Abstract
Background and Aims:
The Ulcerative Colitis Endoscopic Index of Severity (UCEIS) and the Mayo endoscopic score (Mayo ES) are used to evaluate ulcerative colitis (UC) severity. This study compared UCEIS and the Mayo ES for evaluating UC severity and outcomes in patients undergoing remission induction during routine clinical practice with the aim of predicting medium- to long-term prognosis.
Methods:
Forty-one UC patients who received colonoscopy before and after tacrolimus remission induction therapy were included. An index of clinical activity and endoscopic findings scored by both the UCEIS and the Mayo ES were determined. Changes in UCEIS and Mayo ES before and after induction therapy were compared.
Results:
The mean UCEIS improved from 6.2±0.9 to 3.4±2.1 (p < 0.001). Based on the UCEIS, a significant reduction was reached in both the response and the remission groups. In contrast, the Mayo ES did not reflect a significant change in the response group. The discrepancy appeared to be due to ulcers becoming smaller and shallower during the early stages of mucosal healing; the Mayo ES seems to miss these early changes. In other words, whereas the UCEIS indicates improvements when ulcers shrink, the Mayo ES does not distinguish deep ulcers from shallow ulcers and is 3 (severe UC) for both deep and shallow ulcers. Additionally, better UCEIS strata after induction therapy were associated with lower incidences of colectomy (p = 0.0001) or relapse (p = 0.0008).
Conclusions:
The UCEIS accurately reflects clinical outcomes and predicts the medium- to long-term prognosis in UC patients undergoing induction therapy. These findings should support decision-making in clinical practice settings.
Key Words: Ulcerative colitis endoscopic index of severity, tacrolimus, mucosal healing, Mayo endoscopic score
1. Introduction
In patients with inflammatory bowel disease (IBD), endoscopy is essential for the evaluation of disease activity and the efficacy of treatment interventions.1 There are several different scoring systems for the endoscopic evaluation of ulcerative colitis (UC) severity.2–9 Most commonly, the Mayo endoscopic score (Mayo ES)8 is used in clinical trials to describe the degree of endoscopic activity. In clinical trials as well as in clinical practice, a Mayo ES of 0 or 1 is a widely accepted criterion for mucosal healing.1 However, there is wide variation in assessment outcomes among the scoring systems, reflecting the fact that these instruments have not undergone thorough validation.
More recently, the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) has been reported by Travis et al.10 This score was developed as a validated index that captures 90% of the variance in the overall assessment of endoscopic severity. In their second report, a year later, the authors described the UCEIS score,11 and its components showed satisfactory intra- and inter-investigator variability. Further, intra-investigator variability was unaffected by addition of clinical details. However, the threshold for remission or mucosal healing has not yet been determined and there is no report of assessments in a clinical setting of the UCEIS along with another widely applied scoring system.
Further, mucosal healing has recently been viewed as an important treatment goal in IBD. It is widely known that mucosal healing can be regarded as a prognostic marker of long-term outcomes in UC patients.12–16 Regarding treatment of UC patients with tacrolimus, only two reports have evaluated the association of mucosal healing with medium- to long-term prognosis.17,18 Very recently, we demonstrated that Mayo ES 0 and 1 predict long-term prognosis in UC patients undergoing tacrolimus remission induction therapy.18 In the present study, we planned to determine the relevance of the UCEIS in UC patients undergoing tacrolimus induction therapy in a clinical practice setting. We focused on whether or not UCEIS accurately reflects clinical outcomes and has predictive value for the medium- to long-term prognosis.
2. Methods
2.1. Patients
Fifty-three consecutive patients with moderate to severe UC treated at the Centre for Gastroenterology and Inflammatory Bowel Disease, Hamamatsu South Hospital, and the First Department of Medicine, Hamamatsu University School of Medicine, between July 2009 and August 2014 were included in this study. The diagnosis of UC was based on established standard criteria for symptoms and standard radiographic and endoscopic criteria. The extent of colonic involvement was determined by total colonoscopy.
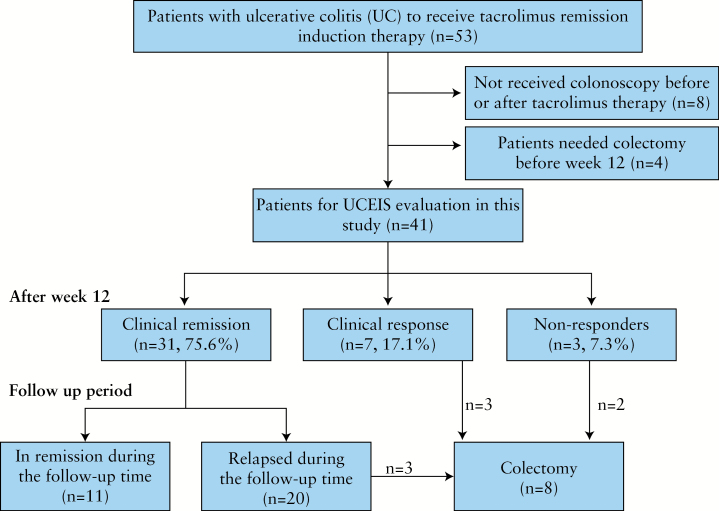
For the determination of UCEIS, the patients who had not received colonoscopy before or after tacrolimus induction therapy (n = 8) and those who needed colectomy within 12 weeks after the start of tacrolimus administration (n = 4) were excluded. Therefore, 41 patients were available for the evaluation of UCEIS, as outlined in Figure 1.
Figure 1.
Treatment design and overall outcomes based on Ulcerative Colitis Endoscopic Index of Severity (UCEIS) scores. Clinical activity index (CAI) ≤4 means clinical remission, while a decrease of ≥4 in the CAI means clinical response. Relapse was defined as an increase in CAI of >4 after achieving clinical remission or the need for switching from tacrolimus to an alternative induction therapy.
Patients were classified as having steroid-refractory, steroid-dependent or steroid-naive UC. According to the definitions described by Ogata et al.,21 steroid refractoriness was defined as lack of response to a systemic daily dose of 1 mg/kg bodyweight or 40 mg or more of prednisolone given over at least 7 consecutive days, or the equivalent of a daily dose of 30 mg of prednisolone or more given over at least 2 weeks. Steroid dependence was defined as a patient who experienced relapse during attempts to taper steroid or who relapsed within 3 months of discontinuing steroids.
2.2. Tacrolimus treatment protocol
Tacrolimus was administered orally at an initial dose of 0.1 mg/kg bodyweight/day in two divided doses. The dosage was chosen to achieve whole-blood trough tacrolimus levels between 10 and 15 ng/ml for inducing remission. After clinical remission was achieved, the dosage was adjusted to produce trough tacrolimus levels between 5 and 10 ng/ml. After 12 weeks of administration, tacrolimus was withdrawn or continued according to clinical requirements under the discretion of the patients’ physicians.
2.3. Clinical efficacy evaluation
Patients were evaluated by using the clinical activity index (CAI) according to Rachmilewitz.6 This indexing system comprises 7 items: stool frequency (0–3); blood in stool (0–4); general well-being (0–3); abdominal discomfort (0–3); fever (0–3); extra-intestinal manifestations (0–9); and laboratory findings (erythrocyte sedimentaion rate and haemoglobin) (0–4). The score is the sum of these 7 items ranging from 0 to 29. Clinical remission was defined as CAI ≤4, while a decrease of ≥4 points in the CAI relative to baseline was defined as clinical response. Relapse was defined as an increase in the CAI score by >4 points after achieving clinical remission or the need for switching from tacrolimus to an alternative induction therapy.
2.4. Endoscopic evaluation
We evaluated the patients by total colonoscopy, whereas in the original determination of the UCEIS score Travis and colleagues10 used sigmoidoscopy. Endoscopy was done in 41 patients before and after tacrolimus remission induction therapy. We used both the UCEIS and the Mayo ES systems to determine endoscopic severity. Patients who had received colonoscopic assessments before the online publication of the UCEIS scoring procedure in October 201110 (n = 14) were scored retrospectively based on this endoscopic reporting system, which included descriptions and photographs. All other cases were evaluated prospectively by the UCEIS and the Mayo ES. The UCEIS consists of the following three descriptors and was calculated as a simple sum: vascular pattern (scored 0–2); bleeding (scored 0–3); and erosions and ulcers (scored 0–3). Therefore, the range of UCEIS scores is 0–8 (Table 1). We stratified the UCEIS scores into four strata: remission (UCEIS 0–1); mild (UCEIS 2–4); moderate (UCEIS 5–6); and severe (UCEIS 7–8). It was assumed that UCEIS 1 in the remission stratum was a descriptor limited to vascular patterns. The Mayo ES has been classified into the following four categories: 0, normal or inactive disease; 1, mild disease with erythema, decreased vascular patterns and mild friability; 2, moderate disease with marked erythema, absence of vascular patterns, friability and erosions; 3, severe disease with spontaneous bleeding and ulceration. Mucosal healing was defined as the remission stratum in the UCEIS and Mayo ES 0 or 1.
Table 1.
Ulcerative Colitis Endoscopic Index of Severity (UCEIS) scores and definitions.
| Descriptor (score of most severe lesions) | Likert scale anchor points | Definition |
|---|---|---|
| Vascular pattern | Normal (0) | Normal vascular patterns with arborization of capillaries clearly defined or with blurring or patchy loss of capillary margins |
| Patchy obliteration (1) | Patchy obliteration of vascular pattern | |
| Obliterated (2) | Complete obliteration of vascular pattern | |
| Bleeding | None (0) | No visible blood |
| Mucosal (1) | Some spots or streaks of coagulated blood on the surface of the mucosa ahead of the scope that can be washed away | |
| Luminal mild (2) | Some free liquid blood in the lumen | |
| Luminal moderate or severe (3) | Frank blood in the lumen ahead of the endoscope or visibly oozing from the mucosa after washing intraluminal blood, or visibly oozing from a haemorrhagic mucosa | |
| Erosions and ulcers | None (0) | Normal mucosa, no visible erosions or ulcers |
| Erosions (1) | Tiny (5mm) defects in the mucosa of a white or yellow colour with a flat edge | |
| Superficial ulcer (2) | Larger (>5mm) defects in the mucosa, which are discrete fibrin- covered ulcers when compared with erosions but remain superficial | |
| Deep ulcer (3) | Deeper excavated defects in the mucosa with slightly raised edge |
Reproduced from Travis et al., Gastroenterology 2013;145:987–95 (reference 11) and Gut 2012;61:535–42 (reference 10).
2.5. Assessments
The primary objective of the present investigation was to better understand the clinical potential of the UCEIS score in UC patients in the clinical practice setting. First, we compared and analysed the UCEIS along with the Mayo ES before and after tacrolimus remission induction therapy to determine which of the two widely used scoring methods (UCEIS and Mayo ES) more accurately reflects clinical outcomes and defines mucosal healing. Next, we focused on the relevance of the UCEIS score as a predictive index for future colectomy or UC relapse. Additionally, we wished to evaluate the short- to long-term efficacy of tacrolimus in UC patients. The endoscopy work was undertaken by physicians who were known to be experienced gastroenterologists; each had >12 years of experience in evaluating patients with gastrointestinal complications, including IBD. Each physician had carried out between 270 and 560 endoscopic procedures per year.
2.6. Ethical considerations
Before initiating this study, our study protocol and the patients’ informed consent forms were reviewed and approved by the institutional review boards at Hamamatsu South Hospital. Patients agreed to participate in this study after being informed of the study purpose, and the nature of the procedures involved. Further, the investigation was conducted in accordance with the Principles of Good Clinical Practice and the ethics standards laid down in the 1964 Helsinki Declaration and its subsequent amendments.
2.7. Statistics
Continuous variables are presented as the mean ± SD and were compared by the t-test or the Mann–Whitney U-test unless stated otherwise. Categorical variables are presented as percentages and were analysed by using Fisher’s exact test. A p-value of <0.05 was considered statistically significant. The comparative values of the UCEIS and the Mayo ES score were determined by using Spearman’s correlation coefficient. The colectomy-free survival and relapse-free survival times were calculated by using Kaplan–Meier estimator graphs, and were compared using the log-rank test.
3. Results
3.1. Patients’ baseline demographic variables
Baseline features of the 41 eligible patients who were evaluated in this study are presented in Table 2. Among the patients who underwent colonoscopic evaluations, 29 were male and 12 were female and mean age at the start of tacrolimus therapy was 42.5 years (range 17–69 years). The mean duration of UC following diagnosis was 5.7 years (range 1 month to 37 years). Thirty-four patients had pancolitis (82.9%) and 7 (17.1%) had left-sided colitis. Ten patients (24.4%) were steroid-refractory, 25 (61%) were steroid-dependent and the remaining 6 (14.6%) were steroid-naive. Twenty-six patients (62.3%) were on corticosteroids at the time of tacrolimus induction therapy, 31 (75.6%) were receiving 5-aminosalicylates and 20 (48.8%) were receiving thiopurines, indicating that some patients were on more than one medication. Leucocytapheresis had been ineffective in 7 patients (17.1%) and in 10 patients (24.4%) biologics had failed. However, all included patients had active UC in spite of receiving the conventional medications mentioned above for an appropriate length of time. However, there was no significant difference between the retrospective group and the prospective group with respect to patients’ baseline demographic variables (Supplementary Table 1).
Table 2.
Baseline demographic variables of 41 patients who received evaluations by the Ulcerative Colitis Endoscopic Index of Severity (UCEIS).
| Variable | Number of patients |
|---|---|
| Average age at initiation of tacrolimus induction therapy, y (range) | 42.5 (17–69) |
| Average duration of ulcerative colitis prior to tacrolimus induction therapy, y (range). | 5.7 (1 month to 37 y) |
| Gender (%) | |
| Male | 29 (70.7) |
| Female | 12 (29.3) |
| Location of colitis (%) | |
| Pancolitis | 34 (82.9) |
| Left-sided | 7 (17.1) |
| Response to corticosteroid (%) | |
| Steroid-refractory | 10 (24.4) |
| Steroid-dependent | 25 (61) |
| Naive | 6 (14.6) |
| Treatment prior to tacrolimus (%) | |
| Prednisolone | 26 (62.3) |
| 5-Aminosalicylates | 31 (75.6) |
| Thiopurines | 20 (48.8) |
| Leucocytapheresis | 7 (17.1) |
| Biologics (%) | 10 (24.4) |
3.2. Clinical efficacy and medium- to long-term outcomes
Figure 1 shows the efficacy and the medium- to long-term outcomes for tacrolimus induction therapy. Thirty-one of the 41 patients (75.6%) achieved clinical remission, 7 (17.1%) had a clinical response within 12 weeks and 3 patients did not respond. The average CAI score decreased from 11.2 at the initiation of tacrolimus therapy to 2.1 at week 12 (p < 0.001). In 26 patients who were on corticosteroids at the start of tacrolimus therapy, the mean dose of prednisolone was 25.6 mg/day. Steroid therapy was discontinued in 21 of the 26 patients (80.8%) and the mean prednisolone dose was reduced from 25.6 to 1.0 mg/day at week 12 in these 26 patients (p < 0.001). During an average observation time of 61.6 weeks (range 12–254 weeks), 8 of 41 patients (19.5%) underwent colectomy. Colectomy was rare in patients who achieved clinical remission (3 of 31 patients, 9.7%). In contrast, the colectomy rate was high (5 of 10 patients, 50%) in the subgroup who did not achieve clinical remission (p = 0.005). Further, in the 31 patients who achieved clinical remission, 11 maintained remission and 20 experienced flare-up during the follow-up time mentioned above.
3.3. Endoscopic findings
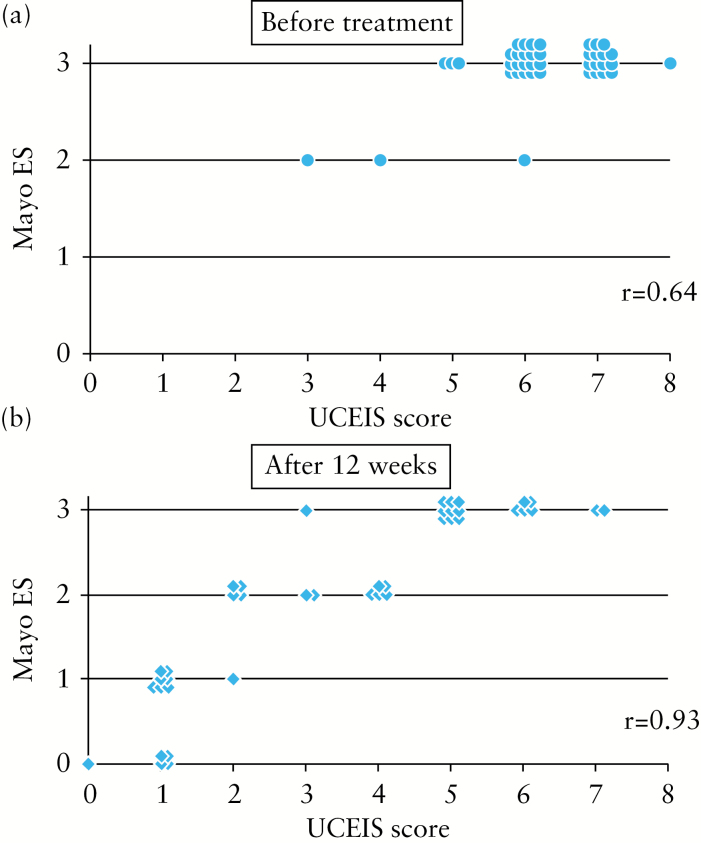
Endoscopic findings assessed with both the UCEIS and the Mayo ES before and after tacrolimus remission induction therapy are shown as a scatter diagram in Figure 2. The UCEIS score showed close correlation with the Mayo ES for the evaluation data before treatment (r = 0.64, p < 0.001, Figure 2a). Similarly, there was a close correlation between the UCEIS score and the Mayo ES for the data after treatment (r = 0.93, p < 0.0001, Figure 2b). However, there was striking discrepancy between the UCEIS and the Mayo ES when the Mayo ES 3 was interpreted. The UCEIS score corresponding to Mayo ES 3 had a wide range: from 5 to 8 before treatment and from 3 to 7 after treatment. This indicated that in patients with severe UC the UCEIS score accurately reflects the true endoscopic findings, while the Mayo ES 3 does not.
Figure 2.
Relationship between the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) and the Mayo endoscopic score (Mayo ES) before and after tacrolimus remission induction. The UCEIS score showed a close correlation with the Mayo ES.
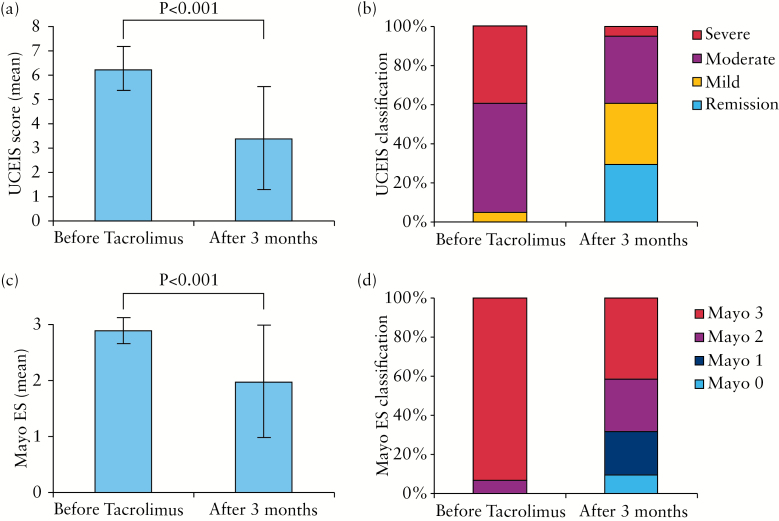
The changes in the mean score and classification before and after tacrolimus remission induction therapy are shown in Figure 3. The mean UCEIS score significantly improved from 6.2±0.9 to 3.4±2.1 (p < 0.001). Similarly, the mean Mayo ES significantly improved from of 2.9±0.3 to 2.0±1.0 (p < 0.001). The UCEIS stratum before tacrolimus remission induction therapy was mild (UCEIS 2–4) in 2 patients (4.9%), moderate (UCEIS 5–6) in 23 patients (56.1%) and severe (UCEIS 7–8) in 16 patients (39%). Unlike the UCEIS, Mayo ES 2 was recorded for only 3 patients (7.3%), while Mayo ES 3 was recorded for 38 patients (92.7%). Therefore, the Mayo ES classified the majority of the patients as having severe UC. Further, the UCEIS stratum after tacrolimus remission induction therapy was remission (UCEIS 0–1) in 12 patients (29.3%), mild (UCEIS 2–4) in 13 patients (31.7%), moderate (UCEIS 5–6) in 14 patients (34.1%) and severe (UCEIS 7–8) in 2 patients (4.9%). Likewise, the Mayo ES was 0 in 4 patients (9.8%), 1 in 9 patients (22%), 2 in 11 patients (26.8%), and 3 in 17 patients (41.5%). It is noteworthy that Mayo ES 3 (severe disease) reflected severe UC in the largest fraction of patients (41.5%). In contrast, the UCEIS strata showed a broad distribution of UC severity level. The remission stratum for UCEIS was close to Mayo ES 0 and 1. Further, to verify the validity of our findings, we separately evaluated our data for the retrospective and prospective groups. There was a close correlation between the UCEIS score and the Mayo ES for the data after treatment in both groups (Supplementary Figure 1). The changes in the average score before and after treatment are shown in Supplementary Figure 2. The mean UCEIS score and the Mayo ES significantly improved in both the retrospective and the prospective group.
Figure 3.
Changes in Ulcerative Colitis Endoscopic Index of Severity (UCEIS) score and Mayo endoscopic score (Mayo ES) before and after tacrolimus induction therapy. In the UCEIS strata, UCEIS 0–1 = remission (as vascular pattern descriptor); UCEIS 2–4 = mild; UCEIS 5–6 = moderate; UCEIS 7–8 = severe ulcerative colitis.
3.4. Association of endoscopic findings with clinical outcomes
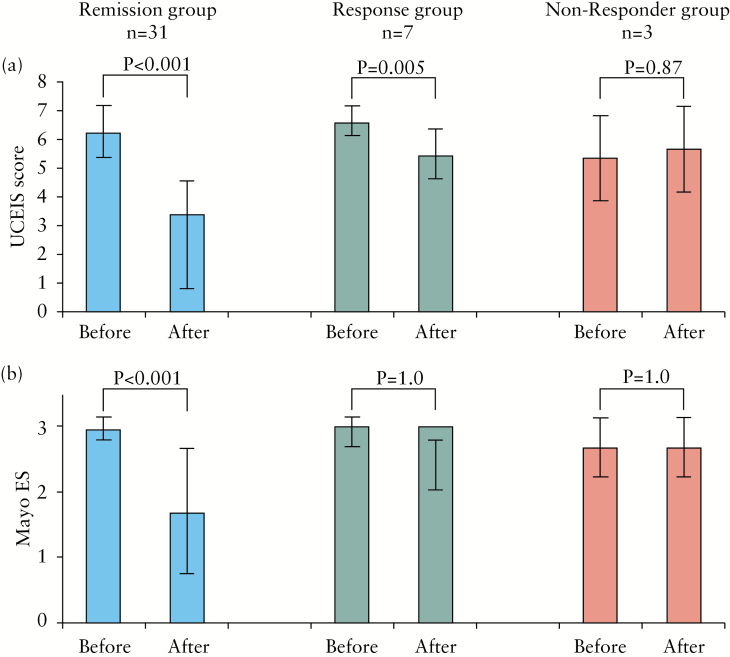
We were interested to see whether the UCEIS score reflected clinical outcomes after tacrolimus induction therapy, i.e. was better than or similar to the Mayo ES. The changes in the UCEIS and the Mayo ES before and after tacrolimus remission induction therapy seen in Figure 4 were compared in the remission group (n = 31), the response group (n = 7) and the non-response group (n = 3). The mean UCEIS score significantly improved in the remission group (from 6.2±0.9 to 2.7±1.9, p < 0.001) and the response group (from 6.6±0.5 to 5.4±0.8, p = 0.005). There was no significant decrease in the non-response group (5.3±1.5 vs 5.7±1.5). In contrast, there was no significant decrease in the Mayo ES even in the response group (from 3 to 3). These findings are in favour of the UCEIS being a more accurate scoring system to assess clinical outcomes after treatment. Further, the Mayo ES may not be able to reflect mucosal improvement seen at an early stage of medical intervention.
Figure 4.
Changes in the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) score and the Mayo endoscopic score (Mayo ES) in relation to clinical outcomes.
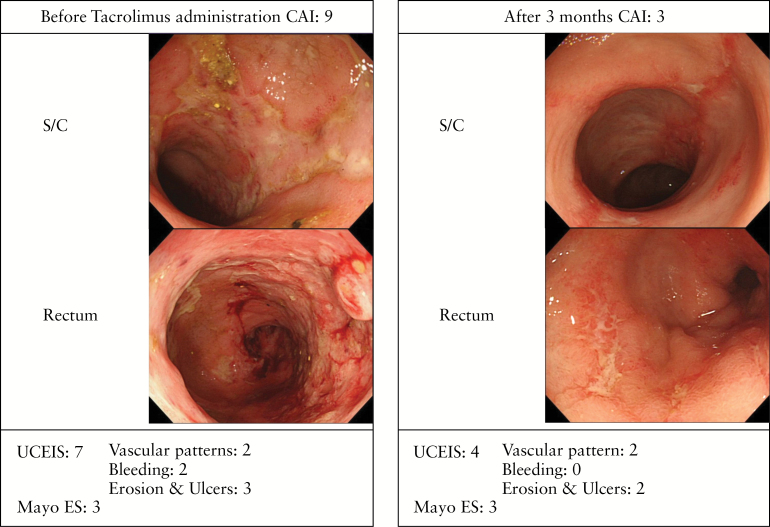
A typical case showing a major difference between the accuracy of the UCEIS and the Mayo ES is presented in Figure 5. The severity of mucosal inflammation was reduced after 3 months of treatment with tacrolimus, but small, shallow ulcers were still seen in the sigmoid colon. The endoscopic score was improved from 7 to 4 by UCEIS (vascular pattern, 2; bleeding, 0; erosions and ulcers, 2), but Mayo ES was still 3.
Figure 5.
Typical case showing a major discrepancy between the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) score and the Mayo endoscopic score (Mayo ES). This 55-year-old female had been diagnosed with total colitis 16 years earlier. Her clinical activity index (CAI) was 9 and total colonoscopy showed mucosal friability and irregular ulcerations in the rectum up to the transverse colon. Before treatment, endoscopic findings were 7 by UCEIS (vascular pattern, 2; bleeding, 2; erosions and ulcers, 3), but 3 (severe ulcerative colitis) by the Mayo ES. After 3 months, small shallow ulcers could still be seen in the sigmoid colon and endoscopic findings were improved from 7 to 4 by UCEIS (vascular pattern, 2; bleeding, 0; erosions and ulcers, 2), but Mayo ES was still 3.
3.5. Colectomy-free and relapse-free survival rates
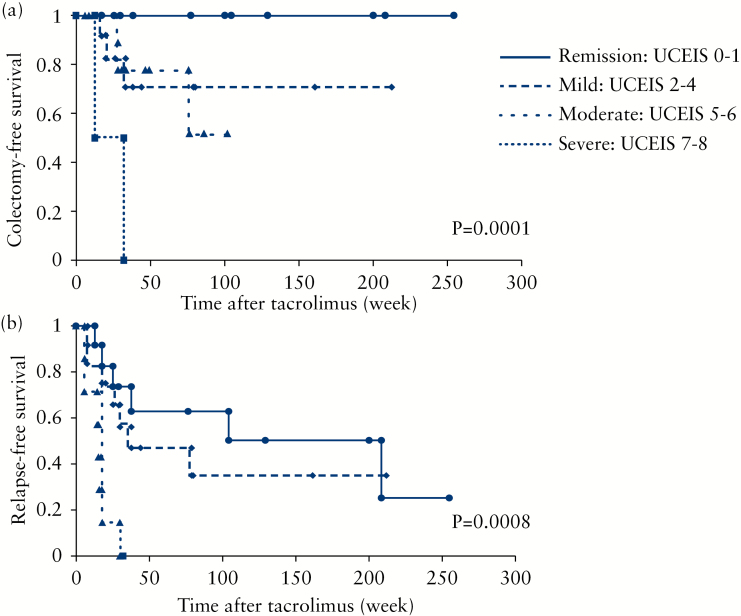
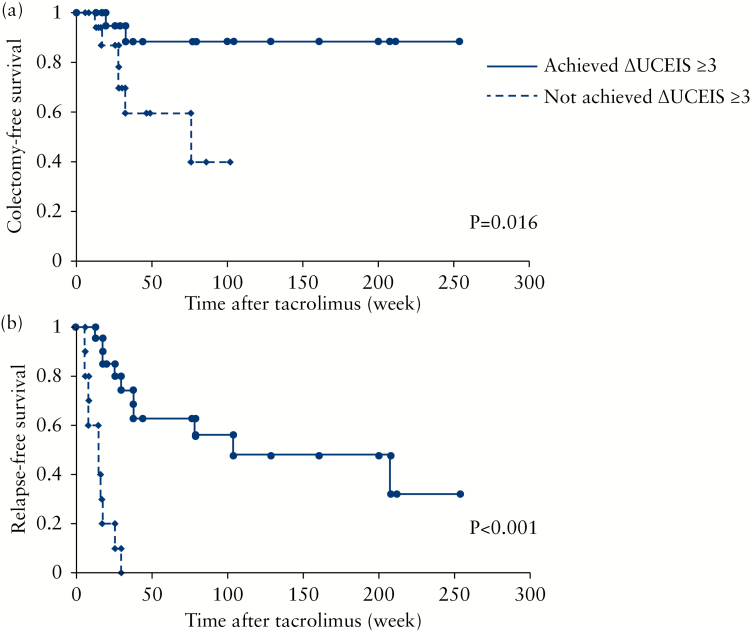
Based on the Kaplan–Meier survival estimator graphs (Figure 6a), the overall cumulative colectomy-free survival rates at 6 months, 1 year and 3 years were 91, 77 and 51%, respectively. Better UCEIS strata were significantly associated with a lower incidence of future colectomy (p = 0.0001, log-rank test). Relapse was seen in a relatively large number of cases who had achieved clinical remission, especially patients who discontinued tacrolimus soon after achieving remission. The cumulative relapse-free survival at 6 months, 1 year and 3 years was estimated to be 61, 42 and 32%, respectively (Figure 6b). Similar to colectomy-free survival, better UCEIS strata were significantly associated with lower incidences of future relapse (p = 0.0008, log-rank test). Then, we looked at the association of endoscopic improvement with medium- to long-term outcomes. We were interested to see whether or not achieving an improvement of ≥3 points in the UCEIS (ΔUCEIS ≥3) had an impact on medium- to long-term prognosis. Twenty-three of the 41 patients had achieved ΔUCEIS ≥3. Achieving ΔUCEIS ≥3 was significantly more frequent in the remission group (71%, 22 of 31 patients) than in the non-remission group (10%, 1 of 10 patients, p < 0.001). The patients who achieved ΔUCEIS ≥3 showed significantly better colectomy-free and relapse-free survival rates compared with the patients who did not (p = 0.016 and p < 0.001, respectively) (Figures 7a and b).
Figure 6.
Kaplan–Meier estimator graphs showing medium- to long-term prognosis of patients who received tacrolimus induction therapy. (a) Colectomy-free time (n = 41); (b) relapse-free time of patients based on the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) stratum (n = 31).
Figure 7.
Colectomy-free and relapse-free time in patients who achieved and those who did not achieve a change in Ulcerative Colitis Endoscopic Index of Severity (UCEIS) of ≥3. (a) Colectomy-free time (n = 41 patients); (b) relapse-free time (n = 31 patients). ΔUCEIS, change in the UCEIS score at 3 months relative to baseline.
4. Discussion
In the management of patients with active IBD, endoscopy has an essential role in viewing and evaluating the severity of disease activity in the intestinal mucosa as well as assessing the efficacy of treatment modalities. Accordingly, a reliable endoscopic scoring system is needed for determining the true severity of the mucosal damage in patients with IBD. There was no validated endoscopic scoring system before the development and publication of the UCEIS system.10,11 This scoring system has significantly increased options for assessing IBD endoscopically. However, to our knowledge, this is the first investigation that was aimed at assessing the clinical relevance of the UCEIS scoring system along with the widely used Mayo ES in a clinical practice setting. The Mayo ES has been in use for a number of years in our hospital and therefore, along with the UCEIS, has received validation during routine clinical practice. We focused on reliability and predictive value for future prognosis. Additionally, we were able to suggest an alternative, and perhaps more relevant, definition of mucosal healing based on the UCEIS scoring process.
The overall UCEIS score after tacrolimus induction therapy significantly improved in a manner similar to the Mayo ES. However, in determining whether or not the UCEIS score reflected the true clinical outcome after medical intervention, the mean UCEIS score significantly improved in the remission group as well as in the response group. In contrast, there was no significant change in the Mayo ES in the response group. We consider this discrepancy to be due to ulcers becoming smaller and shallower during the early stages of mucosal healing. The Mayo ES seems to miss these early changes. In other words, the Mayo ES does not distinguish deep ulcers from shallow ulcers, and is 3 (severe UC) for both deep and shallow ulcers. In contrast, the descriptor of erosions and ulcers in the UCEIS is improved from 3 to 2 after tacrolimus induction therapy. Additionally, since the descriptor of bleeding is clearly defined in the UCEIS scoring system, the score reliably reflects improvement of endoscopic appearance, but the Mayo ES does not. Therefore, we can say that the UCEIS score reflects the true clinical outcomes after a medical intervention very much better than the Mayo ES does.
In the present study, we stratified the UCEIS score into four strata – remission (UCEIS 0–1); mild (UCEIS 2–4); moderate (UCEIS 5–6); and severe (UCEIS 7–8) – and defined mucosal healing as the remission stratum that corresponds to UCEIS 0 or 1. Further, we limited the UCEIS score 1 to a vascular pattern descriptor. Score 1 of the bleeding descriptor means a small amount of blood on the surface of the mucosa and score 1 of the erosions and ulcers descriptor means the presence of erosions. To ensure that such findings do not mean mucosal healing, we limited UCEIS score 1 to a vascular pattern descriptor. On the basis of this definition, the remission stratum of UCEIS was almost equivalent to Mayo ES 0 and 1, which is widely taken as representing mucosal healing in clinical trials. Therefore, our definition of mucosal healing in the UCEIS system appeared to be reasonable. When the UCEIS was divided into 4 strata, like the Mayo ES, we wondered whether UCEIS score 4 related to the mild or the moderate subgroup. However, all cases in UCEIS score 4 appeared in the vascular pattern descriptor 2, the bleeding descriptor 1 and the erosions and ulcers descriptor 1. Additionally, all cases with UCEIS score 5 appeared in the erosions and ulcers descriptor 2 or 3. Therefore, by relating the UCEIS score 4 to the mild stratum, cases with erosions were classified in the mild stratum, while those with ulcers were classified in the moderate or severe stratum.
We were also interested to know the potential of the UCEIS scoring mechanism for predicting future colectomy or relapse. Very recently, Corte et al.19 stated that a severe UCEIS score before induction therapy was associated with worse outcomes in acute severe colitis. The authors report that patients with UCEIS ≥5 on admission required more rescue therapy beyond steroids and underwent colectomy during follow-up. In this study, there was no association between the UCEIS score ‘before’ tacrolimus induction therapy and future relapse or colectomy, but the UCEIS score ‘at 3 months’ following the initiation of tacrolimus induction therapy predicted medium- to long-term outcomes, and lower UCEIS strata were significantly associated with a lower incidence of future colectomy or relapse. As another index of therapeutic efficacy, we focused on the level of reduction in the UCEIS score following initiation of induction therapy, ≥3 points relative to baseline. We divided the patients into two groups: those who achieved ΔUCEIS ≥3 and those who did not do so. Our analyses showed that the subgroup who achieved ΔUCEIS ≥3 had significantly better colectomy-free and relapse-free survival rates than those who did not. This is to say that even if complete mucosal healing was not achieved within 3 months after initiating medical therapy, cases who achieved significant mucosal healing had a better prognosis compared with patients who did not. This is not possible to determine with the Mayo ES, as in this study the largest fraction of patients had a Mayo ES of 3 even after tacrolimus induction therapy. Further, in these patients the timing of endoscopy was set at 3 months after starting induction therapy with tacrolimus. This allowed us to evaluate the degree of mucosal healing at an early stage during medication. In clinical settings, colonoscopic assessment at an early stage should allow appropriate changes to be made in the therapeutic plan.
Travis et al.20 recently reported that clinical information has minimal impact on the endoscopic scoring of disease activity as determined by the UCEIS scoring system. In the present investigation we evaluated the UCEIS score in a clinical practice setting and therefore with initial clinical knowledge of the patients’ UC. This should mean that clinical knowledge did not influence our UCEIS assessment outcomes. In fact, we found a few cases who showed an improved UCEIS score despite experiencing poor clinical symptoms. In such cases, medium- to long-term outcomes were good. Therefore, when there is a discrepancy between the endoscopic score and symptoms, one may suspect irritable bowel syndrome. Accordingly, we feel that the UCEIS is valuable in routine clinical practice settings.
Oral tacrolimus, a calcineurin inhibitor, has recently appeared as an alternative medication in patients with steroid-refractory or steroid-dependent UC.21–27 A few retrospective studies have shown medium- to long-term outcomes for patients who received tacrolimus induction therapy.28–34 However, there are only two reports that have investigated an association between mucosal healing and the medium- to long-term prognosis.17,18 In this study, tacrolimus therapy showed good short-term clinical outcomes (76% remission, 17% response and a 91% non-colectomy rate at 6 months). However, during the medium- to long-term follow-up, about half of the patients who did not achieve clinical remission required colectomy. Additionally, some patients who achieved clinical remission experienced disease relapse, especially those who discontinued tacrolimus soon after achieving remission. Therefore, even if a patient appears to be clinically in remission, endoscopic evaluation should be done about 3 months after the start of medication. If the UCEIS does not reveal adequate mucosal improvement, colectomy may be considered for patients with active disease, or a change in maintenance therapy should be considered even for patients in clinical remission.
Specific limitations may be cited with respect to this report. Firstly, our cohort included patients who were scored retrospectively based on the UCEIS endoscopic reporting system. The data used to calculate the scores for these patients included descriptions and photographs. Potentially, this might prevent contact bleeding from being distinguished from bleeding without contact. Indeed, Travis et al.11 reported that variation in scoring by the same observer was greatest for bleeding.11 However, as stated above, there was no significant difference between the retrospective group and the prospective group with respect to patients’ baseline demographic variables. Additionally, we evaluated our data separately for the retrospective and prospective groups. There was a close correlation between the two scores, as well as between the changes in the average score before and after treatment. Therefore, we are confident that the outcomes in the smaller retrospective group did not compromise the validity of our findings or favour one of the two indexing systems. Secondly, we subdivided the UCEIS score into four strata. However, because of the small sample size, we could not evaluate the validity of any stratified pattern. We believe that this stratification is reasonable at present, but any future study should determine the thresholds for adequate stratification. Thirdly, our evaluations are derived from UC patients who received tacrolimus induction therapy. Evaluation studies in UC patients receiving alternative medications should strengthen our findings.
In conclusion, this is the first investigation with a major focus on assessing the clinical relevance of the UCEIS scoring system in clinical practice in the UC setting. Based on our thorough evaluation, we can confidently say that the use of the UCEIS scoring system more accurately reflects UC severity in the mucosa as well as the short- and long-term clinical outcomes than the Mayo ES does. Further investigations in larger cohorts of patients are warranted to fully reveal the limitations of the Mayo ES.
Funding
The authors received no external funding for this work; their institutional resources were used to carry out this investigation.
Conflict of Interest
The authors have no conflict of interest to declare in connection with the publication of this manuscript.
Author Contributions
All authors had access to the study data. K. Ikeya and H. Hanai: study concept, treatment design and drafting of the manuscript. All authors: patient management and compilation of clinical and laboratory data. K. Ikeya and H. Hanai: preparation of tables and graphs and statistical analyses of the data. All authors: review and approval of the final manuscript version prior to submission.
References
- 1. D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007;132:763–86. [DOI] [PubMed] [Google Scholar]
- 2. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955;2:1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baron JH, Connell AM, Lennard-Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. Br Med J 1964;1:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powell-Tuck J, Bown RL, Lennard-Jones JE. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scand J Gastroenterol 1978;13:833–7. [DOI] [PubMed] [Google Scholar]
- 5. Sutherland LR, Martin F, Greer S, et al. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology 1987;92:1894–8. [DOI] [PubMed] [Google Scholar]
- 6. Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of ulcerative colitis: randomized trials. Br Med J 1989;298:82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz Endoscopic Activity Index with fecal calprotectin, clinical activity, C-reactive protein and blood leukocytes. Inflamm Bowel Dis 2009;15:1851–8. [DOI] [PubMed] [Google Scholar]
- 8. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- 9. Naganuma M, Ichikawa H, Inoue N, et al. Novel endoscopic activity index is useful for choosing treatment in severe active ulcerative colitis patients. J Gastroenterol 2010;45:936–43. [DOI] [PubMed] [Google Scholar]
- 10. Travis SP, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012;61:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Travis SP, Schnell D, Krzeski P, et al. Reliability and initial validation of the Ulcerative Colitis Endoscopic Index of Severity. Gastroenterology 2013;145:987–95. [DOI] [PubMed] [Google Scholar]
- 12. Froslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007;133:412–22. [DOI] [PubMed] [Google Scholar]
- 13. Ardizzone S, Cassinotti A, Duca P, et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol 2011;9:483–9. [DOI] [PubMed] [Google Scholar]
- 14. Ferrante M, Vermeire S, Fidder H, et al. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis 2008;2:219–25. [DOI] [PubMed] [Google Scholar]
- 15. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011;141:1194–201. [DOI] [PubMed] [Google Scholar]
- 16. Yamamoto T, Umegae S, Matsumoto K. Mucosal healing in patients with ulcerative colitis during a course of selective leukocytapheresis therapy: a prospective cohort study. Inflamm Bowel Dis 2010;16:1905–11. [DOI] [PubMed] [Google Scholar]
- 17. Miyoshi J, Matsuoka K, Inoue N, et al. Mucosal healing with oral tacrolimus is associated with favorable medium- and long-term prognosis in steroid refractory/dependent ulcerative colitis patients. J Crohns Colitis 2013;7:609–14. [DOI] [PubMed] [Google Scholar]
- 18. Ikeya K, Sugimoto K, Kawasaki S, et al. Tacrolimus for remission induction in ulcerative colitis: Mayo endoscopic subscore 0 and 1 predict long-term prognosis. Dig Liver Dis 2015:47;365–71. [DOI] [PubMed] [Google Scholar]
- 19. Corte C, Fernandopulle N, Catuneanu AM, et al. Association between the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) and outcomes in acute severe ulcerative colitis. J Crohns Colitis 2015:9;376–81. [DOI] [PubMed] [Google Scholar]
- 20. Travis SPL, Schnell D, Feagan BG, et al. The impact of clinical information on the assessment of endoscopic activity: characteristics of the Ulcerative Colitis Endoscopic Index Of Severity (UCEIS). J Crohns Colitis 2015:9;607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogata H, Matsui T, Nakamura M, et al. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut 2006;55:1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fellermann K, Ludwig D, Stahl M, David-Walek T, Stange EF. Steroid-unresponsive acute attacks of inflammatory bowel disease: immunomodulation by tacrolimus (FK506). Am J Gastroenterol 1998;93:1860–6. [DOI] [PubMed] [Google Scholar]
- 23. Fellermann K, Tanko Z, Herrlinger KR, et al. Response of refractory colitis to intravenous or oral tacrolimus (FK506). Inflamm Bowel Dis 2002;8:317–24. [DOI] [PubMed] [Google Scholar]
- 24. Hogenauer C, Wenzl HH, Hinterleitner TA, Petritsch W. Effect of oral tacrolimus (FK 506) on steroid-refractory moderate/severe ulcerative colitis. Aliment Pharmacol Ther 2003;18:415–23. [DOI] [PubMed] [Google Scholar]
- 25. Baumgart DC, Wiedenmann B, Dignass AU. Rescue therapy with tacrolimus is effective in patients with severe and refractory inflammatory bowel disease. Aliment Pharmacol Ther 2003;17:1273–81. [DOI] [PubMed] [Google Scholar]
- 26. Ogata H, Kato J, Hirai F, et al. Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm Bowel Dis 2012;18:803–8. [DOI] [PubMed] [Google Scholar]
- 27. Schmidt KJ, Herrlinger KR, Emmrich J, et al. Short-term efficacy of tacrolimus in steroid-refractory ulcerative colitis – experience in 130 patients. Aliment Pharmacol Ther 2013;37:129–36. [DOI] [PubMed] [Google Scholar]
- 28. Baumgart DC, Pintoffl JP, Sturm A, Wiedenmann B, Dignass AU. Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease – a long-term follow-up. Am J Gastroenterol 2006;101:1048–56. [DOI] [PubMed] [Google Scholar]
- 29. Ng SC, Arebi N, Kamm MA. Medium-term results of oral tacrolimus treatment in refractory inflammatory bowel disease. Inflamm Bowel Dis 2007;13:129–34. [DOI] [PubMed] [Google Scholar]
- 30. Benson A, Barrett T, Sparberg M, Buchman AL. Efficacy and safety of tacrolimus in refractory ulcerative colitis and Crohn’s disease: a single-center experience. Inflamm Bowel Dis 2008;14:7–12. [DOI] [PubMed] [Google Scholar]
- 31. Yamamoto S, Nakase H, Mikami S, et al. Long-term effect of tacrolimus therapy in patients with refractory ulcerative colitis. Aliment Pharmacol Ther 2008;28:589–97. [DOI] [PubMed] [Google Scholar]
- 32. Ziring DA, Wu SS, Mow WS, Martín MG, Mehra M, Ament ME. Oral tacrolimus for steroid-dependent and steroid-resistant ulcerative colitis in children. J Pediatr Gastroenterol Nutr 2007;45:306–11. [DOI] [PubMed] [Google Scholar]
- 33. Watson S, Pensabene L, Mitchell P, Bousvaros A. Outcomes and adverse events in children and young adults undergoing tacrolimus therapy for steroid-refractory colitis. Inflamm Bowel Dis 2011;17:22–9. [DOI] [PubMed] [Google Scholar]
- 34. Yamamoto S, Nakase H, Matsuura M, Masuda S, Inui K, Chiba T. Tacrolimus therapy as an alternative to thiopurines for maintaining remission in patients with refractory ulcerative colitis. J Clin Gastroenterol 2011;45:526–30. [DOI] [PubMed] [Google Scholar]