Abstract
Background and Aims:
There is an unexplained association between ulcerative colitis [UC] and primary sclerosing cholangitis [PSC], with the intestinal microbiota implicated as an important factor. The study aim was to compare the structure of the intestinal microbiota of patients with UC with and without PSC.
Methods:
UC patients with PSC [PSC-UC] and without PSC [UC] were identified from biobanks at Oslo University Hospital, Foothills Hospital Calgary and Mount Sinai Hospital Toronto. Microbial DNA was extracted from colonic tissue and sequencing performed of the V4 region of the 16S rRNA gene on Illumina MiSeq. Sequences were assigned to operational taxonomic units [OTUs] using Quantitative Insights Into Microbial Ecology [QIIME]. Microbial alpha diversity, beta diversity, and relative abundance were compared between PSC-UC and UC phenotypes.
Results:
In all, 31 PSC-UC patients and 56 UC patients were included. Principal coordinate analysis [PCoA] demonstrated that city of sample collection was the strongest determinant of taxonomic profile. In the Oslo cohort, Chao 1 index was modestly decreased in PSC-UC compared with UC [p = 0.04] but did not differ significantly in the Calgary cohort. No clustering by PSC phenotype was observed using beta diversity measures. For multiple microbial genera there were nominally significant differences between UC and PSC-UC, but results were not robust to false-discovery rate correction.
Conclusions:
No strong PSC-specific microbial associations in UC patients consistent across different cohorts were identified. Recruitment centre had a strong effect on microbial composition. Future studies should include larger cohorts to increase power and the ability to control for confounding factors.
Key Words: PSC, intestinal microbiota, next generation sequencing
1. Introduction
The human intestinal microbiota is a community of 1011–1014 organisms which reside in the intestinal tract.1 Changes in the composition and function of intestinal microbiota are increasingly being recognised to have an important role in the pathogenesis of the inflammatory bowel diseases [IBD], as well as other chronic inflammatory diseases.2,3 Primary sclerosing cholangitis [PSC] is an immune-mediated, chronic liver disease characterised by inflammation and fibrosis of bile ducts. It is a progressive condition without effective treatment, which in a large proportion of patients progresses to cirrhosis and the need for transplantation.4 The co-occurrence of IBD, predominantly ulcerative colitis [UC], in subjects with PSC is 60–80%, and 2–4% of patients with IBD develop PSC.5,6
The overlap between UC and PSC may in part be explained by shared genetic susceptibility.7.Polymorphisms, including variants associated with both UC and PSC, may alter the abundance of a deleterious intestinal microbiota capable of entero-hepatic intrusion. Alternatively, these polymorphisms may amplify the sensitivity of the biliary epithelium to microbial antigens, resulting in inflammation and fibrosis. Thus far however, genetics has only explained a fraction of disease risk, suggesting that environmental factors are also important. Among a host of potential candidate environmental factors, gut microbial components are of interest as they may mediate intestinal inflammation by local effects and hepatic inflammation through the entero-hepatic circulation of microbial factors, as shown in the cholangitis model in rats with bacterial overgrowth.8 The first treatment trials in PSC were performed with antibiotics and, although so far not proven clinically effective, antibiotics do seem to influence disease activity parameters.9 The aim of this study was to compare the structure of the intestinal microbiota of UC patients with PSC [PSC-UC] and without PSC [UC] in order to identify a PSC-specific microbial profile.
2. Materials and Methods
2.1. Study patients
Three academic medical centres participated in this study: Oslo University Hospital Centre, Oslo [Oslo], Foothills Hospital Calgary [Calgary] and Mount Sinai Hospital Toronto [MSH]. Existing tissue biobanks were reviewed to identify individuals with PSC-UC and UC with available endoscopic colonic biopsies. The diagnosis of UC was made by accepted clinical, endoscopic and pathological criteria.10 The diagnosis of PSC was made by accepted clinical, radiological and pathological criteria including: chronically elevated liver enzymes; standard radiological evidence of PSC on endoscopic retrograde cholangiopancreatography or magnetic resonance cholangiopancreatography; and/or liver biopsy consistent with PSC; and exclusion of a secondary cause of cholangitis.5,11 Subjects were excluded if, at the time of endoscopic biopsy, they were receiving antibiotic, ursodeoxycholic acid, corticosteroid, immunosuppressant or biological therapy. Subjects with a previous orthotopic liver transplant were also excluded. Following review of biobanks and application of exclusion criteria, n = 19 Oslo PSC-UC, n = 18 Oslo UC, n = 12 Calgary PSC-UC and n = 12 Calgary UC subjects were identified. MSH provided a further control group of n = 26 UC subjects [Supplementary Figure 1, available as Supplementary data at ECCO-JCC online]. To minimize the confounding effect of mucosal inflammation on microbial composition only subjects with an endoscopic Mayo score ≤ 1 at the time of biopsy were included in final analyses; Oslo PSC-UC n = 19, Oslo UC n = 9, Calgary PSC-UC n = 12, Calgary UC n = 12, MSH UC n = 18 [Table 1]. Data collected included UC disease duration, current medications, and endoscopic Mayo score at time of biopsy. Subjects in the UC cohort were screened to rule out PSC by review of their history, liver enzymes, liver synthetic function and, where available, imaging studies. Additional data collected from PSC-UC subjects included: PSC disease duration, liver enzyme values, and liver synthetic function. Three additional healthy controls from MSH with snap-frozen, and RNAlater solution [Qiagen, Hilden, Germany] preserved endoscopic biopsies were also included in the study, solely to assess the effect of biopsy preservation method on colonic tissue microbial composition; these subjects were not included in any other analyses.
Table 1.
Baseline characteristics of Oslo, Calgary [Cal], and Mount Sinai [MSH] cohorts.
| Oslo PSC-UC [n=19] | Oslo UC [n=18] | Cal PSC-UC [n=12] | Cal UC [n=12] |
MSH UC
[n=26] |
|
|---|---|---|---|---|---|
| Age at diagnosis* [years] | 43 [19–77] | 32 [16–61] | 39 [24–59] | 34 [8–66] | 26 [14–57] |
| Gender [male] | 15 [75]% | 11 [55%] | 7 [58%] | 6 [50%] | 15 [54%] |
| IBD duration* [years] | 15 [0–50] | 0 | 2.2 [0.2–23] | 10.3 [0.6–51] | 6.2 [0–44] |
| 5-aminosalicylate use | 16 [80%] | 0 [0%] | 4 [33%] | 10 [83%] | 24 [86%] |
| Biopsy preservation method | Snap-frozen | Snap-frozen | RNAlater | RNAlater | Snap-frozen |
| Endoscopic Mayo score ≤ 1 | 19 [100%] | 9 [50%] | 12 [100%] | 12 [100%] | 18 [69%] |
PSC-UC, primary sclerosing cholangitis-ulcerative colitis; Cal, Calgary; MSH, Mount Sinai Hospital; IBD, inflammatory bowel disease.
2.2. Sample collection, handling and DNA extraction
Endoscopic biopsies from the left colon were collected. It was elected to focus on left-sided colonic samples to ensure that all included biopsies were from UC-affected segments of the colon, given a proportion of the cohort had only left-sided colitis. Samples from Oslo and MSH were placed into a sterile, empty freezer vial, snap-frozen in liquid nitrogen, and stored at -80°C. Endoscopic biopsies from Calgary were collected in a sterile vial containing RNAlater [Qiagen, Hilden, Germany] and stored at -80°C. Biospecimens from Oslo and Calgary were then transported under appropriate storage conditions to the MSH tissue biobank for processing. Tissue bacterial DNA was extracted using the Qiagen DNeasy blood and tissue kit [Qiagen, Hilden, Germany], with an additional bead-beating step and proteinase K treatment applied to ensure adequate bacterial cell lysis. DNA extractions for all included biospecimens were performed at a single centre [MSH] by a single technician using an identical protocol and the same Qiagen extraction kit across all samples.
2.3. Taxonomic profiling of gut microbiota
The V4 hypervariable region of bacterial 16S ribosomal RNA [16S rRNA] was sequenced on the Illumina MiSeq platform [Illumina Inc., CA, USA], generating paired-end reads of 175 base pairs in length. Paired-end reads were stitched together [approximately 97 base pair overlap] and further processed in a data curation pipeline implemented in Quantitative Insights Into Microbial Ecology [QIIME] 1.7.0.2 Samples with fewer than 1000 reads after quality filtering were removed. Chimeric sequences were identified and removed using reference based and de novo searches using the USEARCH algorithm in QIIME, which has a built in chimera detection step [http://qiime.org/scripts/pick_otus.html].12,13 Non-chimeric sequences were then clustered into operational taxonomic units [OTUs] at 97.0% sequence similarity using a closed reference-based picking approach with UCLUST software against Greengenes reference database [version 13_5]. OTUs present in less than five samples and without genus or species level resolution were removed from the analysis, reducing the pre-filtering OTU count of 3074 to 76.
2.4. Statistical analysis
Rarefaction [1000 samplings] was performed at a depth of 1000 reads per sample, and these rarefied values were used for all diversity analyses. Mean alpha diversity across all rarefactions was used as an estimate of overall alpha diversity of each sample. Beta diversity was estimated using Bray-Curtis-Faith, weighted Unifrac, Spearman, Euclidian, and unweighted Unifrac indices,14,15 and were visualised using principal coordinates analysis [PCoA] to determine if there were large patters of variability in the data.
Based on preliminary analyses, suggesting large patterns of variability due to geographical origin of samples, analyses were performed comparing phenotypic groups within the Oslo and Calgary cohorts separately, with the MSH samples only included in selected sub-analyses. Raw OTU counts were converted to relative abundance and dichotomised based on whether an OTU was present or absent in a sample. Microbial relative abundance and alpha-diversity values were compared between PSC-UC and UC phenotypes using a non-parametric Kruskal-Wallis test. Fisher’s exact test [FET] was used to analyse dichotomised results. False-discovery rate [FDR] correction for multiple testing was applied to all statistical tests to minimise type I errors and, although nominal p-values < 0.05 are reported, only a corrected p-value of < 0.05 was considered significant. Statistical analysis was performed using R [version 3.1.0].
2.5. Ethical considerations
All subjects provided written informed consent. The study received ethics approval from institutional research ethics boards at all sites.
3. Results
3.1. Baseline demographics of the study cohort
The baseline characteristics of the Oslo, Calgary, and MSH cohorts are described in Table 1 and Supplementary Table 1 [available as Supplementary data at ECCO-JCC online]. All subjects had established UC at the time of endoscopic biopsy collection [disease duration at time of endoscopic biopsies is described in Table 1] except subjects in the Oslo UC cohort who had endoscopic biopsies taken at the time of UC diagnosis. Included subjects were minimally medicated, with a proportion of subjects having 5-aminosalicylate [5ASA] exposure [see Table 1] but none using corticosteroids, immunomodulators or biologicals at the time of endoscopic sampling. The mean numbers of reads per sample in the Oslo, Calgary, and MSH cohorts were 3323, 14103, and 15948, respectively.
3.2. Heterogeneity between recruitment centre study populations
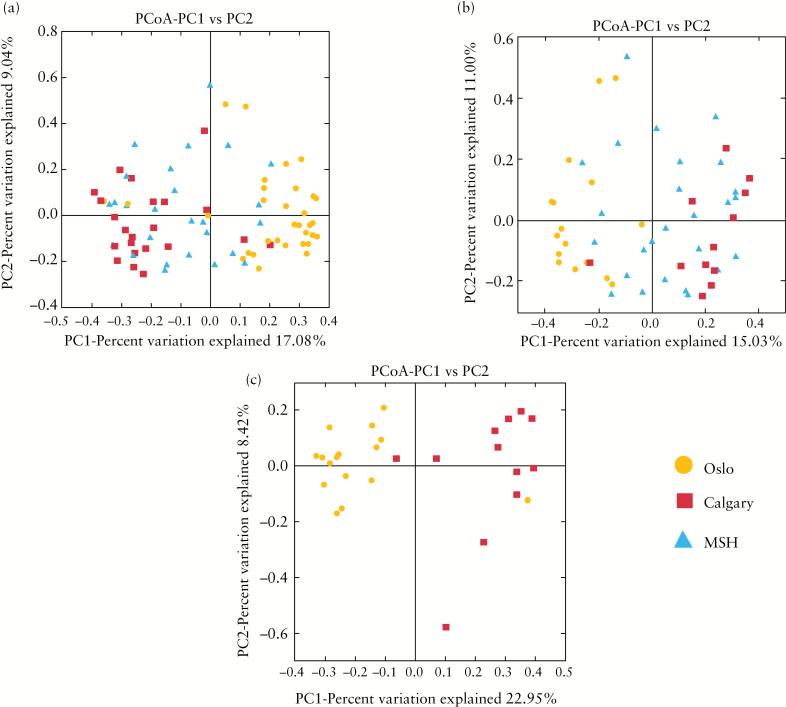
The confounding effect of the geographical origin of samples included in the study on microbial composition was assessed in the combined cohort, as well as the UC and PSC-UC groups, respectively from each of the three recruitment centers. Beta diversity and PCoA demonstrate distinct clustering of samples by recruitment centre in not only the entire study cohort, but also where UC and PSC-UC groups were evaluated separately [Figure 1, Supplementary Figures 2–4, available as Supplementary data at ECCO-JCC online]. At the genus level, several organisms appeared to drive this clustering. Flavobacteria were detected exclusively, although at low relative abundance, in the samples obtained from MSH [p= 3.9 x 10–13]. Alternatively, Pseudomonas was detected at low abundance in all Calgary samples, less frequently in MSH samples [38%] and less frequently again in samples from Oslo [8%], p = 8.7 x 10-10. Eleven additional organisms were detected in significantly different proportions of individuals in the three cohorts. In keeping with this observation, the relative abundance of 17 taxa were varied at each of the centres [nominal p < 0.05]. However, only the relative abundance of Flavobacteria remained significant after correction for multiple testing.
Figure 1.
Effect of recruitment centre on intestinal microbial composition. A. Considering all samples (Oslo, Calgary, and Mount Sinai Hospital [MSH]), beta diversity [Bray Curtis Faith], and principal coordinate analysis [PCoA] demonstrate distinct clustering of samples by recruitment centre. B. Considering only ulcerative colitis [UC] samples [Oslo, Calgary, and MSH], beta diversity [Bray Curtis Faith] and PCoA demonstrate distinct clustering of samples by recruitment centre. C. Considering only primary sclerosing cholangitis [PSC] samples [Oslo and Calgary], beta diversity [Bray Curtis Faith] and PCoA demonstrate distinct clustering of samples by recruitment centre.
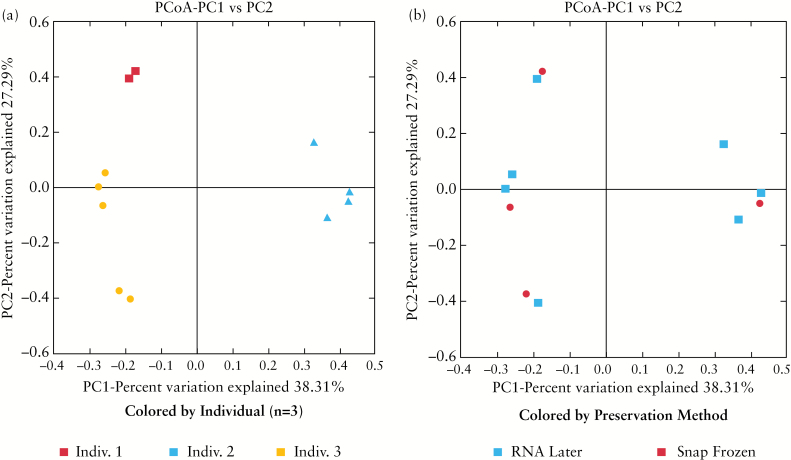
Aside from the effect of geographical origin of samples, there were also differences in biopsy handling between recruitment centres [Table 1]. To evaluate whether these differences represented a significant confounder, we performed a subanalysis in a small cohort of individuals with samples preserved via both snap-freezing and RNAlater [n = 3]. Major clustering occurred at the individual level, with all samples taken from the same biopsy location in the same person appearing to cluster together, suggesting a comparatively small effect of preservation method on the composition of the microbiome [Figure 2].
Figure 2.
Effect of biopsy preservation method on intestinal microbial beta diversity [Bray Curtis Faith]. Beta diversity [Bray Curtis Faith] and principal coordinate analysis [PCoA] demonstrated major clustering occurred at the individual level, suggesting a comparatively small effect of preservation method on the composition of the microbiome. Indiv., individual.
3.3. Comparison of alpha and beta diversity between phenotypic groups
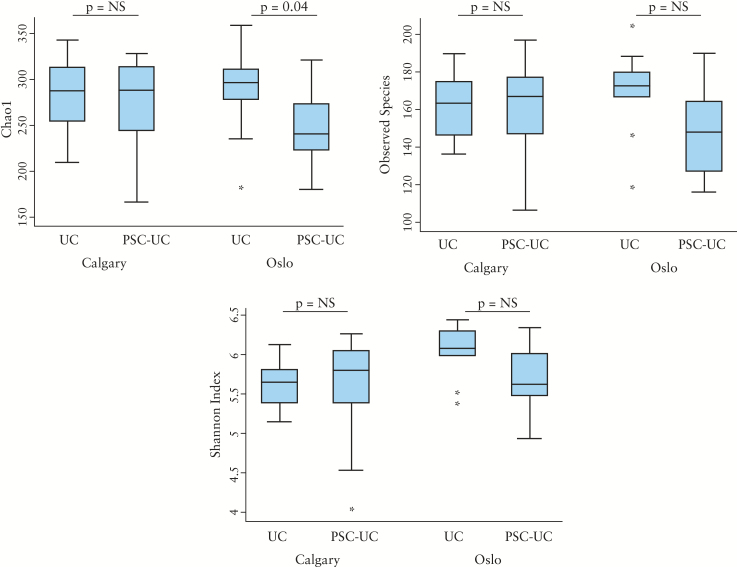
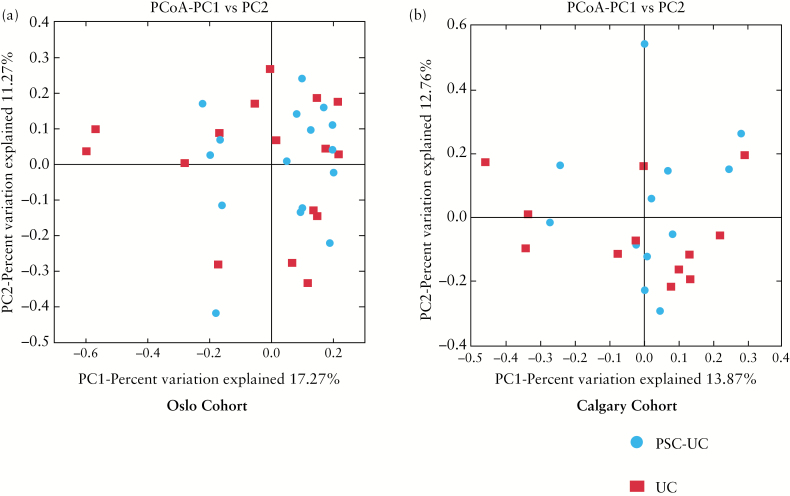
Microbial diversity was investigated in Oslo and Calgary centres separately. In the Oslo cohort, the Chao 1 index was modestly decreased in PSC-UC compared with UC [p = 0.04], but in the Calgary cohort differences in this diversity index between groups did not reach statistical significance. Other measures of alpha diversity [Shannon, observed species] did not suggest a significant difference in microbial diversity between the primary outcome groups [Figure 3]. No apparent clustering was observed using any of the measured beta diversity indices in PCoA analysis between the phenotypic groups [Figure 4, Supplementary Figures 5–7, available as Supplementary data at ECCO-JCC online].
Figure 3.
Comparison of alpha diversity indices between PSC-UC and UC. A, B, and C display alpha diversity expressed as Chao1, observed species, and Shannon index, respectively. Each figure shows Oslo and Calgary cohorts separately and compares alpha diversity in PSC-UC and UC. The Chao1 index was modestly decreased in Oslo PSC-UC compared with UC [p = 0.04]. However, in the Calgary cohort differences in this diversity index between groups did not reach statistical significance. Other measures of alpha diversity [Shannon, observed species] did not differ between phenotypic groups.
Figure 4.
Effect of primary sclerosing cholangitis [PSC] phenotype on microbial beta diversity [Bray Curtis Faith] in Oslo and Calgary cohorts. Beta diversity [Bray Curtis Faith] and principal coordinate analysis [PCoA] did not demonstrate clustering between PSC-UC and UC phenotypic groups in either Oslo [A] or Calgary cohorts [B].
3.4. Comparisons of genus-level taxonomy between phenotypic groups
Comparison of taxonomic groups at the genus level were made between PSC-UC and UC groups from Oslo and Calgary [Table 2]. A number of genera differed in relative abundance between PSC-UC and UC with nominal statistical significance [p < 0.05], but these differences did not remain significant after correction for multiple testing. Additionally, organisms found to nominally associate with outcome were not consistent between the cohorts.
Table 2.
Genera which showed a nominal association with primary sclerosing cholangitis [PSC] phenotype with p-value < 0.05.
| Oslo Cohort | Calgary Cohort | ||
|---|---|---|---|
| Organism | p-Value* | Organism | p-Value* |
| Acinetobacter | 0.006 | Acinetobacter | 0.182 |
| Lactobacillus | 0.009 | Lactobacillus | 0.792 |
| Corynebacterium | 0.015 | Corynebacterium | 0.630 |
| Veillonella | 0.015 | Veillonella | 0.523 |
| Ralstonia | 0.018 | Ralstonia | 0.630 |
| Rothia | 0.030 | Rothia | 0.538 |
| Sphingomonas | 0.032 | Sphingomonas | -- |
| Lachnospira | 0.453 | Lachnospira | 0.012 |
| Oscillospira | 0.908 | Oscillospira | 0.020 |
| Roseburia | 0.166 | Roseburia | 0.024 |
| Ruminococcus | 0.908 | Ruminococcus | 0.031 |
| Collinsella | 0.355 | Collinsella | 0.038 |
Analysis performed separately on Calgary and Oslo samples. Genera which demonstrated a nominal association [p < 0.05] with PSC phenotype in either Oslo or Calgary cohort analysis are included in the table. Bolded genera demonstrated a nominal association with p-value < 0.05. No results significant across all comparisons following false-discovery rate [FDR] correction.
*Uncorrected p-value; Kruskal-Wallis test.
4. Discussion
In this study of more than 30 PSC-UC patients and 50 UC patients from three centres, we were not able to demonstrate a consistently significant difference in microbial abundance, at the genus level, associated with PSC in our cohorts of UC patients; furthermore, the organisms identified at nominal significance thresholds were not consistent across study populations. Microbial alpha diversity was modestly decreased in Oslo PSC-UC compared with UC subjects assessed by the Chao1 index, suggesting that the composition of the microbiome may be different among our outcome groups; however, we likely lacked statistical power to detect organism-level differences. One of the more important observations in this report is the strong effect of recruitment centre on microbiome composition. Our data demonstrate that the geographical origin of samples markedly effects the composition of the intestinal microbiome, the effects of which far obscure any disease-specific effects in our cohorts.
The well-documented strong association of IBD and PSC suggests a common pathogenic agent or inflammatory pathway that initiates and/or perpetuates intestinal and hepatic inflammation. Recent studies in mice have implicated the intestinal microbiota as one such candidate.4 Human studies have added weight to this hypothesis, demonstrating that microbial antigens are present in specimens from PSC livers and that bacteria may be detected in biliary cultures from PSC patients.16 A recent report from a singe centre, examining a small cohort of PSC, UC, and non-inflammatory controls using a phylogenetic microarray demonstrated decreased microbial diversity and richness in PSC compared with UC subjects. In addition, the relative abundance of uncultured Clostridiales II was demonstrated to be significantly lower in PSC compared with UC, a finding we did not replicate.17 Despite this initial report, to date studies of the contribution of the intestinal microbiota to PSC have been few. A contributory factor to the paucity of data in this area has been the difficulty in characterising the intestinal microbiota by culture-dependent techniques. Recent advances in next-generation sequencing technologies have allowed the characterisation of complex microbial communities in a culture-independent manner. These techniques use bacterial DNA sequences as signatures, allowing the estimation of organism identity, relative abundance, and function; 16S ribosomal RNA [rRNA] gene sequencing has become the widely accepted method used to most accurately determine intestinal bacterial composition.18
The absence of a strong microbial association with PSC in this study may reflect a true lack of a differential microbial contribution in UC associated with PSC. However, we believe that this absence is more likely to reflect a number of study design and technical factors resulting in reduced statistical power. Recruiting an adequate number of patients for translational studies such as ours can be challenging, particularly when studying low-prevalence diseases such as PSC. Although a number of large academic centres collaborated in the conduct of this study, the number of subjects included remained relatively small as we sought to recruit minimally medicated subjects with minimal or absent intestinal inflammation—with the result that our study may have been underpowered to detect significant microbial associations with PSC. We focused on the recruitment of homogeneous case and control cohorts to reduce potential confounding; however, despite these efforts, significant heterogeneity between study populations may have hampered our ability to detect associations. Study cohorts significantly differed by location of recruitment sites, IBD disease duration, proportion of 5-ASA use and biopsy preservation method, all of which may have hindered our ability to detect microbial associations with PSC phenotype in UC. From the technical point of view, although we used state-of-the-art next-generation sequencing technology [Illumina sequencing by synthesis], it is possible that a greater depth of sequencing might have discovered lower-abundance organisms associated with PSC.
Mouse studies evaluating the intestinal microbiota have long demonstrated a strong ‘cage effect’, with animals housed together having significantly more similar gut microbiota than those housed apart even where diet and other environmental factors are controlled.19 Given most human microbiota studies cannot be performed under such controlled conditions, and our study comprised populations from diverse geographical regions, we were concerned that recruitment centre would represent a significant confounder. This was what we observed, with city of recruitment having a strong effect on intestinal microbial composition. These differences are likely driven by multiple factors including horizontal and vertical transmission of distinct intestinal microbiota in each region, dietary factors, environmental factors, and differing host genetics between regions.
We also assessed another important confounder in our study, namely method of biopsy preservation. Biopsy specimens from Oslo and MSH were snap-frozen and stored at -80°C, whereas specimens from Calgary were stored in RNAlater solution at -80°C. To better understand the effect of tissue preservation method on microbial composition, we examined the effect of biopsy preservation technique on microbial composition using three subjects who had available snap-frozen and RNAlater-preserved biopsy specimens. We did not observe any significant effect of biopsy preservation technique on intestinal microbiota composition, in concordance with previous data addressing this question.20 The characterization of these important experimental parameters significantly influenced study design, as we elected to focus our case-control analyses on the Oslo and Calgary cohorts separately and to exclude MSH samples from primary analyses.
We believe this multicentre study examining the intestinal microbiota in UC subjects with and without PSC provides important lessons for future studies addressing this issue. It demonstrates the challenges associated with microbiota studies due to the remarkable variation in the microbiome driven by a variety of factors including body site, age, location, lifestyle, diet, and host genetics, and in addition the importance of standardisation of phenotype and sample processing in these studies.21,22 Mirroring the early experience in genetic association studies, current microbiota studies may not be adequately powered to detect significant microbiota–phenotype associations, and future studies are likely to require larger numbers. We demonstrated the strong effect of recruitment site on intestinal microbial composition in this study. This effect will be important to take account of in the design of future multicentre microbiota studies, and suggests that care will have to be exercised in generalising results from individual studies to the general PSC population. Accurate phenotyping of PSC subjects with and without UC in future studies will be essential to ensure case and control populations are as homogeneous as possible, and this will be an ongoing challenge. Whether the more general intestinal microbial profile of faecal samples or the more specific, localised profile of endoscopic tissue samples is optimal for the study of microbiota in PSC remains an open question. Information in this regard is provided by a report focusing on a treatment-naïve cohort of Crohn’s disease patients, which demonstrated that stool samples did not reflect microbial dysbiosis in a way similar to that of mucosal tissue samples at either taxonomic or functional levels. Mucosa-associated organisms, however, were not restricted to any particular intestinal location and were readily observed in faecal and tissue samples, although at differing abundances.2 Until consensus emerges on the optimal biospecimen type for characterization of the intestinal microbiota, in our opinion future studies should provide for the collection of both biospecimen types in their study design. Next-generation sequencing technologies continue to advance with a corresponding decrease in cost, allowing an increase in the number of sequences per sample to be generated. The result of this change is that deeper sequencing will be feasible in future studies, with the consequence that lower-abundance organisms may be characterised, potentially uncovering novel microbial associations with PSC not observed in this study. Whereas characterizing the composition of the intestinal microbiota in UC subjects with and without PSC was an important first step, greater mechanistic insights into PSC pathogenesis may be derived by the determination of microbial function in PSC cases and controls.
In conclusion, this study characterized intestinal microbiota in UC-affected individuals with and without PSC. It demonstrated nominally significant associations between a number of intestinal microbiota and PSC phenotype in UC. However, the associated organisms differed between the European and North American cohorts studied. Although nominal associations were demonstrated, no robust PSC-associated microbial signal could be confirmed. We have, however, gained important insight into experimental factors which have been underappreciated in microbiome studies to date and which will influence the design of future studies. Large, well-designed, prospective studies, with more refined characterization of the intestinal microbiota, are required to define the contribution of the intestinal microbiota to PSC pathogenesis.
Funding
This study is funded by PSC Partners and the International Organization for the Study of Inflammatory Bowel Disease [IOIBD]. DK is a recipient of a Canadian Institutes of Health Research [CIHR] / Canadian Association of Gastroenterology [CAG] / Abbvie Inflammatory Bowel Disease Fellowship. MSS is supported by the Gale and Graham Wright Chair in Digestive Diseases at Mount Sinai Hospital and by grants from Crohn’s and Colitis Canada [CCC] and the National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK] [DK-062423].
Conflicts of Interest
The authors have no conflicts [financial, professional, or personal] to disclose.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
Acknowledgments
There are no additional acknowledgements associated with this article.
Conference presentation: oral presentation, American Association for the Study of Liver Diseases [AASLD], Washington DC, USA, November 2013.
References
- 1. Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010;90:859–904. [DOI] [PubMed] [Google Scholar]
- 2. Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014;15:382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gill N, Finlay BB. The gut microbiota: challenging immunology. Nat Rev Immunol 2011;11:636–7. [DOI] [PubMed] [Google Scholar]
- 4. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet 2013;382:158–799. [DOI] [PubMed] [Google Scholar]
- 5. Chapman R, Fevery J, Kalloo A, et al. American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660–78. [DOI] [PubMed] [Google Scholar]
- 6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology 2011;53:1590–9. [DOI] [PubMed] [Google Scholar]
- 7. Liu JZ, Hov JR, Folseraas T, et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet 2013;45:670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lichtman SN, Keku J, Clark RL, Schwab JH, Sartor RB. Biliary tract disease in rats with experimental small bowel bacterial overgrowth. Hepatology 1991;13:766–72. [PubMed] [Google Scholar]
- 9. Sinakos E, Lindor K. Treatment options for primary sclerosing cholangitis. Expert Rev Gastroenterol Hepatol 2010;4:473–88. [DOI] [PubMed] [Google Scholar]
- 10. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19:5A –36A. [DOI] [PubMed] [Google Scholar]
- 11. Kaplan GG, Laupland KB, Butzner D, Urbanski SJ, Lee SS. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol 2007;102:1042–9. [DOI] [PubMed] [Google Scholar]
- 12. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010;26:2460–1. [DOI] [PubMed] [Google Scholar]
- 14. Moreno CE, Rodriguez P. A consistent terminology for quantifying species diversity? Oecologia 2010;163:279–82. [DOI] [PubMed] [Google Scholar]
- 15. Lozupone CA, Hamady M, Kelley ST, Knight R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol 2007;73:1576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tabibian JH, Talwalkar JA, Lindor KD. Role of the microbiota and antibiotics in primary sclerosing cholangitis. Biomed Res Int 2013;2013:389537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rossen NG, Fuentes S, Boonstra K, et al. The musosa-associated microbiota of PSC patients is characterized by a low diversity and a low abundance of uncultured Clostridiales II. J Crohns Colitis 2015;9:342–8. [DOI] [PubMed] [Google Scholar]
- 18. Tyler AD, Smith MI, Silverberg MS. Analyzing the human microbiome: a ‘how to’ guide for physicians. Am J Gastroenterol 2014;109:983–93. [DOI] [PubMed] [Google Scholar]
- 19. Hildebrand F, Nguyen TL, Brinkman B, et al. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol 2013;14:R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Franzosa EA, Morgan XC, Segata N, et al. Relating the metatranscriptome and metagenome of the human gut. Proc Natl Acad Sci U S A 2014;111:E2329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huttenhower C, Knight R, Brown CT, et al. Advancing the microbiome research community. Cell 2014;159:227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huttenhower C, Kostic AD, Xavier RJ. Inflammatory bowel disease as a model for translating the microbiome. Immunity 2014;40:843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]