Abstract
In this study, we identified the phlebotomine sandfly vectors involved in the transmission of American Cutaneous Leishmaniasis (ACL) in Assis Brasil, Acre, Brazil, which is located on the Brazil-Peru-Bolivia frontier. The genotyping of Leishmania in phlebotomines was performed using polymerase chain reaction (PCR) and PCR-restriction fragment length polymorphism. A total of 6,850 sandflies comprising 67 species were captured by using CDC light traps in rural areas of the municipality. Three sandfly species were found in the state of Acre for the first time: Lutzomyia georgii, Lu. complexa and Lu. evangelistai. The predominant species was Lu. auraensis/Lu. ruifreitasi and Lu. davisi (total 59.27%). 32 of 368 pools were positive for the presence of Leishmania DNA (16 pools corresponding to Lu. davisi, and 16 corresponding to Lu. auraensis/Lu. ruifreitasi), with a minimal infection prevalence of 1.85% in Lu. davisi and 2.05% in Lu. auraensis/Lu. ruifreitasi. The Leishmania species found showed maximum identity with L. (Viannia) guyanensis and L. (V.) braziliensis in both phlebotomine species. Based on these results and similar scenarios previously described along the Brazil/Peru/Bolivia tri-border, the studied area must take into consideration the possibility of Lu. davisi and Lu. auraensis/Lu. ruifreitasi as probable vectors of ACL in this municipality.
Keywords: sandflies, Leishmania, disease vectors, Assis Brasil Municipality, Acre, Brazil
The Amazon basin probably has the highest diversity of phlebotomine sandflies worldwide (Alves et al. 2012). Besides that, the Amazon basin has a high species endemicity (Ramos et al. 2014, Pereira Jr et al. 2015). In spite of this diversity, information about sandfly fauna in the state of Acre in the Brazilian Amazon, is still scarce (Teles et al. 2013b, Araujo-Pereira et al. 2014, Oliveira et al. 2015). These insects are relatively well studied in several places worldwide, due to their importance as vectors of protozoans, bacteria and viruses (WHO 2015).
In Brazil, seven Leishmania species are etiological agents of different clinical forms of American Cutaneous Leishmaniasis (ACL): Leishmania (Viannia) braziliensis, L. (V.) guyanensis, L. (V.) lainsoni, L. (V.) naiffi, L. (V.) shawi and L. (V.) lindenbergi representatives of the L. (V.) braziliensis complex; and L. (L.) amazonensis from the L. mexicana complex. All of these Leishmania species have been registered in the Brazilian Amazon (Lainson 2010).
In northern Brazil there is a wide range of geographically different places where sandflies can be found and also a variety of reservoirs and vector species from the subgenera Nyssomyia; Psychodopygus; Lutzomyia and Trichophoromyia (Castellón 2009, Feitosa et al. 2012). In the state of Pará, the species that stand out are Lutzomyia complexa, Lu. wellcomei and Lu. longipalpis (Aguiar & Medeiros 2003). In Amazonas the main vectors are Lu. umbratilis, Lu. anduzei, Lu. flaviscutellata and Lu. wellcomei (Aguiar & Medeiros 2003, Guerra et al. 2006).
The findings of different epidemiological profiles of leishmaniasis, together with records of outbreaks both in the wild as well as in peri-urban environments (WHO 2015), reinforces the hypothesis of the possible adaptation of vectors to changing environments and also the incorporation of domestic animals as reservoirs (Teles et al. 2013a, Araujo-Pereira et al. 2014).
The municipality of Assis Brasil, in the state of Acre, an area that borders Peru and Bolivia, located in the microregion of Brasiléia, is one of the most important ACL endemic areas of Brazil with high rates of the disease (43.6% of the ACL cases in Acre between 2011-2012 were concentrated in this municipality). In Assis Brasil, during the period from 2007-2012, the average detection rate was 98.2 cases/10,000 inhabitants, with 21.4% being mucosal form cases (SINAN 2014). In addition to the high rate of detection, this municipality has some important epidemiological characteristics like the permanence of a significant percentage (40%) of the population living in rural areas (IBGE 2014). Furthermore, after the opening of the Pacific Highway, migration across the triple border and the growth of tourism in the region increased (Cesário et al. 2011). This scenario may play an important role in the spread of ACL.
According to Teles et al. (2015), the most prevalent Leishmania species in humans from this municipality are L. braziliensis and L. shawi. Little is known about the vectors of this region, but some species suspected of or implicated in transmitting Leishmania spp. were reported in the state such as Lutzomyia davisi, Lu. whitmani, Lu. antunesi, Lu. ubiquitalis and Lu. umbratilis (Azevedo et al. 2008, Silva-Nunes et al. 2008). Sandfly studies in Acre are still scarce. Some entomological studies have been published to date, describing the sandfly fauna involving the cities of Cruzeiro do Sul, Feijó, Bujari, Xapuri, Rio Branco, Acre and Assis Brasil (Martins & Silva 1964, Arias & Freitas 1982, Azevedo et al. 2008, Silva-Nunes et al. 2008, Teles et al. 2013b, Araujo-Pereira et al. 2014, Oliveira et al. 2015).
Assis Brasil is one of the main epicenters of Amazonian ACL; however, there are information gaps on the epidemiological, clinical, vectorial and special aspects of Leishmania spp. circulating in this and various microregions of the state. The study reports for the first time, vectors and etiological agents from this border region in Acre.
MATERIALS AND METHODS
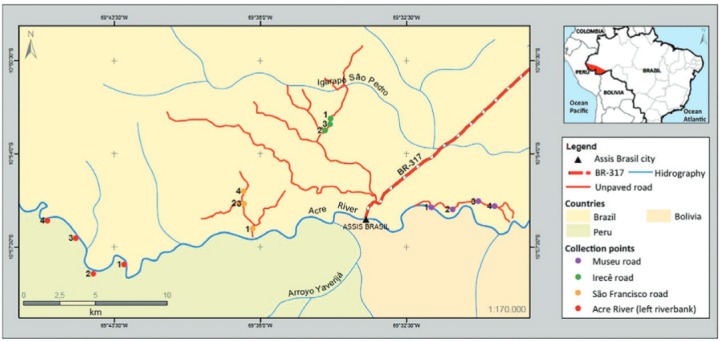
Study area - The municipality of Assis Brasil (Fig. 1) occupies an area of 4.974 km2 in the state of Acre, Brazil, in the mesoregion of the Acre valley, Brasiléia microregion (10º56’29”S and 69º34’01”W). This area of Brazil borders Bolivia and Peru (Acre-Brazil, Madre de Dios-Peru and Pando-Bolivia). The Acre River marks the border between Assis Brasil and Bolivia. A population of 6,610 inhabitants occupies the area. The study area has a landscape formed by a mosaic of indigenous lands, extractive reserves, riverine communities, and small and large settlements. The local economy is focused on rubber, Brazil nuts, wood, vegetable oils and wild fruit. There are some small crop and livestock farms (Acre 2008).
Fig. 1. : Map of Brazil highlighting the state of Acre, location of the district headquarters of Assis Brasil municipality and points of sandfly capture (legends 1, 2, 3 and 4). Irecê road: point 1 (10º53’40.55”S/ 69º36’2.53”W); point 2 (10º53’3.35”S/ 69º35’25.95”W); point 3 (10º52’46.11”S/ 69º35’12.81”W). São Francisco road: point 1 (10º56’49.5”S/ 69º38’17.4”W); point 2 (10º55’49.40”S/ 69º38’49.96”W); point 3 (10º55’52.4”S/ 69º38’38.6”W); point 4 (10º55’22.6”S/ 69º38’36.0”W). Museu road: point 1 (10º56’0,95”S/ 69º31’33,54”W); point 2 (10º56’4.49”S/ 69º30’45.71”W); point 3 (10º55’46.97”S/ 69º29’47.54”W); point 4 (10º55’58.30”S/ 69º29’11.07”W). Riparian Forest: point 1 (10º58’09.4”S/ 69º43’07.8”W); point 2 (10º56’4.49”S/ 69º30’45.71”W); point 3 (10º55’46.97”S/ 69º29’47.54”W); point 4 (10º55’58.30”S/ 69º29’11.07”W).
The equatorial weather is hot and humid and the average annual temperature is 26.5ºC; the relative humidity is 80-90% all year round. There are two very distinct seasons: a wet season between November-April, and a dry season between May-October (IBGE 2014).
Phlebotomine sandfly survey - The phlebotomine sandfly surveys were done between August 2009-June 2010 with three or five CDC light traps/localities, placed approximately 150 cm above the ground with a distance of approximately 200 metres between them. Collections were carried out on five to eight consecutive nights from 18:00-06:00; a total of 780 captures were performed. During the dry season 26 visits were carried out totaling 448 traps installed in peridomiciliary environment (rural areas - Irecê road - capture August/2009; São Francisco road - capture September/2009; and Museu road - capture April and June/2010) according to the accessibility of roads and reports of recent cases of ACL (Figs 1-2). During the rainy season, the collections were held at four fixed points in riparian environments along the left bank of the river where 23 visits were carried out totaling 332 traps (capture November and December/2009 and February/2010).
Fig. 2. : landscape aspect of the sandfly collection areas, Assis Brasil Municipality, Acre state, Brazil. The satellite images were obtained through Google Earth (May 2010).
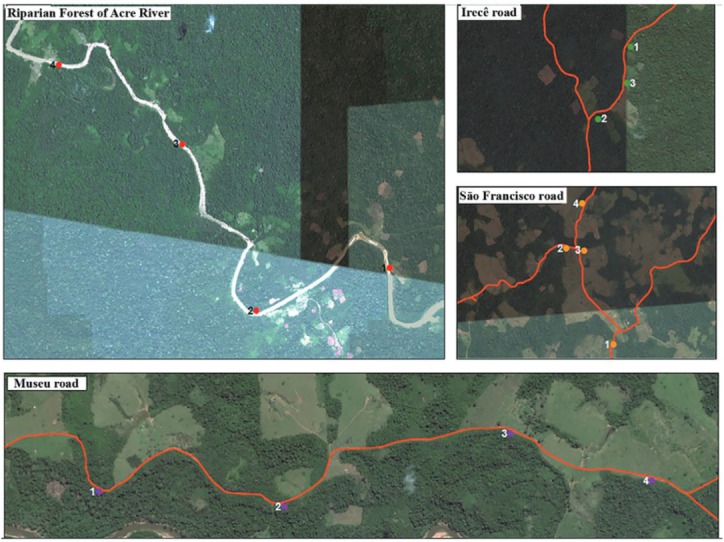
The choice of the collection areas within each area of road were defined as follows: households with recent cases of ACL, within peridomicile areas possessing livestock, a fruitgarden and with primary or secondary fragments of forest. A transect of at least 800 metres was done; and the light traps were distributed throughout this transect. Points 1-3 of the riparian environment of the Acre River had native vegetation consisting of continuous rainforest and point 4 was in a peridomicile environment located in a fragment of forest near a riverside residence.
The sandflies collected were stored in 70% ethanol and then identified by the taxonomic key according to Young and Duncan (1994). The captured females were treated as follows: the head and the last three segments of the abdomen were clarified and mounted in the dorsal-ventral position in Berlese fluid; and the remainder of the body was stored in a microtube containing 70% ethanol for later molecular analysis. The males were slide mounted normally and then identified. Recently, Lu. (Thrychophoromyia) ruifreitasi was described after the identification from individuals collected for this study (Oliveira et al. 2015), and due to the fact that the females of Lu. auraensis and Lu. ruifreitasi are probably indistinguishable, we decided to temporarily name them Lu. auraensis/Lu. ruifreitasi.
Detection of natural infection of sandflies by Leishmania sp. - The collected females were assembled in groups (2-20 specimens per group) in micro test tubes. The single pool is composed with belonging the same sandfly species, date and place of capture. Total DNA was extracted from each pool based on the method adapted by Mukhopadhyay et al. (2000) and Paiva et al. (2007). After the extraction they were stored at 20ºC for polymerised chain reaction (PCR) in order to identify the genus Leishmania, complexes and Leishmania species.
PCR targeted the mkDNA gene - The PCR assay for the genus Leishmania (mkDNA gene PCR) was performed following Oliveira et al. (2005); the described primers 5’-GGG(GT)AGGGGCGTTCT(G/C)CGAA-3’ and 5’-(G/C)(G/C)(G/C)(A/T)CTAT(A/T)TTACACCAACCCC3’ target the conserved region of the kinetoplast DNA minicircles (mkDNA) (120 pb) among all the species of Leishmania sp.
Multiplex PCR targeted the RNA mini-exon spliced leader (SL) gene - In order to determine the three complexes of New World L. (V.) braziliensis, L. (L.) mexicana and L. (L.) donovani, the DNA from samples positive for mkDNA gene PCR were analysed through a second PCR, Multiplex PCR assays, with the specific primers for the SL RNA (mini-exon) gene (Harris et al. 1998). SL RNA Multiplex PCR uses simultaneously three specific primers for each complex: L. braziliensis complex LB-3C 5’-CGT(C/G)CCGAACCCCGTGTC-3’; L. mexicana complexLM-3A 5’-GCACCGCACCGG(A/G)CCAC-3’; L. donovani complex (LC-3L) 5’-GCCCGCG(C/T)GTCACCACCAT-3’ and one oligonucleotide primer which were each conserved in all Leishmania species LU-5A 5’-TTTATTATGCGAAACTTC-3’.
The size of the expected SL RNA Multiplex PCR product was 146 to 149 bp for the L. braziliensis complex, 218 to 240 bp for the L. mexicana complex, and 351 to 397 bp for the L. donovani complex.
hsp70 gene PCR-restriction fragment length polymorphism (RFLP) and DNA sequencing - In order to identify the Leishmania species from female sandflies, the hsp70 region was amplified (hsp70 PCR) with primers hsp 70cF 5’-GGACGAGATCGAGCGCATG GT-3’ and hsp 70cR 5’-TCCTTCGACGCCTCCTGGTTG-3’ to amplify the 240 bp fragment (da Graça et al. 2012).
The final products amplified using mkDNA gene PCR, SL RNA Multiplex PCR and hsp70 PCR were analysed on a 2% agarose gel stained with GelRed and examined under UV light.
For the RFLP analysis from the hsp70 amplicons of 240 bp, the restriction enzymes HaeIII (Invitrogen®, USA) and BstUI (Bio Labs®, New England) were used in independent reactions according to the manufacturer’s instructions. The resulting products of the restriction digestion were analysed on 12% silver-stained polyacrylamide gels.
Negative controls such DNA from male sandflies was used for each PCR. The following DNA reference strains of the Leishmania collection from the Instituto Oswaldo Cruz (CLIOC) were used, namely: L. (V.) braziliensis (IOCL 566), L. (V.) guyanensis (IOCL 565), L. (V.) lainsoni (IOCL 1023), L. (V.) naiffi (IOCL 1365), L. (V.) shawi (IOCL 1545); L. (L.) amazonensis (IOCL 575) and L. (L.) infantum (IOCL 0579).
Sequencing of Leishmania DNA copies was carried out with a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems Brazil, São Paulo, Brazil) in an Applied Biosystems3130xl DNA sequencer from Genomic Company (São Paulo, Brazil). The hsp70 genes of the Leishmania species were compared with the sequences obtained from analysis with reference sequences deposited in GenBank. Comparisons were done using BLAST program searches (Basic Local Alignment Search Tool, NCBI) (http://blast.ncbi.nlm.nih.gov).
Pool screening - Prevalence studies of infection in the sandflies by Leishmania spp. was assessed by estimating the prevalence of infection in positive pools using the Pool Screening Program (http://www.soph.uab.edu/bst/poolscreen) Version 2.0 (Katholi et al. 1995).
RESULTS
A total of 6,850 phlebotomine were captured, 3,480 males (50.8%) and 3,370 females (49.2%), with a female/male ratio of 0.97. In all, 67 species were identified belonging to two genus, Brumptomyia (73 individuals, 1.08%) and Lutzomyia (6,777, 98.92%). The distribution and percentage of specimens of the genus Lutzomyia are presented in Table I, they belong to the following subgenera: Trichophoromyia (39.51%), Psychodopygus (27.34%), Pressatia (9.89%), Nyssomyia (8.58%). The other subgenera and species group made up less than 13.6%.
TABLE I. Abundance of phlebotomine sandflies collected with CDC light traps in Assis Brasil Municipality, state of Acre, Brazil, between August of 2009-June of 2010.
| Genus | Subgenera/Groups | Species | Environments | Total | (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Peridomiciliary environment | Riparian environment | |||||||||
|
|
|
|||||||||
| ♀ | ♂ | Total | ♀ | ♂ | Total | |||||
| Brumptomyia | Brumptomyia avellari | 0 | 13 | 13 | 0 | 2 | 2 | 15 | 0,22 | |
| B. pentacantha | 3 | 41 | 44 | 2 | 1 | 3 | 47 | 0,7 | ||
| B. sp. | 11 | 0 | 11 | 0 | 0 | 0 | 11 | 0,16 | ||
| Lutzomyia | Evandromyia | Lutzomyia monstruosa | 6 | 1 | 7 | 0 | 0 | 0 | 7 | 0,1 |
| Lu. georgii a | 7 | 0 | 7 | 0 | 0 | 0 | 7 | 0,1 | ||
| Lu. tarapacaensis | 17 | 6 | 23 | 7 | 4 | 11 | 34 | 0,5 | ||
| Lutzomyia | Lu. evangelistai a | 4 | 0 | 4 | 2 | 0 | 2 | 6 | 0,09 | |
| Lu. flabellata | 0 | 6 | 6 | 0 | 0 | 0 | 6 | 0,09 | ||
| Lu. sherlocki | 56 | 98 | 154 | 13 | 2 | 15 | 169 | 2,46 | ||
| Micropygomyia | Lu. micropyga | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0,01 | |
| Nyssomyia | Lu. antunesi b | 77 | 28 | 105 | 31 | 0 | 31 | 136 | 2 | |
| Lu. flaviscutellata b | 1 | 6 | 7 | 2 | 4 | 6 | 13 | 0,19 | ||
| Lu. reducta b | 0 | 4 | 4 | 1 | 1 | 2 | 6 | 0,09 | ||
| Lu. richardwardi | 10 | 0 | 10 | 35 | 1 | 36 | 46 | 0,67 | ||
| Lu. shawi | 87 | 53 | 140 | 43 | 1 | 44 | 184 | 2,7 | ||
| Lu. umbratilis b | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0,01 | ||
| Lu. whitmani b | 67 | 56 | 123 | 6 | 0 | 6 | 129 | 1,89 | ||
| Lu. yuilli yuilli | 51 | 17 | 68 | 0 | 0 | 0 | 68 | 1 | ||
| Lu. christenseni | 0 | 2 | 2 | 0 | 0 | 0 | 2 | 0,03 | ||
| Pintomyia | Lu. calcarata | 1 | 1 | 2 | 0 | 6 | 6 | 8 | 0,12 | |
| Pressatia | Lu. choti | 127 | 35 | 162 | 131 | 380 | 511 | 673 | 9,83 | |
| Lu. triacantha | 1 | 3 | 4 | 0 | 0 | 0 | 4 | 0,06 | ||
| Psathyromyia | Lu. abonnenci | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0,01 | |
| Lu. campbelli | 1 | 1 | 2 | 0 | 0 | 0 | 2 | 0,03 | ||
| Lu. dendrophyla | 12 | 77 | 89 | 2 | 0 | 2 | 91 | 1,33 | ||
| Lu. lutziana | 8 | 30 | 38 | 0 | 2 | 2 | 40 | 0,58 | ||
| Lu. punctigeniculata | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0,01 | ||
| Lu. shannoni | 5 | 6 | 11 | 0 | 0 | 0 | 11 | 0,16 | ||
| Psychodopygus | Lu. amazonensis | 11 | 9 | 20 | 5 | 0 | 5 | 25 | 0,36 | |
| Lu. ayrozai b | 2 | 0 | 2 | 2 | 0 | 2 | 4 | 0,06 | ||
| Lu. bispinosa | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0,03 | ||
| Lu. carrerai | 20 | 7 | 27 | 15 | 1 | 16 | 43 | 0,63 | ||
| Lu. davisi b | 317 | 130 | 447 | 729 | 267 | 996 | 1443 | 21,07 | ||
| Lu. geniculata | 23 | 2 | 25 | 65 | 22 | 87 | 112 | 1,64 | ||
| Lu. hirsuta hirsuta b | 23 | 20 | 43 | 36 | 11 | 47 | 90 | 1,31 | ||
| Lu. llanosmartinsi | 0 | 2 | 2 | 0 | 2 | 2 | 4 | 0,06 | ||
| Lu. lainsoni | 1 | 0 | 1 | 5 | 1 | 6 | 7 | 0,1 | ||
| Lu. paraensis b | 3 | 1 | 4 | 1 | 0 | 1 | 5 | 0,07 | ||
| Lu. yucumensis | 3 | 0 | 3 | 71 | 62 | 133 | 136 | 2 | ||
| Lu. complexa a,b | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0,01 | ||
| Sciopemyia | Lu. preclara | 3 | 1 | 4 | 6 | 11 | 17 | 21 | 0,3 | |
| Lu. servulolimai | 12 | 1 | 13 | 4 | 0 | 4 | 17 | 0,25 | ||
| Lu. sordellii | 1 | 2 | 3 | 0 | 3 | 3 | 6 | 0,08 | ||
| Lutzomyia | Trichophoromyia | Lu. auraensis /Lu. ruifreitasi b** | 232 | 229 | 461 | 665 | 1490 | 2155 | 2616 | 38,2 |
| Lu. melloi | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0,01 | ||
| Lu. ubiquitalis b | 33 | 51 | 84 | 4 | 0 | 4 | 88 | 1,3 | ||
| Viannamyia | Lu. furcata b | 6 | 2 | 8 | 0 | 0 | 0 | 8 | 0,11 | |
| Group Aragaoi | Lu. abunaensis | 1 | 6 | 7 | 0 | 1 | 1 | 8 | 0,11 | |
| Lu. aragaoi | 31 | 66 | 97 | 14 | 41 | 55 | 152 | 2,22 | ||
| Lu. brasiliensis | 0 | 3 | 3 | 0 | 0 | 0 | 3 | 0,04 | ||
| Group Dreisbachi | Lu. dreisbachi | 3 | 1 | 4 | 0 | 0 | 0 | 4 | 0,06 | |
| Group Migonei | Lu. andersoni | 5 | 6 | 11 | 0 | 0 | 0 | 11 | 0,16 | |
| Lu. bacula | 1 | 4 | 5 | 0 | 0 | 0 | 5 | 0,07 | ||
| Lu. migonei b | 1 | 1 | 2 | 0 | 1 | 1 | 3 | 0,04 | ||
| Lu. sallesi | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 0,03 | ||
| Lu. termitophila | 9 | 6 | 15 | 0 | 0 | 0 | 15 | 0,22 | ||
| Lu. walkeri | 0 | 2 | 2 | 3 | 0 | 3 | 5 | 0,07 | ||
| Lu. williamsi | 2 | 1 | 3 | 0 | 0 | 0 | 3 | 0,04 | ||
| Group Oswaldoi | Lu. longipennis | 3 | 2 | 5 | 0 | 2 | 2 | 7 | 0,1 | |
| Lu. peresi | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0,01 | ||
| Lu. villelai* | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0,01 | ||
| Group Saulensis | Lu. saulensis | 45 | 12 | 57 | 3 | 1 | 4 | 61 | 0,89 | |
| Lu. wilsoni | 46 | 11 | 57 | 1 | 0 | 1 | 58 | 0,85 | ||
| Group Verrucarum | Lu. nevesi | 18 | 22 | 40 | 34 | 10 | 44 | 84 | 1,2 | |
| Lu. serrana | 17 | 57 | 74 | 1 | 2 | 3 | 77 | 1,12 | ||
| Lu. naiffi | 0 | 2 | 2 | 0 | 0 | 0 | 2 | 0,03 | ||
| Total | 1427 | 1146 | 2573 | 1943 | 2334 | 4277 | 6850 | 100 | ||
a: new occurrence to Acre state; b: species are incriminated/suspected vectors (Rangel & Lainson 2009); b**: species are suspected vectors according to these study’s findings; *: taxon considers a synonym of Lu. trinidadensis by Young and Duncan (1994).
Among the 67 species, three were reported in the state of Acre for the first time: Lu. georgii, Lu. complexa and Lu. evangelistai. The most abundant taxa were Lu. auraensis/Lu. ruifreitasi (38.2%), Lu. davisi (21.07 %), Lu. choti (9.83%) and Lu. shawi (2.7%), which represent 71.8% of the total. The less abundant species were: Lu. micropyga, Lu. umbratilis, Lu. abonnenci, Lu. punctigeniculata, Lu. complexa, Lu. melloi, Lu. peresi and Lu. villelai (synonymy of Lu. trinidadensis by Young & Duncan 1994), representing 0.08% (Table I).
In the peridomiciliary environment 2,573 sandflies were collected, distributed among 63 species. The most abundant were Lu. auraensis/Lu. ruifreitasi (17.91%), Lu. davisi (17.37%), Lu. choti (6.3%), Lu. sherlocki (5.98%), Lu. shawi (5.44%), Lu. whitmani (4.78%) and 56 other species that occurred in small numbers for a total of 42.22%.
In the riparian forest environments, 4,277 sandflies were collected and distributed among 44 species. The most abundant were Lu. auraensis/Lu. ruifreitasi (50.38%), Lu. davisi (23.28%), Lu. choti (11.94%), Lu. yucumensis (3.1%), Lu. geniculata (2.03%), Lu. aragaoi (1.28%) and 38 other species amounting to 7.99%.
12 sandfly species are incriminated/suspected vectors of ACL (Rangel & Lainson 2009): Lu. davisi, Lu. antunesi and Lu. whitmani being the most abundant species, whereas the less abundant were Lu. flaviscutellata, Lu. reducta, Lu. umbratilis, Lu. ayrozai, Lu. hirsuta hirsuta; Lu. paraensis, Lu. furcata, Lu. ubiquitalis, Lu. migonei and Lu. complexa (Table I).
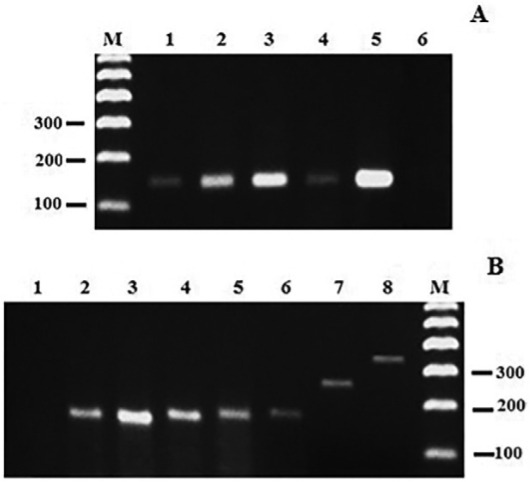
A total of 3,218 (95.48%) females were grouped into 368 pools and 32 (8.7%) were positive for the mkDNA gene PCR specific for the Leishmania genus (16 pools corresponding to Lu. davisi and 16 to Lu. auraensis/Lu. ruifreitasi) (Table II). The minimal infection prevalence for Lu. davisi was 1.85% (95% CI = 1.08% - 2.99%, n = 89 pools with 73 negatives) while Lu. auraensis/Lu. ruifreitasi was 2.05% (95% CI = 1.13% - 3.36%, n = 58 pools with 42 negatives). The Multiplex PCR technique identified all samples as related to the L. braziliensis complex (Fig. 3).
TABLE II. Distribution of positive sandfly pools for mkDNA gene polymerase chain reaction (PCR) according to species and collection environment, in Assis Brasil, AC.
| Positive pools/Environment | hsp70 PCR-restriction fragment length polymorphism | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Museu road | Forest Riparian | ||||||||||
| Species (specimes - total pool) | P1 | P2 | P3 | P4 | P1 | P2 | P3 | P4 | Total | (%) | (nº pools) Leishmania identification |
| Lutzomyia auraensis/Lu. ruifreitasi (1026-58) | 3 | 1 | - | - | 9 | 1 | - | 2 | 16 | 50 | (1) L. (Viannia) braziliensis |
| (3) L. (V.) guyanensis | |||||||||||
| (4) L. (V.) guyanensis/ L. (V.) braziliensis | |||||||||||
| Lu. davisi (853-89) | - | - | - | - | - | 3 | 1 | 12 | 16 | 50 | (1) L. (V.) guyanensis/ L. (V.) braziliensis |
| (6) L. (V.) guyanensis | |||||||||||
| Total | 3 | 1 | 0 | 0 | 9 | 4 | 1 | 14 | 32 | 100 | |
P: points of phlebotomine collection.
Fig. 3. : mkDNA gene polymerase chain reaction (PCR) products and SL RNA Multiplex PCR resulting from sandfly pools. 2% agarose gel stained with GelRed. (A) mkDNA gene PCR. Lines 1-3: samples from Lutzomyia davisi pools; Line 4: Lu. auraensis/Lu. ruifreitasi pool; Line 5: Leishmania (Leishmania) amazonensis reference strain and Line 6: males of Lutzomyia sp. (B) Multiplex PCR for the Leishmania complex. Line 1: males of Lutzomyia sp.; Lines 2, 5: samples from Lu. davisi pools; Lines 3, 4: samples from Lu. auraensis/Lu. ruifreitasi pools; Lines 6-8 Leishmania spp. reference strains; Line 6: L. (Viannia) braziliensis; 7: L. (L.) amazonensis; 8: L. (L.) infantum chagasi. M: 100 bp size marker (Invitrogen).
Most of the positive pools were found in riparian environments of the Acre River (28 pools - 87.0% covering all points), of which 14 pools (44.0%) were collected near riverside houses (point 4 of the riparian environment). Another four positive pools (13.0%) were collected at points 1 and 2 along Museu road (Table II).
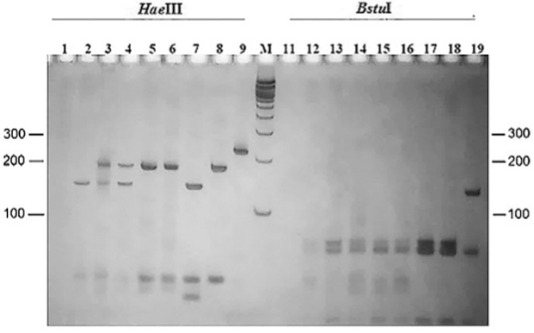
In this study, the hsp70 PCR-RFLP analysis confirmed the Leishmania species of 15 phlebotomine pools (Table II), with two Leishmania species identified with restriction patterns equal to L. (V.) guyanensis (approximately HaeIII 190 bp/BstuI 50 bp) and L. (V.) braziliensis (HaeIII 160 bp/BstuI 50 bp) (Fig. 4). Eight of the 15 hsp70 PCR-RFLP from the positive pools were composed of Lu. auraensis/Lu. ruifreitasi: L. (V.) guyanensis (three pools), L. (V.) braziliensis (one) and L. (V.) braziliensis/L. (V.) guyanensis (four). Seven additional positive pools were composed of Lu. davisi: L. (V.) guyanensis (six) and L. (V.) guyanensis/L. (V.) braziliensis (one). The others sandfly samples did not yield any amplification product for hsp70 PCR and it could not indicate the Leishmania species. However, all samples were positive for the L. braziliensis complex thought of Multiplex PCR.
Fig. 4. : polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) for identification of Leishmania species in sandflies. The hsp70 PCR products (234 bp) amplified within pools were digested by the enzymes HaeIII and BstuI. 12% silver-stained polyacrylamide gel. Lines 1, 11: Negative control of male Lutzomyia spp.; Lines 2, 12: Lu. auraensis/Lu. ruifreitasi pool I; Lines 3, 13: Lu. davisi pool II; Lines 4, 14: Lu. auraensis/Lu. ruifreitasi pool III; Lines 5, 15: Lu. auraensis/Lu. ruifreitasi pool IV; Lines 6, 16: Lu. davisi pool V; Lines 7, 17: positive control of L. (Viannia) braziliensis; Lines 8, 18: positive control of L. (V.) guyanensis; Lines 9, 19: positive control of L. (L.) amazonensis. M: 100 bp size marker (Invitrogen).
Five samples with higher amplification yield by hsp70 PCR were confirmed by genetic sequencing. Of these, three pools corresponding to Lu. davisi and two Lu. auraensis/Lu. ruifreitasi confirmed the presence and similarity (95-100%) of L. (V.) guyanensis deposited in GenBank under code number GU368213.1.
DISCUSSION
Of the 67 captured species, three were recorded for the first time in Acre: Lu. georgii, Lu. complexa and Lu. evangelistai. The first species mentioned is considered endemic in northern Brazil. According to past records and this study, there are 92 known species for the state of Acre which corresponds to 34.6% of the registered species in Brazil (Aguiar & Medeiros 2003, Teles et al. 2013b, Galati 2014, Oliveira et al. 2015).
The number of species found in Assis Brasil was higher than that reported by other authors in Acre: Martins and Silva (1964) recorded 30 species of sandflies with a predominance of Lu. nevesi in Rio Branco and Arias and Freitas (1982), in a study carried out in the cities of Cruzeiro do Sul, Feijó and Rio Branco, identified 50 species, the most abundant being Lu. auraensis. Azevedo et al. (2008) identified 52 species in the municipalities of Bujari, Xapuri and Rio Branco, 67% of them being Lu. auraensis, Lu. antunesi, Lu. whitmani and Lu. davisi. Silva-Nunes et al. (2008) in the municipality of Acrelândia observed that Lu. antunesi (45%) and Lu. whitmani (15%) were the most abundant of the 40 collected specimens. Araujo-Pereira et al. (2014) in Rio Branco captured 23 species of sandflies with 53.3% Lu. auraensis and 18.8% Lu. whitmani. These studies indicated that knowledge of sandfly fauna is still scarce compared to other Brazilian states of the Amazon Basin.
The abundance the subgenera of Trichophoromyia, Psychodopygus, Pressatia and Nyssomyia found in this study is consistent with these studies performed in Acre (Arias & Freitas 1982, Azevedo et al. 2008, Silva-Nunes et al. 2008), with a high abundance of Lu. (Trichophoromyia) auraensis, Lu. (Psychodopygus) davisi and Lu. (Pressatia) choti. These three species amounted to 85.62% of total individuals caught in the riparian environment and 41.58% in the rural area. This profile of few dominant species and many species with few individuals has been reported in sandfly fauna studies in the north Brazil (Azevedo et al. 2008, Feitosa et al. 2012, Ramos et al. 2014).
We highlighted the findings of Lu. davisi and Lu. auraensis/Lu. ruifreitasi containing DNA tracks of Leishmania from the L. braziliensis complex. The minimal infection prevalence observed for these two species were: Lu. davisi with 1.85% and Lu. auraensis/Lu. ruifreitasi with 2.05%. To date, only Valdivia et al. (2012) observed Lu. davisi and Lu. auraensis through positive qPCR diagnosis in Madre de Dios department, Peru (0.9% and 0.6% respectively), associated with the transmission of L. (V.) lainsoni and L. (V.) braziliensis. Other studies confirming infection by Leishmania spp. in Lu. davisi were based on a conventional method of identifying flagellates using monoclonal antibodies (Grimaldi Jr et al. 1991, Gil et al. 2003, Souza et al. 2010).
This is the first report of infection of Lu. auraensis/Lu. ruifreitasi by L. (V.) guyanensis and L. (V.) braziliensis in Brazil. Teles et al. (2015) found both Leishmania species in tissue biopsies from local patients with ACL. Lutzomyia auraensis is present in northern Brazil with low abundance in Amazonas and Rondônia (Castellón et al. 1994, Gil et al. 2003, Teles et al. 2013a), and high abundance in Acre (Arias & Freitas 1982, Azevedo et al. 2008, Araujo-Pereira et al. 2014). Araujo-Pereira et al. (2014) reported the abundance of Lu. auraensis in peri-urban (city parks) and peridomiciliary environments in Rio Branco. However, no studies incriminating this sandfly as a vector have been conducted in Brazil, and its epidemiological role has not been well investigated so far. This species has also demonstrated a high abundance and anthropophilic behavior in Peru (Arístides 1999, Quispe et al. 2008, Valdivia et al. 2012). This evidence combined with molecular detection of Leishmania from the L. braziliensis complex bring to light the question of the role of Lu. auraensis/Lu. ruifreitasi as a suspect in the transmission of ACL.
The finding of Lu. davisi infected by Leishmania reinforces the importance of this species in the region, since it is considered a possible vector of L. (V.) braziliensis and L. (V.) naiffi in Rondônia (Grimaldi Jr et al. 1991, Gil et al. 2003) and in Pará (Souza et al. 2010). Despite its wild habitat, Lu. davisi has been found in peridomestic locations with the occurrence of ACL (Gomes et al. 2013, Teles et al. 2013a). In this study, most of the positive pools of Lu. davisi containing Leishmania DNA were collected near riparian forest. Lu. davisi is anthropophilic and the relationship between the density of this species, its possible adaptation to anthropogenic environments, together with the finding of natural infection of L. (V.) braziliensis and L. (V.) guyanensis in this study, reinforce the hypothesis of its involvement in enzootic and zoonotic cycles in the Amazon Region (Grimaldi Jr et al. 1991, Gil et al. 2003, Souza et al. 2010, Valdivia et al. 2012).
The local circulation of L. (V.) braziliensis and L. (V.) guyanensis has important epidemiological considerations since these species are endemic and responsible for cases of mucosal leishmaniasis in the Amazon Region (Guerra et al. 2006, Teles et al. 2015). Leishmania (V.) braziliensis has a higher diversity of potential vectors incriminated in its transmission in the literature (i.e. Lu. choti, Lu. yucumensis, and Lu. carrerai carrerai noted in this study); however, the principal described vector of L. (V.) guyanensis is Lu. umbratilis whose density was low in this study. The identification by hsp70 PCR-RFLP of L. (V.) guyanensis in 14 pools (14/15) at different collection points and the high density of Lu. auraensis/Lu. ruifreitasi do not exclude the possibility that these sandflies participates in the transmission cycle of this parasite in the border area. In this sense, further studies are needed to assess the vector capacity such as through dissection of the sandflies digestive tube for detection of the parasite; an anthropophilic behavior study and one on blood feeding in other vertebrate hosts (Killick-Kendrick 1990).
The record of clinical and epidemiological samples in Assis Brasil from other Leishmania species: L. (V.) shawi and L. (L.) amazonensis (Teles et al. 2015) as well as L. (V.) lainsoni and L. (V.) naiffi in humans from the microregion of Rio Branco (Tojal da Silva et al. 2006) confirm the epidemiological importance of potential vectors found in the fauna reported in this study, such as: Lu. paraensis, Lu. ayrozai and Lu. hirsuta hirsuta, which have been found naturally infected by L. (V.) naiffi (Rangel & Lainson 2009); Lu. flaviscutellata which is considered the main vector of L. (L.) amazonensis, an etiological agent of the diffuse form of cutaneous leishmaniasis (Lainson et al. 1994, Lainson 2010); and Lu. ubiquitalis, a potential vector of L. (V.) lainsoni in Pará (Silveira et al. 1991, Lainson 2010).
Another significant fact in this study is the prevalence of the species Lu. whitmani, Lu. antunesi and Lu. ubiquitalis in the peridomiciliary environment, while in the riparian environment these species were collected in smaller quantities. These species adapt to changing environments (Rebêlo et al. 1999, Araujo-Pereira et al. 2014) and are important in the transmission of L. (V.) braziliensis (Queiroz et al. 1994), L. (V.) guyanensis (Freitas et al. 2002) and L. (V.) shawi (Lainson et al. 1994). The correlation of such species density and their presence in anthropogenic environments as observed by Azevedo et al. (2008), Silva-Nunes et al. (2008) and Araujo-Pereira et al. (2014) in Acre indicate Lu. whitmani, along with Lu. antunesi, as suspicious insect vector species in the region.
Lu. antunesi has been associated with wild and peridomestic environments (Rebêlo et al. 1999, Silveira et al. 2002, Ramos et al. 2014) and occurs in Peru and Bolívia (Galati 2014). This species was associated with visceral leishmaniasis outbreaks and the transmission of L. (V.) lindenbergi in Pará (Ryan et al. 1984, Silveira et al. 2002). Recently, Trujillo et al. (2013) highlighted the epidemiological importance of Lu. antunesi in the transmission of Leishmania spp. in an endemic area in Colombia for its high infection rate (1.6%), abundance and adaptation in intra and peridomiciliary environments.
Regarding Lu. ubiquitalis, this species has been related to a variety of habitats including areas surrounding homes and forested areas in the Amazon (Ramos et al. 2014). Despite its low anthropophily in natural environments, Lu. ubiquitalis is the first representative of the subgenus Trichophoromyia implicated as a potential vector of L. (V.) lainsoni in Pará (Silveira et al. 1991, Lainson 2010).
The record of Lu. shawi in this study (frequency of 2.7%) deserves attention when considering the epidemiological surveillance of leishmaniasis around the border. This species has recently been implicated in the dissemination of Leishmania in Bolivia supported by evidence of its anthropophilic character, its occurrence and abundance in endemic areas of ACL and in peridomiciliary environments, as well as its proven infection by L. (V.) braziliensis and L. (V.) guyanensis (Garcia et al. 2007, Bustamante et al. 2012).
Thus, the data from this study demonstrate the great diversity of sandflies species with potential involvement in the leishmaniasis transmission cycle in Assis Brasil. In addition, the abundance of Lu. davisi and Lu. auraensis/Lu. ruifreitasi with several positive pools for the L. braziliensis complex increases the data about vector suspects in the north Brazil and suggests the need for new studies proving such species as vectors in the ACL cycle.
ACKNOWLEDGEMENTS
To Tony Hiroshi Katsuragawa and Marcelo Zagonel-Oliveira researchers who created the map.
Footnotes
Financial support: FAPESP (Project nº 08/11319-0).
REFERENCES
- Acre [cited 2012 Dez 12];Bacia do Alto Acre na Regional de Desenvolvimento do Alto Acre. 2008 Internet. www.mp.ac.gov.br/wp-content/files/Imagem04.pdf.
- Aguiar GM, Medeiros WM. Rangel EF, Lainson R.orgs . Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz; 2003. Distribuição e hábitats; pp. 207–255. [Google Scholar]
- Alves VR, Freitas RA, Santos FL, Oliveira AFJ, Barrett TV, Shimabukuro PHF. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012;56(2):220–227. [Google Scholar]
- Araujo-Pereira T, Fuzari AA, Andrade JD, Filho, Pita-Pereira D, Britto C, Brazil RP. Sand fly fauna (Diptera: Psychodidae: Phlebotominae) in an area of leishmaniasis transmission in the municipality of Rio Branco, state of Acre, Brazil. Parasit Vectors. 2014;7(360):2–5. doi: 10.1186/1756-3305-7-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias JR, Freitas RA. The know geographical distribution of sand flies in the state of Acre, Brazil (Diptera: Psychodidae) Acta Amaz. 1982;12:401–408. [Google Scholar]
- Arístides H. La leishmaniasis tegumentaria en el Alto Tambopata, Departamento de Puno, Perú. Rev Peru Med Exp Salud Publica. 1999;15(1-2):15–24. [Google Scholar]
- Azevedo ACR, Costa SM, Pinto MCG, Souza JL, Cruz HC, Vidal J, et al. Studies on the sandfly fauna (Diptera: Psychodidae: Phlebotominae) from transmission areas of American Cutaneous Leishmaniasis in state of Acre, Brazil. Mem Inst Oswaldo Cruz. 2008;103(8):760–767. doi: 10.1590/s0074-02762008000800003. [DOI] [PubMed] [Google Scholar]
- Bustamante M, Díaz M, Espinoza J, Parrado R, Reithinger R, García AL. Sand fly fauna in Chapare, Bolivia: an endemic focus of Leishmania (Viannia) braziliensis. J Med Entomol. 2012;49(5):1159–1162. doi: 10.1603/me12013. [DOI] [PubMed] [Google Scholar]
- Castellón EG, Arias JR, Freitas RA, Naiff RD. Os flebotomíneos da região Amazônica, estrada Manaus - Humaitá, estado do Amazonas, Brasil (Diptera: Psychodidae: Phlebotominae) Acta Amaz. 1994;24(1-2):91–102. [Google Scholar]
- Castellón EG. Lutzomyia sand flies in the Brazilian Amazon Basin (Diptera: Psychodidae) Manaus: INPA; 2009. 202 [Google Scholar]
- Cesário M, Cesário RR, Andrade-Morraye M. Environmental change and health impacts in Amazonia. Human Health and Global Environmental Change. 2011;1(1):26–33. [Google Scholar]
- Graça GC, Volpini AC, Romero GAS, Oliveira MP, Neto, Hueb M, Porrozzi R, et al. Development and validation of PCR-based assays for diagnosis of American cutaneous leishmaniasis and identification of the parasite species. Mem Inst Oswaldo Cruz. 2012;107(5):664–674. doi: 10.1590/s0074-02762012000500014. [DOI] [PubMed] [Google Scholar]
- Feitosa MAC, Julião GR, Costa MDP, Belém B, Pessoa FAC. Diversity of sand flies in domiciliary environment of Santarém, state of Pará, Brazil: species composition and abundance patterns in rural and urban areas. Acta Amaz. 2012;42(4):507–514. [Google Scholar]
- Freitas RA, Naiff RD, Barrett TV. Species diversity and flagellate infections in the sand fly fauna near Porto Grande, state of Amapá, Brazil (Diptera: Psychodidae. Kinetoplastida: Trypanosomatidae) Mem Inst Oswaldo Cruz. 2002;97(1):53–59. doi: 10.1590/s0074-02762002000100008. [DOI] [PubMed] [Google Scholar]
- Galati EAB. Phlebotominae (Diptera, Psychodidae): classificação, morfologia, terminologia e identificação de adultos. Vol. 1. São Paulo: USP; 2014. 127 apostila. [Google Scholar]
- Garcia AL, Tellez T, Parrado R, Rojas E, Bermudez H, Dujardin JC. Epidemiological monitoring of American tegumentar leishmaniasis: molecular characterization of a peridomestic transmission cycle in the Amazonian lowlands of Bolivia. Trans R Soc Trop Med Hyg. 2007;12(101):1208–1213. doi: 10.1016/j.trstmh.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Gil LHS, Basano SA, Souza AA, Silva MGS, Barata I, Ishikawa EA, et al. Recent observations on the sand fly (Diptera: Psychodidae) fauna of the state of Rondônia, Western Amazônia, Brazil: the importance of Psychodopygus davisi as a vector of zoonotic cutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 2003;98(6):751–755. doi: 10.1590/s0074-02762003000600007. [DOI] [PubMed] [Google Scholar]
- Gomes LHM, Albuquerque MIC, Rocha LC, Pinheiro FG, Franco AMR. Diversity and distribution of sandflies (Diptera: Psychodidae) in a military area in the state of Amazonas, Brazil. Mem Inst Oswaldo Cruz. 2013;108(5):651–656. doi: 10.1590/0074-0276108052013018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G, Jr, Momem H, Naiff RD, Pratt DMM, Banet TV. Characterization and classification of leishmanial parasites from humans, wild mammals, and sand flies in the Amazon Region of Brazil. Am J Trop Med Hyg. 1991;44(6):645–661. doi: 10.4269/ajtmh.1991.44.645. [DOI] [PubMed] [Google Scholar]
- Guerra JAO, Ribeiro JAS, Coelho L, Barbosa MGV, Paes GP. Epidemiologia da leishmaniose tegumentar na comunidade São João, Manaus, Amazonas, Brasil. Cad Saude Publica. 2006;22(11):2319–2327. doi: 10.1590/s0102-311x2006001100006. [DOI] [PubMed] [Google Scholar]
- Harris E, Kropp G, Belli A, Rodriguez B, Agabian N. Single-step multiplex PCR assay for characterization of new world Leishmania complexes. J Clin Microbiol. 1998;36(7):1989–1995. doi: 10.1128/jcm.36.7.1989-1995.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBGE - Instituto Brasileiro de Geografia e Estatística [cited 2014 Out 13];Anuário Estatístico do Brasil. 2014 Internet. www.ibge.gov.br.
- Katholi CR, Toè L, Merriwether A, Unnasch TR. Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. J Infect Dis. 1995;172(5):1414–1417. doi: 10.1093/infdis/172.5.1414. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R. Phlebotomine vectors of leishmaniasis: a review. Med Vet Entomol. 1990;4(1):1–24. doi: 10.1111/j.1365-2915.1990.tb00255.x. [DOI] [PubMed] [Google Scholar]
- Lainson R, Shaw JJ, Silveira FT, Souza AAA, Braga RR, Ishikawa EAY. The dermal leishmaniases of Brazil, with special reference to the Eco-epidemiology of the disease in Amazonia. Mem Inst Oswaldo Cruz. 1994;89(3):435–443. doi: 10.1590/s0074-02761994000300027. [DOI] [PubMed] [Google Scholar]
- Lainson R. The Neotropical Leishmania species: a brief historical review of their discovery, ecology and taxonomy. Rev Pan-Amaz Saude. 2010;1(2):13–32. [Google Scholar]
- Martins AV, Silva JE. Notas sobre os flebotomíneos do estado do Acre, com a descrição de duas espécies novas (Diptera: Psychodidae) Rev Brasil Biol. 1964;24:127–138. [PubMed] [Google Scholar]
- Mukhopadhyay J, Ghosh K, Braig HR. Identification of cutaneous leishmaniasis vectors, Phlebotomus papatasi e P. duboscqi using random amplified polymorphic DNA. Acta Trop. 2000;2(76):277–283. doi: 10.1016/s0001-706x(00)00130-3. [DOI] [PubMed] [Google Scholar]
- Oliveira AFJ, Teles CBG, Medeiros FM, Camargo LMA, Pessoa FAC. Description of Trichophoromyia ruifreitasi, a new phlebotomine species (Diptera, Psychodidae) from Acre state, Brazilian Amazon. Zookeys. 2015;526:65–73. doi: 10.3897/zookeys.526.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JGS, Novais FO, Oliveira CI, Cruz AC, Jr, Campos LF, Rocha AV, et al. Polymerase chain reaction (PCR) is highy sensitive for diagnosis of mucosal leishmaniasis. Acta Trop. 2005;94(1):55–59. doi: 10.1016/j.actatropica.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Paiva BR, Secundino NFC, Pimenta PFP, Galati EAB, Andrade HF, Júnior, Malafronte RS. Standardization of conditions for PCR detection of Leishmania spp. DNA in sandflies (Diptera, Psychodidae) Cad Saude Publica. 2007;23(1):87–94. doi: 10.1590/s0102-311x2007000100010. [DOI] [PubMed] [Google Scholar]
- Pereira AM, Jr, Teles CBG, Santos APA, Rodrigues MD, Marialva EF, Pessoa FAC, et al. Ecological aspects and molecular detection of Leishmania DNA Ross (Kinetoplastida: Trypanosomatidae) in phlebotomine sandflies (Diptera: Psychodidae) in terra firme and várzea environments in the Middle Solimões Region, Amazonas state, Brazil. Parasit Vectors. 2015;8(180) doi: 10.1186/s13071-015-0789-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz RG, Vasconcelos IA, Vasconcelos AW, Pessoa FA, Souza RN, David JR. Cutaneous leishmaniasis in Ceará state in Northeasten Brazil: incrimination of Lutzomyia whitmani (Diptera: Psychodidae) as a vector of Leishmania braziliensis in Baturité municipality. Am J Trop Med Hyg. 1994;50(6):693–698. doi: 10.4269/ajtmh.1994.50.693. [DOI] [PubMed] [Google Scholar]
- Quispe D, Rado D, Quispe W, Pacheco R. Sandfly fauna (Diptera: Psychodidae) of intra and peri - domicilliary environments in aguas calientes, la convención, Cusco, Perú; International Symposium on Phlebotomine Sandflies; 2008; Lima, Perú, Miraflores. 2008. 131 Oct 27-31. [Google Scholar]
- Ramos WR, Medeiros JF, Julião GR, Ríos-Velásquez CM, Marialva EF, Desmouliére SJM, et al. Anthropic effects on sand fly (Diptera: Psychodidae) abundance and diversity in an Amazonian rural settlement, Brazil. Acta Trop. 2014;139:44–52. doi: 10.1016/j.actatropica.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Rangel EF, Lainson R. Proven and putative vectors of American cutaneous leishmaniasis in Brazil: aspects of their biology and vectorial competence. Mem Inst Oswaldo Cruz. 2009;104(7):937–954. doi: 10.1590/s0074-02762009000700001. [DOI] [PubMed] [Google Scholar]
- Rebêlo JMM, Araújo JAC, Carvalho ML, Barros VLL, Silva FS, Oliveira ST. Flebótomos (Diptera, Phlebotominae) da Ilha de São Luís, zona do Golfão Maranhense, Brasil. Rev Soc Bras Med Trop. 1999;32(3):247–253. [PubMed] [Google Scholar]
- Ryan L, Silveira FT, Lainson R, Shaw JJ. Leishmanial infections in Lutzomyia longipalpis and Lu. antunesi (Diptera: Psychodidae) on the Island of Marajó, Pará state, Brazil. Trans R Soc Trop Med Hyg. 1984;78(4):547–548. doi: 10.1016/0035-9203(84)90081-6. [DOI] [PubMed] [Google Scholar]
- Silva-Nunes M, Cavasini CE, Silva NS, Galati EAB. Epidemiologia da leishmaniose tegumentar e descrição das populações de flebotomíneos no município de Acrelândia, Acre, Brasil. Rev Bras Epidemiol. 2008;11(2):241–251. [Google Scholar]
- Silveira FT, Ishikawa EA, Souza AA, Lainson R. An outbreak of cutaneous leishmaniasis among soldiers in Belém, Pará state, Brazil, caused by Leishmania (Viannia) lindenbergi n. sp. A new leishmanial parasite of man in the Amazon Region. Parasite. 2002;9(1):43–50. doi: 10.1051/parasite/200209143. [DOI] [PubMed] [Google Scholar]
- Silveira FT, Souza AAA, Lainson R, Shaw JJ, Braga RR, Ishikawa EEA. Cutaneous leishmaniasis in the Amazon Region: natural infection of the sandfly Lutzomyia ubiquitalis (Psychodidae: Phlebotominae) by Leishmania (Viannia) lainsoni in Pará state, Brazil. Mem Inst Oswaldo Cruz. 1991;86(1):127–130. doi: 10.1590/s0074-02761991000100021. [DOI] [PubMed] [Google Scholar]
- SINAN [cited 2014 Set 28];Sistema de Informações de Agravos de Notificação. 2014 Internet. http://dtr2004.saude.gov.br/sinanweb/index.php?saude=http%3A%2F%2Fdtr2004.saude.gov.br%2Fsinanweb%2Findex.php&botaook=OK&obj=http%3A%2F%2Fdtr2004.saude.gov.br%2Fsinanweb%2Findex.php.
- Souza AAA, Silveira FT, Lainson R, Barata IR, Silva MGS, Lima JAN, et al. Phlebotominae fauna in Serra dos Carajás, Pará state, Brazil, and its possible implications for the transmission of American tegumentar leishmaniasis. Rev Pan-Amaz Saude. 2010;1:45–51. [Google Scholar]
- Teles CBG, Basano AS, Zagonel-Oliveira M, Campos JJ, Oliveira AFJ, Freitas RA, et al. Epidemiological aspects of American cutaneous leishmaniasis and phlebotomine sandfly population, in the municipality of Monte Negro, state of Rondônia, Brazil. Rev Soc Bras Med Trop. 2013a;46(1):60–66. doi: 10.1590/0037-868216062013. [DOI] [PubMed] [Google Scholar]
- Teles CBG, Freitas RA, Oliveira AFJ, Ogawa GM, Cavalcante EA, Medeiros JF, et al. Description of a new phlebotomine species (Diptera: Psychodidae, Phlebotominae) and new records of sand flies from the state of Acre, Northern Brazil. Zootaxa. 2013b;3609(1):85–90. doi: 10.11646/zootaxa.3609.1.6. [DOI] [PubMed] [Google Scholar]
- Teles CBG, Medeiros JF, Santos APA, Freitas LAR, Katsuragawa TH, Cantanhêde LM, et al. Molecular characterization of American cutaneous leishmaniasis in the tri-border area of Assis Brasil, Acre state, Brazil. Rev Inst Med Trop Sao Paulo. 2015;57(4):343–347. doi: 10.1590/S0036-46652015000400012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojal da Silva AC, Cupolillo E, Volpini AC, Almeida R, Romero GAS. Species diversity causing human cutaneous leishmaniasis in Rio Branco, state of Acre, Brazil. Trop Med Int Health. 2006;11(9):1388–1398. doi: 10.1111/j.1365-3156.2006.01695.x. [DOI] [PubMed] [Google Scholar]
- Trujillo AV, Reina AEG, Orjuela AG, Suárez EP, Palomares JE, Alvarez LSB. Seasonal variation and natural infection of Lutzomyia antunesi (Diptera: Psychodidae: Phlebotominae), an endemic species in the Orinoquia region of Colombia. Mem Inst Oswaldo Cruz. 2013;108(4):463–469. doi: 10.1590/0074-0276108042013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia HO, los Santos MB, Fernández R, Baldeviano GC, Zorrilla VO, Vera H, et al. Natural Leishmania infection of Lutzomyia (Trichophoromyia) auraensis in Madre de Dios, Peru, detected by a fluorescence resonance energy transfer-based real-time polymerase chain reaction. Am J Trop Med Hyg. 2012;87(3):511–517. doi: 10.4269/ajtmh.2012.11-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO - World Health Organization [cited 2015 Nov 12];Health topies: Leishmaniasis. 2015 Internet. http://www.who.int/topies/leishmaniasis/en/
- Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae) 881American Entomological Institute. 1994;54 [Google Scholar]


