Abstract
Background and Aims:
Reports on imaging of active Crohn’s disease (aCD) using contrast-enhanced ultrasound (CEUS) are encouraging. However, the statistical power of most published papers is limited due to the small size of the patient groups included. This study was performed to verify the diagnostic value of CEUS in detecting aCD.
Methods:
A systematic literature search was performed by two independent reviewers for articles on the test characteristics of CEUS for the identification of aCD. The quality of the analysed studies was evaluated using a quality assessment tool for diagnostic accuracy studies (QUADAS-2). Pooling was performed using a diagnostic random-effect model and bivariate analysis.
Results:
Eight articles were included in the final analysis, with a total of 332 patients. There was no significant publication bias. Significant heterogeneity was found regarding CEUS methodology and sonographic definitions of aCD. In a bivariate analysis, pooled sensitivity was 0.94 (95% CI 0.87–0.97) and pooled specificity was 0.79 (95% CI 0.67–0.88). Spearman correlation statistics presented no significant diagnostic threshold effect (r = 0.12, p > 0.9). Subgroup analysis showed that relative intestine wall enhancement had the highest diagnostic value (area under the curve 94%), while the presence of enhancement and analysis of the slope were less useful (area under the curve 91 and 90%, respectively).
Conclusions:
CEUS presents good sensitivity and moderate specificity in the detection of the aCD. Large-scale randomized trials with quantitative evaluation of CEUS images are necessary to promote this technique in clinical practice.
Key Words: Crohn’s disease, CEUS, meta-analysis
1. Introduction
Crohn’s disease (CD) is a chronic idiopathic inflammatory disorder that can affect all sections of the gastrointestinal tract, and most often involves the terminal ileum. It is characterized by cycles of remission and exacerbation.1. The prevalence of CD in Europe varies from <10 to about 150 per 100 000 inhabitants.2 The chronic and recurrent nature of the disease requires repeated endoscopic, biochemical and imaging examinations in order to decide the most appropriate treatment and to determine the prognosis.
Prompt detection of the active phase of CD (aCD) is crucial to the introduction of more aggressive treatment and to the avoidance of serious complications. However, due to the heterogeneous clinical presentation and disseminated nature of CD, there is no gold standard for the diagnosis and evaluation of disease activity. Therefore, a comprehensive assessment of CD activity requires clinical, endoscopic, histological, radiological and biochemical investigations.3 Over the years, many clinical scales have been proposed to determine the degree of disease activity. Currently, the most commonly used is the CD activity index (CDAI).4,5 However, the CDAI is not a perfect measure of CD activity since it is, to some extent, based on indirect measures and subjective reporting by the patient. Moreover, the practical value of the CDAI system is limited by insufficient accuracy in detecting cases of fistulizing and stenosing CD, low usefulness in patients after extensive ileocolonic resections or stoma, and by significant interobserver variability.3,6,7 Therefore, in many cases additional studies are needed to assess the degree of disease activity.
The gold standard in the diagnosis of inflammatory bowel disease located in the colon is colonoscopy.8 However, colonoscopy is invasive and is not well tolerated by patients with active disease. Instead, imaging methods such as computed tomography (CT), magnetic resonance imaging (MRI) and transabdominal ultrasonography (US) are performed, but all of these have disadvantages. CT exposes the patient to a high dose of ionizing radiation, which is important when repeated examinations are necessary9. MRI is expensive and of limited availability. Conventional and Doppler US are highly operator-dependent and present limited sensitivity.10
Contrast-enhanced ultrasonography (CEUS) is a relatively new technique that can provide information on local tissue vascularization and perfusion; it involves microvessel passage of an intravenously administrated microbubble contrast agent.11 Increased bowel wall perfusion generally indicates active inflammation, which can be confirmed by histopathological studies. Therefore, CEUS may be useful in determining the degree of disease activity.
Reports on imaging of acute CD using CEUS are encouraging. However, most of the published papers are based on small groups of patients and therefore the statistical power of such reports is limited. The purpose of the present study was to systematically review the literature concerning the role of CEUS using intravenously administrated microbubble contrast media in the imaging of aCD. We also aimed to estimate the diagnostic accuracy of CEUS in the detection of aCD.
2. Materials and methods
2.1. Literature search
We performed a systematic review of the literature indexed in PubMed (US National Library of Medicine), EMBASE (Elsevier) and Web of Science (Thomson Reuters) up to June 30, 2014. The following keywords were used: CEUS, contrast-enhanced ultrasound, contrast-enhanced sonography, Crohn. These terms were exploded in EMBASE and Web of Science or were used as medical subject headings (referred to as MeSH) in MEDLINE. The search was restricted to studies in English, German, Spanish and French. In order to screen for eligibility, two reviewers (ZS, MB) independently reviewed all abstracts and retrieved full-text articles on inclusion criteria using a standardized data extraction form. Retrieved article references were also searched for additional eligible studies.
2.2. Eligibility and data extraction
Inclusion criteria were as follows: (1) CEUS examination to detect aCD; (2) verification vs clinical scoring system (CDAI or equivalent) or histopathology; (3) research paper (not case report) including at least 10 patients; (4) original study (not a letter, review or editorial) published in a peer-reviewed journal. If both CDAI and histopathology were used as a method of reference, results of microscopic assessment were used for calculations. If there was suspicion that the same study population was presented in more than one publication, data from the article presenting the largest group were used.
Two reviewers (ZS, MB) independently extracted relevant data from the studies that met the inclusion criteria and placed it in predefined tables. These two reviewers were blinded to the authors of original studies. Data extracted from the papers analysed included demographic characteristics, reference method, study outcome, technical parameters of US scanning, contrast medium dosage and postprocessing method.
2.3. Quality appraisal of studies and statistical analysis
The results of this systematic review were reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA Statement).12 The quality of the analysed studies was evaluated by two reviewers (ZS, MB) in consensus using a quality assessment tool for diagnostic accuracy studies (QUADAS-2).13 The risk of publication bias and concerns regarding applicability of studies were then assessed by visually inspecting QUADAS-2 plots. The presence of publication bias was verified using Deeks’s funnel plot, where significant asymmetry (p < 0.1) indicated the presence of this bias.14
For data pooling, the Cochrane Q statistic was calculated with the I 2 index to assess the heterogeneity of the included studies. For p values <0.10, the assumption of homogeneity was deemed not valid and the diagnostic random-effect model (DerSimonian–Laird method) was used to calculate the pooled negative likelihood ratio (NLR), positive likelihood ratio (PLR) and diagnostic odds ratio (DOR) with their 95% confidence intervals (CIs). For sensitivity and specificity pooling, two hierarchical logistic regression models were used: a bivariate analysis (maximum likelihood) and a hierarchical summary receiver operating characteristic (HSROC) model. The bivariate model was used to calculate summary points for sensitivity and specificity. The HSROC model was used to plot a summary line representing the relation between sensitivity and specificity. Apart from the heterogeneity analysis described above, p values <0.05 were considered statistically significant. Statistical analyses were performed using OpenMeta[Analyst] (Tufts University, Boston, MA, USA).15
3. Results
3.1. Characteristics of studies
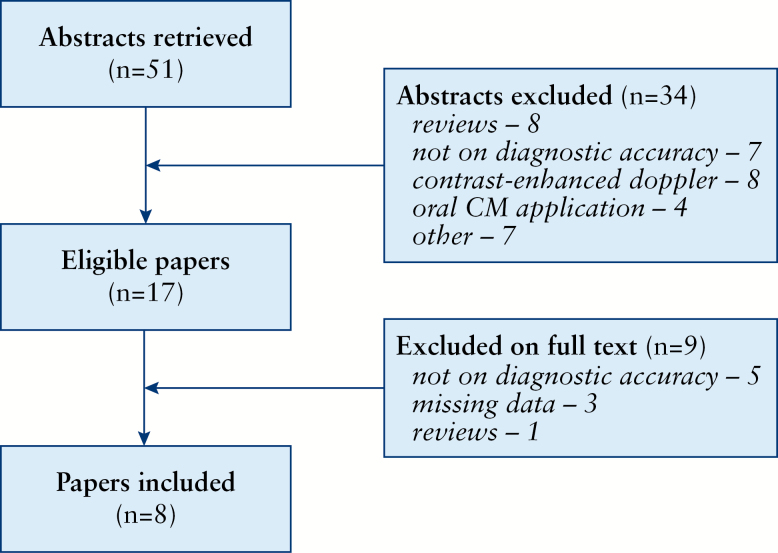
The flow chart of the literature search is presented in Figure 1. Finally, we included 8 studies with a total of 332 patients (mean 47.4 subjects, range 28–61).16–23 In the case of paper by Ripollés et al.,23 we included the given number of analysed intestine segments, not patients.
Figure 1.
Flow chart of the literature search.
Detailed characteristics of the analysed studies are presented in Tables 1 and 2. Out of 8 studies, 4 were declared as prospective, 1 was retrospective, and in 3 the design was not stated. None of the papers included any description of sample size calculation. In all but 2 studies,19,21 inclusion was restricted to subjects with proven CD. In 2 studies, specific patient populations were tested: subjects after ileocolic anastomosis22 and those who were scheduled for elective bowel resection.23 Only in 2 papers16,18 were data provided regarding the current medication given to subjects.
Table 1.
Characteristics of included primary studies.
| Study | Declared design | Study group | Mean or median age (y) | Reference method | Reference definition of CD activity | Site evaluated by CEUS | CEUS threshold/ outcome |
|---|---|---|---|---|---|---|---|
| Robotti, 200416 | Retrospective | 52 | NR | Laboratory/ clinical | NR | Small intestine | Presence of enhancement |
| Giangregorio, 200917 | Prospective | 30 | 41.9 | Endoscopy | Histology | Ileum/colon | Full thickness or double- layer enhancement, AUC (>15), IMA (>10) |
| Migaleddu, 200918 | NR | 47 | 38 | Endoscopy | Histology | Bowel | Presence of enhancement |
| Ripollés, 200919 | NR | 61 | 39 | Endoscopy | SES-CD (grade 3–4) | Ileum/colon | Relative enhancement >46% |
| De Franco, 201220 | Prospective | 54 | 35 | Endoscopy /clinical |
CICDA | Terminal ileum | Maximum peak intensity (>24 VI) and β coefficient of the slope (>4.5 VI/s) |
| Quaia, 201221 | Prospective | 28 | 48.5 | Endoscopy | Histology | Terminal ileum | Relative enhancement (>40.5%), TTP (≤9.44), AUC (>1024.82) |
| Paredes, 201322 | prospective | 60 | 39 | Endoscopy | Rutgeerts scale (grade 1–4) | Ileum | Relative enhancement >34.5% |
| Ripolles, 201323 | NR | 25 | 37.3 | Resection | Histology | Ileum/colon | Relative enhancement >65% |
NR, not reported; SES-CD, simple endoscopic score for Crohn’s disease; CICDA, composite index of Crohn’s disease activity; VI, video intensity; TTP, time to peak; AUC, area under the curve; IMA, mean intensity of AUC
Table 2.
Characteristics of contrast-enhanced ultrasonography (CEUS) acquisition and image analysis.
| Study | Probe (MHz) | MI | CM dose (mL) | Scan delay/ scan time (s) | Operators | Ultrasonographic device | Software | Mean interval reference/CEUS (range; d) |
|---|---|---|---|---|---|---|---|---|
| Robotti, 200416 | NR | 0.09 | 4.8 | NR | 2 | Esatune, Esoate | NR | NR |
| Giangregorio, 200917 | Linear (7.5) | NR | 4.8 | 180/NR | NR | Technos MPX, Esaote | Logiq works, Logiq 9, GEHC | NR (0–31) |
| Migaleddu, 200918 | NR (5–7) | NR | 2.4–5.0 | NR | 3 | Esatune, Esaote | NR | NR (0–5) |
| Ripolles, 200919 | Convex (3–6) | <0.1 | 1.2 | NR/40 | 2 | Aplio 80, Toshiba | Built-in | 11 (1–30) |
| De Franco, 201220 | Linear (4–8) | 0.08 | 4.8 | NR | 2 | Philips iU22 | QLAB 4.1.2., Philips | NR (0–14) |
| Quaia, 201221 | Convex (2–5) | 0.06–0.08 | 2.4 | 5/30 | 1 | Siemens Sequoia 512 | Q-contrast 4.0, e-AMID, IT | NR |
| Paredes, 201322 | Convex (3–4) | <0.1 | 1.2 | NR/40 | NR | Aplio 80, Toshiba | Built-in | 3 |
| Ripolles, 201323 | Convex (3–4) | <0.1 | 1.2 | NR/40 | NR | Aplio 80, Toshiba | Built-in | 35 (10–59) |
NR, not reported; MI, mechanical index; CM, contrast medium
Reference methods were mostly endoscopic. Only Robotti et al.16 did not apply any colonoscopic evaluation as a reference, basing their study on roughly described laboratory and clinical parameters. On the other hand, De Franco et al.20 used a very specific composite index of CD activity (CICDA). In their study, CD was classified as active when at least 3 of the following 4 criteria were met: (1) CDAI at least 150; (2) C-reactive protein level >5 mg/dL, white blood cell count >10000/µl and fibrinogen level >400 mg/dL; (3) presence of ileal ulceration at retrograde ileoscopy; and (4) small-bowel enema or small-bowel follow-through examination showing aphthous or linear ulcers, cobblestone mucosa, sinus tracts, fistulas with extraluminal fluid collections or fold thickening, and/or CT or magnetic resonance enterography showing mucosal hyperenhancement, mural stratification, comb sign, ulceration, fistulas with extraluminal fluid collections and perienteric fat with increased attenuation on CT and/or high signal intensity on T2-weighted MRI.
The mean/median age of patients ranged from 35 to 49 years. In all studies SonoVue (Bracco, Milan, Italy) was used as a sonographic contrast medium at doses ranging from 1.2 to 5.0 ml. The interval between the reference and CEUS examinations ranged from 0 to 59 days. The authors used both convex and linear probes with a frequency range of 2–8 MHz and a mechanical index range of 0.06–0.1. Scan delay and scan time were described precisely in 1 paper only.21 Variability of results was tested in only 1 study.20
3.2. Quality of studies and risk of bias
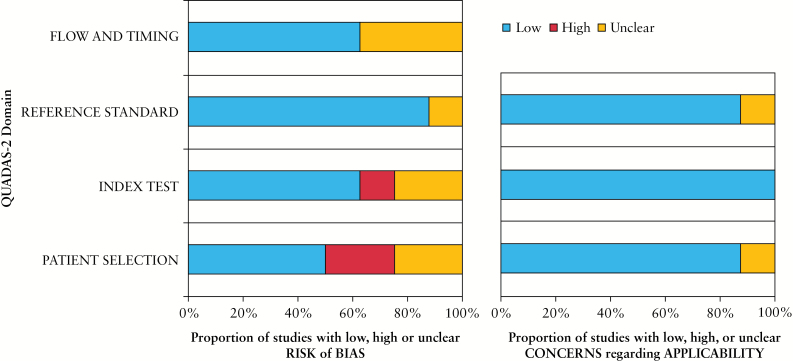
Results of QUADAS-2 evaluation are shown in Figure 2. Overall, the studies presented a low to moderate risk of bias and low concerns about applicability. Only 3 studies had scores showing low bias in all 4 domains of the QUADAS-2 system. The highest risk of bias was related to patient selection. Considering concerns regarding applicability, only two studies were scored. The Deeks funnel plot asymmetry test showed that there was no significant publication bias (r = –0.12, p = 0.7696).
Figure 2.
Results of QUADAS-2 quality assessment of original studies.
3.3. Test characteristics of CEUS
Thresholds for diagnosing aCD using CEUS that were used in the included studies are listed in Table 1. In 3 papers, 2 or more alternative methods for quantification of enhancement quantification were used. Giangregorio et al.17 used a semi-quantitative enhancement pattern analysis and a time–intensity curve. The pattern of enhancement was classified into 4 types: (1) enhancement of the entire intestinal wall; (2) enhancement of mucosa with submucosa; (3) enhancement of submucosa only; and (4) no enhancement of any wall layers. The first 2 patterns were considered indicative of aCD. From a spectrum of time–intensity curve measures, the total area under the curve (AUC) and the mean intensity of AUC (IMA) were chosen. De Franco et al.20 determined the maximum peak intensity (MPI), which reflected the strength of intestine perfusion, and the β-coefficient of the slope that correlated with time to peak enhancement, and evaluated the dynamics of blood flow. Finally, Quaia et al. calculated test characteristics based on relative maximal enhancement, time to peak of the slope, and AUC.21 For general pooling, from the above-mentioned studies we chose methods presenting the highest DOR.
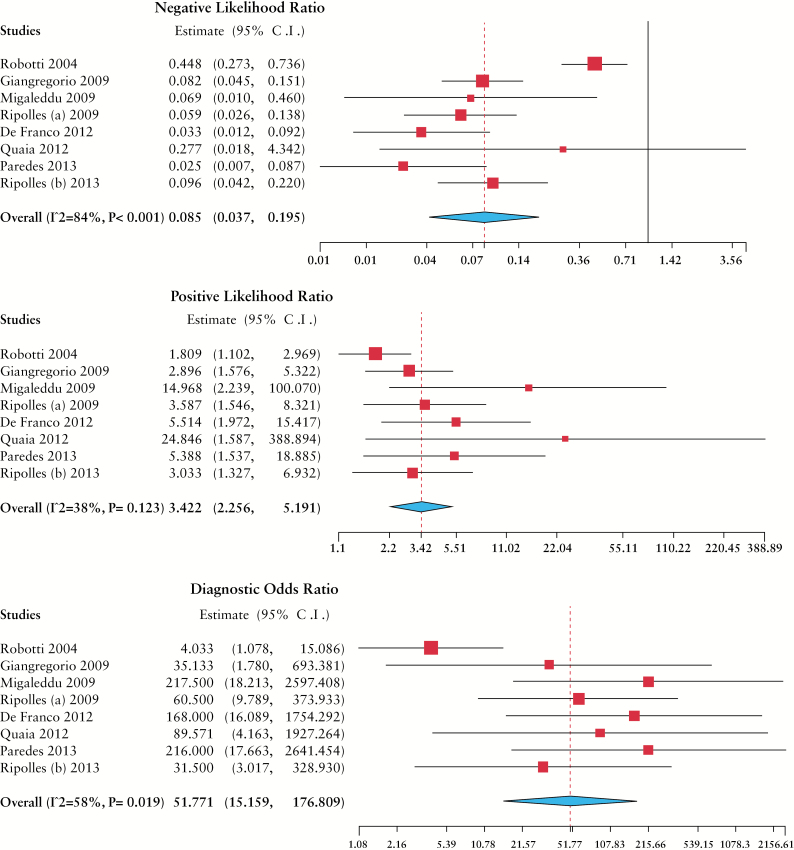
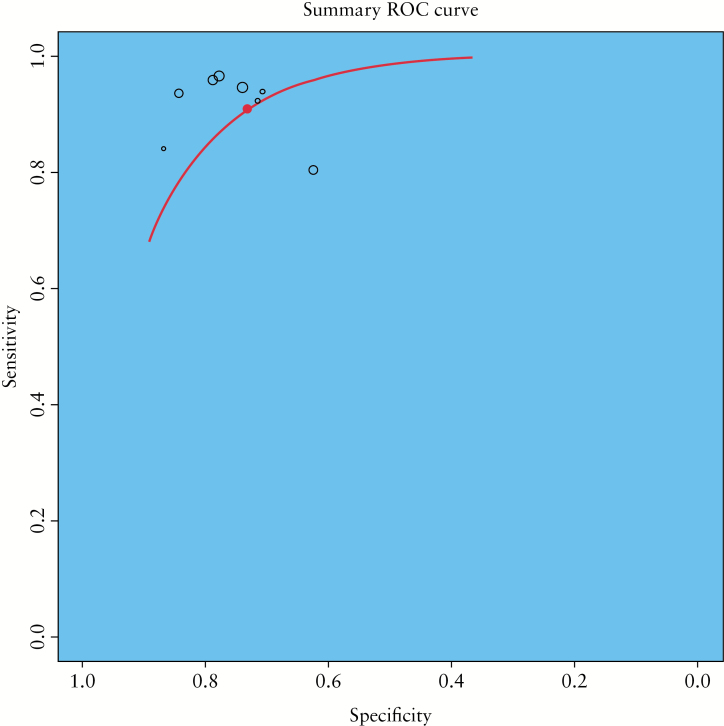
Results of NLR, PLR and DOR calculations are summarized in Table 3 and Figure 3. Heterogeneity was substantial in the case of NLR (I 2 = 84%, p < 0.001), borderline but significant for DOR (I 2 = 58%, p < 0.02) and low for PLR (I 2 = 38%, p > 0.1). In bivariate analysis, pooled sensitivity was 0.94 (95% CI 0.87–0.97) and pooled specificity was 0.79 (95% CI 0.67–0.88). Spearman correlation statistics showed no significant diagnostic threshold effect (r = 0.12, p > 0.9). The summary ROC curve based on HSROC analysis is presented in Figure 4. A separate analysis was devoted to specific subgroups of studies. Table 3 presents a comparison of CEUS test characteristics calculated for all included reports, studies testing the presence of intestinal wall enhancement or its pattern,16,17,21 studies based on slope analysis17,20,21 and studies designed to detect a specific level of relative intestine enhancement (threshold range 34.5–65.0%).19,22,23 Interestingly, this last group of studies was conducted by investigators from 1 centre. This subgroup analysis showed that relative intestine wall enhancement had the highest diagnostic value, while the presence of enhancement and analysis of the slope were less useful.
Table 3.
Test characteristics of contrast-enhanced ultrasonography (CEUS) with their 95% confidence intervals in all studies and selected subgroups.
| Characteristic | All studies16–23 | Presence of enhancement16–18 | Relative enhancement19, 21–23 | Enhancement curve 17,20, 21 |
|---|---|---|---|---|
| Sensitivitya | 0.94 (0.87–0.97) | 0.87 (0.66–0.96) | 0.95 (0.89–0.98) | 0.86 (0.75–0.93) |
| Specificitya | 0.79 (0.67–0.88) | 0.70 (0.49–0.85) | 0.70 (0.57–0.81) | 0.83 (0.63–0.93) |
| NLRb | 0.09 (0.04–0.20) | 0.16 (0.04–0.73) | 0.07 (0.03–0.16) | 0.19 (0.06–0.61) |
| PLRb | 3.42 (2.26–5.19) | 3.10 (1.21–7.90) | 3.09 (2.04–4.67) | 3.71 (1.68–8.18) |
| DORb | 51.8 (15.2–176.8) | 26.4 (1.9–366.4) | 50.9 (17.0–152.0) | 22.8 (5.1–101.6) |
| AUC | 94% | 91% | 94% | 90% |
aBivariate analysis (maximum likelihood).
bRandom-effect model; p < 0.05 for all characteristics.
NLR, negative likelihood radio; PLR, positive likelihood ratio; DOR, diagnostic odds ratio; AUC, area under the curve.
Figure 3.
Negative likelihood ratio, positive likelihood ratio and odds radio and their forest plots for the diagnostic value of CEUS in detecting Crohn’s disease activity.
Figure 4.
Summary receiver operating characteristic plot presenting test performance of CEUS in detecting Crohn’s disease activity.
Only 4 primary studies that were included in our meta-analysis reported the value of B-mode or Doppler US for the diagnosis of aCD.17–19,22 In the study by Giangregorio et al.17 there was a significant difference in Doppler resistance index between inflamed and non-inflamed bowel loops. There was also a moderate difference in bowel wall thickness (8.1 and 7.1 mm, respectively) but its statistical significance was not given. The presence of a typical 3-layer appearance of the bowel wall had sensitivity of 25% and specificity of 65%. Similarly, peri-intestinal findings were not useful for the diagnosis of aCD in that study.17 Migaleddu et al.18 measured the diagnostic accuracy of sonographic scores based on the length of involved bowel segments, wall layer appearance, wall thickness and vascularity determined using colour Doppler. Accuracy values for those scores were 74, 89, 91 and 91%, respectively, and were lower than the accuracy calculated for CEUS (94%). Comparable results were presented by Ripollés et al.19 In their study, diagnostic accuracy of CEUS reached 90% and was higher than that of wall thickness measurement (79%) and colour Doppler assessment (69%). Finally, in the study by Paredes et al.22 CEUS presented higher diagnostic accuracy than a combined classic US index (wall thickness >3 mm and/or colour Doppler flow): 88 and 95%, respectively.
4. Discussion
To our knowledge this is the first systematic review and meta-analysis specifically focused on the role of CEUS using intravenously administrated microbubble contrast medium in the detection of the acute phase of CD. We found that the pooled sensitivity and specificity of CEUS in this regard were 0.94 (95% CI 0.87–0.97) and 0.79 (95% CI 0.67–0.88), respectively.
The reliability of the clinical assessment of CD activity, as well as the value of laboratory markers of inflammation, is increasingly being disputed.24 Active disease can occur with very poorly expressed symptoms, which in turn may lead to a lack of or delay in the initiation of intensive treatment. On the other hand, the coexistence of irritable bowel syndrome symptoms often causes unnecessary and sometimes harmful intensification of aggressive therapy.25 The active phase of CD is characterized by intense neovascularization and angiogenesis,26 which results in increased regional perfusion and may be related to contrast enhancement. Non-invasive transsectional imaging modalities, including CT, MRI and CEUS, are able to assess both the inflammatory activity, which is considered as thickening and contrast enhancement of the intestinal wall, and the extent of disease outside the intestine.27–29 A meta-analysis by Horsthuis et al.30 has confirmed that MRI and CT are effective methods for the diagnosis of inflammatory bowel diseases (IBD), including CD. Mean sensitivity for the diagnosis of IBD on a per-patient basis was 0.93 and 0.84 for MRI and CT, respectively, while mean per-patient specificity was 0.93 for MRI and 0.95 for CT. The test characteristics calculated on a per bowel segment basis were slightly lower. Therefore, because of their accuracy and non-invasiveness, these modalities are of greatest interest for routine follow-up. US as a cost-effective, readily available and non-invasive modality has become the standard in the diagnostics and monitoring of disease activity.31 In centres specializing in the treatment of patients with IBD, US is becoming the method of choice and its outcome affects treatment decisions.32 B-mode US has been used to evaluate CD since the early 1990s. Hence, the improvement of US systems and the advent of high-resolution, broadband linear array transducers has led to more common use of this imaging modality in clinical practice.33 US is able to visualize bowel wall thickening and the associated transmural and perivisceral inflammation. However, it is also known that these abilities are highly reliant on the experience of the operator and on the bowel segment involved by the disease. The diagnostic performance of B-mode US is extremely variable among different studies; its sensitivity has been reported to range between 0.7734 and 0.9635 and its specificity between 0.5734 and 1.36–38 In a recent meta-analysis, Dong et al.33 evaluated the performance of bowel wall thickness measured by B-mode US for the diagnosis of aCD. The threshold for the diagnosis reported in primary studies varied between 3 and 7 mm. The meta-analysis showed that the pooled sensitivity of US was 0.88, the pooled specificity was 0.97 and the pooled accuracy was 0.94, although data regarding specificity and accuracy were affected by significant heterogeneity. Therefore, compared with our results, B-mode US seems to be less sensitive and more specific than CEUS. Doppler US has also been proposed as a method of aCD detection,39,40 but again the subjectivity of the analysis prevented the setting of any widely accepted standard of flow analysis.31 Instead, combined sonographic scores, including bowel wall thickness, wall echogenicity and vascularity, have become popular.18,19,22
US with intravenous administration of microbubble contrast medium has been proposed as a modality for aCD diagnosis for many years.16 Recent guidelines by the European Federation of Societies for Ultrasound in Medicine and Biology indicate that adding CEUS to the routine diagnostic protocol improves reliability in estimating the activity of CD and that quantitative measurements of enhancement obtained by CEUS also correlate with activity.41 Moreover, the authors of the guidelines suggest that evaluation of intestinal wall enhancement during biological therapy (e.g. with anti-tumour necrosis factor [TNF] agents) seems to be a useful and relatively cheap imaging modality for the clinical monitoring of CD activity. The overall Recommendation Level for the use of CEUS for estimation of activity in inflammatory bowel disease was B;1b.41 However, no widely accepted consensus regarding enhancement parameters for the diagnosis of aCD was proposed. Without such an arrangement, a reasonable determination of the diagnostic value of CEUS is not possible.
Recently, a systematic review published by Ma et al.42 had an aim similar to that of our study, i.e. to assess the overall performance of CEUS in the diagnosis of active and quiescent CD. Based on their meta-analysis, the authors concluded that CEUS ‘can be a great measurement tool differentiating active from quiescent CD’. However, the review included somewhat obsolete contrast media (Levovist and oral PEG-3350), which reduced the practical value of the paper. Moreover, the methodology of the review by Ma et al. excluded 3 papers pooled in our meta-analysis.16,20,21 Finally, the results of the study by Paredes et al.22 were calculated using a joint sonographic score that included both enhancement and bowel wall thickening; this approach resulted in an artificial increase in the CEUS pooled diagnostic value. Considering all the criticisms mentioned above, we consider our meta-analysis an important contribution to the discussion of the role of CEUS in the diagnostics of aCD.
We found that the most important problem with the current evidence regarding imaging of aCD with the use of CEUS is the technical and methodological quality of the studies. In several cases, although the studies were declared as prospective, diagnostic outcomes were not strictly defined in the study design. Moreover, all studies were based on small patient groups without any previous sample statistical calculations. Another problem was the observed great variability in diagnostic thresholds used. Those included the simple presence of intestinal wall enhancement,16–18 a range of relative enhancement values19,21–23 and a mathematical slope analysis. 17,20,21 The first method appeared fairly robust, probably because of the subjectivity of estimation. Arrival of single SonoVue bubbles at the intestinal wall takes place even in normal bowel segments and therefore the determination of a significant signal increase is difficult. On the other hand, CD activity determination based on a fixed relative enhancement value presented the highest DOR in our meta-analysis. However, this result may be biased since the particular thresholds used in primary studies varied significantly (34.5–65.0%) and were established retrospectively based on raw data. Interestingly, an advanced enhancement curve analysis using dedicated software was of limited value. Such software packages offer a variety of perfusion measures, including the analysis of contrast medium wash-in and wash-out dynamics as well as enhancement intensity.43,44 They also offer several tools to standardize measurements, such as US device-specific calibration, linearization of video files, curve-fitting modelling and motion compensation. Still, although aCD detection based on slope analysis seems to be promising, current evidence indicates its limited accuracy. Thus, further standardized investigation is necessary in this regard.
There are limitations to this study that should be addressed. Firstly, only articles that were published in peer-reviewed journals, were indexed in 3 main databases and were written in 4 main languages were included in this systematic review, which might have introduced publication bias. Moreover, since CEUS in CD is usually an above-standard but also a technically non-demanding procedure, there is the possibility that many single-centre studies have not been published. However, although a peer-review system does not guarantee optimal scientific value of included articles, its formal requirements should improve the quality of publications. Secondly, we included studies with a wide range of reference standards, including histopathological specimen evaluation, endoscopic visual assessment and CDAI. Therefore, the proper classification of CD activity might have been biased. Thirdly, definition of aCD was either not given in the papers or differed significantly among the studies, which underlines the limited quality of current evidence on the subject. Finally, the authors of the reviewed studies used very different enhancement thresholds to diagnose aCD, which seems to be the most important weakness of this meta-analysis. This situation is a result of the lack of widely accepted consensus regarding the use of CEUS in CD. Hopefully, the introduction of advanced postprocessing software will result in the setting of some strictly defined parameters of intestinal wall enhancement that will be both reproducible and objective.
In conclusion, the role of CEUS in the detection of the active phase of Crohn’s disease seems to be promising, considering its significant test characteristics and non-invasiveness. However, evidence for the routine use of CEUS was weak, since the published studies were based on small study groups and present significant methodological heterogeneity. There is still a need for a large prospective study on the role of CEUS in active CD detection that would help in introducing the method into everyday practice. We would also emphasize the need for a widely accepted diagnostic threshold for the diagnosis of active CD that would promote CEUS as an objective modality.
Funding
This study did not receive funding from any source and writing assistance was not obtained.
Conflict of interest
No conflict of interest exists for any authors with regard to the content of this study.
Author Contributions
ZS was involved in the conception and design, statistical analysis, data interpretation and manuscript drafting. MB and AB performed data acquisition and manuscript drafting. LMS was involved in the statistical analysis and manuscript revision. MK participated in the conception and design and revised the manuscript. All authors read and approved the final manuscript.
This work has been conducted within the framework of the Network for Assessment of Imaging in Medicine (EuroAIM), research platform of the European Institute for Biomedical Research (http://www.eibir.org/scientific-activities/joint-initiatives/euroaim/).
A part of this work was presented at the European Congress of Radiology, Vienna, 2015 (abstract No. B-0457).
References
- 1. Park SJ, Kim WH, Cheon JH. Clinical characteristics and treatment of inflammatory bowel disease: a comparison of Eastern and Western perspectives. World J Gastroenterol 2014;20:11525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hovde Ø, Moum BA. Epidemiology and clinical course of Crohn’s disease: results from observational studies. World J Gastroenterol 2012;18:1723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Assche G Dignass A Panes J et al. , . European Crohn’s and Colitis Organisation (ECCO). . The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis 2010;4:7–27. [DOI] [PubMed] [Google Scholar]
- 4. Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- 5. Jørgensen L, Fredholm L, Hyltoft Petersen P, Hey H, Munkholm P, Brandslund I. How accurate are clinical activity indices for scoring of disease activity in inflammatory bowel disease (IBD)? Clin Chem Lab Med 2005;43:403–11. [DOI] [PubMed] [Google Scholar]
- 6. Sostegni R, Daperno M, Scaglione N, Lavagna A, Rocca R, Pera A. Review article: Crohn’s disease: monitoring disease activity. Aliment Pharmacol Ther 2003;17:11–7. [DOI] [PubMed] [Google Scholar]
- 7. De Dombal FT, Softley A. IOIBD report no. 1: Observer variation in calculating indices of severity and activity in Crohn’s disease. Gut 1987;28:474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goetz M, Neurath MF. Imaging techniques in inflammatory bowel disease: recent trends, questions and answers. Gastroenterol Clin Biol 2009;33:S174–82. [DOI] [PubMed] [Google Scholar]
- 9. Allen BC, Baker ME, Einstein DM, et al. Effect of altering automatic exposure control settings and quality reference mAs on radiation dose, image quality, and diagnostic efficacy in MDCT enterography of active inflammatory Crohn's disease. Am J Roentgenol 2010;195:89–100. [DOI] [PubMed] [Google Scholar]
- 10. Poza-Cordón J, Ripollés-González T. Utility of abdominal ultrasonography in the diagnosis and monitoring of inflammatory bowel disease. Rev Esp Enferm Dig 2014;106:395–408. [PubMed] [Google Scholar]
- 11. Ripollés T, Martinez-Perez MJ, Blanc E, et al. Contrast-enhanced ultrasound (CEUS) in Crohn’s disease: technique, image, interpretation and clinical applications. Insights Imaging 2011;2:639–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- 13. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- 14. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–93. [DOI] [PubMed] [Google Scholar]
- 15. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw 2012;49:1–15. [Google Scholar]
- 16. Robotti D, Cammarota T, Debani P, Sarno A, Astegiano M. Activity of Crohn disease: value of color-power-Doppler and contrast-enhanced ultrasonography. Abdom Imaging 2004;29:648–52. [DOI] [PubMed] [Google Scholar]
- 17. Giangregorio F, Bertone A, Fanigliulo L, et al. Predictive value of time-intensity curves obtained with contrast-enhanced ultrasonography (CEUS) in the follow-up of 30 patients with Crohn’s disease. J Ultrasound 2009;12:151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Migaleddu V, Scanu AM, Quaia E, et al. Contrast-enhanced ultrasonographic evaluation of inflammatory activity in Crohn’s disease. Gastroenterology 2009;137:43–52. [DOI] [PubMed] [Google Scholar]
- 19. Ripollés T, Martínez MJ, Paredes JM, Blanc E, Flors L, Delgado F. Crohn disease: correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology 2009;253:241–8. [DOI] [PubMed] [Google Scholar]
- 20. De Franco A, Di Veronica A, Armuzzi A, et al. Ileal Crohn disease: mural microvascularity quantified with contrast-enhanced US correlates with disease activity. Radiology 2012;262:680–8. [DOI] [PubMed] [Google Scholar]
- 21. Quaia E, De Paoli L, Stocca T, Cabibbo B, Casagrande F, Cova MA. The value of small bowel wall contrast enhancement after sulfur hexafluoride-filled microbubble injection to differentiate inflammatory from fibrotic strictures in patients with Crohn’s disease. Ultrasound Med Biol 2012;38:1324–32. [DOI] [PubMed] [Google Scholar]
- 22. Paredes JM, Ripollés T, Cortés X, et al. Contrast-enhanced ultrasonography: usefulness in the assessment of postoperative recurrence of Crohn’s disease. J Crohns Colitis 2013;7:192–201. [DOI] [PubMed] [Google Scholar]
- 23. Ripollés T, Rausell N, Paredes JM, Grau E, Martínez MJ, Vizuete J. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn’s disease: a comparison with surgical histopathology analysis. J Crohns Colitis 2013;7:120–128. [DOI] [PubMed] [Google Scholar]
- 24. Falvey JD, Hoskin T, Meijer B, et al. Disease activity assessment in IBD. Inflamm Bowel Dis 2015;21:824–31. [DOI] [PubMed] [Google Scholar]
- 25. Lahiff C, Safaie P, Awais A, et al. The Crohn’s disease activity index (CDAI) is similarly elevated in patients with Crohn’s disease and in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2013;37:786–94. [DOI] [PubMed] [Google Scholar]
- 26. Spalinger J, Patriquin H, Miron MC, et al. Doppler US in patients with Crohn disease: vessel density in the diseased bowel reflects disease activity. Radiology 2000;217:787–91. [DOI] [PubMed] [Google Scholar]
- 27. Koh DM, Miao Y, Chinn RJ, et al. MR imaging evaluation of the activity of Crohn’s disease. Am J Roentgenol 2001;177:1325–32. [DOI] [PubMed] [Google Scholar]
- 28. Wold PB, Fletcher JG, Johnson CD, Sandborn WJ. Assessment of small bowel Crohn disease: noninvasive peroral CT enterography compared with other imaging methods and endoscopy – feasibility study. Radiology 2003;229:275–81. [DOI] [PubMed] [Google Scholar]
- 29. Katulska K, Wykrętowicz M, Stajgis P, Krokowicz Ł, Banasiewicz T, Stajgis M. Usefulness of magnetic resonance imaging in diagnosis and monitoring of treatment of perianal fistulas. Pol Przegl Chir 2014;86:383–90. [DOI] [PubMed] [Google Scholar]
- 30. Horsthuis K, Bipat S, Bennink RJ, Stoker J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008;247:64–79. [DOI] [PubMed] [Google Scholar]
- 31. Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis 2013;7:556–85. [DOI] [PubMed] [Google Scholar]
- 32. Novak K, Tanyingoh D, Petersen F, et al. Clinic-based point of care transabdominal ultrasound for monitoring Crohn’s disease: impact on clinical decision making. J Crohns Colitis 2015;795–801. [DOI] [PubMed] [Google Scholar]
- 33. Dong J, Wang H, Zhao J, et al. Ultrasound as a diagnostic tool in detecting active Crohn’s disease: a meta-analysis of prospective studies. Eur Radiol 2014;24:26–33. [DOI] [PubMed] [Google Scholar]
- 34. Paredes JM, Ripolles T, Cortes X, et al. Non-invasive diagnosis and grading of postsurgical endoscopic recurrence in Crohn’s disease: usefulness of abdominal ultrasonography and (99m)tc-hexamethylpropylene amineoxime-labelled leucocyte scintigraphy. J Crohns Colitis 2010;4:537–45. [DOI] [PubMed] [Google Scholar]
- 35. Calabrese E, La Seta F, Buccellato A, et al. Crohn’s disease: a comparative prospective study of transabdominal ultrasonography, small intestine contrast ultrasonography, and small bowel enema. Inflamm Bowel Dis 2005;11:139–45. [DOI] [PubMed] [Google Scholar]
- 36. Cittadini G, Giasotto V, Garlaschi G, de Cicco E, Gallo A, Cittadini G. Transabdominal ultrasonography of the small bowel after oral administration of a non-absorbable anechoic solution: comparison with barium enteroclysis. Clin Radiol 2001;56:225–30. [DOI] [PubMed] [Google Scholar]
- 37. Miao YM, Koh DM, Amin Z, et al. Ultrasound and magnetic resonance imaging assessment of active bowel segments in Crohn’s disease. Clin Radiol 2002;57:913–8. [DOI] [PubMed] [Google Scholar]
- 38. Limberg B, Osswald B. Diagnosis and differential diagnosis of ulcerative colitis and Crohn’s disease by hydrocolonic sonography. Am J Gastroenterol 1994;89:1051–7. [PubMed] [Google Scholar]
- 39. Esteban J M, Maldonado L, Sachiz V, Minguez M, Benages A. Activity of Crohn's disease assessed by colour Doppler ultrasound analysis of the affected loops. Eur Radiol 2001;11:1423–8. [DOI] [PubMed] [Google Scholar]
- 40. Neye H, Voderholzer W, Rickes S, Weber J, Wermke W, Lochs H. Evaluation of criteria for the activity of Crohns disease by power Doppler sonography. Dig Dis 2004;22:67–72. [DOI] [PubMed] [Google Scholar]
- 41. Piscaglia F, Nolsøe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012;33:33–59. [DOI] [PubMed] [Google Scholar]
- 42. Ma X, Li Y, Jia H, Zhang J, Wang G, Liu X, Song Y. Contrast-enhanced ultrasound in the diagnosis of patients suspected of having active Crohn’s disease: meta-analysis. Ultrasound Med Biol 2015;41:659–68. [DOI] [PubMed] [Google Scholar]
- 43. Białecki M, Białecka A, Laskowska K, et al. Contrast-enhanced ultrasonography for the determination of Crohn’s disease activity – preliminary experience. Pol J Radiol 2014;79:70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Payen T, Coron A, Lamuraglia M, et al. Echo-power estimation from log-compressed video data in dynamic contrast-enhanced ultrasound imaging. Ultrasound Med Biol 2013;39:1826–37. [DOI] [PubMed] [Google Scholar]