budesonide or cyclophosphamide, and biological therapy (tumour necrosis factor [TNF]-inhibitors, vedolizumab) were also recorded in all patients. For quantification of current and previous systemic glucocorticoid treatment, the administration of high-dose therapy [≥ 5mg prednisolone equivalent daily] exceeding 3 months in pulse treatment and long-term treatment was assessed and summed. Patients were divided into three groups: group I having received 0–3 pulses of glucocorticoids, group II having received 4–10 pulses, and group III represents patients with high-dose glucocorticoid treatment with more than 10 pulses or continuous treatment for at least 1 year.
History of previous fractures after inadequate trauma, diagnosis of osteoporosis, bisphosphonate therapy [oral and intravenous] ,and oral supplementation with calcium and 25[OH]-vitamin D3 were recorded. Disease activity indices, Harvey-BradshawIndex [HBI] for CD and clinical partial MayoScore for UC, respectively, were obtained from specialists at the Department of Gastroenterology [RA, SH, and MN]. Definition of clinical remission was based on these two disease activity scores with an HBI of ≤ 4 or a clinical subscore of Mayo Score ≤ 1. Medical history regarding previous bowel resection [resection of terminal ileum, ileoanal pouch] was obtained.
2.2. High-resolution peripheral quantitative computed tomography
HR-pQCT measurements of IBD patients and 50 healthy controls of comparable age and sex were performed at the ultra-distal radius of the dominant hand with an Xtreme CT scanner [Scanco, Bruettisellen, Switzerland] using the manufacturer’s standard in vivo protocol. Daily cross-calibrations with a standardised control phantom [Moehrendorf, Germany] were conducted to standardise measurements. All measurements and evaluations were performed using the manufacturer’s standard software. The hand was immobilised in a carbon-fibre cast for scanning. The reference line was set manually. The region of interest was defined using the anteroposterior scout view. The first CT slice was 9.5mm proximal to the reference line, and 110 slices [82-µm voxel size] were carried out. The effective dose equivalent for the scan was lower than 3 μSv for each patient and the measurement time was 2.8min. Motion grading [one to five] of scans was assessed using Scanco SOP scale, and scans graded higher than 3 were excluded from analysis.
2.3. Bone structure analysis
HR-pQCT allows the assessment of BMD and bone microstructure and geometry.13 It provides three-dimensional volumetric BMD [vBMD] of the entire distal radius [total BMD, mg hydroxyapatite/cm3] and selectively also of its cortical [Dcomp, mg HA/cm3] and trabecular compartment [Dtrab, mg HA/cm3]. In addition, trabecular BMD adjacent to bone cortex [Dmeta, mg HA/cm3] and central medullary trabecular BMD [Dinn, mg HA/cm3] can be recorded. Bone microstructural parameters are similar to those used in bone histology. They include trabecular bone volume fraction [BV/TV, %], trabecular number [Tb.N, mm-1], trabecular thickness [Tb.Th, µm], trabecular separation [Tb.Sp, µm], the inhomogeneity of the trabecular network [µm], cortical thickness [Ct.Th, µm], cortical porosity [Ct.Po, %], cortical pore volume [mm3], and cortical pore diameter [µm]. Furthermore, bone geometry parameters including total, cortical, and trabecular bone area [mm2] can be measured by HR-pQCT.
2.4. Statistical analysis
Statistical analysis included a comparison of demographical and disease-related characteristics among the subgroups of interest. Inferential comparisons comprised chi-square tests for categorical variables [indicated by N [%] in the tables] to check for deviations of observed from expected frequencies as well as Kruskal-Wallis and Mann-Whitney U-tests to compare data coming from interval scales. The predefined a priori criterion for interpretation of linear regression results was a proportion of at least 30% of the dependent variable’s variance [adjusted R2] to be accounted for by the set of predictors. From the characteristics that were screened for regression [i.e. total bone mineral density, cortical bone mineral density, cortical area, and cortical thickness] only cortical area fulfilled the predefined criterion. In order to investigate potential relations of the cortical area to demographical and disease-related characteristics, we computed a multiple linear regression with a forced entry procedure including all predictors at a single step, and incorporating the following predictors: diagnosis of IBD [either CD or UC], sex, age, BMI, and smoking status [currently or previous]. Two further linear regressions, using an identical approach, were used to investigate whether demographical and disease-related characteristics are related to the outcomes of cortical area. The set of predictors in both models was identical with the exception of vitamin D3 level, which was included in one model whereas current treatment with biologicals was incorporated in the other. The set of common predictors in both models comprised: diagnosis of CD vs UC, sex, disease duration, age, BMI, remission status, cumulative numbers of glucocorticoid pulses during IBD treatment (group 1: 0-3 glucocorticoid pulses, group 2: 4–10 glucocorticoid pulses, group 3 [which was designated the reference]: more than 10 glucocorticoid pulses). All descriptive or inferential tests were computed using IBM SPSS software version 21, whereas p-values ≤ 0.05 were considered statistically significant. All results are presented in median [25th;75th percentile] if not stated otherwise.
3. Results
3.1. Demographic characteristics of IBD patients
Table 1 summarises demographic characteristics of IBD patients and healthy controls. HR-pQCT scans of 98 patients were eligible for analysis after motion grading. IBD patients and controls were comparable in age (median [25th;75th percentile]: 44.4 [31.2;54.4] vs 42.6 [30.3;56.6] years, p = 0.712]) and sex distribution [57.1% vs 58.0% females, p = 0.921]. In the present cohort, no differences in height, weight or body mass index were observed between IBD patients and controls; However, 42.9% of IBD patients were current or previous smokers in contrast to 19.1% of the controls [p = 0.005]. When comparing demographic and disease-specific characteristics of patients with CD and UC, no differences were observed [Table 1].
Table 1.
Demographic and disease-specific characteristics of patients with inflammatory bowel diseases [IBD] and healthy controls.
| IBD | CD | UC | CO | IBD vs CO | CD vs UC | |
|---|---|---|---|---|---|---|
| N = 98 | N = 59 | N = 39 | N = 50 | p | p | |
| Demographic characteristics | ||||||
| Sex [male/female] | 42/56 | 23/36 | 19/20 | 21/29 | 0.921 | 0.340 |
| Age [years] | 44.4 [31.2;54.4] | 42.8 [30.3;54.1] | 44.8 [32.7;55.0] | 42.6 [30.3;56.6] | 0.712 | 0.591 |
| Height [m] | 1.70 [1.63;1.78] | 1.70 [1.62;1.78] | 1.70 [1.65;1.80] | 1.70 [1.64;1.80] | 0.770 | 0.354 |
| Weight [kg] | 75.0 [62.0;85.3] | 78.0 [62.0;85.0] | 75.0 [62.0;90.0] | 73.0 [63.5;83.0] | 0.418 | 0.752 |
| BMI [kg/m2] | 25.5 [22.0;29.1] | 25.9 [21.1;29.4] | 24.5 [22.8;26.9] | 24.1 [22.1;27.9] | 0.169 | 0.495 |
| Current or previous smoking, N [%] | 42 [42.9] | 27 [45.8] | 15 [38.5] | 9 [18.0] | 0.005 | 0.475 |
| Disease-specific characteristics | ||||||
| Duration of disease [years] | 10.0 [4.0;22.3] | 11.0 [4.0;25.0] | 9.0 [4.0;19.0] | - | - | 0.416 |
| Activity index HBI / pMayo-Score | - | 4.0 [2.0;8.0] | 1.0 [1.0;3.0] | - | - | - |
| Disease remission, N [%] | 57 [58.2] | 33 [55.9] | 24 [61.5] | - | - | 0.582 |
| Ileocoecal resection, N [%] | 30 [30.6] | 30 [50.8] | 0 [0] | - | - | < 0.001 |
| Total colectomy, N [%] | 2 [2.0] | 0 [0] | 2 [5.1] | - | - | 0.156 |
| CRP [mg/l] | 3.5 [1.6;8.2] | 3.5 [1.5;8.3] | 3.6 [1.9;8.6] | - | - | 0.723 |
| 25[OH]-Vitamin D3 [ng/ml] | 23.1 [14.4;34.3] | 20.1 [14.0;32.3] | 26.4 [15.5;42.6] | - | - | 0.116 |
| Non-traumatic fractures, N [%] | 7 [7.1] | 3 [5.1] | 4 [10.3] | - | - | 0.431 |
| Treatment modalities | ||||||
| Current biological therapy, N [%]* | 65 [66.3] | 42 [71.2] | 23 [59.0] | - | - | 0.211 |
| N current and previous biologicals* | 1.0 [0.0;1.0] | 1.0 [0.0;1.0] | 1.0 [0.0;1.0] | - | - | 0.715 |
| Current IS therapy, N [%]** | 44 [44.9] | 20 [33.9] | 24 [61.5] | - | - | 0.012 |
| N of current and previous IS** | 1.0 [1.0;2.0] | 1.0 [1.0;2.0] | 2.0 [1.0;2.0] | - | - | 0.508 |
| Current systemic GC, N [%]† | 14 [14.3] | 7 [11.9] | 7 [17.9] | - | - | 0.572 |
| Previous GC – Group I, N [%]‡ | 28 [28.6] | 13 [22.0] | 15 [38.5] | - | - | 0.078 |
| Previous GC – Group II, N [%]‡ | 21 [21.4] | 14 [23.7] | 7 [17.9] | - | - | 0.495 |
| Previous GC – Group III, N [%]‡ | 49 [50] | 32 [54.2] | 17 [43.6] | - | - | 0.302 |
| Current or previous budesonide > 3 months, N [%] |
13 [13.3] | 6 [10.2] | 7 [17.9] | 0.266 | ||
| Current calcium supplementation, N [%] | 27 [27.6] | 16 [27.1] | 11 [28.2] | - | - | 0.906 |
| Current 25[OH]vitamin D3 supplementation, N [%] | 40 [40.8] | 24 [40.7] | 16 [41.0] | - | - | 0.973 |
| Current or previous bisphosphonates, N [%] | 5 [5.1] | 4 [6.8] | 1 [2.6] | - | - | 0.645 |
IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; CO, controls; BMI, body mass index; HBI, Harvey-BradshawIndex, activity index for Crohn’s Disease; pMayo Score, partial Mayo Subscore [clinical], activity index for ulcerative colitis; disease remission defined as HBI < 5 for Crohn’s disease and pMayo Subscore ≤ 1; CRP, C-reactive protein; IS, immunosuppressive therapy; GC, glucocorticoid* tumour necrosis factor inhibitors, vedolizumab and cyclophosphamide; **azathioprine, methotrexate, 6-mercaptopurine, mesalazine and olsalazine; †current glucocorticoids ≥ 5mg prednisolone for at least 3 months; ‡summation of previous systemic glucocorticoid pulse therapy for at least 3 months: group I 0–3 pulses, group II 4–10 pulses, group III > 10 pulses or continuous treatment > 1 year; results are median [25th;75th percentile] or absolute values and percentage. Bold indicates significant differences (p < 0.05).
3.2. Disease-specific characteristics of IBD patients
Median [25th;75th percentile] disease duration was 10.0 [4.0;22.3] years. According to disease activity scores, 55.9% of CD patients and 61.5% of UC patients were in clinical disease remission; 50.8% of CD patients had previous resection of the ileum, whereas 5.1% of UC patients had an ileoanal pouch. Biological therapy was common in the study population [71.2% CD vs 59.0% UC], whereas orally administered immunosuppressive treatment was more prevalent in UC [61.5% vs 33.9%, p = 0.012]. At time of assessment, 14.3% of IBD patients were on glucocorticoid treatment with > 5mg equivalent to prednisolone daily. Previous high-dose glucocorticoid pulse therapies [> 5mg equivalent to prednisolone daily ≥ 3 months] were common in the cohort: 28.6% of IBD patients received 0-3 pulse therapies [group I], 21.4% 4-10 pulses [group II], and 50% of patients had previous high exposures with more than 10 pulses or continuous treatment > 1 year [group III].
Serum C-reactive protein level was slightly elevated in both disease cohorts. The percentage of patients with supplementation with calcium and 25[OH]-vitamin D3 was similar between CD and UC. Median serum vitamin D level was in the normal range in IBD patients, but with a broad range with low levels in patients without supplementation (mean ± standard deviation [SD], 29.6±23.5ng/ml). Low-trauma fractures occurred in 7.1% of IBD patients. All fractures were self-reported peripheral and vertebral fractures. Only 5.1% of the patients were on current or previous anti-resorptive treatment with bisphosphonates.
3.3. Volumetric bone mineral density and microstructure in IBD patients
In the first part of the analysis, we compared patients with IBD with healthy controls. Total volumetric BMD was significantly different between IBD patients and healthy controls, with lower values in IBD patients (IBD vs controls: 299 [251;335] vs 326 [302;368] mg HA/cm3, p = 0.001). Significant bone loss was found in both cortical bone (811 [771;851] vs 868 [828;892]; p ≤ 0.001) and trabecular bone(163 [130;189] vs179 [147;208]; p = 0.034). All results on bone parameters are summarised in Table 2.
Table 2.
Bone microstructure in IBD patients assessed by high-resolution peripheral quantitative CT [HR-pQCT].
| IBD | CD | UC | CO | IBD vs CO | CD vs UC vs CO | CD vs CO | UC vs CO | |
|---|---|---|---|---|---|---|---|---|
| p | p-Value | p-Value | p-Value | |||||
| Bone geometry | ||||||||
| Total bone area [mm2] | 300 [257;373] | 293 [247;362] | 310 [261;376] | 310 [259;369] | 0.976 | 0.709 | - | - |
| Ct. area [mm2] | 54 [45;62] | 50 [39;60] | 57 [50;65] | 59 [54;75] | 0.001 | < 0.001 | < 0.001 | 0.174 |
| Tb. area [mm2] | 241 [198;300] | 241 [193;296] | 240 [201;302] | 229 [202;286] | 0.558 | 0.786 | - | - |
| Volumetric bone mineral density | ||||||||
| Total BMD [HA/ cm3] | 299 [251;335] | 286 [241;332] | 304 [285;344] | 326 [302;368] | 0.001 | < 0.001 | < 0.001 | 0.115 |
| Ct. BMD [HA/cm3] | 811 [771;851] | 803 [760;849] | 820 [783;853] | 868 [828;892] | < 0.001 | < 0.001 | < 0.001 | 0.003 |
| Tb. BMD [HA/cm3] | 163 [130;189] | 151 [122;188] | 170 [153;192] | 179 [147;208] | 0.034 | 0.022 | 0.008 | 0.477 |
| Tb. meta BMD [HA/ cm3] | 222 [191;246] | 212 [183;247] | 226 [210;246] | 241 [211;267] | 0.005 | 0.005 | 0.002 | 0.115 |
| Tb. inn BMD [HA/ cm3] | 124 [89;150] | 113 [81;148] | 131 [110;154] | 132 [100;173] | 0.097 | 0.047 | 0.022 | 0.782 |
| Bone microstructure | ||||||||
| BV/TV [%] | 13.6 [10.8;15.8] | 12.6 [10.2;15.7] | 14.2 [12.8;16.0] | 14.9 [12.3;17.4] | 0.033 | 0.022 | 0.008 | 0.469 |
| Tb. N [mm-1] | 2.00 [1.87;2.22] | 1.96 [1.81;2.22] | 2.01 [1.89;2.23] | 2.09 [1.93;2.24] | 0.118 | 0.216 | - | - |
| Tb. Th [µm] | 64 [59;75] | 63 [55;73] | 68 [62;78] | 68 [62;80] | 0.058 | 0.017 | 0.009 | 0.763 |
| Tb. Sp [µm] | 429 [376;481] | 443 [375;492] | 420 [376;456] | 402 [369;454] | 0.083 | 0.141 | - | - |
| Inhomogeneity [µm] | 175 [152;201] | 179 [151;207] | 173 [152;194] | 170 [144;190] | 0.118 | 0.193 | - | - |
| Ct. Th [µm] | 730 [595;815] | 690 [570;810] | 745 [650;840] | 830 [760;930] | < 0.001 | < 0.001 | < 0.001 | 0.015 |
| Ct. Pm [mm] | 74.1 [68.9;83.0] | 74.1 [66.2;81.7] | 76.9 [70.1;85.6] | 74.4 [68.6;82.1] | 0.758 | 0.476 | - | - |
| Ct. Po [%] | 2.2 [1.3;3.2] | 2.2 [1.3;3.2] | 2.2 [1.3;3.3] | 1.9 [1.2;2.8] | 0.203 | 0.444 | - | - |
| Ct. pore volume [mm3] | 10.2 [6.3;17.8] | 10.1 [6.2;17.2] | 10.8 [6.4;19.4] | 11.7 [6.2;16.2] | 0.917 | 0.722 | - | - |
| Ct. pore Dm [µm] | 156 [147;172] | 154 [146;169] | 161 [148;176] | 154 [145;161] | 0.225 | 0.245 | - | - |
Bone geometry, microstructure and volumetric bone mineral density [BMD] by high resolution peripheral quantitative CT [HR-pQCT] at the ultradistal radius. Kruskal-Wallis Test for Crohn’s disease vs ulcerative colitis vs controls; only parameters with significant differences in Kruskal-Wallis-Test calculated with Mann Whitney U-test [comparison CD vs CO and UC vs CO]. Results are median [25th;75th percentile]. Bold indicates significant differences (p < 0.05).
IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; CO, controls; Ct., cortical; Tb., trabecular; Tb. meta BMD, peripheral trabecular density adjacent to cortex; Tb. inn BMD, central medullary trabecular density; BV/TV, trabecular bone volume; N, number, Th, thickness; Sp, separation; Pm, perimeter; Po, porosity; Dm, diameter.
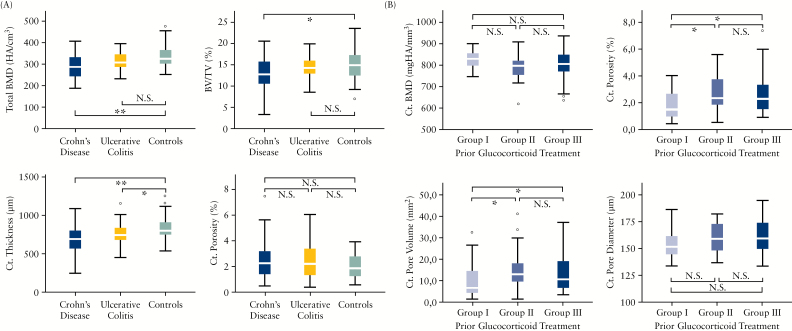
Geometrical analysis of cortical bone revealed a significant difference in cortical area (IBD vs controls: 54 [45;62] vs 59 [54;75] mm2; p = 0.001). A similar pattern was found with respect to cortical thickness (730 [595;815] and 830 [760;930] µm, p < 0.001) in the microstructure analysis. No differences were found with respect to cortical pores, cortical pore volume, or diameter. Within the IBD group, however, the intensity of glucocorticoid treatment affected cortical porosity and cortical pore volume [Figure 1]. Further analysis of bone microstructure showed that IBD patients and controls differed in total trabecular bone volume (BV/TV, %; IBD: 13.6 [10.8;15.8], controls: 14.9 [12.3;17.4], p = 0.03) and trabecular thickness by trend (Tb.Th, µm; 64 [59;75] and 68 [62;80], p = 0.058). In contrast, no differences in trabecular number, separation, or the inhomogeneity index were found.
Figure 1.
Differences in bone microarchitecture in inflammatory bowel disease [IBD] patients. [A] Changes of total bone mineral density, trabecular bone volume, cortical thickness and cortical porosity between Crohn’s disease, ulcerative colitis, and controls. [B] Patients with inflammatory bowel disease [IBD, Crohn’s disease, ulcerative colitis] were divided into three groups according to previous administration of high-dose therapy [≥ 5mg prednisone equivalent daily] exceeding 3 months in pulse treatment and long-term treatment: group I, 0–3 pulses; group II, 4–10 pulses; group III, more than 10 pulses or continuous treatment for at least 1 year. Ct, cortical; BMD, bone mineral density; BV/TV, bone volume over total volume, N.S. = not significant; *p < 0.05; **p < 0.001.
3.4. Comparison of bone microstructure between CD, UC, and controls
When dissecting bone changes of CD and UC, significant differences in lower cortical area, cortical thickness, and BV/TV, as well as total, cortical, and trabecular BMD, were found in CD compared with UC and healthy controls. These results are summarised in Table 2. Comparing CD and healthy controls, significant differences in BMD were observed, with lower values in all compartments: total [p < 0.001], cortical [p < 0.001], trabecular [p = 0.008], trabecular area adjacent to cortex [p = 0.002], and intramedullary [p = 0.022]. Furthermore, cortical and trabecular microstructure seemed to be deteriorated in CD, with decreased cortical area and thickness [p < 0.001 for both] as well as lower trabecular bone volume [BV/TV: p = 0.008] and trabecular thickness [p = 0.009].
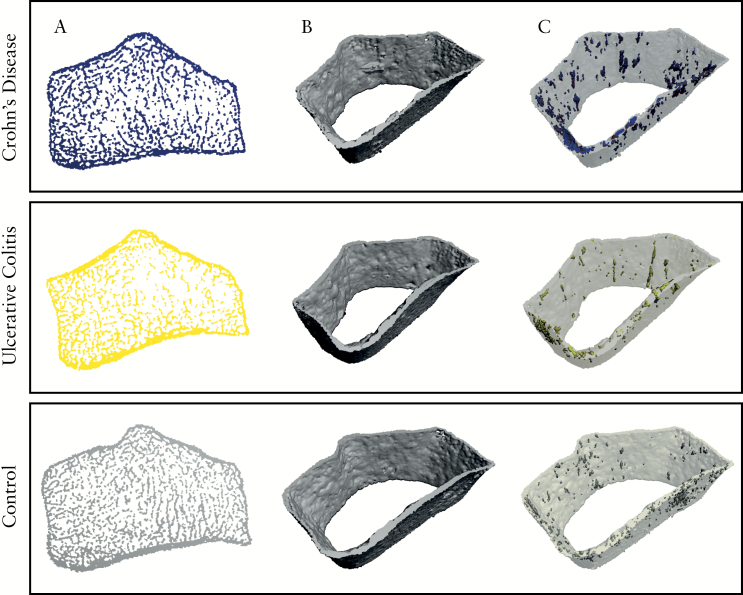
Previous ileocoecal resection in CD patients did not impact on the majority of bone structure parameters except cortical pore diameter [p = 0.005]. Analysis of the effects of ileoanal pouch in UC was not performed, due to small patient numbers. Overall, UC patients showed much milder bone changes than CD: only cortical thickness [p = 0.015] and cortical BMD [p = 0.003] were different, with smaller values in the UC patients than controls. However, no differences were found in in trabecular or endocortical compartments [Figure 2].
Figure 2.
High-resolution peripheral quantitative CT scans of the ultra-distal radius of patients with Crohn’s disease, ulcerative colitis, and healthy controls. [A] Axial view of ultra-distal radius, [B] three-dimensional [3D] reconstruction of cortical bone of total scan region, and [C] -D reconstruction of cortical bone of total scan region including cortical pores.
3.5. Predictors for microstructural deterioration in IBD patients
Among the several bone structure parameters, only the regression model for cortical area fulfilled the predefined criteria of variance, suggesting an important contribution of independent variables. In a multivariate regression model analysing predictive factors for low cortical area in IBD patients, female sex and lower BMI had been identified as significant predictors, with no influence of age and smoking [Table 3]. In the next step, we set up a regression model containing disease entity [CD or UC], demographic variables [age, sex, BMI], duration of disease, remission state [according to consecutive clinical activity scores for CD and UC], serum level of vitamin D, and glucocorticoid treatment groups [I, II, III].
Table 3.
Predictors for reduced cortical area in IBD patients.
| Cortical area [mm 2 ] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI low | 95% CI high | T | p-Value | |||||||
| IBD* vs controls | 0.326 | 0.188 | 0.464 | 4.739 | < 0.001 | ||||||
| Sex [female vs male*] | -0.504 | -0.638 | -0.370 | -7.532 | < 0.001 | ||||||
| Age | -0.091 | -0.232 | 0.050 | -1.298 | 0.196 | ||||||
| BMI | 0.219 | 0.076 | 0.362 | 3.067 | 0.003 | ||||||
| Smoking [yes vs no*] | -0.026 | -0.165 | 0.113 | -0.382 | 0.703 | ||||||
| Intercept | - | - | - | 8.334 | < 0.001 | ||||||
| R2 adjusted | - | - | - | 0.365 | - | ||||||
| Cortical area [mm 2 ] | Cortical area [mm 2 ] | ||||||||||
| β | 95% CI low | 95% CI high | T | p-Value | β | 95% CI low | 95% CI high | T | p-Value | ||
| CD* vs UC | 0.223 | 0.046 | 0.400 | 2.520 | 0.014 | CD* vs UC | 0.223 | 0.049 | 0.397 | 2.569 | 0.012 |
| Sex [female vs male*] | -0.455 | -0.627 | -0.283 | -5.288 | < 0.001 | Sex [female vs male*] | -0.453 | -0.621 | -0.285 | -5.382 | < 0.001 |
| Disease duration | -0.107 | -0.325 | 0.111 | -0.980 | 0.330 | Disease duration | -0.116 | -0.327 | 0.095 | -1.094 | 0.277 |
| Age | -0.175 | -0.403 | 0.053 | -1.539 | 0.127 | Age | -0.167 | -0.383 | 0.049 | -1.544 | 0.126 |
| BMI | 0.200 | 0.017 | 0.383 | 2.178 | 0.032 | BMI | 0.200 | 0.020 | 0.380 | 2.219 | 0.029 |
| Remission vs no remission* | 0.200 | 0.024 | 0.376 | 2.278 | 0.025 | Remission vs no remission* | 0.176 | 0.003 | 0.349 | 2.035 | 0.045 |
| 25[OH]-vitamin D3 | -0.007 | -0.184 | 0.170 | -0.079 | 0.937 | Biological vs no biological therapy* | -0.063 | -0.236 | 0.110 | -0.729 | 0.468 |
| GC I vs III* | 0.057 | -0.143 | 0.257 | 0.570 | 0.570 | GC I vs III* | 0.032 | -0.166 | 0.230 | 0.326 | 0.745 |
| GC II vs III* | 0.030 | -0.155 | 0.215 | 0.320 | 0.750 | GC II vs III* | 0.026 | -0.155 | 0.207 | 0.285 | 0.776 |
| Intercept | - | - | - | 7.195 | < 0.001 | Intercept | - | - | - | 7.340 | < 0.001 |
| R2 adjusted | - | - | - | 0.331 | - | R2 adjusted | - | - | - | 0.344 | - |
Remission defined as Harvey-Bradshaw Index < 5 for Crohn’s disease and clinical partial Mayo-Subscore ≤ 1. Smoking, current or previous. GC, sum of pulses of glucocorticoids ≥ 5mg prednisolone > 3 months; group I, 0–3 pulses; group II, 4–10 pulses; group III, > 10 pulses or continuous treatment > 1 year. Bold indicates significant differences (p < 0.05).
IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; BMI, body mass index;
*Reference category for analysis; 95% CI, 95% confidence intervals for standardized betas.
Diagnosis of CD [β = 0.223, p = 0.014], female sex [β = -0.455, p < 0.001], lower BMI [β = 0.200, p = 0.032], and lack of remission [β = 0.200, p = 0.025] were identified as independent predictors for reduced cortical area [Figure 1]. In a next step, we exchanged vitamin D level with ongoing biological treatment. Like in the previous model, diagnosis of CD [β = 0.223, p = 0.012], female sex [β = -0.453, p < 0.001], lower BMI [β = 0.200, p = 0.029], and lack of remission status [β = 0.176, p = 0.045] were identified as predictors for lower cortical area, whereas biological drug therapy, glucocorticoid therapy, age, and disease duration did not reach significance [Table 3]. Very similar and significant results were obtained when regressions models for cortical thickness were calculated [data not shown]. However, since this parameter did not fulfil the predefined criterion for interpretation of linear regression [see Methods section], the regression models are presented for cortical area only.
4. Discussion
IBD patients combine several risk factors for bone loss.5,16,17 Chronic inflammation shifts bone homeostasis towards increased bone resorption. This process is based on the induction of RANKL, an osteoclast differentiation and activation factor, by proinflammatory cytokines.18 For instance, Ghosh and colleagues showed enhanced prevalence of osteoporosis in patients with Crohn’s disease [CD] before starting immunosuppressive treatment.19 Apart from inflammation, glucocorticoids are an important enhancer of bone loss. These drugs are frequently used for the treatment and prevention of relapses in IBD.20,21 Hence, more than 50% of IBD patients receive steroids within 5 years of diagnosis and 20% face cumulative doses of more than 3g prednisolone equivalent within 1 year of disease.22 Malabsorption is the third potential key player for bone loss in IBD. Up to 65% of patients are considered deficient in vitamin D3.23,24,25 Interestingly, supplementation of calcium and vitamin D3 failed to improve BMD in premenopausal women with IBD,26 albeit an association of vitamin D3 level and BMD in IBD has been reported.27 Finally, resection of the terminal ileum in CD or ileoanal pouch in ulcerative colitis [UC] may pose additional risks to bone in IBD patients.28,29,30,31
Herein, we provide an in-depth analysis of bone structure of patients with CD and UC by using state-of-the-art bone analysis with HR-pQCT analysis. We show a significant deterioration of total, cortical, and trabecular bone micro-architecture in IBD patients. The detrimental effect of IBD on the cortical bone compartment is particularly remarkable. All the main parameters determining cortical bone strength, such as cortical BMD, cortical thickness, and cortical area, were significantly reduced in IBD patients. Since the majority of load is carried by cortical bone, weakening of this compartment affects the biomechanical properties of bone and reduces bone strength.32 A reduction of cortical bone parameters is associated with increased fracture risk, even in healthy premenopausal women, and is a well-known phenomenon in postmenopausal women with decreased BMD, as a consequence of microstructural deterioration caused by ageing. 33,34 Data on fracture risk in IBD patients are conflicting.2,3,35,36 Targownik and colleagues reported an increased risk for hip fractures in IBD patients after controlling for co-founding risk factors; taking into account that the femoral neck is built on substantial amounts of cortical bone, cortical thinning may indeed represent an important factor for reduced bone strength in IBD.37 Moreover in our cohort, the prevalence of previous non-traumatic peripheral and vertebral fractures was as high as 7.1%, despite the young age of the individuals with a median of 44.4 years.
Our data show that CD has a more profound impact on bone compared with UC. CD is characterised by strongly enhanced endocortical resorption, resulting in an enlargement of the medullary cavity, decreased cortical thickness, and loss of trabecular bone. Whereas patients with CD showed significant loss of BMD in all compartments as well as deterioration of cortical and trabecular bone microstructure, UC only affected cortical BMD and cortical thickness. Some of these changes were also found in one histological study of bone in CD patients.38 Differences in total BMD between the two diseases have also been suggested by DXA studies.39 These findings suggest differences in the pathophysiology between CD and UC. For instance, it is conceivable that the more widespread inflammation in CD, often resembling transmural affection of the intestinal wall, exposes the body to higher systemic cytokine concentrations, precipitating a higher rate of bone loss. Moreover, resorption problems resulting from the affection of the small intestine in CD may further negatively affect bone in CD patients compared with UC patients.
Chronic inflammatory states contribute per se to bone loss, as previously described in IBD and other diseases.19,40 Zanchetta et al. recently published data on patients with coeliac disease and showed cortical and trabecular deterioration of bone, even in newly diagnosed patients.41 These results underline the importance of disease control to reduce proinflammatory cytokines and consecutively reduce bone resorption due to increased osteoclast activity. In the present study, the diagnosis of CD, lower BMI, the absence of remission, and female sex identified IBD patients with more pronounced cortical bone loss. In contrast, factors such as glucocorticoid treatment, disease duration, and vitamin D status were not independently associated with bone changes. Importantly, however, median serum vitamin D level was within the normal range due to oral supplementation in over 40% of patients. Due to this effective supplementation, a potential impact of vitamin D deficiency on bone loss in IBD patients is difficult to assess in this cohort. Another limitation of this study is that the sample size is not powered for the analysis of different subgroups of IBD patients. Since IBD patients are heterogeneous with respect to disease activity and anti-inflammatory treatment, such analysis appears to be valuable. Ideally, disease activity over time would have been interesting in this context; however, this information has not been available due to the cross-sectional design of the study.
Glucocorticoid treatment selectively affected cortical porosity among the different bone parameters in patients with IBD. Patients with no or low previous exposure to glucocorticoids showed significantly less cortical porosity than those with moderate to high exposure. The negative impact of TNF inhibitor treatment does not appear to be independently linked to better bone architecture in IBD patients. Indeed, a potential positive effect of TNF inhibitors on the bone may be compensated by the more severe disease course in IBD patients receiving these drugs. Therefore, the results underline the importance of alertness to bone disease in patients with IBD; particularly those with CD, patients are at risk for developing structural bone deficits despite reduced mineralisation.
5. Conclusion
This detailed study on bone composition in IBD patients by HR-pQCT revealed a significant decrease of both cortical and trabecular volumetric bone mineral density associated with an impairment of bone microstructure in IBD. Bone changes in CD were generally more severe than in UC and affected virtually all bone compartments. In contrast, significant bone changes in UC were confined to cortical bone. Diagnosis of CD, female sex, lower BMI, and failure to reach remission state were identified as independent factors associated with bone loss in IBD. Hence, especially CD patients require close monitoring of concomitant bone disease.
Funding
The study was supported by the Bundesministerium fuer Bildung und Forschung [BMBF project Metarthros], the Deutsche Forschungsgemeinschaft [SPP1468 and CRC1181], the Marie Curie project Osteoimmune, and the IMI-funded project BTCure. CPF was supported by Ciencias sem Fronteiras from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico [CNPq], Brazil.
Conflict of Interest
None.
Author Contributions
JH, SH, AK, and LS collected and analysed the data. JH and ME performed the statistical analysis. JH, SH, AKrnd Kleyer, ME, FF, DS, CPF, CM, RK, HR, RA, MFN, JR, and GS interpreted the data and revised the manuscript. JH and GS wrote the manuscript.
Acknowledgment
The authors would like to thank Fabian Stemmler for technical support and assistance with scan evaluation.
References
- 1. Targownik LE, Bernstein CN, Nugent Z, et al. Inflammatory bowel disease and the risk of fracture after controlling for FRAX. J Bone Miner Res 2013;28:1007–13. [DOI] [PubMed] [Google Scholar]
- 2. van Staa TP, Cooper C, Brusse LS, et al. Inflammatory bowel disease and the risk of fracture. Gastroenterology 2003;125:1591–7. [DOI] [PubMed] [Google Scholar]
- 3. Card T, West J, Hubbard R, et al. Hip fractures in patients with inflammatory bowel disease and their relationship to corticosteroid use: a population based cohort study. Gut 2004;53:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bjarnason I, Macpherson A, Mackintosh C, et al. Reduced bone density in patients with inflammatory bowel disease. Gut 1997;40:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ali T, Lam D, Bronze MS, et al. Osteoporosis in inflammatory bowel disease. Am J Med 2009;122:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Targownik LE, Bernstein CN, Nugent Z, et al. Inflammatory bowel disease has a small effect on bone mineral density and risk for osteoporosis. Clin Gastroenterol Hepatol 2013;11:278–85. [DOI] [PubMed] [Google Scholar]
- 7. Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 2004;34:195–202. [DOI] [PubMed] [Google Scholar]
- 8. Muschitz C, Patsch J, Buchinger E, et al. Prevalence of vertebral fracture in elderly men and women with osteopenia. Wien Klin Wochenschr 2009;121:528–36. [DOI] [PubMed] [Google Scholar]
- 9. Seeman E, Delmas PD. Bone quality - the material and structural basis of bone strength and fragility. N Engl J Med 2006;354:2250–61. [DOI] [PubMed] [Google Scholar]
- 10. Stein EM, Kepley A, Walker M, et al. Skeletal structure in postmenopausal women with osteopenia and fractures is characterized by abnormal trabecular plates and cortical thinning. J Bone Miner Res 2014;29:1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engelke K, Libanati C, Fuerst T, et al. Advanced CT based in vivo methods for the assessment of bone density, structure, and strength. Curr Osteoporos Rep 2013;11:246–55. [DOI] [PubMed] [Google Scholar]
- 12. Cheung AM, Adachi JD, Hanley DA, et al. High-resolution peripheral quantitative computed tomography for the assessment of bone strength and structure: a review by the Canadian Bone Strength Working Group. Curr Osteoporos Rep 2013;11:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boutroy S, Bouxsein ML, Munoz F, et al. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 2005;90:6508–15. [DOI] [PubMed] [Google Scholar]
- 14. Amstrup AK, Jakobsen NF, Moser E, et al. Association between bone indices assessed by DXA, HR-pQCT and QCT scans in post-menopausal women. J Bone Miner Metab 2015, Aug 21. PMID: 26293682. [DOI] [PubMed] [Google Scholar]
- 15. Vico L, Zouch M, Amirouche A, et al. High-resolution pQCT analysis at the distal radius and tibia discriminates patients with recent wrist and femoral neck fractures. J Bone Miner Res 2008;23:1741–50. [DOI] [PubMed] [Google Scholar]
- 16. Targownik LE, Bernstein CN, Leslie WD. Risk factors and management of osteoporosis in inflammatory bowel disease. Curr Opin Gastroenterol 2014;30:168–74. [DOI] [PubMed] [Google Scholar]
- 17. Bernstein CN. Inflammatory bowel diseases as secondary causes of osteoporosis. Curr Osteoporos Rep 2006;:116–23. [DOI] [PubMed] [Google Scholar]
- 18. Schett G, David JP. The multiple faces of autoimmune-mediated bone loss. Nat Rev Endocrinol 2010;:698–706. [DOI] [PubMed] [Google Scholar]
- 19. Ghosh S, Cowen S, Hannan WJ, et al. Low bone mineral density in Crohn’s disease, but not in ulcerative colitis, at diagnosis. Gastroenterology 1994;107:1031–9. [DOI] [PubMed] [Google Scholar]
- 20. Ford AC, Bernstein CN, Khan KJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011;10:590–9; quiz 600. [DOI] [PubMed] [Google Scholar]
- 21. van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002;13:777–87. [DOI] [PubMed] [Google Scholar]
- 22. Targownik LE, Nugent Z, Singh H, et al. Prevalence of and outcomes associated with corticosteroid prescription in inflammatory bowel disease. Inflamm Bowel Dis 2014;20:622–30. [DOI] [PubMed] [Google Scholar]
- 23. Bours PH, Wielders JP, Vermeijden JR, et al. Seasonal variation of serum 25-hydroxyvitamin D levels in adult patients with inflammatory bowel disease. Osteoporos Int 2011;22:2857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Driscoll RH, Jr, Meredith SC, Sitrin M, et al. Vitamin D deficiency and bone disease in patients with Crohn’s disease. Gastroenterology 1982;83:1252–8. [PubMed] [Google Scholar]
- 25. Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. J Parenter Enteral Nutr 2007;31:311–9. [DOI] [PubMed] [Google Scholar]
- 26. Bernstein CN, Bector S, Leslie WD. Lack of relationship of calcium and vitamin D intake to bone mineral density in premenopausal women with inflammatory bowel disease. Am J Gastroenterol 2003;98:2468–73. [DOI] [PubMed] [Google Scholar]
- 27. Leslie WD, Miller N, Rogala L, et al. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Am J Gastroenterol 2008;103:1451–9. [DOI] [PubMed] [Google Scholar]
- 28. Navaneethan U, Shen L, Venkatesh PG, et al. Influence of ileal pouch anal anastomosis on bone loss in ulcerative colitis patients. J Crohns Colitis 2011;5:415–22. [DOI] [PubMed] [Google Scholar]
- 29. van Hogezand RA, Banffer D, Zwinderman AH, et al. Ileum resection is the most predictive factor for osteoporosis in patients with Crohn’s disease. Osteoporos Int 2006;17:535–42. [DOI] [PubMed] [Google Scholar]
- 30. Silvennoinen JA, Karttunen TJ, Niemela SE, et al. A controlled study of bone mineral density in patients with inflammatory bowel disease. Gut 1995;37:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta S, Shen B. Bone loss in patients with the ileostomy and ileal pouch for inflammatory bowel disease. Gastroenterol Rep 2013;1:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pistoia W, van Rietbergen B, Ruegsegger P. Mechanical consequences of different scenarios for simulated bone atrophy and recovery in the distal radius. Bone 2003;33:937–45. [DOI] [PubMed] [Google Scholar]
- 33. Chevalley T, Bonjour JP, van Rietbergen B, et al. Fracture history of healthy premenopausal women is associated with a reduction of cortical microstructural components at the distal radius. Bone 2013;55:377–83. [DOI] [PubMed] [Google Scholar]
- 34. Kawalilak CE, Johnston JD, Olszynski WP, et al. Characterizing microarchitectural changes at the distal radius and tibia in postmenopausal women using HR-pQCT. Osteoporos Int 2014;25:2057–66. [DOI] [PubMed] [Google Scholar]
- 35. Bernstein CN, Blanchard JF, Leslie W, et al. The incidence of fracture among patients with inflammatory bowel disease. A population-based cohort study. Ann Intern Med 2000;133:795–9. [DOI] [PubMed] [Google Scholar]
- 36. Loftus EV, Jr, Achenbach SJ, Sandborn WJ, et al. Risk of fracture in ulcerative colitis: a population-based study from Olmsted County, Minnesota. Clin Gastroenterol Hepatol 2003;1:465–73. [DOI] [PubMed] [Google Scholar]
- 37. Bohr H, Schaadt O. Bone mineral content of the femoral neck and shaft: relation between cortical and trabecular bone. Calcif Tissue Int 1985;37:340–4. [DOI] [PubMed] [Google Scholar]
- 38. Oostlander AE, Bravenboer N, Sohl E, et al. Histomorphometric analysis reveals reduced bone mass and bone formation in patients with quiescent Crohn’s disease. Gastroenterology 2011;140:116–23. [DOI] [PubMed] [Google Scholar]
- 39. Jahnsen J, Falch JA, Aadland E, et al. Bone mineral density is reduced in patients with Crohn’s disease but not in patients with ulcerative colitis: a population based study. Gut 1997;40:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang XL, Griffith JF, Qin L, et al. SLE disease per se contributes to deterioration in bone mineral density, microstructure and bone strength. Lupus 2013;22:1162–8. [DOI] [PubMed] [Google Scholar]
- 41. Zanchetta MB, Costa F, Longobardi V, et al. Significant bone microarchitecture impairment in premenopausal women with active celiac disease. Bone 2015;76:149–57. [DOI] [PubMed] [Google Scholar]