Abstract
Background and Aims:
Evidence from mouse colitis models indicates that cytotoxic CD8+ T cells [CTL] play a key role in the initiation of gut lesions. We investigated whether changes in CD8+ CTL in blood or lamina propria [LP] of the neoterminal ileum were associated with postoperative endoscopic recurrence of Crohn’s disease [CD].
Methods:
A total of 37 CD patients with ileocolonic resection were endoscopically followed up at 6 and 12 months post-surgery. CD8+ T cells were analysed by flow cytometry in blood and ileal LP.
Results:
Granzyme B- and perforin-producing CD8+ T cells were significantly increased at 6 months in blood and in ileum LP in patients with endoscopic recurrence, as compared with those in remission. At a cutoff point of 45% of CD8+ CTL, the overall accuracies of the frequency of blood granzyme B+ or perforin+ CD8+ T cells to identify patients with postoperative endoscopic recurrence were 77% and 83%, respectively. Interestingly, patients with endoscopic recurrence at 12 months were those showing the highest mucosal CD8+ CTL frequency at 6 months, while still in remission.
Conclusions:
Enrichment of cytotoxic CD8+ T cells in blood and ileal mucosa coincides with CD postoperative endoscopic recurrence. This underscores that CD8 CTL may play a pathophysiological role in the initiation of gut lesions during CD.
Key Words: Postoperative recurrence, Crohn’s disease, CD8+ cytotoxic T cells
1. Introduction
Crohn’s disease [CD] is a chronic inflammatory bowel disease that is mediated by T cells and features an imbalance between effector and regulatory subsets. Although CD4+ T cells encompassing both Th1- and Th17-type cells accumulate in the intestinal mucosa of patients with active CD, several studies in experimental models of colitis have emphasised a role for CD8+ T cells as initiators of the inflammatory process and gut lesions. Accordingly, it has been proposed that CD8+ T cells may play an earlier role in CD development or relapse than CD4+ T cells.1 We documented in two different antigenic models that interferon gamma [IFNγ]-producing granzyme B+ cytotoxic CD8+ T cells [CTL] are delayed-type hypersensitivity effectors that initiate relapsing episodes of colitis upon recruitment into the lamina propria [LP] and induction of epithelial cell apoptosis.2 , 3 Others studies using double transgenic mice confirmed that CD8+ T cells specific for an antigen targeted on intestinal epithelial cells induced spontaneous enteropathy.4 Finally, a role for CD8+ T cells in driving interleukin [IL]-23/IL-17 mediated intestinal inflammation was also highlighted in mice overexpressing tumour necrosis factor alpha [TNFα]5 and in a T-cell transfer model of colitis in RAG2-/- mice.6 However, whether a rise in CD8+ CTL-expressing granzyme B or perforin may be an early indicator of the inflammatory process in CD is unknown. Post-surgical recurrence of CD is the most relevant clinical setting in which to address this issue, as it is assumed that the immune processes involved in CD recurrence following ‘curative’ ileocolonic resection reproduce those involved in the early phase of disease. Furthermore, although operated patients undergo clinical remission, endoscopic survey during the year after surgery detects macroscopic lesions of the neoterminal ileum in 70% of patients, who are at high risk to develop clinical flare.7,8,9 Since a significant proportion of operated patients are in endoscopic remission 6 months post-surgery, identification of immune markers anticipating endoscopic signs of inflammation could improve patients’ clinical and therapeutic management.
In this study we aimed to assess changes in CD8+ CTL in blood or LP of the neoterminal ileum of operated CD patients in order to determine their pathophysiological relevance to endoscopic recurrence. As mouse colitis models indicate that CD8+ CTL play a key role in the initiation of gut lesions, we hypothesised that this subset of T cells could be an early indicator of the inflammatory process in the clinical setting of postoperative CD recurrence.
2. Patients and Methods
2.1. Patients and controls
A total of 37 consecutive CD patients who underwent a non-urgent ‘curative’ ileocolonic resection were enrolled. At the time of surgery, all macroscopically involved parts of the intestine were removed. The neoterminal ileum was anastomosed to the right or transverse colon using a side-to-side anastomosis, and the ileal resection margin was systematically checked and found microscopically free of active lesions. Patients with a short bowel syndrome or a gut stoma were excluded from the study. Patient’s characteristics including location and behaviour of disease, according to the Montreal classification, are summarizsd in Table 1. The non-CD control group consisted of seven subjects undergoing a screening colonoscopy for colorectal cancer, who were free of any endoscopic abnormalities. All patients and controls gave their written informed consent for participation in the study, which was previously approved by the Ethics Committee of the University of Lyon [number 2010-042-AM1].
Table 1.
Characteristics of patients according to their post-surgical endoscopic status.
| CD patients in endoscopic remission at M12 [n = 11] | CD patients in endoscopic recurrence at M6 [n = 12] | CD patients in endoscopic recurrence at M12 [n = 14] |
P a | Non-CD controls [n = 7] | |
|---|---|---|---|---|---|
| Sex, male [%] | 6 [55] | 3 [25] | 5 [36] | 0.51 | 3 [43] |
| Age [years] mean, [range] | 38 [21-75] | 36 [24-75] | 39 [28-64] | 0.87 | 32 [24-55] |
| Smoking n [%] | 1 [6] | 5 [42] | 4 [29] | 0.01 | 2 [28] |
| Disease duration [years] mean, [range] | 12 [3-28] | 10 [4-21] | 13 [5-31] | 0.73 | |
| Illness behaviour, n [%] | |||||
| B1 [non-stricturing, non-penetrating] | 1 [9] | 0 [0] | 0 [0] | 0.45 | |
| B2 [stricturing] | 6 [55] | 9 [75] | 9 [64] | 0.19 | |
| B3 [penetrating] | 4 [36] | 3 [25] | 5 [36] | 0.74 | |
| CD location, n [%] | |||||
| L1 [ileal] | 1 [9] | 4 [33] | 4 [29] | 0.02 | |
| L2 [colonic] | 0 [0] | 0 [0] | 0 [0] | - | |
| L3 [ileocolonic] | 10 [91] | 8 [67] | 10 [71] | 0.17 | |
| History of previous surgery, n [%] | 3 [27] | 2 [17] | 1 [7] | 0.62 | |
| Perianal lesions, n [%] | 6 [55] | 3 [25] | 3 [21] | 0.72 | |
| Postoperative therapy, n [%] | |||||
| No | 4 [36] | 4 [33] | 3 [21] | 0.51 | |
| Mesalamine | 1 [9] | 2 [17] | 2 [14] | 0.43 | |
| Thiopurines | 3 [27] | 4 [33] | 4 [29] | 0.48 | |
| Anti-TNFa: | 4 [36] | 4 [33] | 6 [43] | 0.32 | |
| Monotherapy | 3 [27] | 2 [17] | 5 [36] | 0.21 | |
| Combotherapy | 1 [9] | 2 [17] | 1 [7] | 0.58 | |
CD, Crohn’s disease; M, months; TNF, tumour necrosis factor.
aChi square test.
2.2. Study design
Patients were prospectively followed after surgery by clinical, endoscopic and biological assessment (C-reactive proein [CRP], haematocrit, haemoglobin). Disease activity was monitored at each visit using the Crohn’s Disease Activity Index [CDAI]. An ileocolonoscopy under anaesthesia was performed in all patients at 6 months post-surgery and also at 12 months unless endoscopic recurrence was evidenced at 6 months. All the endoscopies were performed by three senior gastroenterologists [GB, DM, SN] who were experienced in grading endoscopic severity of the neoterminal ileum, using the validated Rutgeerts score.8 Absence of endoscopic recurrence was defined as a score of i0 [no lesion] and i1 [≤5 aphthous lesions]; moderate recurrence was defined as a score of i2 [>5 aphthous lesions with normal mucosa between the lesions, or skip areas of larger lesions or lesions confined to the ileocolonic anastomosis]; severe recurrence included scores of i3 [diffuse aphthous ileitis with diffusely inflamed mucosa] and i4 [diffuse inflammation with larger ulcers, nodules, and/or narrowing].8 Since this study was designed to investigate whether CD8+ CTL may be associated with early gut lesions and the postoperative endoscopic recurrence, and not to evaluate the risk of disease recurrence, it was left to the discretion of each investigator to manage postoperative therapy.
Ten endoscopic biopsies from the neoterminal ileum close to the site of anastomosis were harvested in RPMI 1640 medium supplemented with penicillin and 10% fetal calf serum [FCS] at +4°C, pooled and immediately processed for immunological analysis. Two additional biopsies were included in paraffin for routine pathological examination after staining with haematoxylin and eosin. All the investigators involved in the assessment of cytotoxic CD8+ T cell infiltration were blinded to the results of the endoscopic status during the whole period of the study.
2.3. Isolation of neoterminal ileum LP and blood leukocytes
After an extensive washing with phosphate-buffered saline [PBS], pooled biopsies were incubated for 30min at room temperature in PBS containing 1mM ethylene diamine tetraacetic acid [EDTA] and vortexed every 5min. Biopsies were extensively washed to eliminate epithelial cells and consequently the intra-epithelial lymphocytes; the remaining tissue was incubated for 40min at 37°C in RPMI 1640 medium containing 1mg/ml of collagenase IV plus 0.1mg/ml of DNAse I. The resulting cell suspension was incubated 15min in RPMI 1640 plus 10% fetal calf serum [FCS] and cells collected were centrifuged, washed and re-suspended in PBS. Heparinised venous blood samples were collected from patients and controls, diluted 1:3 with PBS and layered on a Ficoll-Hypaque density gradient, then centrifuged for 30min at 900g, and peripheral blood [PB] leukocytes were collected from the interface.
2.4. Flow cytometry analysis
LP and PB leukocytes were suspended in PBS and initially incubated for 15min at room temperature with human serum [pooled from normal donor] to reduce non-specific staining. Leukocytes were then incubated for 30min with specific fluorochrome-conjugated monoclonal antibodies directed against CD3 and CD8α [BD Biosciences, San Jose, CA]. For intracellular staining, cells were fixed and permeabilised and then incubated with mouse anti-human fluorescein isothiocyanate FITC-conjugated or Alexa-Fluor 647-conjugated antibodies specific for granzyme B [clone GB11, BD Pharmingen, San Jose, CA] or perforin [clone deltaG9, Invitrogen], respectively, or relevant isotype controls. Stainings were analysed by flow cytometry on a LSRII analyser [BD Biosciences] and by using FlowJo software [Tree Star, Ashland, OR].
2.5. Statistical analysis
Statistical analyses were performed using the Statistical Package GraphPad software Inc. [San Diego, CA]. Results of numerical data are presented as mean percentage ± standard deviation [SD] of granzyme B- or perforin-positive CD8+ T cells among total CD8+ T cells. The non-parametric Mann-Whitney test and the one-way analysis of variance [ANOVA] test with Bonferroni correction were used as appropriate. Chi square test was used to compare patients’ characteristics in Table 1; P-values < 0.05 were considered statistically significant. Receiver operating characteristic [ROC] curve analysis was generated to determine the best cutoff point [defined as the maximum sum of sensitivity plus specificity] of the frequencies of circulating granzyme B- or perforin-expressing CD8+ T cells for discriminating between patients with or without endoscopic recurrence. The reliability of cytotoxic CD8+ T cells as a marker for diagnosing endoscopic postoperative recurrence in CD patients was expressed in terms of sensitivity, specificity, positive predictive value, negative predictive value, and overall accuracy. The overall accuracy was calculated by addition of the true-positive and true-negative test results divided by all tests [true-positive + true-negative + false-positive + false-negative].
3. Results
3.1. Characteristics of patients
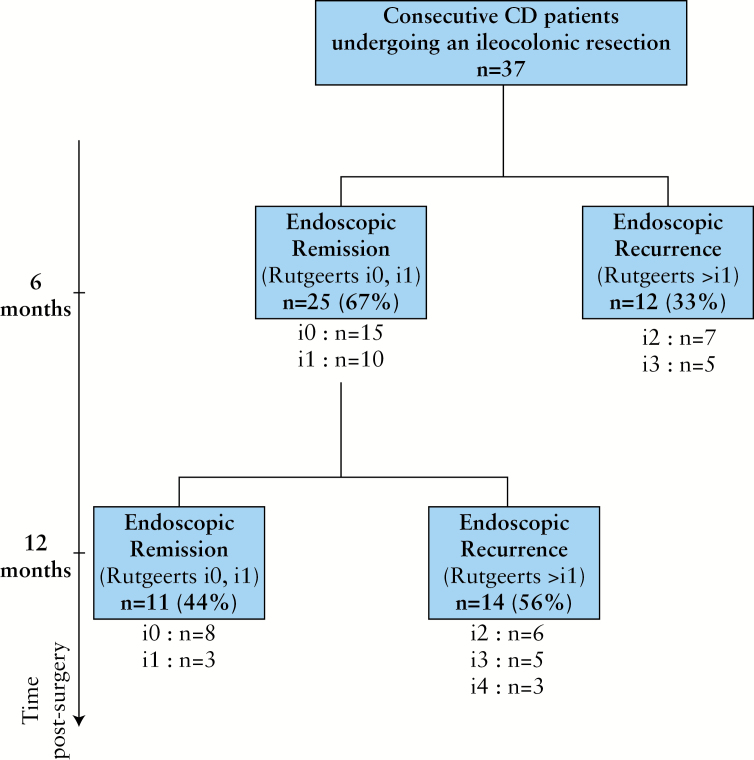
A total of 37 patients (14 males [37.8%], mean age 39 years, range 21–75 yrs) undergoing a ‘curative’ ileocolonic resection for CD were studied prospectively for 1 year. The characteristics of CD patients and the seven control subjects (three males [42.9%], mean age 32 years, range 24–55 years) are summarized in Table 1. The flow chart of the population studied with reference to the endoscopic status within the year following intestinal resection is represented in the Figure 1. Overall, an endoscopic recurrence was identified in 26 out of 37 [70%] patients within 1 year of postoperative follow-up. Among them, an early recurrence, defined as the occurrence of macroscopic mucosal lesions at 6 months after surgery, was evidenced in 12 patients [33%] with a Rutgeerts score classified i2 and i3 in seven and five patients, respectively. Among the 26 patients with endoscopic recurrence, seven developed macroscopic lesions affecting both the ileum and colon, but none had lesions exclusively located in the colon. No clinical sign of recurrence [defined as CDAI < 150] was evidenced within 12-months follow-up.
Figure 1.
Flow chart of 1-year postoperative endoscopic follow-up of patients with Crohn’s disease [CD] undergoing ileocolonic resection.
3.2. Enrichment of cytotoxic CD8+ T cells in ileal LP and in PB leukocytes is associated with early endoscopic recurrence of CD at 6 months post-surgery
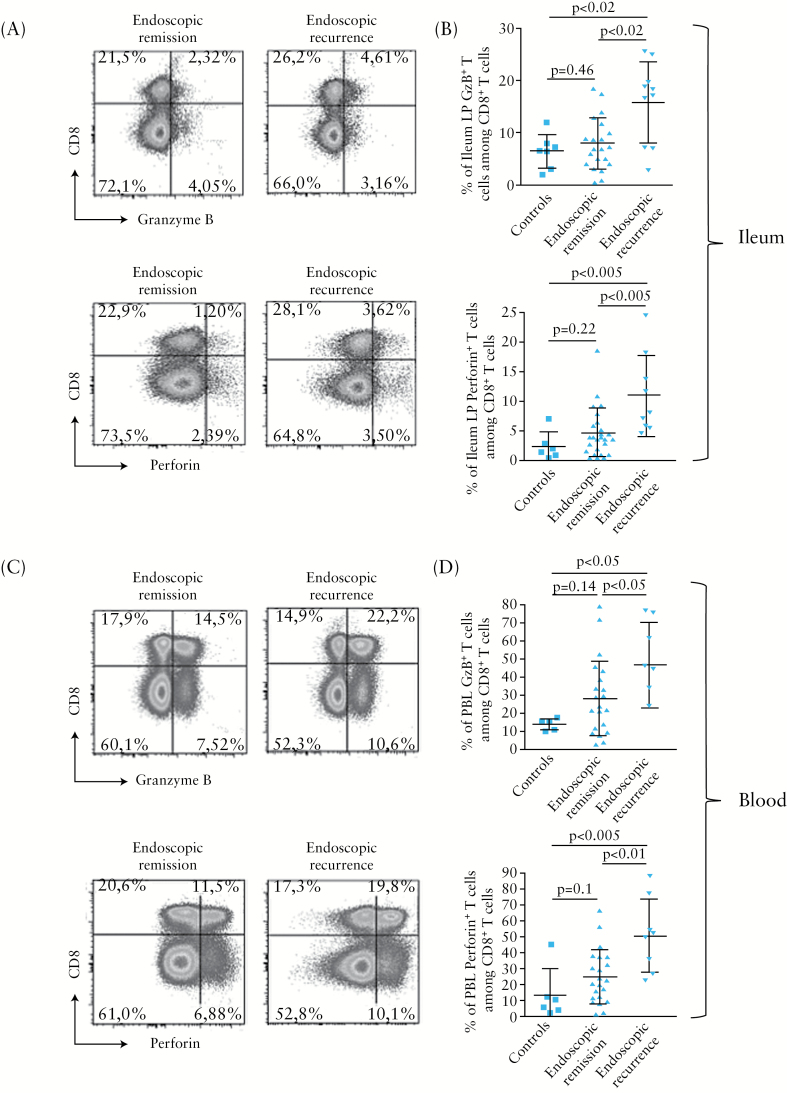
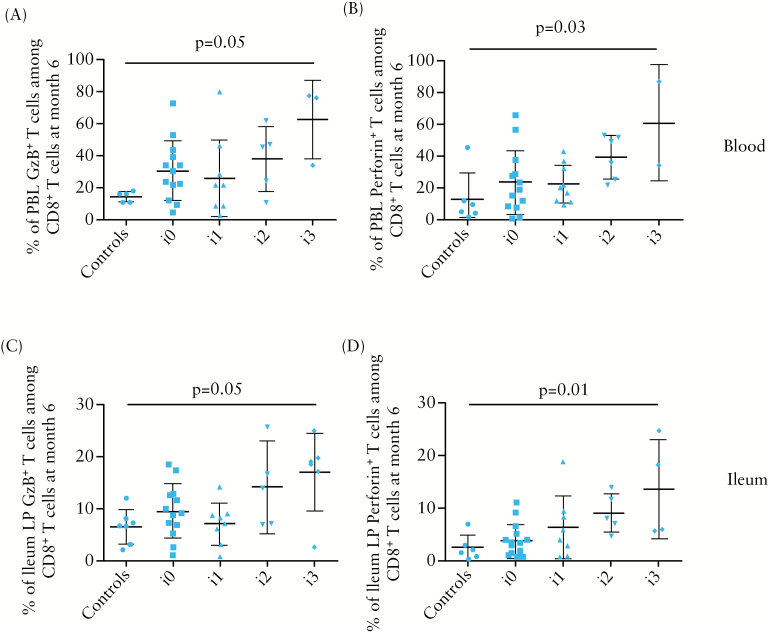
At 6 months post-surgery, the proportion of granzyme B- or perforin-expressing T cells among total CD8+ T cells was significantly higher [p < 0.02] in ileal LP from CD patients who were in endoscopic recurrence [15.87±7.78% and 11.16±6.74%, respectively, n = 12], compared with those who were in endoscopic remission [8.04±4.87% and 4.70±4.34%, respectively, n = 25] or those of non-CD control gut samples [6.38±3.29% and 2.50±2.38%, respectively, n = 7] [Figure 2A and B]. There was no significant difference in CD8+ CTL frequency in ileal LP of patients with [n = 3] or without [n = 9] colonic lesions [data not shown]. Likewise, a statistically significant increase [p = 0.05] in granzyme B or perforin CD8+ T cells was observed in blood of CD patients with endoscopic recurrence [47.05±23.94% and 50.08±22.94%, respectively], compared with those in remission [28.74±20.65% and 24.35±16.87%, respectively] and to controls [14.34±3.29% and 13.00±16.29%, respectively] [Figure 2C and D]. In addition, there was a gradual increase of the frequency of granzyme B- or perforin-expressing T cells among total CD8+ T cells in blood and ileal LP with regard to the postoperative endoscopic severity assessed by the Rutgeerts score [Figure 3].
Figure 2.
Flow cytometry analysis of cytotoxic CD8+ T cells in ileal biopsies and blood of Crohn’s disease [CD] patients. A, C. Representative dot plots showing the frequency of granzyme B [GzB, top panels]- or perforin- [bottom panels] expressing CD8+ T cells from ileal lamina propria [LP] [A] and PB leukocytes [C] of CD patients in endoscopic remission [n = 12] [left] or endoscopic recurrence [n = 25] [right] at 6 months post-surgery. B, D. Frequencies of granzyme B [GzB, top panels]- or perforin- [bottom panels] expressing cells in gated CD8+ T cells from ileal LP [B] and blood [D] of CD patients with endoscopic recurrence [n = 12] [black bars] or endoscopic remission [n = 25] [white bars] at 6 months post-surgery and in non-CD controls [n = 7] [grey bars].
Figure 3.
Frequency of granzyme B [GzB]- or perforin-expressing CD8+ T cells among total CD8+ T cells in blood [A, B] or ileal LP [C, D] among CD patients according to the endoscopic score of severity [Rutgeerts score] at 6 months after ileocolonic resection and in non-CD controls.
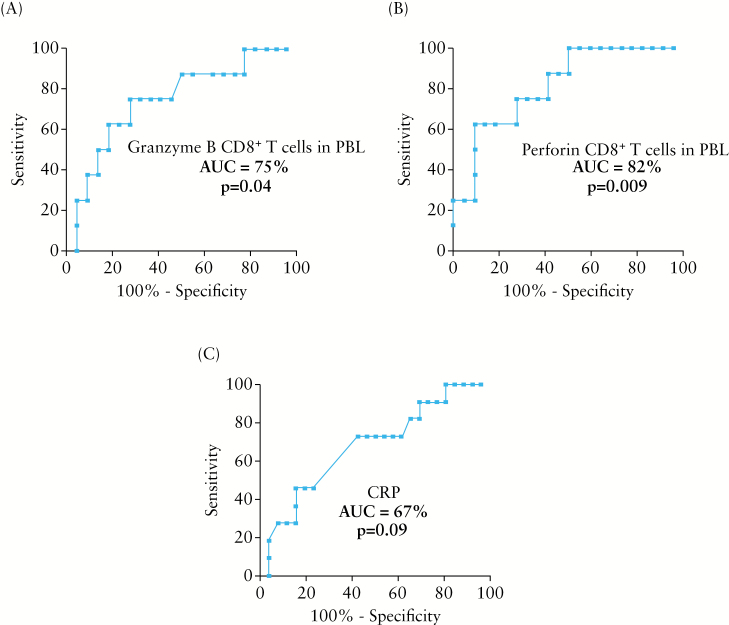
To determine whether such a rise in circulating cytotoxic CD8+ T cells represented a marker of endoscopic recurrence and to define its diagnostic accuracy, ROC curves were built and a cutoff was determined for each marker to obtain the optimal sensitivities [Sen], specificities [Spe], positive [PPV], and negative [NPV] predictive values [Table 2]. At a cutoff of 45%, the overall accuracy of circulating granzyme B CD8+ T cells and that of perforin CD8+ T cells in the detection of endoscopic recurrence were 77% and 83%, respectively. The areas under ROC curves were 75% and 82% for circulating granzyme B CD8+ T cells and for perforin CD8+ T cells, respectively [Figure 4 A and B]. We also determined the optimal cutoff of 5mg/l as an upper limit for CRP concentration using the ROC curves. At this cutoff, the area under ROC curve was 67% for CRP [Figure 4C]. Sensitivity [Sen], specificity [Spe], overall accuracy, positive [PPV], and negative [NPV] predictive values of CRP were summarised in Table 2.
Table 2.
Sensitivities, specificities, predictive values, and overall accuracies of the frequencies of circulating granzyme or perforin CD8+ T cells and CRP as biomarkers capable to discriminate CD patients with or without postoperative ileal endoscopic recurrence within 6 months post-surgery.
|
Granzyme B
among CD8+ T cells [cutoff > 45%] |
Perforin
among CD8+ T cells [cutoff > 45%] |
CRP
[cutoff > 5mg/l] |
|
|---|---|---|---|
| Sensitivity | 63% | 63% | 43% |
| Specificity | 82% | 91% | 86% |
| Positive predictive value | 56% | 71% | 69% |
| Negative predictive value | 86% | 87% | 59% |
| Overall accuracy | 77% | 83% | 62% |
Figure 4.
Receiver operating characteristics [ROC] curves of granzyme B- [A] and perforin- [B] expressing CD8+ T cells in peripheral blood leukocytes [PBL] and C-reactive protein [CRP] [C] of CD patients in endoscopic recurrence [n = 12] or remission [n = 25] at 6 months post-surgery. AUC, area under the ROC curve.
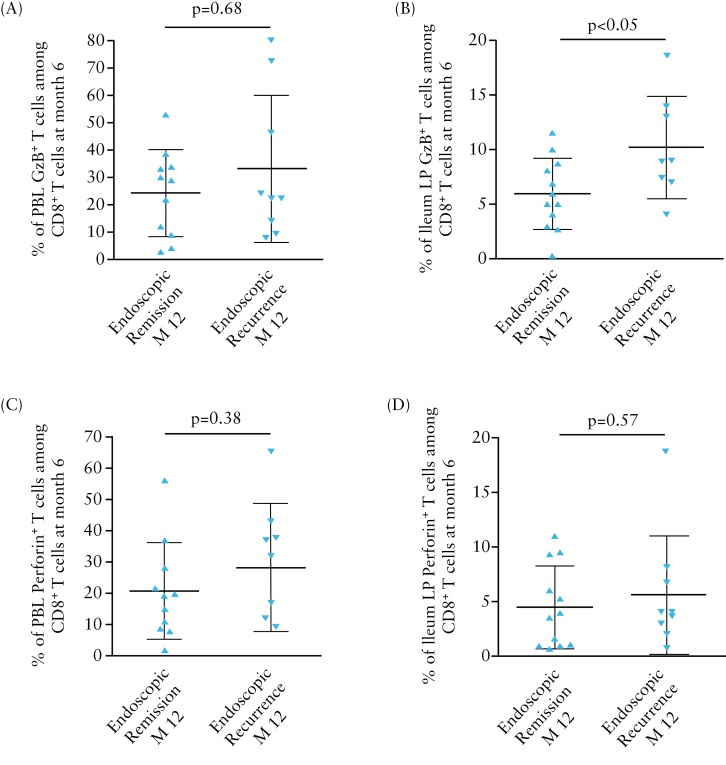
3.3. Enrichment of granzyme B CD8+ T cells in ileal LP of patients in endoscopic remission at 6 months precedes endoscopic recurrence at 12 months post-surgery
Finally, we investigated whether early cytotoxic CD8+ T cell enrichment in either blood or mucosa at 6 months post-surgery could identify among patients in endoscopic remission those at a high risk of endoscopic recurrence at 12 months. For that purpose, the frequencies of granzyme B- or perforin-positive CD8+ T cells from ileum LP or blood leukocytes in patients who were in endoscopic remission at 6 months post-surgery [n = 25] were compared according to their endoscopic status at 12 months post-surgery. Among the 25 patients analysed, the frequency of granzyme B-expressing CD8+ T cells was significantly higher in ileal LP at 6 months post-surgery in patients who subsequently experienced an endoscopic recurrence at 12 months [n = 14], as compared with that of patients who remained in endoscopic remission [n = 11] within 1 year post-surgery [10.24±4.67% versus 5.98±3.28 %, respectively; p < 0.05] [Figure 5]. In addition, higher frequencies of circulating granzyme B+ or perforin+ CD8+ T cells and also of ileal LP perforin+ CD8+ T cells were observed in patients who experienced an endoscopic recurrence 6 months later, when compared with those who did not, but this did not reach statistical significance [Figure 5].
Figure 5.
Frequency of granzyme B [GzB]- or perforin-expressing CD8+ T cells among total CD8+ T cells in blood [A, C] or ileal LP [B, D] of CD patients in endoscopic remission at 6 months post-surgery according to their subsequent endoscopic status at 12 months post-surgery [n = 25].
4. Discussion
In the present study, we found that early post-surgical endoscopic recurrence was associated with a rise in the frequency of CD8+ CTL expressing granzyme B or perforin in ileal LP and peripheral blood leukocytes. At 6 months post-surgery, a frequency in circulating cytotoxic CD8+ T cell subsets above 45% [with a predictive negative value of 86–87 %] appeared to accurately identify patients undergoing endoscopic recurrence. In addition, there was a gradual increase in the frequency of cytotoxic CD8+ T cells in ileal LP and peripheral blood leucokytes according to the severity of endoscopic recurrence. Last, the frequency of cytotoxic CD8+ T cells in ileal LP and peripheral blood was higher in patients who experienced an endoscopic recurrence 6 months later than in patients still in endoscopic remission, but the difference reached a statistically significant level only for the frequency of granzyme B-expressing CD8+ T cells in ileal mucosa.
In our study, a rise in cytotoxic CD8+ T cells [both in blood and in ileal LP] coincides with the onset of gut lesions in the model of post-surgical CD relapse. Although this setting is assumed to reproduce the pre-symptomatic phase of CD, whether our data translate to non-operated patients warrants further investigation in a larger patients cohort with graded severity and duration of disease. The diagnostic value of CD8+ CTL in blood is better than that of serum CRP to detect patients in post-surgical endoscopic recurrence, but must be evaluated in comparison with other challenging inflammatory markers, such as faecal calprotectin.10,11 The fact that a rise in mucosal cytotoxic CD8+ T cells precedes by 6 months the onset of macroscopic lesions supports the suggestion that immunological profiling of different cytotoxic T cell subsets may help to characterise subgroups of patients more prone to disease relapse.
Whether changes in CD8+ T cells play a role either in ongoing inflammation or in disease pathogenesis, cannot be inferred from our data. Previous reports suggest that CD8+ T cells may play a role in CD pathogenesis. Indeed, an accumulation of CD8+ T cells in the ileal crypt region of infants with inflammatory bowel disease [IBD] at first presentation has been reported.12 In situ hybridisation detected the presence of perforin and granzyme A cytotoxic granules co-localising with CD8+ T cells, in the vicinity of the gut epithelium.12,13 Moreover, granzyme B-expressing CD8+ T cells and apoptotic cells were observed together in the focal mucosal lesions of CD,14 suggesting that cytotoxicity represents a potential mechanism of initiation or perpetuation of the inflammatory process. Along these lines, other studies reported the presence of autoreactive class I-restricted cytotoxic T cells in blood of CD patients.15 Interestingly, transcriptional profiling of circulating T cells from IBD patients has recently identified a prognostic gene expression signature in CD8+ [but not CD4+] T cells in a subgroup of patients who experienced a more aggressive disease course with higher incidence of flares and treatment failure.16 Considering the colitogenic effector function of cytotoxic CD8+ CTL documented in several mouse models of colitis,2,3,4 these data suggest that cytotoxic CD8+ T cells may play a role in the initiation of intestinal lesions and underscore the suggestion that these cells might constitute a potential novel therapeutic target in CD.
It should be however emphasised that beyond CD8+ T cells, CD4+ T cells endowed with cytolytic properties might also contribute to CD pathogenesis. Indeed, active CD patients harbour circulating and mucosal CD4+ T cells performing natural-killer group 2, member D [NKG2D]-mediated cytotoxicity against inflamed epithelial cells upregulating the NKG2D]ligand major histocompatibility complex class I-related chain A/B [MICA/B].17 Moreover, co-engagement of NKG2D and the T-cell receptor significantly increased production of IL-17 and TNFα by these CD4+ T cells.18 Interestingly, T cell repertoire analysis revealed oligoclonal expansions of both CD8+ and CD4+ T cells in blood and intestinal mucosa from active and also macroscopically inactive areas of patients with CD, suggesting that both these T cell subsets might contribute to recurrence of gut lesions.19
In conclusion, we show that endoscopic recurrence is associated with a rise in circulating and mucosal CD8+ CTL and that mucosal accumulation of CD8+ CTL identifies, among CD patients in endoscopic remission, those at high risk of subsequent recurrence.
Funding
This work was supported by institutional grants from the Institut National de la Santé et de la Recherche Médicale [INSERM], Hospices Civils de Lyon [HCL] ,and the François Aupetit Association [AFA].
Conflict of Interest
None for all the authors.
Author Contributions
Study design [SN, BF, DK]; acquisition of data, analysis and interpretation of data [GB, SN, DK, BF]; drafting of the manuscript [GB, SN, BF, DK]; critical review of the manuscript [EC, DM, YF].
Acknowledgments
We are grateful to the Cytometry plateform of the UMR5308 for help with FACS analysis.
References
- 1. Cheroutre H. In IBD eight can come before four. Gastroenterology 2006;131:667–70. [DOI] [PubMed] [Google Scholar]
- 2. Nancey S, Holvoet S, Graber I, et al. CD8+ cytotoxic T cells induce relapsing colitis in normal mice. Gastroenterology 2006;131:485–96. [DOI] [PubMed] [Google Scholar]
- 3. Nancey S, Boschetti G, Hacini F, et al. Blockade of LTB[4] /BLT[1] pathway improves CD8[+] T-cell-mediated colitis. Inflamm Bowel Dis 2011;17:279–88. [DOI] [PubMed] [Google Scholar]
- 4. Westendorf AM, Fleissner D, Deppenmeier S, et al. Autoimmune-mediated intestinal inflammation-impact and regulation of antigen-specific CD8+ T cells. Gastroenterology 2006;131:510–24. [DOI] [PubMed] [Google Scholar]
- 5. Kontoyiannis D, Boulougouris G, Manoloukos M, et al. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn’s-like inflammatory bowel disease. J Exp Med 2002;196:1563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tajima M, Wakita D, Noguchi D, et al. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J Exp Med 2008;205:1019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olaison G, Smedh K, Sjodahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut 1992;33:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990;99:956–63. [DOI] [PubMed] [Google Scholar]
- 9. Walters TD, Steinhart AH, Bernstein CN, et al. Validating Crohn’s disease activity indices for use in assessing postoperative recurrence. Inflamm Bowel Dis 2011;17:1547–56. [DOI] [PubMed] [Google Scholar]
- 10. Kopylov U, Rosenfeld G, Bressler B, Seidman E. Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory bowel disease. Inflamm Bowel Dis 2014;20:742–56. [DOI] [PubMed] [Google Scholar]
- 11. Boschetti G, Laidet MH, Moussata D, et al. Levels of fecal calprotectin are associated with the severity of postoperative endoscopic recurrence in asymptomatic patients with Crohn’s disease. Am J Gastroenterol 2015;110:865–72. [DOI] [PubMed] [Google Scholar]
- 12. Muller S, Lory J, Corazza N, et al. Activated CD4+ and CD8+ cytotoxic cells are present in increased numbers in the intestinal mucosa from patients with active inflammatory bowel disease. Am J Pathol 1998;152:261–8. [PMC free article] [PubMed] [Google Scholar]
- 13. Kappeler A, Mueller C. The role of activated cytotoxic T cells in inflammatory bowel disease. Histol Histopathol 2000;15:167–72. [DOI] [PubMed] [Google Scholar]
- 14. Jenkins D, Seth R, Kummer JA, Scott BB, Hawkey CJ, Robins RA. Differential levels of granzyme B, regulatory cytokines, and apoptosis in Crohn’s disease and ulcerative colitis at first presentation. J Pathol 2000;190:184–9. [DOI] [PubMed] [Google Scholar]
- 15. Okazaki K, Morita M, Nishimori I, et al. Major histocompatibility antigen-restricted cytotoxicity in inflammatory bowel disease. Gastroenterology 1993;104:384–91. [DOI] [PubMed] [Google Scholar]
- 16. Lee JC, Lyons PA, McKinney EF, et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J Clin Invest 2011;121:4170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allez M, Tieng V, Nakazawa A, et al. CD4+NKG2D+ T cells in Crohn’s disease mediate inflammatory and cytotoxic responses through MICA interactions. Gastroenterology 2007;132:2346–58. [DOI] [PubMed] [Google Scholar]
- 18. Pariente B, Mocan I, Camus M, et al. Activation of the receptor NKG2D leads to production of Th17 cytokines in CD4+ T cells of patients with Crohn’s disease. Gastroenterology 2011;141:217–26, 26 e1–2. [DOI] [PubMed] [Google Scholar]
- 19. Camus M, Esses S, Pariente B, et al. Oligoclonal expansions of mucosal T cells in Crohn’s disease predominate in NKG2D-expressing CD4 T cells. Mucosal Immunol 2014;7:325–34. [DOI] [PubMed] [Google Scholar]