Dear Sir,
Anti-tumour necrosis factor (TNF) therapy, such as infliximab, is an effective treatment for Crohn’s disease (CD). However, based on potential cost and safety concerns, de-escalation strategies for anti-TNF therapy may be considered, especially in inflammatory bowel disease (IBD) patients with low relapse risk.1 It was recently shown that de-escalation of infliximab maintenance therapy, based on therapeutic drug monitoring, may be feasible in IBD patients who are in deep remission or who have a stable clinical response and supra-therapeutic infliximab trough concentrations, as the risk of relapse is relatively low.2,3 Faecal calprotectin (FC) may also potentially guide therapeutic decisions in IBD patients on stable maintenance infliximab therapy, although data are limited.4,5 Thus, based on serial FC measurements, we investigated the risk of relapse after infliximab de-escalation from an 8-week to a 10-week dosing interval in consecutive patients with luminal, inflammatory CD in composite deep remission for at least 3 years on infliximab maintenance therapy (5 mg/kg every 8 weeks), treated between 2010 and 2012.
This single-centre pilot study included 16 patients (10 males, age 25 [range 18–48] years, L1 [ileal; n = 5], L2 [colonic; n = 4], L3 [ileocolonic; n = 7]), who were prospectively followed on 5 mg/kg infliximab for 2 years. We defined composite deep remission as the combination of clinical (Harvey–Bradshaw index [HBI] ≤4), biological (C-reactive protein [CRP] ≤0.5 mg/dl) and endoscopic remission (no ulcers at ileocolonoscopy performed up to 3 months prior to study entry) as well as FC level <100 µg/g of faecal tissue (FT) (quantitative method; Quantum Blue, Bühlmann, Switzerland). Patients were followed by clinical examination (HBI) and serological (CRP) assessments every 10 weeks, while FC was measured before every other infliximab infusion. If FC was >200 µg/g FT, patients were followed closely by repeated FC measurements and if CRP became abnormal (>0.5 mg/dl) with or without clinical symptoms, patients underwent ileocolonoscopy to assess endoscopic recurrence of CD. Relapse was confirmed by composite markers of clinical (HBI >4), biological (CRP >0.5 mg/dl) and endoscopic (mucosal ulceration) recurrence of CD.
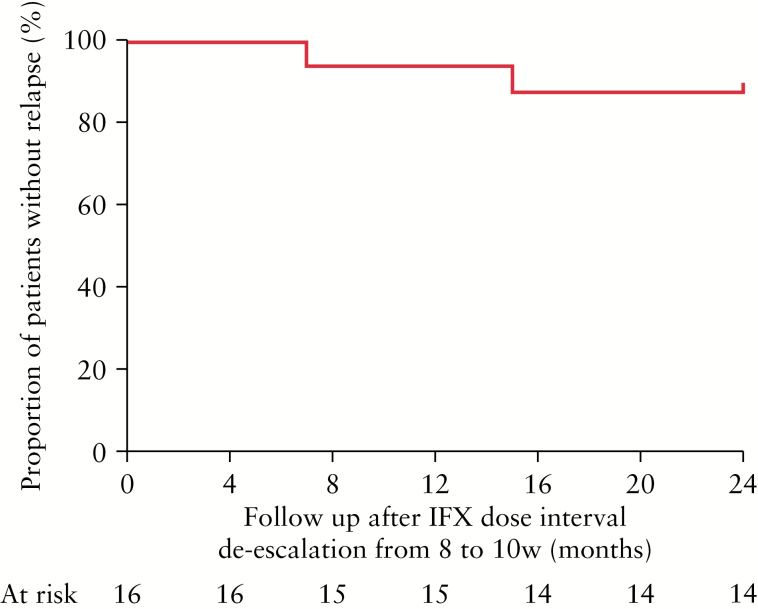
At the end of follow-up, only 2 out of 16 (12.5%) patients had relapsed, 7 and 15 months after the infliximab de-escalation, respectively. The 12- and 24-month cumulative probability (standard error) of relapse was 7.1 (0.069) and 14.3% (0.094), respectively (Figure 1). Infliximab trough concentrations or antibodies to infliximab were not measured; this reflects current real-life clinical practice at the majority of IBD centres.
Figure 1.
Kaplan–Meier survival curve of the proportion of patients without relapse after de-escalation of infliximab (IFX) maintenance therapy from an 8- to a 10-week dosing interval based on serial faecal calprotectin measurements in Crohn’s disease patients with composite deep remission. w, weeks.
In conclusion, this single-centre pilot study indicates that de-escalation of the infliximab dose interval from every 8 to every10 weeks is feasible in CD patients in composite deep remission when FC levels are maintained within the normal range, as the risk of relapse is relatively low. Large, prospective studies are certainly warranted before any clinical recommendations can be made for tailoring anti-TNF maintenance therapy in IBD patients achieving deep remission.
Funding
This study received no funding.
Conflict of Interest
GJM has received honoraria for lectures, consultations and advisory boards from Abbott International, AbbVie, MSD Hellas, MSD International, Danon-Hellas and Astra-Zeneca, and for lectures from Ferring International and the Falk Foundation OMEGA Pharma and Angelini, and has participated as primary investigator in AbbVie, MSD, FalkPharma, GSK, Pfizer, Millennium, Amgen, Hoffmann-La Roche and Astellas clinical trials. The remaining authors disclose no conflict of interest.
Author Contributions
KP: study design, data collection, analysis and interpretation, manuscript writing. PK: data collection, analysis and interpretation. GJM: study design, FC measurements, data collection and critical revision of the manuscript.
References
- 1. Pariente B, Laharie D. Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2014;40:338–53. [DOI] [PubMed] [Google Scholar]
- 2. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9. [DOI] [PubMed] [Google Scholar]
- 3. Paul S, Roblin X, Peyrin-Biroulet L. Letter: infliximab de-escalation based on trough levels in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2015;42:939–40. [DOI] [PubMed] [Google Scholar]
- 4. Huang VW, Prosser C, Kroeker KI, et al. Knowledge of fecal calprotectin and infliximab trough levels alters clinical decision-making for IBD outpatients on maintenance infliximab therapy. Inflamm Bowel Dis 2015;21:1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Molander P, Färkkilä M, Ristimäki A, et al. Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J Crohns Colitis 2015;9:33–40. [DOI] [PubMed] [Google Scholar]