Abstract
Aims
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13, is a metalloprotease that cleaves von Willebrand factor (VWF). There is considerable evidence that VWF levels increase and ADAMTS13 levels decrease in ST-elevation myocardial infarction (STEMI) patients. It is unclear whether this contributes to no reflow, infarct size, and intramyocardial haemorrhage (IMH). We aimed to determine the role of ADAMTS13 in STEMI patients and to investigate the benefits of recombinant ADAMTS13 (rADAMTS13) in a porcine model of myocardial ischaemia-reperfusion.
Methods and results
In 49 consecutive percutaneous coronary intervention (PCI)-treated STEMI patients, blood samples were collected directly after through 7 days following PCI. Cardiac magnetic resonance was performed 4–6 days after PCI to determine infarct size and IMH. In 23 Yorkshire swine, the circumflex coronary artery was occluded for 75 min. rADAMTS13 or vehicle was administered intracoronary following reperfusion. Myocardial injury and infarct characteristics were assessed using cardiac enzymes, ECG, and histopathology. In patients with IMH, VWF activity and VWF antigen were significantly elevated directly after PCI and for all subsequent measurements, and ADAMTS13 activity significantly decreased at 4 and 7 days following PCI, in comparison with patients without IMH. VWF activity and ADAMTS13 activity were not related to infarct size. In rADAMTS13-treated animals, no differences in infarct size, IMH, or formation of microthrombi were witnessed compared with controls.
Conclusions
No correlation was found between VWF/ADAMTS13 and infarct size in patients. However, patients suffering from IMH had significantly higher VWF activity and lower ADAMTS13 activity. Intracoronary administration of rADAMTS13 did not decrease infarct size or IMH in a porcine model of myocardial ischaemia-reperfusion. These data dispute the imbalance in ADAMTS13 and VWF as the cause of no reflow.
Keywords: von Willebrand factor, ADAMTS13, Acute myocardial infarction, Ischemia-reperfusion injury, No reflow
1. Introduction
In up to 40% of ST-elevation myocardial infarction (STEMI) patients, imperfections in myocardial blood flow are observed despite reperfusion at the epicardial level.1 This phenomenon, also referred to as no reflow, is occasionally observed angiographically directly after percutaneous coronary intervention (PCI). In a majority of cases, it is established several days later by cardiac magnetic resonance (CMR) as a hypoenhanced area within the hyperenhanced infarcted myocardium.2,3 Both angiographic no reflow and CMR-defined no reflow are related to increased cardiac failure and death.4–6 Various mechanisms for this phenomenon have been proposed including tissue oedema, distal embolization of atherosclerotic debris, and also local microthrombi causing occlusion of capillaries leading to larger infarct size and worse outcome.7 Recently, it was shown that CMR-defined no reflow actually contains intramyocardial haemorrhage (IMH) and complete microvascular destruction.8–10
von Willebrand factor (VWF) is an important factor in primary haemostasis. It attracts platelets to damaged endothelium, causing platelet adhesion; and it thereby initiates and stabilizes platelet aggregation. VWF is released from endothelium during vessel injury in the form of ultra-large VWF multimers, which contain several platelet binding sites and are therefore more prothrombotic. ADAMTS13, a disintegrin and metalloprotease with a thrombospondin type 1 repeats-13, is a metalloprotease that cleaves VWF, reducing the size of VWF multimers and diminishing their prothrombotic features.11 In STEMI patients, VWF levels are increased,12,13 but it is not known whether this is related to infarct size and occurrence of no reflow and IMH. In parallel with the increase in VWF, ADAMTS13 decreases in STEMI patients.12,13 Recently, it was shown that ADAMTS13 knockout mice developed larger myocardial infarctions after coronary occlusion and showed decreased left ventricular function when compared with wild-type mice. Also, treatment with recombinant ADAMTS13 (rADAMTS13) reduced infarct size in wild-type mice.14–16 However, the potential beneficial effects of rADAMTS13 on infarct size and infarct characteristics have never been tested in a large animal model of myocardial ischaemia-reperfusion.
In the present study, the relationship between ADAMTS13 and VWF levels and CMR-derived infarct size as well as occurrence of IMH was determined prospectively in STEMI patients. Also, in a porcine model of myocardial ischaemia-reperfusion, closely resembling the clinical scenario of acute myocardial infarction (AMI) treated with primary PCI, intracoronary infusion of rADAMTS13 was tested for its effects on infarct size, formation of microthrombi, and IMH.
2. Methods
2.1. Patient study
Sixty consecutive patients with acute STEMI, presenting at the catheterization laboratory within 6 h after onset of symptoms and successfully treated by primary PCI, were included in a prospective study as reported earlier.17 For the present analysis, only patients who underwent CMR at Days 4–6 after PCI were selected (n= 49). The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the ethics committee of the VU University Medical Center. Upon arrival, daily administration of 100 mg acetylsalicylic acid and 10 mg prasugrel was started. Bivalirudin was administered as a single bolus, followed by 0.25 mg/kg intravenously during 4 h. Blood was withdrawn directly after (T0) and at 1, 4, and 7 days (T1, T4, and T7) after PCI. VWF activity, VWF antigen, VWF propeptide, ADAMTS13 activity, fibrinogen, and D-dimer were measured. Further details on methods such as exclusion criteria and measurement of ST-resolution can be found in the Supplementary material online.
2.2. Cardiovascular magnetic resonance imaging
CMR was performed between 4 and 6 days after PCI using a 1.5Tesla MR-scanner (Avanto, Siemens, Erlangen, Germany). IMH was identified on T2w-images as hypointense areas within the hyperintense signal of infarct-related oedema. Cine images were analysed by tracing the endocardial and epicardial myocardial borders in both end-diastolic and end-systolic phases, providing myocardial volumes and ejection fraction. Quantification of infarct size was performed on short-axis late gadolinium-enhanced images. Further details on acquisition and analysis and definitions of CMR parameters are specified in the Supplementary material online.
2.3. Porcine ischaemia-reperfusion model
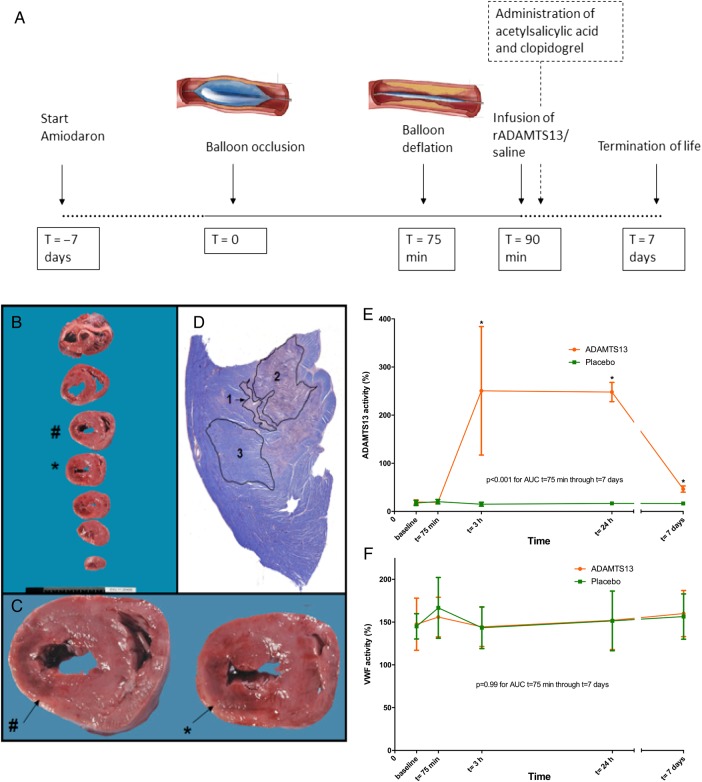
Approval was obtained from the local Animal Ethics Committee of the VU University Medical Center. The animal procedures that were performed conform the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. A full description of animal experimental procedures, including continuous 12-lead electrocardiograms and transthoracic echocardiography, can be found in the Supplementary material online. See also Figure 4A for the study flow chart. In brief, 23 female Yorkshire swine were included; 12 animals received vehicle and 11 were given rADAMTS13. An over-the-wire balloon was placed in the proximal left circumflex artery and inflated for 75 min. During coronary occlusion, animals received a bolus of 5000 IU of unfractionated heparin and the same amount after deflation of the balloon. After reperfusion, 300 mg acetylsalicylic acid and 300 mg clopidogrel were administered. All animals were given daily doses of 80 mg acetylsalicylic acid and 75 mg clopidogrel until their planned sacrifice 7 days after ischaemia-reperfusion. rADAMTS13 (400 U/kg body weight = 320 μL/kg body weight, Baxter Innovations, Vienna, Austria) or a comparable amount of vehicle were administered intracoronary in one single bolus 15min after reperfusion by an investigator blinded for treatment.
Figure 4.
Study flow chart of the comprehensive study protocol, histopathological methods in the porcine ischaemia-reperfusion model, and ADAMTS13 and VWF activity in the porcine ischaemia-reperfusion model. (A) Study flow chart. (B) Post-mortem sectioning of a porcine heart. (C) Macroscopically visible infarcted area (indicated by # and *) was fixed and embedded. (D) PTAH staining, which stains non-haemorrhagic infarction pink (1), haemorrhagic infarction dark purple (2), and viable tissue blue (3).44 (E) ADAMTS13 activity (%) measured before and after 75 min of balloon occlusion (T = 75 min) of the left circumflex artery (median and IQR). rADAMTS13-treated animals demonstrated elevated ADAMTS13 activity levels compared with controls (P < 0.001). (F) VWF activity (%) measured before and after 75 min of balloon occlusion (T = 75 min) of the left circumflex artery (median and IQR). rADAMTS13-treated animals had similar VWF activity levels compared with controls directly after reperfusion (T = 75 min). P= 0.99 for rADAMTS13. n = 11, 10 (rADAMTS13, control)/group. Mean AUCs were compared using independent samples t-tests.
2.4. Histopathological analysis
Detailed information on histopathological methods can be found in the Supplementary material online. In brief, on the seventh day after experimental AMI, animals were sedated and killed. Total infarct size and IMH were determined macroscopically and microscopically (Figure 4C). Anti-CD31 staining was performed to detect (micro-)thrombi and (micro-)vessels. For both treatment arms, microthrombi and the number of vessels and thrombi per mm² were calculated. To assess inflammatory involvement, myocardial tissue was stained for MPO and CD45.18,19
2.5. Laboratory measurements performed in the patient and porcine study
Laboratory measurements are described in detail in the Supplementary material online. VWF factor activity (Innovance VWF Ac) was determined on a Behring Coagulation System according to protocols from the manufacturer (Siemens Healthcare Diagnostics, Marburg, Germany). ADAMTS13 activity was determined as described earlier.20 VWF antigen and VWF propeptide levels were measured by ELISA using commercial antibodies (DAKO, Denmark and Sanquin, The Netherlands, respectively). Data are presented in percentage activity or percentage of antigen. The reference value is derived from normal pooled plasma. Our pool is composed of >200 healthy volunteers. D-dimer levels were determined with a particle-enhanced immunoturbidimetric assay (Innovance D-dimer, Siemens Healthcare Diagnostics). Fibrinogen concentration was derived from the change in optical signal during prothrombin time determination. Fibrinogen antigen was determined by ELISA using antibodies from DAKO (Glostrup, Denmark). The ability of rADAMTS13 to cleave porcine VWF was determined in vitro by measuring porcine VWF activity in plasma of animals before and after addition of rADAMTS13.
2.6. Statistical analysis
Categorical data are presented as frequencies (percentage) and continuous data as mean ± standard error (SE) or median with interquartile range (IQR). For the patient study, missing values for coagulation parameters at specific time points were imputed using multiple imputation. The imputation model included age, sex, and all coagulation parameters, and 20 data sets were created. Area under the receiver operator curve (AUC) for levels of D-dimer, VWF activity, VWF antigen, VWF propeptide, and ADAMTS13 was determined using blood measurements taken at T0, T1, T4, and T7 (separately for each of the imputed data sets). Mean AUCs of patients with and without IMH were compared using independent samples t-tests. For comparisons of coagulation parameters at different time points, repeated measures ANOVA was used with IMH/no IMH as a between-factor and time point as a within-factor. To determine the correlation between continuous variables, Pearson's R was calculated. Estimated mean differences and P-values of the tests were pooled over the imputations using the standard pooling procedures for multiple imputed data sets available in SPSS. For the porcine study, differences between treatment groups were compared with Mann–Whitney U tests for unpaired and Wilcoxon signed-rank tests for paired non-parametric analysis. Pearson's χ2 test was performed on categorical variables, and ANOVA was used for regression. Plots of means were drawn using GraphPad Prism (GraphPad Software 6.00, San Diego, CA, USA). Statistical analyses were performed using SPSS software package (IBM SPSS Statistics 22.0, Chicago, IL, USA).
3. Results
3.1. General characteristics of the patient study
Forty-nine STEMI patients underwent CMR between 4 and 6 days after PCI. Clinical demographics and CMR parameters of these patients are shown in Table 1.
Table 1.
Clinical demographics, angiographic characteristics, and functional parameters in the patient study
| Characteristic | STEMI patients (n = 49) |
|---|---|
| Male sex | 37 (76%) |
| Age (years) | 59 ± 9 |
| Weight (kg) | 85 ± 14 |
| BMI (kg/m2) | 27 ± 3 |
| CAD risk factors | |
| Diabetes | 7 (14%) |
| Hypertension | 38 (78%) |
| Hypercholesterolaemia | 8 (16%) |
| Smoking history | 41 (84%) |
| Family history | 33 (67%) |
| Functional parameters assessed by CMR at 4–6 days | |
| LVEDV (mL) | 90.5 ± 18.5 |
| LVESV (mL) | 45.5 ± 16.8 |
| LVEF (%) | 50.9 ± 8.2 |
| Infarct size (% of the LV) | 17.5 ± 12.3 |
| Time to reperfusion (h) | 2.6 ± 1.3 |
| CK-MB peak (U/L) | 176 ± 183 |
| Infarct-related artery | |
| LAD | 27 (55%) |
| LCx | 5 (10%) |
| RCA | 17 (35%) |
| Thienopyridine | 49 (100%) |
| Platelet glycoprotein IIb/IIIa inhibitors | 14 (29%) |
| TIMI 3 flow grade post-PCI | 46 (94%) |
| Incomplete (≤70%) ST-segment resolution post-PCI | 28 (57%) |
Data are n or mean ± SD.
BMI, body mass index; CAD, coronary artery disease; CMR, cardiovascular magnetic resonance; LV, left ventricle; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; CK-MB, creatine kinase-myocardial band; PCI, percutaneous coronary intervention.
3.2. IMH associated with higher VWF activity and lower ADAMTS13 activity
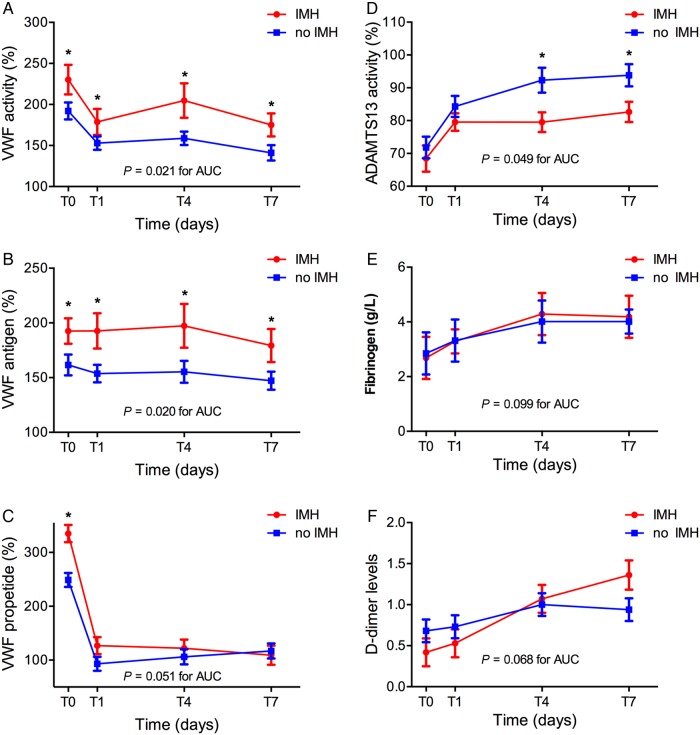
CMR-defined IMH was found in 19 patients (39%). No significant interaction between the presence of IMH and time was found for VWF activity, VWF antigen levels, ADAMSTS13 activity, and Fibrogen levels (P = 0.61, 0.83, 0.058, and 0.229, respectively), implying a similar difference between the mean levels of patients with and without IMH at all time points. Mean VWF activity (Figure 1A ) and VWF antigen (Figure 1B) levels were found to be consistently higher at all time points in patients with IMH (mean difference IMH—no IMH ± SE; VWF activity: 35.8 ± 14.8, P = 0.020, VWF antigen levels: 35.2 ± 15.3, P = 0.026). A significant interaction between the presence of IMH and time was found for VWF propeptide levels (P = 0.008), with mean VWF propeptide levels at T0 being significantly higher for patients with IMH, but not significantly different at other time points (IMH vs. no IMH; mean ± SE, P-value; T0: 335 ± 16% vs. 249 ± 13%, P < 0.001, T1: 127 ± 16% vs. 93 ± 13%, P = 0.11, T4: 122 ± 16% vs. 106 ± 14%, P = 0.45, T7: 109 ± 18% vs. 117 ± 14%, P = 0.75) (Figure 1C). ADAMTS13 activity was lower in patients with IMH (Figure 1D) (P = 0.049). No difference was found in fibrinogen (Figure 1E) levels between patients with and without IMH (P = 0.83). A significant interaction between the presence of IMH and time was found for D-dimer levels (P = 0.001), with mean D-dimer levels at T0 and T1 being lower in IMH group and mean levels at T4 and T7 being higher in IMH group but with post hoc tests for comparison of mean levels between groups at T0, T1, T4 and T7 separately revealing no significant differences (IMH vs. no IMH; mean ± SE, P-value; T0: 0.42 ± 0.17 vs. 0.68 ± 0.14, P = 0.26, T1: 0.53 ± 0.17 vs. 0.73 ± 0.14, P = 0.38, T4: 1.07 ± 0.17 vs. 1.00 ± 0.14, P = 0.75, T7: 1.36 ± 0.18 vs. 0.94 ± 0.14, P = 0.064) (Figure 1F).
Figure 1.
VWF activity, VWF antigen, VWF propeptide, ADAMTS13 activity, and fibrinogen and D-dimer levels in STEMI patients treated with PCI. (A) VWF activity (%), (B) VWF antigen (%), (C) VWF propeptide (%), (D) ADAMTS13 activity (%), (E) fibrinogen level (g/L), and (F) D-dimer levels, measured directly after PCI (T0) and at 1, 4, and 7 days following PCI (T1, T4, T7). VWF activity (P = 0.021 for AUC) and VWF antigen levels (P = 0.020 for AUC) were significantly higher in patients with IMH compared with patients without IMH (n = 19, 30 for IMH, no IMH/group). Mean AUCs were compared using independent samples t-tests.
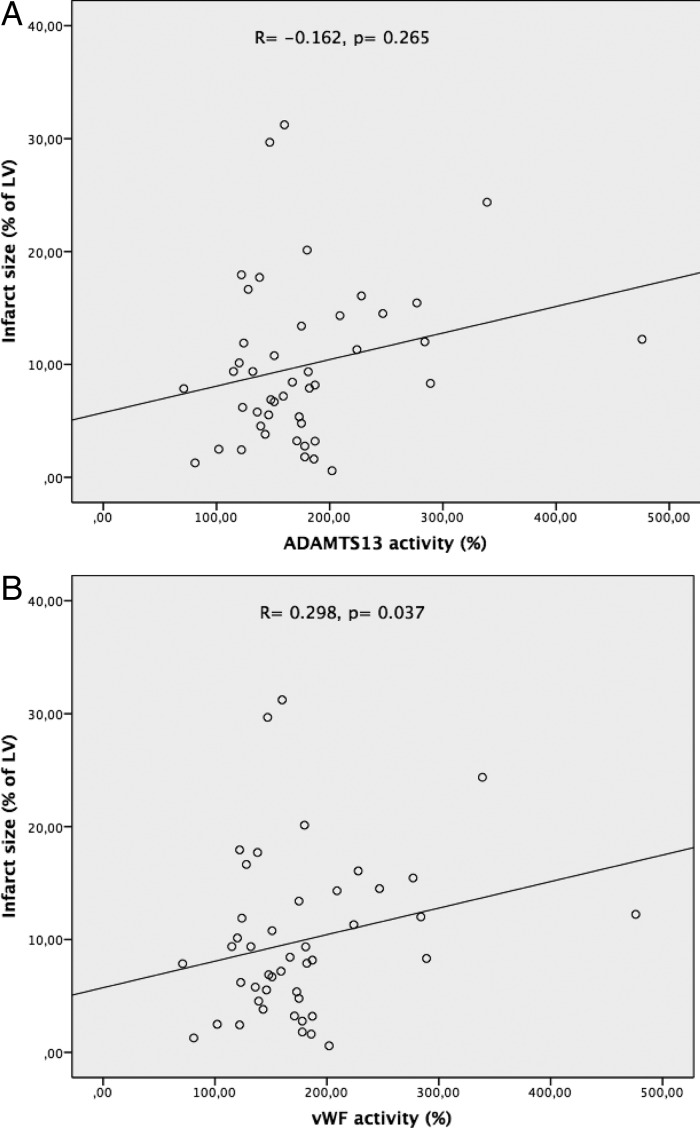
3.3. No strong correlation between VWF activity, ADAMTS13 activity, and infarct size
Mean infarct size [as percentage of the left ventricle (LV)] in STEMI patients was 17.5 ± 12.3% and did not correlate substantially to ADAMTS13 activity measured directly after PCI (R= −0.162, P= 0.265) or to any other subsequent measurement (Figure 2A). VWF activity correlated statistically significant with infarct size (Figure 2B), but only at T4, and the correlation was weak (R= 0.298, P= 0.037). Also, myocardial salvage did not correlate significantly to ADAMTS13 activity at T0 (R= 0.032, P = 0.831) or to measurements at other time points. The same held true for the relationship between myocardial salvage and VWF activity at T0 (R= −0.159, P = 0.285) as well as T1, T4, and T7.
Figure 2.
Correlation between ADAMTS13 and VWF activity. Mean infarct size (as percentage of the LV) in STEMI patients did not correlate substantially to ADAMTS13. VWF levels correlated statistically significant with infarct size.
3.4. General characteristics of the porcine study
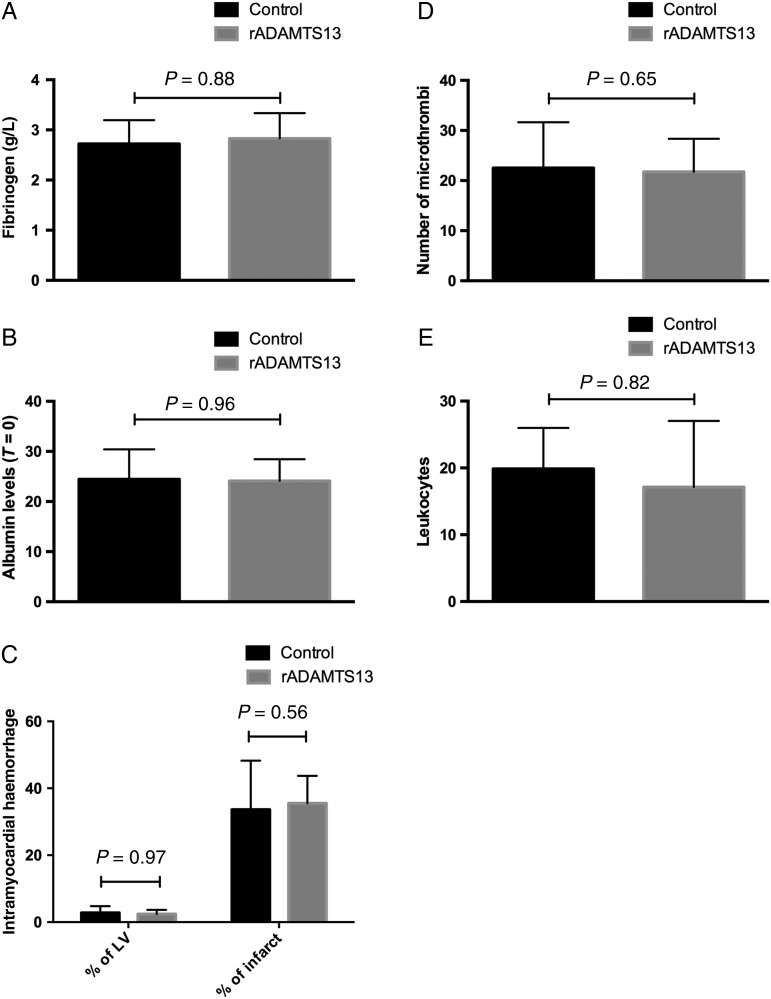
In the present study, 23 animals were included (median age 83 days, IQR 79–91; median weight 30 kg, IQR 27–34); 12 animals received vehicle and 11 were given rADAMTS13. Two animals from the vehicle arm died within 24 h after the intervention. In ex vivo experiments, when adding increasing concentrations of rADAMTS13 to porcine plasma, a corresponding decrease in VWF activity was measured. In the porcine model, at T = 75 min after reperfusion fibrinogen (Figure 3A) decreased in comparison with baseline values. As all animals received a substantial amount of intravenous fluids, albumin levels (Figure 3B) at baseline and after release of the balloon were measured, showing clear evidence of dilution (percentage decrease albumin at T = 75 min: 18%, P < 0.001, no differences between treatment groups). This percentage was used to correct measurements of all coagulation assays performed at T = 75 min.
Figure 3.
Fibrinogen and albumin levels, IMH, microthrombi, and leukocytes in the animal model. (A) Fibrinogen levels (T = 75 min), (B) albumin levels (T = 0), (C) IMH in percentage of the LV and as percentage of infarct size (T = 7), (D) number of microthrombi (T = 7), and (E) number of leukocytes (T = 7) in rADAMTS13-treated animals compared with controls. n = 11, 10 (rADAMTS13, control)/group.
3.5. VWF and ADAMTS13 levels in experimental myocardial ischaemia-reperfusion
Median baseline VWF activity for all swine was 150% (IQR 127–172). After correction for dilution, there was a trend towards increased VWF activity directly following the intervention (median 165%, IQR 123–183, P = 0.056). ADAMTS13 activity did not change after the induced AMI (baseline vs. T = 75 min: 18%, IQR 15–4 vs. 18%, IQR 16–24, P = 0.57). Following intracoronary infusion of rADAMTS13 at 3 h after AMI, ADAMTS13 activity increased significantly to 324% (IQR 117–384, P = 0.003 in comparison with baseline, P < 0.001 vs. control group that had a median level of 15%, IQR 11–19). ADAMTS13 activity remained increased in the rADAMTS13-treated group on all following measurements (P < 0.001 for repeated measures ANOVA in comparison with control group). There was no difference in VWF activity after infusion between both treatment groups for all following measurements (P = 0.99 for repeated measures ANOVA) (Figure 4E and F).
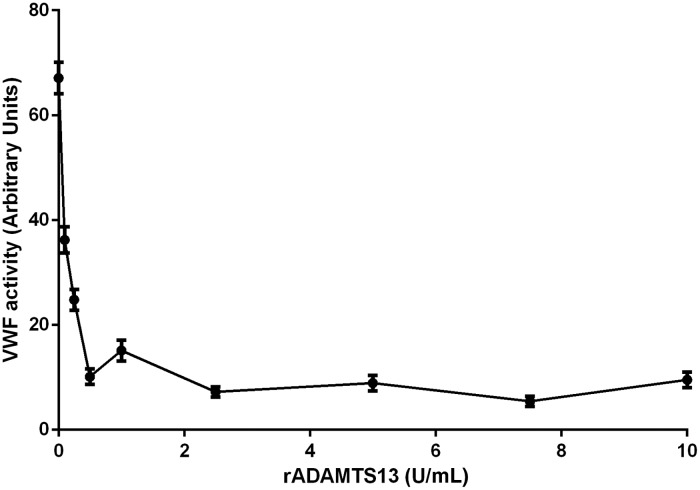
Induction of myocardial infarction had no direct influence on fibrinogen levels (baseline vs. T = 75 min: 3.3 g/L, IQR 3.0–3.5 vs. 3.3 g/L, IQR 2.7–3.7; P = 0.88). However, fibrinogen levels were increased at 1 and 7 days after induction of ischaemia-reperfusion (6.9, IQR 5.7–7.5 and 4.8, IQR 4.0–5.6, respectively; P < 0.001 vs. baseline for both) compared with baseline levels. There were no differences in fibrinogen measurements throughout the experiment between treated animals and controls (P= 0.80 for repeated measures ANOVA). There were no differences in red blood cell count and platelet count between rADAMTS13- and vehicle-treated animals throughout the study (data not shown). In vitro studies proved the efficacy of cleavage of porcine VWF by rADAMTS13 at a dosage of <1 U/mL (Figure 5).
Figure 5.
Cleavage of porcine VWF by rADAMTS13. In vitro studies show that adding rADAMTS13 to porcine plasma results in a dose-dependent decrease in VWF activity.
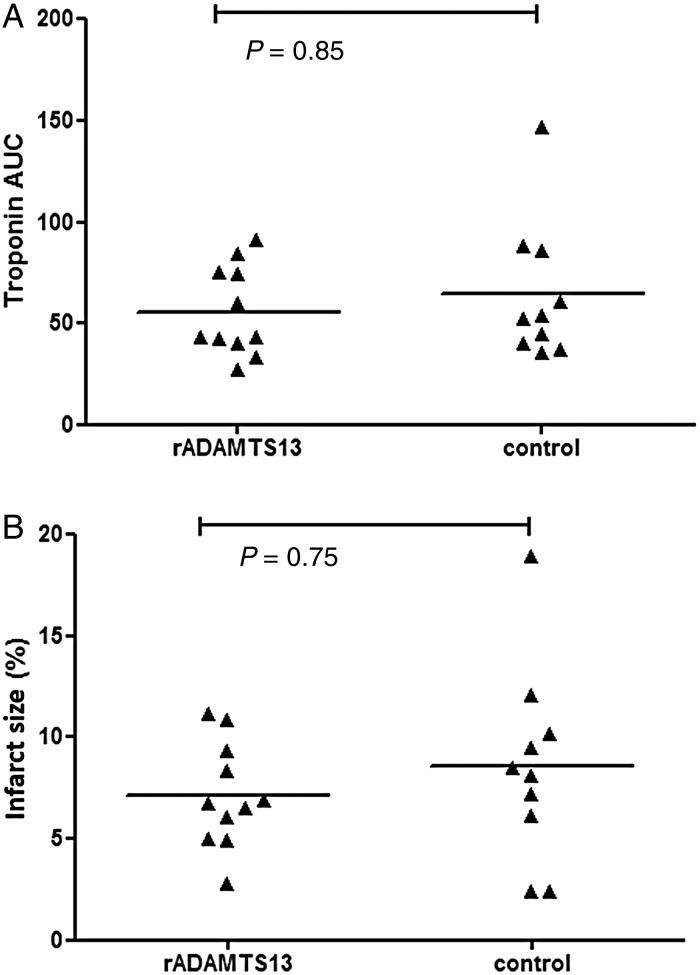
3.6. No effects of rADAMTS13 on ST-segment resolution, infarct size, and cardiac function
ST-segment resolution and cardiac enzymes were similar between treatment groups (see also Figure 6A and the Supplementary material online). Microscopic infarct size as measured by PTAH staining was comparable between rADAMTS13-treated group (6.7%, IQR 5.0–9.4) and control group (8.2%, IQR 2.4–10.2; P = 0.75, see also Figure 6B). Macroscopic estimation of infarct size from photographed cross sections of the heart showed no differences between rADAMTS13-treated animals and controls (rADAMTS13 vs. controls: 25.1%, IQR 20.6–26.6 vs. 22.2%, IQR 20.0–29.3; P= 0.97). rADAMTS13-treated animals had an increased wall motion score index (WMSI) at 60 min after reperfusion (P= 0.009 for absolute values and P= 0.02 for delta 2-1, see also Supplementary material online, Table S1). No other differences were observed between first and final echocardiogram for all echocardiography parameters, when comparing rADAMTS13-treated animals and controls (see also the echocardiographic analysis in the Supplementary material online, Results and Table S1).
Figure 6.
Results on infarct size from the porcine study. (A) Cardiac enzyme troponin T (μg/L) was measured at 3, 6, and 24 h after the balloon occlusion. Infarct size was calculated as the AUC of troponin T.45 (B) Histopathological findings, using PTAH staining.44 Differences between treatment groups were compared with Mann–Whitney U tests for unpaired and Wilcoxon signed-rank tests for paired non-parametric analysis.
3.7. No effects of intracoronary rADAMTS13 infusion on IMH or formation of microthrombi
Histopathological findings showed that the core of all infarctions contained haemorrhage. The size of IMH as a percentage of LV was similar between treatment groups (rADAMTS13 vs. controls: 2.4%, IQR 1.2–3.7 vs. 2.1%, IQR 0.8–4.8, P= 0.97). Same results were found for IMH as percentage of infarct size (rADAMTS13 vs. controls: 37.0%, IQR 27.3–43.7 vs. 30.1%, IQR 19.0–48.3, P= 0.56) (Figure 3C).
The total number of microthrombi in the infarct tissue did not differ between groups (rADAMTS13 vs. controls: 19.0 per section, IQR 17.0–27.5 vs. 21.5 per section, IQR 17.5–28.5; P = 0.65) (Figure 3D).
Leukocytes as detected with CD45 and MPO staining were found more often in the border zone (97.5, IQR 63.8–135.8) in comparison with infarct tissue (17.8, IQR 11.1–28.9, P < 0.001) (Figure 3E). There was no difference between rADAMTS13-treated and control animals with regard to the presence of leukocytes in the infarcted tissue, the border zone, or the non-affected myocardium.
4. Discussion
There is ample proof of the association between VWF/ADAMTS13 levels and myocardial infarction,12,13 and rADAMTS13 has been effective in reducing myocardial injury in rodent models.21 In the present study, VWF/ADAMTS13 levels were assessed in 49 STEMI patients and IMH was determined using CMR. Furthermore, rADAMTS13 was administered with the aim to limit myocardial injury in a porcine myocardial ischaemia-reperfusion model. The main findings are the following: (i) IMH was associated with higher VWF activity and (eventually) lower ADAMTS13 activity; (ii) intracoronary administration of rADAMTS13 did not reduce infarct size in a porcine myocardial ischaemia-reperfusion model on a background of dual antiplatelet therapy and heparin.
4.1. VWF and ADAMTS13 levels in patients with IMH: cause or consequence?
STEMI patients with IMH had higher VWF activity immediately after PCI and in all subsequent measurements. VWF propeptide, a cleavage product of VWF, was significantly higher only in patients with IMH for the first day. This could be explained by its short half-life of 2–3 h.22 Directly after PCI, there were no differences in ADAMTS13 activity between patients with and without IMH. Lower ADAMTS13 activity was found in patients with IMH at 4 and 7 days following STEMI. These findings are in line with Zhao et al. who showed that VWF levels in STEMI patients were higher immediately after PCI in patients with no reflow, whereas ADAMTS13 levels did not differ between patients with and without no reflow, until the seventh day after PCI.23
The present findings that VWF levels are increased in patients with IMH and ADAMTS13 levels decreased are novel and counter-intuitive at first. However, a similar pattern has been shown in patients after hip surgery, and also patients with subarachnoid bleeding display increased levels of VWF.24–26 VWF is believed to be an acute phase protein, and its levels increase in various inflammatory diseases.27–29 Possibly, the rise in VWF following AMI is an epiphenomenon or a necessary compensation for IMH.
VWF and ADAMTS13 may nevertheless serve as diagnostic markers to detect patients at risk of developing IMH. Especially, given that no reflow is detected angiographically in a minority of cases3 and that CMR, although more effective in detecting no reflow,2,3 cannot be performed directly in acute STEMI patients. Other frequently used markers of coagulation, D-dimer and fibrinogen, were similar in both patient groups.
4.2. No effect of rADAMTS13 in reducing myocardial injury
Treatment with rADAMTS13 reduced infarct size in a murine stroke model by 30% without signs of increased bleeding.30 Furthermore, rADAMTS13 exerted antithrombotic effects in a model of vessel damage in which ADAMTS13 decreased occlusion time of mesenteric arterioles after FeCl3-induced thrombus formation.31 In open chest myocardial infarction models, a therapeutic benefit of rADAMTS13 was shown in both ADAMTS13 knockout mice and wild-type mice treated with rADAMTS13.14–16 In all murine AMI studies, rADAMTS13 led to a decrease in neutrophil accumulation in the infarcted myocardium. Interestingly, there was no evidence of any antithrombotic effect of rADAMTS13. For instance, there was no reduction of thrombotic occlusions in myocardial microvessels. Therefore, its therapeutic properties were deemed to be anti-inflammatory rather than antithrombotic.14–16 The inflammatory response may have been caused by the use of an open chest model, unlike the closed chest approach in our study. Possibly, a beneficial effect of rADAMTS13 was not found in our study due to the fact that the model used was not inflammatory or thrombotic enough. VWF activity did not increase substantially after the infarction, and fibrinogen did not increase until 24 h. However, the model implemented in the present analysis has been established and proved effective in detecting treatment effect in myocardial injury models.32–36 Furthermore, the medical regime used in our study was according to the ESC guidelines for STEMI patients, mimicking the treatment of patients with AMI realistically. In the rodent studies, no antiplatelet agent, heparin, or any other anticoagulant was applied besides rADAMTS13.14–16,37 Assessment of area at risk would have attributed to a more standardized assessment of ischaemia-reperfusion injury. However, assessment of area at risk directly after the PCI was not possible, as the pigs would have to have been killed immediately after the intervention.38 For the porcine study, the choice was made to kill the swine 7 days after the intervention, to better assess myocardial infarct size. All findings from this study from several parameters (cardiac enzymes, continuous 12-lead electrocardiograms, echocardiography, and histopathological analysis) did point in the same direction, and this underlines the robustness of the data.
In the rodent studies and the murine stroke model, rADAMTS13 was administered prior to reperfusion. In the present porcine myocardial ischaemia-reperfusion model, rADAMTS13 was applied only 15 min following reperfusion. However, because rADAMTS13 primarily works as an antagonist for VWF, it seems unlikely that its efficacy would be less 15 min following reperfusion.11 Furthermore, patients with AMI will likely be treated only with a therapeutic regime against no reflow, following PCI.
4.3. High dosage, timing, and lack of efficacy of rADAMTS13
ADAMTS13 levels increased >15-fold immediately after rADAMTS13 administration and remained elevated throughout the following 7 days. It seems likely therefore that enough rADAMTS13 was applied. Furthermore, in vitro studies proved the efficacy of cleavage of porcine VWF by rADAMTS13 at a dosage of <1 U/mL (see also Figure 5). Pigs have an average circulating blood volume of 65 mL/kg body weight, and pigs in the present study had a median weight of 30 kg, adding up to a median circulating blood volume of 1950 mL. If 1 U/mL rADAMTS13 would be effective in vitro, 1950 U would be effective per pig. The 400 U/kg body weight rADAMTS13 dosage as applied, adding up to a median of (400 × 30 kg) 12 000 U per pig should have to have been sufficient. Perhaps an overdose of rADAMT13 may have caused a potential direct effect on myocardial performance. Even though the difference in WMSI was the only statistically significant finding, all echocardiographic parameters showed a decrease between the first and the second echocardiogram for the rADAMTS13 group, followed by a recovery from the second to the third (final) echocardiography (Supplementary material online, Table S1). This may indicate a negative effect of rADAMTS13 on cardiac function that would not have occurred with lower dosages. However, treatment did not lead to an increased bleeding tendency or increased IMH. Because the first minutes of reperfusion are believed to be highly relevant for the development of ischaemia-reperfusion injury, perhaps rADAMTS13 could have been more effective when applied prior to the termination of the myocardial infarction.
4.4. No reflow and IMH
An important finding in this study was provided by results from anti-CD31 staining. Previously, it has been postulated that in no reflow, distal embolization of atherosclerotic debris and local microthrombi cause occlusion of capillaries.7 In contrast with this assumption, we witnessed no microthrombi and very few microvessels in the core of the infarction. Instead, obstruction of the microvasculature was more evident in the border zone, whereas the core of the infarction contained IMH. These results are in line with previous animal studies,8–10,37,39–43 and recent reports suggesting that the pathophysiology behind no reflow is IMH rather than microvascular obstruction.8–10 These insights in the pathophysiology behind no reflow may explain why few therapeutic regimens have been effective in reducing the morbidity and mortality caused by no reflow.2 This may also explain why IMH size was similar in rADAMTS13-treated animals vs. controls and why rADAMTS13 was not beneficial in the porcine study because of the lack of a causal relationship between ADAMTS13 levels and IMH.
4.5. Limitations
The exclusion of patients with TIMI 0–1 flow is possibly a limitation of the present study. For the primary study in which this patient cohort is reported,17 solely patients with TIMI 2 or 3 flow were included. However, very few patients were actually excluded due to TIMI 0–1 flow (n = 3).
In the porcine model, anaesthesia was maintained using sevoflurane, an agent reported to have cardioprotective effects. Even though both treatment groups were given the same anaesthesia, this may have caused smaller infarct size in the total study group. MRI would have been favourable to echocardiography. Unfortunately, due to logistic reasons, it was not possible to perform MRI in all pigs.
We did not measure area at risk. Although total occlusion time and anatomic site of occlusion were standardized, it cannot be excluded that there were differences between the two groups in area at risk. Also, we were not able to calculate total myocardial salvage in the present study but only total infarct size. Potentially, administration of rADAMTS13 prior to initiation of myocardial infarction does have a beneficial effect. However, in clinical practice, this is not feasible and therefore was not tested in the present pre-clinical study.
5. Conclusions
This is the first study to show that IMH as measured by CMR is associated with increased VWF levels and decreased ADAMTS13 levels in STEMI patients. Administration of rADAMTS13 was not effective in reducing infarct size and no reflow in a porcine model of myocardial ischaemia-reperfusion.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by an unrestricted research grant from Baxter Innovations, Vienna, Austria, who also supplied the recombinant ADAMTS13. Funding to pay the Open Access publication charges for this article was provided by ZonMW funding: Publishing negative or neutral animal experimental data.
Acknowledgements
We thank Klaas Walter Meyer and Paul Sinnige for their assistance during the animal experiments; and personnel of the Department of Experimental Vascular Medicine of the AMC for their laboratory analyses. We thank Hanspeter Rottensteiner and Alexandra Schiviz from Baxter Innovations, Vienna, Austria, for helpful discussions and critical reading of the manuscript.
Conflict of interest: P.W.K. has served as a consultant for Bayer, Boehringer, CSL Behring, and Ablynx; and has received investigator-initiated research grants from Bayer, LeoPharma, Pfizer, and CSL Behring. N.v.R. received educational grants from Baxter and Biotronik.
References
- 1.Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 2008;117:3152–3156. [DOI] [PubMed] [Google Scholar]
- 2.Wu KC. CMR of microvascular obstruction and hemorrhage in myocardial infarction. J Cardiovasc Magn Reson 2012;14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison RW, Aggarwal A, Ou FS, Klein LW, Rumsfeld JS, Roe MT, Wang TY. Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol 2013;111:178–184. [DOI] [PubMed] [Google Scholar]
- 4.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 1998;97:765–772. [DOI] [PubMed] [Google Scholar]
- 5.Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, Matsui H, Toki Y, Ito T, Hayakawa T. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol 2000;36:1202–1209. [DOI] [PubMed] [Google Scholar]
- 6.Kaul S, Ito H. Microvasculature in acute myocardial ischemia: part I: evolving concepts in pathophysiology, diagnosis, and treatment. Circulation 2004;109:146–149. [DOI] [PubMed] [Google Scholar]
- 7.Ito H. No-reflow phenomenon and prognosis in patients with acute myocardial infarction. Nat Clin Pract Cardiovasc Med 2006;3:499–506. [DOI] [PubMed] [Google Scholar]
- 8.Robbers LF, Eerenberg ES, Teunissen PF, Jansen MF, Hollander MR, Horrevoets AJ, Knaapen P, Nijveldt R, Heymans MW, Levi MM, van Rossum AC, Niessen HW, Marcu CB, Beek AM, van RN. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J 2013;34:2346–2353. [DOI] [PubMed] [Google Scholar]
- 9.Betgem RP, de Waard GA, Nijveldt R, Beek AM, Escaned J, van RN. Intramyocardial haemorrhage after acute myocardial infarction. Nat Rev Cardiol 2015;12:156–167. [DOI] [PubMed] [Google Scholar]
- 10.Bekkers SC, Smulders MW, Passos VL, Leiner T, Waltenberger J, Gorgels AP, Schalla S. Clinical implications of microvascular obstruction and intramyocardial haemorrhage in acute myocardial infarction using cardiovascular magnetic resonance imaging. Eur Radiol 2010;20:2572–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawley JT, de GR, Xiang Y, Luken BM, Lane DA. Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor. Blood 2011;118:3212–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutten B, Maseri A, Cianflone D, Laricchia A, Cristell N, Durante A, Spartera M, Ancona F, Limite L, Hu D, Li H, Uren N, de GP, Mannucci P, Roest M. Plasma levels of active von Willebrand factor are increased in patients with first ST-segment elevation myocardial infarction: a multicenter and multiethnic study. Eur Heart J Acute Cardiovasc Care 2015;4:64–74. [DOI] [PubMed] [Google Scholar]
- 13.Matsukawa M, Kaikita K, Soejima K, Fuchigami S, Nakamura Y, Honda T, Tsujita K, Nagayoshi Y, Kojima S, Shimomura H, Sugiyama S, Fujimoto K, Yoshimura M, Nakagaki T, Ogawa H. Serial changes in von Willebrand factor-cleaving protease (ADAMTS13) and prognosis after acute myocardial infarction. Am J Cardiol 2007;100:758–763. [DOI] [PubMed] [Google Scholar]
- 14.Doi M, Matsui H, Takeda Y, Saito Y, Takeda M, Matsunari Y, Nishio K, Shima M, Banno F, Akiyama M, Kokame K, Miyata T, Sugimoto M. ADAMTS13 safeguards the myocardium in a mouse model of acute myocardial infarction. Thromb Haemost 2012;108:1236–1238. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi C, Motto DG, Jensen M, Lentz SR, Chauhan AK. ADAMTS13 deficiency exacerbates VWF-dependent acute myocardial ischemia/reperfusion injury in mice. Blood 2012;120:5224–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Meyer SF, Savchenko AS, Haas MS, Schatzberg D, Carroll MC, Schiviz A, Dietrich B, Rottensteiner H, Scheiflinger F, Wagner DD. Protective anti-inflammatory effect of ADAMTS13 on myocardial ischemia/reperfusion injury in mice. Blood 2012;120:5217–5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teunissen PF, de Waard GA, Hollander MR, Robbers LF, Danad I, Biesbroek PS, Amier RP, Echavarria-Pinto M, Quiros A, Broyd C, Heymans MW, Nijveldt R, Lammertsma AA, Raijmakers PG, Allaart CP, Lemkes JS, Appelman YE, Marques KM, Bronzwaer JG, Horrevoets AJ, van Rossum AC, Escaned J, Beek AM, Knaapen P, van RN. Doppler-derived intracoronary physiology indices predict the occurrence of microvascular injury and microvascular perfusion deficits after angiographically successful primary percutaneous coronary intervention. Circ Cardiovasc Interv 2015;8:e001786. [DOI] [PubMed] [Google Scholar]
- 18.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol 2005;77:598–625. [DOI] [PubMed] [Google Scholar]
- 19.Saunders AE, Johnson P. Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell Signal 2010;22:339–348. [DOI] [PubMed] [Google Scholar]
- 20.Kostousov V, Fehr J, Bombeli T. Novel, semi-automated, 60-min-assay to determine von Willebrand factor cleaving activity of ADAMTS-13. Thromb Res 2006;118:723–731. [DOI] [PubMed] [Google Scholar]
- 21.Sgueglia GA, Niccoli G, Spaziani C, Cosentino N, Russo E, Andreotti F, Lanza GA, Landolfi R, Crea F. Baseline von Willebrand factor plasma levels and no-reflow phenomenon after primary percutaneous coronary intervention for ST segment elevation myocardial infarction. Int J Cardiol 2010;145:230–232. [DOI] [PubMed] [Google Scholar]
- 22.Federici AB. VWF propeptide: a useful marker in VWD. Blood 2006;108:3229–3230. [Google Scholar]
- 23.Zhao B, Li J, Luo X, Zhou Q, Chen H, Shi H. The role of von Willebrand factor and ADAMTS13 in the no-reflow phenomenon: after primary percutaneous coronary intervention. Tex Heart Inst J 2011;38:516–522. [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Ling J, Ma Z, Yuan Q, Pan J, Yang H. Changes in von Willebrand factor and ADAMTS-13 in patients following arthroplasty. Mol Med Rep 2015;11:3015–3020. [DOI] [PubMed] [Google Scholar]
- 25.Vergouwen MD, Bakhtiari K, van GN, Vermeulen M, Roos YB, Meijers JC. Reduced ADAMTS13 activity in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 2009;29:1734–1741. [DOI] [PubMed] [Google Scholar]
- 26.Frijns CJ, Rinkel GJ, Castigliego D, Van GJ, Sixma JJ, Fijnheer R. Endothelial cell activation after subarachnoid hemorrhage. Neurosurgery 2002;50:1223–1229. [DOI] [PubMed] [Google Scholar]
- 27.Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood 2004;104:100–106. [DOI] [PubMed] [Google Scholar]
- 28.Zezos P, Papaioannou G, Nikolaidis N, Vasiliadis T, Giouleme O, Evgenidis N. Elevated plasma von Willebrand factor levels in patients with active ulcerative colitis reflect endothelial perturbation due to systemic inflammation. World J Gastroenterol 2005;11:48–7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferlitsch M, Reiberger T, Hoke M, Salzl P, Schwengerer B, Ulbrich G, Payer BA, Trauner M, Peck-Radosavljevic M, Ferlitsch A. von Willebrand factor as new noninvasive predictor of portal hypertension, decompensation and mortality in patients with liver cirrhosis. Hepatology 2012;56:1439–1447. [DOI] [PubMed] [Google Scholar]
- 30.Zhao BQ, Chauhan AK, Canault M, Patten IS, Yang JJ, Dockal M, Scheiflinger F, Wagner DD. von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood 2009;114:3329–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chauhan AK, Motto DG, Lamb CB, Bergmeier W, Dockal M, Plaimauer B, Scheiflinger F, Ginsburg D, Wagner DD. Systemic antithrombotic effects of ADAMTS13. J Exp Med 2006;203:767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Shao DB, Zhang FX, Zhang J, Yuan W, Man YL, Du W, Liu BX, Wang DW, Li XR, Cao KJ. Establishment and evaluation of a swine model of acute myocardial infarction and reperfusion-ventricular fibrillation-cardiac arrest using the interventional technique. J Chin Med Assoc 2013;76:491–496. [DOI] [PubMed] [Google Scholar]
- 33.Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DP. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 2007;1:129–137. [DOI] [PubMed] [Google Scholar]
- 34.Gyongyosi M, Posa A, Pavo N, Hemetsberger R, Kvakan H, Steiner-Boker S, Petrasi Z, Manczur F, Pavo IJ, Edes IF, Wojta J, Glogar D, Huber K. Differential effect of ischaemic preconditioning on mobilisation and recruitment of haematopoietic and mesenchymal stem cells in porcine myocardial ischaemia-reperfusion. Thromb Haemost 2010;104:376–384. [DOI] [PubMed] [Google Scholar]
- 35.Buszman PP, Wojakowski W, Milewski K, Debinski M, Pajak J, Aboodi MS, Jackiewicz W, Kawka M, Bochenek A, Prats J, Granada JF, Kaluza GL, Buszman PE. Controlled reperfusion with intravenous bivalirudin and intracoronary abciximab combination therapy in the porcine myocardial infarction model. Thromb Res 2011;130:265–272. [DOI] [PubMed] [Google Scholar]
- 36.Ekelof S, Rosenberg J, Jensen JS, Gogenur I. Pharmacological attenuation of myocardial reperfusion injury in a closed-chest porcine model: a systematic review. J Cardiovasc Transl Res 2014;7:570–580. [DOI] [PubMed] [Google Scholar]
- 37.Steg PG, James SK, Gersh BJ. 2012 ESC STEMI guidelines and reperfusion therapy: evidence-based recommendations, ensuring optimal patient management. Heart 2013;99:1156–1157. [DOI] [PubMed] [Google Scholar]
- 38.Uitterdijk A, Yetgin T, te Lintel HM, Sneep S, Krabbendam-Peters I, van Beusekom HM, Fischer TM, Cornelussen RN, Manintveld OC, Merkus D, Duncker DJ. Vagal nerve stimulation started just prior to reperfusion limits infarct size and no-reflow. Basic Res Cardiol 2015;110:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaman AK, French CJ, Spees JL, Binbrek AS, Sobel BE. Vascular rhexis in mice subjected to non-sustained myocardial ischemia and its therapeutic implications. Exp Biol Med (Maywood) 2011;236:598–603. [DOI] [PubMed] [Google Scholar]
- 40.Campbell CD, Takanashi Y, Laas J, Meus P, Pick R, Replogle RL. Effect of coronary artery reperfusion on infarct size in swine. J Thorac Cardiovasc Surg 1981;81:288–296. [PubMed] [Google Scholar]
- 41.Garcia-Dorado D, Theroux P, Solares J, Alonso J, Fernandez-Aviles F, Elizaga J, Soriano J, Botas J, Munoz R. Determinants of hemorrhagic infarcts. Histologic observations from experiments involving coronary occlusion, coronary reperfusion, and reocclusion. Am J Pathol 1990;137:301–311. [PMC free article] [PubMed] [Google Scholar]
- 42.Ganame J, Messalli G, Dymarkowski S, Rademakers FE, Desmet W, Van de Werf F, Bogaert J. Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J 2009;30:1440–1449. [DOI] [PubMed] [Google Scholar]
- 43.Husser O, Monmeneu JV, Sanchis J, Nunez J, Lopez-Lereu MP, Bonanad C, Chaustre F, Gomez C, Bosch MJ, Hinarejos R, Chorro FJ, Riegger GA, Llacer A, Bodi V. Cardiovascular magnetic resonance-derived intramyocardial hemorrhage after STEMI: influence on long-term prognosis, adverse left ventricular remodeling and relationship with microvascular obstruction. Int J Cardiol 2013;167:2047–2054. [DOI] [PubMed] [Google Scholar]
- 44.Van Dijk A, Krijnen PA, Vermond RA, Pronk A, Spreeuwenberg M, Visser FC, Berney R, Paulus WJ, Hack CE, van Milligen FJ, Niessen HW. Inhibition of type 2A secretory phospholipase A2 reduces death of cardiomyocytes in acute myocardial infarction. Apoptosis 2009;14:753–763. [DOI] [PubMed] [Google Scholar]
- 45.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]