Abstract
Background and Aims:
In Western studies, one-third of patients with Crohn’s disease have stricturing or penetrating disease at presentation and one-half will progress to complicated disease in 20 years. Asian studies indicate that the Asian disease phenotype may be different. Our aim was to study the disease behaviour in Indian patients with Crohn’s disease.
Methods:
In this hospital-based study, we analysed [Montreal classification] disease phenotype, presence of perianal disease, need for intestinal surgery, and changes in the Montreal classification over time in Crohn’s disease patients from our database.
Results:
In the 178 patients (median age 35, interquartile range [IQR] 21 years; 97 males) with Crohn’s disease, the proportion of various featureswas as follows. More patients had ileo-colonic[L3: 43.8%] than ileal[L1: 27.5%] or colonic[L2: 28.7%] disease. Perianal disease was seen in 11.8% at baseline. Non-stricturing, non-fistulising disease[B1] was seen in 74.7%, 65.7%, 50%, and 44.4% at baseline, at 5, 10 and 15 years, respectively. Stricturing disease[B2] was seen in 21.4%, 21.9%, 28.9%, and 33.3%; penetrating disease[B3] in 3.9%, 11.4%, 21%, and 16.7%; and intestinal surgery was required in 10.7%, 20%, 34.2%, and 55.5%, respectively. KaplanMeier analysis showed no association between progression of disease and patient age or the location of the disease.
Conclusions:
Gender distribution and predominant ileo-colonic location of disease were similar to earlier Asian reports on Crohn’s disease. Perianal disease was less frequent than reported in Western and other Asian studies. One-fourth of Indian patients had aggressive disease at diagnosis, but the tendency to progress towards aggressive disease over time was less pronounced than in Western patients.
Key Words: Crohn’s disease, India, Montreal classification, natural history
1. Introduction
Studies from the West have shown that the location of disease in Crohn’s disease [CD] tends to be stable over time but its behaviour tends to vary not only between patients but also in the same patient at different times.1 After 15–20 years, the disease is reported to progress to a more aggressive phenotype in about half the patients.2 The stricturing and penetrating phenotypes are associated with a more complicated disease course, resulting in higher morbidity and surgery rates.3
The first attempt to classify disease behaviour was the Vienna classification4 which was later modified as the Montreal classification;5 the latter is now commonly used for classifying disease phenotype in CD. For paediatric patients, the Paris modification6 is used; this incorporates growth retardation and has the option of combining B2 and B3 disease.
The few Asian studies, from China7 and Korea,8 suggest that Asian patients with CD may behave differently from Western patients. There are epidemiological and phenotypic differences in CD between Chinese and Caucasian patients, including male predominance, lack of familial clustering, higher proportion of upper gastrointestinal tract involvement, and lower frequency of ileal disease in Chinese patients.7 Similarly Korean patients differed in gender distribution, disease location, and occurrence of perianal fistula; they may also have a more favourable clinical course, as indicated by a lower intestinal resection rate.8
No data are available on the phenotype and disease behaviour of Indian patients with CD, in whomwe studied the disease behaviour pattern using the Montreal classification and changes over time.
2. Patients and Methods
This retrospective analysis used the Crohn’s disease patient database of the Division of Gastroenterology in Mumbai, maintained since 2005. The diagnosis of CD was based on combination of clinical, histological and radiological, surgical, and serological parameters9 or the Lennard Jones criteria.10 Patients with uncertain diagnosis or incomplete data or follow-up were excluded. Records of 216 patients were screened [last patient in May 2014] and 178 were included in the study. Follow-up was done at scheduled visits or by telephone calls, letters, or email. Age at diagnosis, gender, baseline behaviour, location of disease, perianal involvement, and treatment including surgery were noted. The disease phenotype was classified as per the Montreal classification. Their subsequent course was noted at 5, 10, 15, and 20 years, and changes in Montreal class were noted along with requirement for surgery.
The study design was approved by the institutional review board.
2.1. Statistical methods
Data were entered into a spreadsheet. Chi-square test was used to compare proportions. KaplanMeier survival analysis was used to study change in behaviour over time and compare it, based on age at diagnosis and location of disease. Log rank test was used to compare survival curves.
3. Results
Of 216 patients screened, 38 were excluded for the following reasons: not fulfilling diagnostic criteria [15 patients], incomplete data [3 patients], incomplete follow-up [4 patients], or change in diagnosis later [16 patients]. The 178 patients included [median age 35, range 4.5 to 76 years, IQR 25 to 46 years; 97 males] had median duration of follow-up of 5 [IQR 1 to 7] years, 105 patients had follow-up of 5 years, 38 patients 10 years, and 18 patients 15 years.
Of the 178 patients, 59 [21.9 %] had received anti-tuberculous treatment and were later diagnosed as having CD. Tissue Mycobacterium culture was done from all these patients to rule out drug-resistant tuberculosis, before final confirmation as CD.
More than half the patients [55.1%] were in the age group 17 to 40 years [Montreal A2], followed by A3 [37.6%], and A1 [7.3%] [Table 1]. More patients had ileo-colonic [L3; 43.8%] than ileal disease [L1; 27.5%] or colonic [L2; 28.7%] disease. Perianal disease [p] was seen in 11.8 % of patients at baseline. Three-fourths of patients had non-stricturing, non-penetrating [inflammatory] phenotype, 21.4 % had stenotic, and 3.9% had fistulising phenotype at diagnosis. Upper gastrointestinal [GI] endoscopy was not routinely performed.
Table 1.
Montreal classification at diagnosis [n = 178], data as n [%].
| A1 | A2 | A3 | |||
| 13 [7.3 %] | 98 [55.1%] | 67 [37.6%] | |||
| L1 | L2 | L3 | |||
| 49 [27.5%] | 51 [28.7%] | 78 [43.8%] | |||
| B1 | B1p | B2 | B2p | B3 | B3p |
| 112 [62.9] | 21 [11.8] | 38 [21.4] | 0 | 7 [3.9] | 0 |
p, perianal.
Table 2 and Figures 1 and 2 show the disease behaviour, perianal disease, and surgeries at baseline and at 5, 10, and 15 years. At baseline one-fourth of patients had B2/B3 disease; after 10 and 15 years, the corresponding figures were 50% and 56%. However, this trend was not statistically significant. Table 3 shows the location of disease; this was stable over time.
Table 2.
Disease behaviour, perianal disease, and surgery. at baseline and at 5, 10, and 15 years’ follow-up, data as n [%].
| Disease behaviour | B0a | B1 | B2 | B3 | Perianal disease | Surgery |
|---|---|---|---|---|---|---|
| Baseline, n = 178 | 0 | 133 [74.7] | 38 [21.3] | 7 [3.9] | 21 [11.8] | 19 [10.7] |
| Cohort with 5 years’ data [n = 105] | ||||||
| [p = 0.14] | [p = 0.09] | [p = 0.51] | ||||
| At presentation | 0 | 77 [73.3] | 24 [22.8] | 4 [3.8] | 9 [8.6] | 17 [16.1] |
| 5 years | 1 [0.9] | 69 [65.7] | 23 [21.9] | 12 [11.4] | 17 [16.2] | 21 [20] |
| Cohort with 10 years’ data [n = 38] | ||||||
| [p = 0.58] | [p = 0.15] | [p = 0.40] | ||||
| At presentation | 0 | 24 [63.1] | 11 [28.9] | 3 [7.8] | 3 [7.8] | 8 [21] |
| 5 years | 0 | 21 [55.2] | 11 [28.9] | 6 [15.7] | 8 [21] | 12 [31.5] |
| 10 years | 0 | 19 [50] | 11 [28.9] | 8 [21] | 9 [23.6] | 13 [34.2] |
| Cohort with 15 years’ data [n = 18] | ||||||
| [p = 0.61] | [p = 0.5] | [p = 0.58] | ||||
| At presentation | 0 | 12 [66.6] | 6 [33.3] | 0 | 0 | 6 [33.3] |
| 5 years | 0 | 11 [61.1] | 6 [33.3] | 1 [5.5] | 2 [11.1] | 8 [44.4] |
| 10 years | 0 | 10 [55.5] | 6 [33.3] | 2 [11.1] | 2 [11.1] | 9 [50] |
| 15 years | 1 [5.5] | 8 [44.4] | 6 [33.3] | 3 [6.6] | 1 [5.5] | 10 [55.5] |
p-Values were calculated using chi-square test to compare parameters [behaviour, surgery, perianal disease] at baseline vs 5, 10, and 15 years.
aPatients had prolonged complete remission without medications.
Figure 1.
Behaviour at diagnosis, and at 5, 10, and 15 years’ follow-up.
Figure 2.
Proportion of patients in B1, B2, and B3 at diagnosis, and at 5, 10, and 15 years’ follow-up.
Table 3.
Location of disease at baseline and at 5, 10, and 15 years’ follow-up, data as n [%].
| Disease location | L0a | L1 | L2 | L3 | p-Value [vs at diagnosis] | L4 modifier |
|---|---|---|---|---|---|---|
| Baseline [n = 178] | 49 [27.5] | 51 [28.7] | 78 [43.8] | 12 [6.7] | ||
| Cohort with 5 years’ data [n = 105] | ||||||
| At presentation | 0 | 29 [27.6] | 30 [28.5] | 46 [43.8] | 5 [4.7] | |
| 5 years | 1 [0.9] | 31 [29.5] | 29 [27.6] | 44 [41.9] | 0.94 | 8 [7.6] |
| Cohort with 10 years’ data [n = 38] | ||||||
| At presentation | 11 [28.9] | 12 [31.5] | 15 [39.4] | 2 [5.2] | ||
| 5 years | 14 [36.8] | 10 [26.3] | 14 [36.8] | 0.74 | 2 [5.2] | |
| 10 years | 14 [36.8] | 8 [21.0] | 16 [42.2] | 0.56 | 2 [5.2] | |
| Cohort with 15 years’ data [n = 18] | ||||||
| At presentation | 7 [38.8] | 6 [33.3] | 5 [27.7] | 2 [11.1] | ||
| 5 years | 8 [44.4] | 6 [33.3] | 4 [22.2] | 0.91 | 2 [11.1] | |
| 10 years | 8 [44.4] | 4 [22.2] | 6 [33.3] | 0.75 | 2 [11.1] | |
| 15 years | 1 [5.5] | 8 [44.4] | 4 [22.2] | 5 [27.7] | 0.80 | 1 [5.5] |
p-Values were calculated using chi square test, to compare location from baseline vs 5, 10, and 15 years.
aPatients had prolonged complete remission without medications.
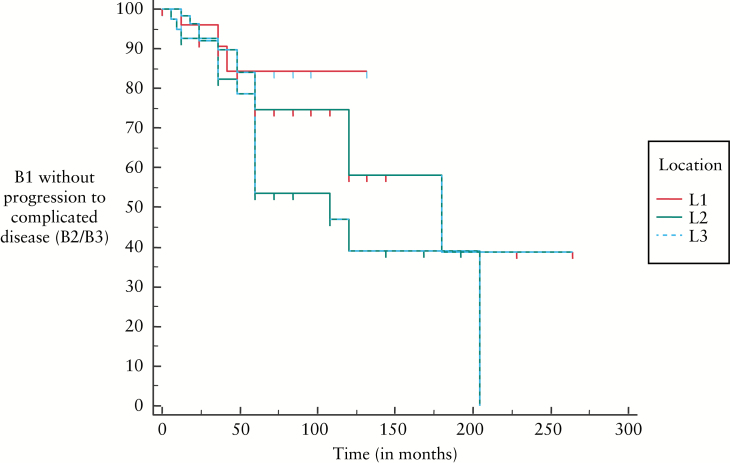
KaplanMeier curves [Figures 3 and 4] show that change from B1 to B2/B3 disease was not different between the age groups or between the locations of disease at time of diagnosis.
Figure 3.
KaplanMeier curves showing change from B1 to B2/B3 disease in relation to age at diagnosis [p = 0.56].
Figure 4.
KaplanMeier curves showing change from B1 to B2/B3 disease in relation to location of disease at diagnosis [p = 0.08].
3.1. Surgery
Of 178 patients, 19 [10.7%] presented with complications requiring surgery which led to the diagnosis of Crohn’s disease. After 5 years, 21 of 105 [20%] patients; after 10 years, 13 of 38 [34.2%] patients; and after 15 years, 10 of 18 [55.5%] patients had undergone intestinal surgery. [These figures do not include perianal surgeries.] Seven patients underwent more than one surgery.
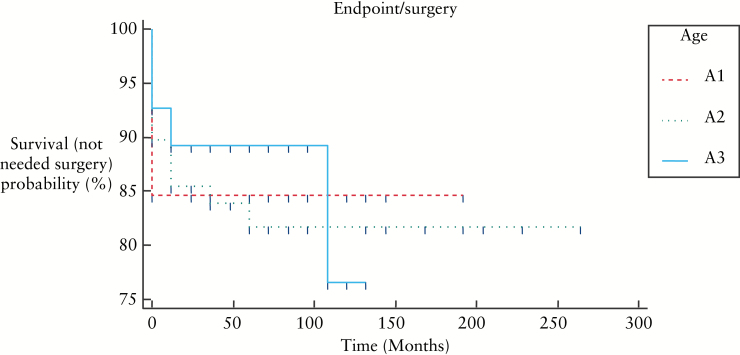
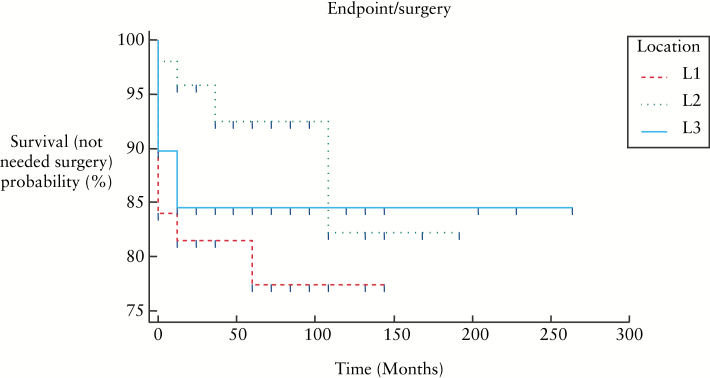
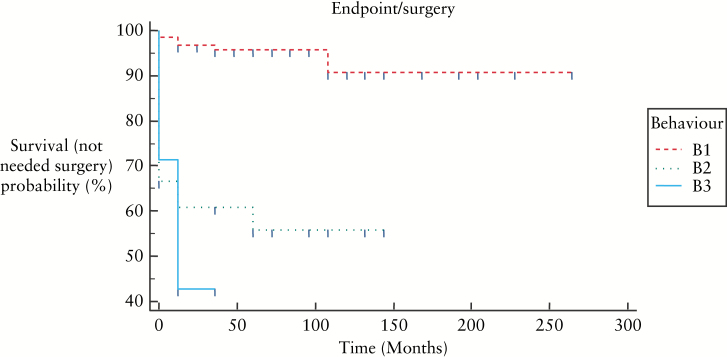
Figure 5, 6, and 7 show KaplanMeier curves revealing no association between need for surgery and age or disease location; however B2 and B3 behaviours were associated with need for surgery: B1 vs B2: p < 0.0001, B1 vs B3: p < 0.0001, B2 vs B3: p = 0.461.
Figure 5.
KaplanMeier curves showing need for surgery in relation to age at the time of diagnosis [p = 0.76].
Figure 6.
KaplanMeier curves showing need for surgery in relation to location of disease at the time of diagnosis [p = 0.177].
Figure 7.
KaplanMeier curves showing need for surgery in relation to behaviour of disease at the time of diagnosis [p < 0.0001].
4. Discussion
This is the first study reporting changes in Montreal phenotype from India. We report the temporal change in Montreal phenotype over 15 years in 178 patients with Crohn’s disease.
Our data show certain similarities to those reported in other Asian studies; the age of onset in our study was 31–40 years in more than half of our patients and there was no second peak. Some Western studies11,12,13 have reported two peaks, whereas others have not found this14,15; Asian studies have shown either a smaller second peak7,16 or no second peak.17,18 Our cohort had nearly equal gender distribution [males:females 1.2:1]. In most Western reports, women are equal or higher in proportion12,13,19; one European study20 and most Asian studies18,21,22 have shown male predominance [1.7:1:2.9:1]. We found the ileo-colonic region to be the most commonly affected site, as noted in other Asian studies3,8,16,23; Western studies report isolated colonic disease as the most common type.19,24,25
Perianal disease at the time of diagnosis was present in 12% of our patients, which is similar to other reports from India.26,27 In contrast, other Asian studies have reported much higher occurrence of perianal disease at diagnosis [Hong Kong 33%,[3] Korea 37%,[8] China 59%[22]]. Figures from Western studies vary from 16% to 27%.28,29
At baseline, the disease behaviour phenotype [75% B1, 21% B2, 4% B3] is similar to that seen in most Western studies and in a national survey by the Indian Society of Gastroenterology.27 A multicentre Indian study by Das et al.30 reported a more complicated phenotype [51% B1, 24% B2, 25% B3], but both these Indian studies27,30 indicated disease behaviour at inclusion, not at baseline.
Western studies have shown that a large proportion of patients change their behaviour pattern to a more aggressive type [B2 and B3] after 5, 10, and 15 years of follow-up.2 In the older studies based on the Vienna classification, more patients were reported as having B3 as this category included perianal disease. Table 4 shows studies on disease phenotype based on the Montreal classification. The study from Korea by Ye et al.8 reported similar behaviour phenotype at time of diagnosis as our study. Chow et al.3 reported more patients with aggressive disease at baseline and more likelihood of disease progression than in our study. Lakatos et al. 31 noted a change in disease behaviour in 31% of patients with initial non-stricturing non-penetrating disease, after mean disease duration of 9±7 years; we noted change to more aggressive disease at 10 years in only 20% of patients. Western studies28,32 have also shown faster progression as compared with our data. A limitation of our study was the small number of patients at 10 and 15 years and a shorter median duration of follow-up.
Table 4.
Studies on natural history of Crohn’s disease based on Montreal classification.
| Baseline | 5 years | 10 years | 15 years | ||||
|---|---|---|---|---|---|---|---|
| Present study | B1 | 74.7 % | 65 % | 50 % | 44.4 % | ||
| B2 | 21.3 % | 21 % | 28.9 % | 33.3 % | |||
| B3 | 3.9 % | 11 % | 21% | 16.6 % | |||
| Chow et al.3
2007 |
B1 | 67 % | 50 % | 43 % | |||
| B2 | 30 % | 45 % | 43 % | ||||
| B3 | 3 % | 5 % | 14 % | ||||
| Thia et al.29
2010 |
B1 | 81 % | 64% | 58% | |||
| B2 | 5 % | 12% | 15% | ||||
| B3 | 14% | 24% | 17% | ||||
| Tarrant et al.
200828 |
B1 | 73% | 56% | 44 % | |||
| B2 | 17 % | 25% | 31 % | ||||
| B3 | 10 % | 20% | 25 % | ||||
| Lovasz et. al.32 | B1 | 57% | |||||
| B2 | 20% | ||||||
| B3 | 23% | ||||||
| Solberg et al.36 | B1 | 62 | 47 | ||||
| B2 | 27 | 31 | |||||
| B3 | 11 | 22 | |||||
| EE | WE | A | |||||
| Vegh et al.
37
[2011] |
B1 | 57% | 73% | 79% | |||
| B2 | 27% | 17% | 11% | ||||
| B3 | 16% | 10% | 10% | ||||
| Burisch et al.38
[2010] |
B1 | 68% | 68% | ||||
| B2 | 19% | 21% | |||||
| B3 | 13% | 12% | |||||
EE, Eastern Europe; WE, Western Europe; A, Australia.
In our study, surgery rates are 10.7 %, 20%, 34.2%, and 55.5% at diagnosis, at 5 years, at 10 years, and at 15 years, respectively. Surgery rates have been described in wide ranges in various studies, but overall they are less in Asian studies7,8 as compared with Western studies.33,34,35
In summary, there are similarities in our data with other Asian studies: age of onset, absence of a second peak, gender distribution, and location of disease. Our patients differed from Western and other Asian patients in lower occurrence of perianal disease. The location of disease in our patients remained stable over time. One-fourth of our patients with Crohn’s disease had aggressive behaviour at presentation; however, fewer patients progressed to stricturing or penetrating behaviour than those seen in Western reports. The surgical rates were also less than in a majority of reported studies.
Funding
None received.
Conflict of Interest
Nothing to declare.
References
- 1. Louis E, Collard A, Oger AF, Degroote E, El Yafi FAN, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut 2001;49:777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behaviour of Crohn’s disease. Inflamm Bowel Dis 2002;8:244–50. [DOI] [PubMed] [Google Scholar]
- 3. Chow DKL, Leong RWL, Lai LH, et al. Changes in Crohn’s disease phenotype over time in the Chinese population: validation of the Montreal classification system. Inflamm Bowel Dis 2008;14:536–41. [DOI] [PubMed] [Google Scholar]
- 4. Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis 2000;6:8–15. [DOI] [PubMed] [Google Scholar]
- 5. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19[Suppl A]:5A–36A. [DOI] [PubMed] [Google Scholar]
- 6. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- 7. Leong RWL, Lau JY, Sung JJY. The epidemiology and phenotype of Crohn’s disease in the Chinese population. Inflamm Bowel Dis 2004;10:646–51. [DOI] [PubMed] [Google Scholar]
- 8. Ye BD, Yang S-K, Cho YK, et al. Clinical features and long-term prognosis of Crohn’s disease in Korea. Scand J Gastroenterol 2010;45:1178–85. [DOI] [PubMed] [Google Scholar]
- 9. Van Assche G, Dignass A, Panes J, et al. The second European Evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis 2010;4:7–27. [DOI] [PubMed] [Google Scholar]
- 10. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl 1989;170:2–6; discussion 16–9. [DOI] [PubMed] [Google Scholar]
- 11. Ekbom A, Helmick C, Zack M, Adami HO. The epidemiology of inflammatory bowel disease: a large, population-based study in Sweden. Gastroenterology 1991;100:350–8. [DOI] [PubMed] [Google Scholar]
- 12. Loftus CG, Loftus E V, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis 2007;13:254–61. [DOI] [PubMed] [Google Scholar]
- 13. Loftus EV, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn’s disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology.1998;114:1161–8. [DOI] [PubMed] [Google Scholar]
- 14. Shivananda S, Lennard-Jones J, Logan R, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease [EC-IBD]. Gut 1996;39:690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernández A, Hernández V, Martínez-Ares D, et al. Incidence and phenotype at diagnosis of inflammatory bowel disease. Results in Spain of the EpiCom study. Gastroenterol Hepatol 2015;38:534 40. [DOI] [PubMed] [Google Scholar]
- 16. Yang S-K, Yun S, Kim J-H, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: A KASID study. Inflamm Bowel Dis 2008;14:542–9. [DOI] [PubMed] [Google Scholar]
- 17. Ouyang Q, Tandon R, Goh KL, et al. Management consensus of inflammatory bowel disease for the Asia-Pacific region. J Gastroenterol Hepatol 2006;21:1772–82. [DOI] [PubMed] [Google Scholar]
- 18. Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol 2009;44:659–65. [DOI] [PubMed] [Google Scholar]
- 19. Sjöberg D, Holmström T, Larsson M, et al. Incidence and clinical course of Crohn’s disease during the first year results from the IBD Cohort of the Uppsala Region [ICURE] of Sweden 2005–2009. J Crohns Colitis 2014;8:215–22. [DOI] [PubMed] [Google Scholar]
- 20. Burisch J, Pedersen N, Čuković-Čavka S, et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut 2014;63:588–97. [DOI] [PubMed] [Google Scholar]
- 21. Moon CM, Park D, Kim ER, et al. Clinical features and predictors of clinical outcomes in Korean patients with Crohn’s disease: a Korean association for the study of intestinal diseases multicenter study. J Gastroenterol Hepatol 2014;29:74–82. [DOI] [PubMed] [Google Scholar]
- 22. Zeng Z, Zhu Z, Yang Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol 2013;28:1148–53. [DOI] [PubMed] [Google Scholar]
- 23. Oriuchi T, Hiwatashi N, Kinouchi Y, et al. Clinical course and longterm prognosis of Japanese patients with Crohn’s disease: predictive factors, rates of operation, and mortality. J Gastroenterol 2003;38:942–53. [DOI] [PubMed] [Google Scholar]
- 24. Lapidus A. Crohn’s disease in Stockholm County during 1990–2001: an epidemiological update. World J Gastroenterol 2006:12:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolters FL, Russel MG, Sijbrandij J, et al. Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut 2006;55:1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goel A, Dutta AK, Pulimood AB, Eapen A, Chacko A. Clinical profile and predictors of disease behaviour and surgery in Indian patients with Crohn’s disease. Indian J Gastroenterol 2013;32:184–9. [DOI] [PubMed] [Google Scholar]
- 27. Makharia GK, Ramakrishna BS, Abraham P, et al. Survey of inflammatory bowel diseases in India. Indian J Gastroenterol 2012;31:299–306. [DOI] [PubMed] [Google Scholar]
- 28. Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn’s disease phenotype results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol 2008;103:3082–93. [DOI] [PubMed] [Google Scholar]
- 29. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010;139:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Das K, Ghoshal UC, Dhali GK, Benjamin J, Ahuja V, Makharia GK. Crohn’s Disease in India: A Multicenter Study from a Country Where Tuberculosis Is Endemic. Dig Dis Sci 2009;54:1099–107. [DOI] [PubMed] [Google Scholar]
- 31. Lakatos PL, Czegledi Z, Szamosi T, et al. Perianal disease, small bowel disease, smoking, prior steroid or early azathioprine/biological therapy are predictors of disease behaviour change in patients with Crohn’s disease. World J Gastroenterol 2009;15:3504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lovasz BD, Lakatos L, Horvath A, et al. Evolution of disease phenotype in adult and pediatric onset Crohn’s disease in a population-based cohort. World J Gastroenterol 2013;19:2217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hellers G. Crohn’s disease in Stockholm county 1955–1974. A study of epidemiology, results of surgical treatment and long-term prognosis. Acta Chir Scand Suppl 1979;490:1–84. [PubMed] [Google Scholar]
- 34. Lakatos PL, Golovics PA, David G, et al. Has there been a change in the natural history of Crohn’s disease? Surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977–2009. Am J Gastroenterol 2012;107:579–88. [DOI] [PubMed] [Google Scholar]
- 35. Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota [1970–2004]. Am J Gastroenterol 2012;107:1693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Solberg IC, Vatn MH, Høie O, et al. IBSEN Study Group. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol 2007;5:1430–8. [DOI] [PubMed] [Google Scholar]
- 37. Vegh Z, Burisch J, Pedersen N, et al. Incidence and initial disease course of inflammatory bowel diseases in 2011 in Europe and Australia: results of the 2011 ECCO-EpiCom inception cohort. J Crohns Colitis 2014;8:1506–15. [DOI] [PubMed] [Google Scholar]
- 38. Burisch J, Pedersen N, Cukovic-Cavka S, et al. Initial Disease Course and Treatment in an Inflammatory Bowel Disease Inception Cohort in Europe. Inflamm Bowel Dis 2014;20:36–46. [DOI] [PubMed] [Google Scholar]