Abstract
Bacterial vaginosis (BV) affects almost a quarter of US women, making it a condition of major public health relevance. Key questions remain regarding the etiology of BV, mechanisms for its association with poor reproductive health outcomes, and reasons for high rates of treatment failure. New model systems are required to answer these remaining questions, elucidate the complex host-microbe and microbe-microbe interactions, and develop new, effective interventions. In this review, we cover the strengths and limitations of in vitro and in vivo model systems to study these complex intercellular interactions. Furthermore, we discuss advancements needed to maximize the translational utility of the model systems. As no single model can recapitulate all of the complex physiological and environmental conditions of the human vaginal microenvironment, we conclude that combinatorial approaches using in vitro and in vivo model systems will be required to address the remaining fundamental questions surrounding the enigma that is BV.
Keywords: vaginal microbiome, in vitro vaginal epithelial cell culture models, animal models, microfluidics, biofilm
Bacterial vaginosis (BV) is a clinical condition characterized by a vaginal microbial community comprising diverse anaerobic species and a low abundance of most Lactobacillus species [1]. BV affects >21 million US women and is associated with significant health problems, such as preterm birth, miscarriage, and an increased risk of human immunodeficiency virus acquisition [2–4]. This syndrome was first described in the 1920s by Schröder, but its cause is still not known. Treatment with antibiotics can provide transient success but is associated with frequent failures, with up to 50% of women experiencing recurrence within 1 year [5]. Longitudinal studies that would better define the etiology of this condition are difficult and expensive, highlighting the potential for innovative model systems to further our understanding of the host-microbe and microbe-microbe interactions that underlie BV acquisition and pathogenesis.
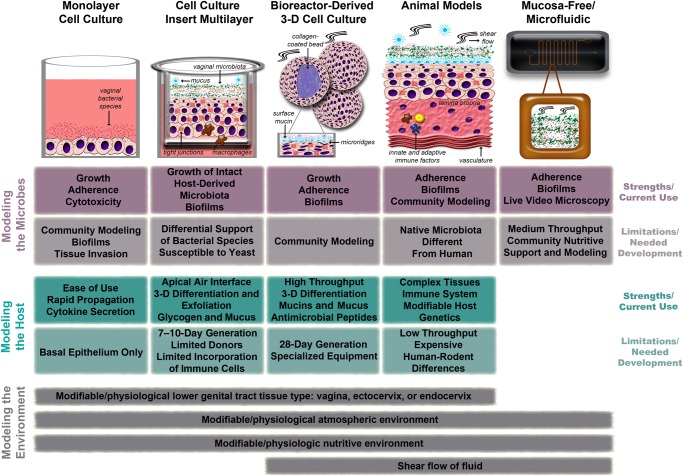
No model system recapitulates all of the complex biological, chemical, and structural features of humans. The goal of a model is to create a system that reflects biological reality but can be perturbed and manipulated to illuminate relevant mechanisms and pathways. In the study of BV, there are 3 major components that likely permit the vaginal microbiota to shift to a dysbiotic state: the microbes, their host, and the vaginal environment (Figure 1). In this review, we will examine the existing model systems for studying host-microbe and intercellular interactions in the female lower genital tract, outlining the strengths and limitations of each system with regard to their physiological resemblance to the human tissue and their ease of manipulation. In addition, we will identify gaps in the field and suggest ways that model systems could be refined or advanced to help illuminate the enigma that is BV.
Figure 1.
Comparison of human cell culture models, animal rodent models, and mucosa-free models of bacterial vaginosis. Key strengths/limitations and current uses/areas of needed development, as well as which aspects of the vaginal environment are recapitulated, are indicated for each model.
THE FEMALE LOWER GENITAL TRACT AND BACTERIAL VAGINOSIS: WHAT IS BEING MODELED?
The microbiology of the human vagina has been well described using both cultivation- and molecular-based methods. The most common dominant microbes in US women are Lactobacillus species, but ethnicity and age influence the composition of the microbial community [6], and, in some African populations, other microbes such as Gardnerella vaginalis or Prevotella species are more often dominant [7]. BV is a heterogeneous syndrome characterized by a paucity of Lactobacillus species, increased community diversity, and, in some cases, a biofilm adherent to the vaginal epithelium [8]. The microbial communities observed in women with BV are heterogeneous, varying from woman to woman and from episode to episode [9]. Several publications have suggested that some women with BV have bacterial biofilms coating the vaginal epithelium, possibly facilitating recurrence of BV after antibiotic therapy [8]. The factors that prompt biofilm formation and dispersal in the vagina remain poorly characterized, and modeling studies could help elucidate these mechanisms. The presence of a biofilm may also affect bacterial growth dynamics, interspecies interactions (ie, metabolic syntrophies or genetic exchange may be facilitated by positioning within biofilm scaffolds), and resistance to low pH and antimicrobials.
The human vagina is characterized by a stratified squamous mucosal epithelium with innate and adaptive immune cells scattered throughout, with increased density in the basal layers [10]. The vaginal epithelium is hormonally responsive—its thickness and glycogen content decrease with less estrogen. The glycogen that is produced by this mucosa serves as a crucial carbon source to support the metabolism of the microbiome, including the production of lactic acid by Lactobacilli, which produces a vaginal pH of ≤4.5.
The vagina is a microaerophilic environment, although menstruation and insertion events transiently increase oxygen levels [11]. The epithelium lining these sites is covered by cervicovaginal fluid (a mixture of cervical mucus and vaginal secretions) that forms a barrier to microbial insults and houses soluble immune mediators. Hundreds of proteins are found in cervicovaginal fluid, many with immunological functions, such as antimicrobial peptides. Genetic polymorphisms in immune response pathways have been shown to increase the risk of outcomes such as preterm birth when BV is present [12], suggesting that host responses to BV may be as important as the microbial composition of BV in determining patient outcomes. The viscosity, pH, and biochemical composition of cervicovaginal fluid changes with routine reproductive events, including hormonal cycling, menstrual bleeding, sexual activity, and menopause [13]. Together these environmental features comprise mechanical, physical, and chemical barriers to invading microbes, including BV-associated species, and are important to consider for model development, advancement, and translational utility. Conversely, these host-produced and -introduced components also are the carbon sources used by the normobiotic and dysbiotic bacterial communities, adding to the complexity of interpretation of clinical studies and the need for sophisticated and controlled modeling systems.
WHAT ARE THE STRENGTHS AND LIMITATIONS OF AVAILABLE MODELS OF THE VAGINAL ENVIRONMENT?
The following sections describe existing model systems for studying host-microbe and intercellular interactions for BV: monolayer cell culture models, cell culture insert multilayer models, rotating wall vessel (RWV) bioreactor–derived 3-D cell culture models, animal models, and mucosa-free/microfluidic models. Each highlights the strengths and limitations of the respective system in the context of studying vaginal microbiota.
Monolayer Vaginal Epithelial Cell Culture Models
Both primary and immortalized vaginal epithelial cell lines have been grown in standard tissue culture plates to generate a monolayer model system of the vaginal epithelial surface. Immortalized lines are transformed with human papillomavirus E6/E7 [14] and express similar Toll-like receptors (TLRs) as primary cells [15], as well as the appropriate cytokeratin markers for epithelial differentiation [14]. These cells have been cultured aerobically and anaerobically, with 95% cell viability after 24 hours in both cases [16, 17]. The monolayer model has been used to evaluate the epithelial immune responses and the impact of substances such as microbicides and seminal plasma on the epithelium. When bacteria are cocultured with vaginal epithelial cells, both cells and bacteria maintain viability, but bacterial growth is minimal [16, 17].
For evaluation of the mucosal innate immune response, the monolayer model in general shows similar patterns to what is seen in vivo. In culture with TLR agonists and individual BV-associated bacterial species such as Gardnerella vaginalis, Prevotella bivia, and Atopobium vaginae, vaginal epithelial monolayers produce cytokines, chemokines, and antimicrobial peptides similar to those reported in clinical samples from women with BV, such as interleukin 1β (IL-1β), interleukin 6, and interleukin 8 (IL-8) [15–17]. Bacterial species associated with the highest levels of clinical inflammation induced the most interleukin 1α, IL-1β, and IL-8 in a monolayer model system [7]. The model has also been used to evaluate microbicide toxicity, and it showed limited inflammatory response when microbicides were applied to cells alone but significantly greater upregulation of nuclear factor κB, RANTES, and IL-8 by cellulose sulfate than by hydroxyethyl cellulose when bacteria were also present, which is consistent with findings from clinical studies [17]. The monolayer model represents parts of the vaginal mucosal immune response quite well, it is easy to use, and experiments can be performed in a short time frame (24–48 hours). However, some immune analytes of interest (IL-1β, tumor necrosis factor α, and interleukin 10) are not produced at high levels by these cells in all studies [16]. In other areas of cell culture research, epithelial cells behave differently in culture when stromal cells are present, suggesting that what is measured in the monolayer culture model may only be part of the picture of what is happening in vivo.
Monolayers have also been used for evaluation of bacterial interaction with or adherence to vaginal epithelial cells (Figure 1). In this model, G. vaginalis has been shown to form a biofilm, Lactobacillus crispatus has been shown to decrease adherence of G. vaginalis to epithelial cells, and Lactobacillus iners has been shown to enhance attachment [18]. These models allow characterization of surface interactions between cells and bacteria and between bacterial species, but they are unable to assess bacterial infiltration of a stratified epithelium or interactions with intraepithelial or subepithelial immune cells.
Cell Culture Insert Multilayer Models
Cell cultures established on insert systems (commonly known as Transwell inserts) have been used widely to produce polarized, differentiated, 3-D multilayer cultures to model a variety of tissues, including vaginal and cervical mucosa. The system uses a plastic insert assembly that contains a porous membrane support with options for pore sizes and surface areas compatible with standard 6-, 12-, 24-, or 96-well dishes. Cells of interest are plated onto the membrane of the cup, which then is placed into an appropriate culture chamber filled with relevant medium. This format creates separated apical and basal chambers that allow study of directional secretion of host products and selective application of test materials. This separation also provides the opportunity for cell migration assays, including those of immune cell types that extravasate via transepithelial migration along chemokine gradients produced in response to pathogenic or experimental insult (Figure 1).
Cells from both lower and portions of the upper female genital tract have been used to create insert system models, enhancing the usefulness of the cell types previously evaluated only as monolayer cultures [19–21]. Culture inserts are a key component of the commonly used commercial EpiVaginal cultures that can be supplemented with additional immune cell types [19]. These cultures are established with primary ectocervical cells that are conditioned to form stratified squamous epithelium to better model the vaginal mucosa. Like other primary culture approaches, these cells have a finite availability, reducing opportunities for reproduction studies and expanded group sizes. Additionally, primary cultures can be affected by the same confounders that complicate clinical research interpretation, including environmental impacts experienced by the donor, hormonal and inflammation state at the time of harvest, and epigenetic alterations of gene expression, that are not tempered through the finite passages in culture. As a result, we have established multilayers of vaginal epithelial cells formed in inserts that more accurately reflect stratified squamous epithelium, with many crucial characteristics, including tight junctions and basal progenitor cell layers [20, 21].
A recent advance in culture insert modeling of the vaginal mucosa recognized that the vagina is a microaerophilic potential space in which the microbiome thrives under conditions that support growth of both obligatory aerobic and anaerobic bacterial species. To better model this aspect of the vaginal environment, after stabilization (for 12–24 hours) of plated immortalized vaginal epithelial cells, the culture fluid is removed from the apical chamber, creating an air-interface. This interface, with CO2-supplemented air, enhances the development of differentiated cell layers that increase to normal tissue thickness, form apically sloughed anucleate cells, and produce substantial levels of glycogen and mucous that gathers on the apical surface of the culture upon maturation (after 7–10 days) [21, 22]. This environment and the vaginal epithelia–produced carbon sources support the growth of individual bacterial species [21], as well as transplanted microbiomes, collected from women during gynecological examinations [22]; this system represents the first culture approach that allows for the controlled study of intact human microbiomes with the associated host tissues. The system is still being validated, but current data have illustrated substantial consistency between the profile of the original microbiome community and that grown in the insert culture, including microbiomes from women with symptomatic BV. Microbiomes representing each of the major community state types have been successfully transplanted and establish biofilms consistent with those observed in vivo [22].
Vaginal epithelial multilayers created on inserts have been established with both primary and immortalized cells from vaginal cuff tissue collected during hysterectomies or from surgical repairs of prolapses. Immortalized vaginal epithelial cells illustrate an impressive consistency in expression of TLRs and responses to pathogenic insult relative to primary cells [15, 20, 21]. More recently, microarray, reverse transcription polymerase chain reaction (PCR), and immunolabeling analyses of multilayer vaginal epithelial cultures showed remarkably similar expression patterns of molecular transporters, further supporting the usefulness of this model system for study of the impact of BV [23]. Finally, to better model the vaginal tissue of origin, vaginal epithelial multilayer cultures have been successfully supplemented with immune cells, including monocyte-derived macrophages, that further enhance the usefulness of the system to study tissue responses to infection and BV [22]. At present, the vaginal multilayer cultures lack the other cell types and underlying structures (eg, lamina propria and vasculature) associated with the vaginal mucosa.
RWV Bioreactor–Derived 3-D Cell Culture Models
The RWV bioreactor has been used to create in vitro human 3-D organotypic models of a variety of mucosal sites, including the vaginal and endocervical epithelium, by providing the necessary low fluid shear (eg, the biomechanical force known to influence cellular differentiation and development) microenvironment to form fully differentiated aggregates that display in vivo–like features often not observed in standard 2-D culture formats [24–26]. These tissue-like aggregates form when human vaginal epithelial cells are combined with collagen-coated microcarrier beads in the fluid-filled RWV bioreactor under constant low fluid shear (the sedimentation of cells is offset by rotating fluid, creating a constant, gentle fall of cells through the medium), allowing the cells to attach, grow, and differentiate into 3-D vaginal aggregates [25]. Once cellular differentiation is completed (in 28 days), the 3-D vaginal aggregates are removed from the bioreactor and seeded into multiwell plate formats for experimental analyses and downstream assays (eg, infection or toxicology studies, microscopy, RNA/protein analysis, and flow cytometry). The physiologically relevant features exhibited by the RWV-derived 3-D vaginal aggregates include cellular differentiation as determined by tight junctions, mucin expression and mucus secretion, microridge formation (which serves to interlock mucus secretions and has not been exhibited by any other in vitro model), immune mediator signaling and secretion (cytokines, chemokines, and antimicrobial peptides), and authentic human responses to external stimuli (Figure 1) [24, 26]. Vaginal aggregates are removed from the bioreactor for downstream infection/colonization assays, as studies have shown that culturing bacteria in the RWV bioreactor can alter bacterial virulence, stress resistance, biofilm formation, and protein expression. Studies are required to determine whether BV-associated bacterial virulence factors and biofilm formation are altered under low-fluid-shear culture conditions in the bioreactor.
Although much can be learned using 2-D formats, these techniques do not provide models that appropriately reflect the ultrastructural and morphological barrier features required for predictive models (Figure 1). The RWV-generated vaginal aggregates express membrane-associated mucins (MUC1, MUC3, MUC4, and MUC16), as well as gel-forming mucins (MUC5AC, MUC5B, and MUC6), that reflect the profile in human tissue and cervicovaginal secretions [24, 27]. These mucins are expressed at low-to-undetectable levels in vaginal epithelial cells grown in 2-D formats [24, 27]. Notably, these well-characterized human models are more resistant to microbicide toxicity (eg, due to nonoxynol 9), cytotoxicity (eg, due to vaginal microbiota), and microbial infection (eg, due to herpes simplex virus type 2 and Mycoplasma genitalium), compared with 2-D monolayers, most likely because of enhanced barrier features (stratified squamous epithelium, microridge formation, and mucus secretion), and have been effectively used to study interactions between the host and sexually transmitted pathogens and between the host and vaginal microbes [24, 25, 27]. Use of the RWV vaginal model revealed that vaginal microbiota, including BV-associated bacteria, alter innate immune mechanisms and epithelial barrier features in a species-specific fashion [27]. With this reductionist approach, a unique microbial signature was induced by individual bacterial species (Lactobacilli and BV-associated bacteria) [27]. Studies are underway to determine the impact of polymicrobial communities and community states on innate immune responses and epithelial barrier properties.
RWV bioreactor–derived models of the vaginal epithelium are excellent tools to dissect the innate immune mechanisms, epithelial barrier function, and epithelial-specific responses to commensal or pathogenic organisms. These 3-D vaginal aggregates allow for the study of mucin and mucus-microbe interactions in the context of the host epithelium, which is a benefit to using this well-characterized model system. Ongoing scanning electron microscopy studies have shown that vaginal microbiota, including G. vaginalis, colocalize with extracellular secretions on the surface of these vaginal aggregates, possibly as a means of adherence and biofilm formation. To date, these 3-D reproductive models have not incorporated immune cells or been used for cellular migration assays, but cocultures have been established using this RWV approach (reviewed by Barilla et al [28]). Drawbacks to this model system are the culture time (28 days) required for full differentiation and the need for specialized bioreactors and associated rotation apparatus (Figure 1). Despite requiring a RWV bioreactor system and extended culture period, this advanced culture system allows for high-throughput, flexible formats for a variety of downstream applications, including “omics” technologies [25]. Since the 3-D aggregates accurately reflect many of the relevant features of the human vaginal epithelium and BV, these models may be valuable tools for predicting the efficacy, toxicity, and pharmacokinetics of new biologics, microbicides, and interventions for BV that can be easily translated in humans.
Animal Models
A robust animal model of BV could advance our understanding of BV pathogenesis, especially with regard to factors such as the role of biofilms, transmission mechanisms, enhancement of other infections, and adverse pregnancy outcomes. In addition, such a system could provide a platform for testing candidate preventive and therapeutic measures. An ideal animal model would combine ease of use, genetic tractability, and a background vaginal microbiota closely resembling that of humans. Such a model does not exist at present.
Prior approaches to in vivo models of BV have been reviewed [29]. Since the original description of the association between G. vaginalis (then called Haemophilus vaginalis) and “non-specific vaginitis” (BV), there have been several attempts to study G. vaginalis–host interactions in vivo. Early human challenge studies [30] yielded conflicting data regarding the pathogenicity of G. vaginalis in vivo. Significant discrepancies in rates of BV following inoculation with pure cultures of G. vaginalis or clinical samples, with the latter being substantially more efficient, foreshadowed later concepts of BV as a polymicrobial or dysbiotic entity. Several attempts to model BV in nonhuman primates have been reported. Johnson et al [31] initiated G. vaginalis vaginal colonization in pig-tailed macaques, but neither clue cells nor alteration of pH was detected, and neither chimpanzees nor tamarins were susceptible to colonization. In grivet monkeys [32], vaginal discharge and prolonged colonization were induced by coadministration of G. vaginalis and long, curved rod-shaped bacteria, presumed to be Mobiluncus. It is notable that the community composition of primate vaginal microbial communities is highly host specific, with that of humans characterized by a relatively low-diversity, Lactobacillus-dominant microbiota. Some nonhuman primates have detectable Lactobacillus species in their vaginal microbiota, but these species are at low abundance and are present as part of a more diverse community [33]. These more recently recognized factors may pose significant limitations to the use of nonhuman primates as model systems to investigate vaginal dysbioses, such as BV.
There are important potential benefits of small-animal model systems to study BV. In addition to logistical advantages over larger animals, a genetically tractable small-animal system might allow for studies of the influence of specific host genes on BV pathogenesis. Dukes and Gardner [34] attempted unsuccessfully to model systemic infection with G. vaginalis in several small-animal systems (ie, mice, guinea pigs, rats, and rabbits). In pregnant rabbits, G. vaginalis induced amnionitis and deciduitis and led to the development of histopathologic lesions in the fetal brain [35]. A 4-day murine model of G. vaginalis colonization was used to test both a potential probiotic and a candidate therapy for BV, with bacterial density and local myeloperoxidase accumulation used as end points [36]. Hymes et al [37] used DNase as an antibiofilm strategy to decrease murine vaginal colonization, and Gilbert et al noted both exfoliation of vaginal epithelial cells with adherent bacteria and increased sialidase activity in G. vaginalis–colonized mice [38]. The murine vaginal microbiota remains incompletely described but is not Lactobacillus predominant, posing difficulties for the development of biologically meaningful models of BV. Teixeira et al used gnotobiotic mice either monoassociated or diassociated with G. vaginalis and Lactobacillus to model carriage, histologic lesions, and potential interbacterial competition [39]. Such models, especially if combined with more-complex bacterial combinations (either defined culture-based mixtures or human samples), represent an intriguing future direction for the field and a potential route to overcome some limitations of the existing systems.
Mucosa-Free and Microfluidic Modeling
Cultivation of microbes in the absence of host cells creates opportunities to investigate their dynamics and interactions under controlled conditions. While most cultivation studies of vaginal bacteria have focused on individual species grown in monoculture, a number of bacterial coculture studies have helped define the antimicrobial properties of vaginal lactobacilli [40] and the metabolic interdependencies among a handful of BV-associated bacteria [41]. However, our knowledge of these and other important aspects of vaginal microbial communities, such as their propensity to form biofilms, survive environmental stress, and exchange genetic material, are far from complete. One of the reasons community cultivation work has progressed slowly is the lack of a suitable culture medium. Although efforts have been made to formulate chemically defined media that simulate vaginal secretions [42], robust growth of most fastidious BV-associated species requires supplementation with undefined components, such as crude mucin preparations, peptone, or serum [41, 42]. Metabolic predictions coming from today's burgeoning metabolomic, metagenomic, and whole-genome sequence data could point the way to a more universal culture medium. Another approach that was recently successful for the oral niche is the use of empirical testing to guide medium development. Growth of oral bacterial community members was monitored in different media, using PCR-based approaches and next-generation sequencing [43]; favorable conditions were combined to yield a universal growth medium for synthetic and host-derived oral microbial communities.
Another note the BV research community might take from investigations of the oral niche is the use of microfluidics models in our experimental repertoire. The vagina is a sloping fibromuscular tube with its walls in frequent contact due to squeezing forces (compliant pressure from neighboring organs and contraction of pelvic floor musculature). Vaginal secretions thus spread and flow across the mucosal surface, creating shear stress at the fluid-epithelial interface [44]. Consequently, organisms that adhere to the epithelium experience hydrodynamic forces that could alter their biofilm-forming behaviors, growth dynamics, and survival strategies. Microfluidics technology has been used to model oral and bronchial biofilms formed in shear flow conditions for over a decade, but to our knowledge, this technology has not yet been used to the study of vaginal biofilms, likely because of a number of technical challenges. One major challenge for the BV research community is the lack of genetic tools that allow for the creation of mutant bacterial strains, including those that stably express fluorescent proteins. The availability of fluorescently tagged strains would enable direct observation of individual species' growth and contribution to the biofilm during live video microscopy experiments.
A second challenge is the choice of which species and strains to target for microfluidic biofilm inquiries and the development of critical genetic tools. Most of our knowledge of the composition of vaginal biofilms comes from fluorescence in situ hybridization studies with probes that detect multiple species within a given family or genus [8], leaving the precise species-level composition of the biofilm an open question. Indeed, one recent study found that many vaginal bacteria displayed some propensity for in vitro biofilm formation but in a manner that was highly dependent on the choice of growth medium [45]. Although there is little question that G. vaginalis is the species that dominates the biofilm in most women [8], its biofilm-forming properties are highly variable among strains, and there currently is no consensus on which strains or genetic factors are critical for BV recurrence or development of sequelae.
ADVANCING CURRENT MODEL SYSTEMS TO BETTER UNDERSTAND INTERCELLULAR INTERACTIONS IN THE FEMALE GENITAL TRACT
Current model systems for BV could be advanced and refined to ask more-complex research questions; however, the best model system depends on the question or hypothesis being tested. For example, while all of the models described here could be used to evaluate candidate antimicrobials, a small-animal model may be best suited to address BV recurrence rates following treatment. Similarly, although each model could be used to evaluate bacterial growth and adherence, the human cell culture and animal models would be appropriate for linking these factors to the host response, whereas the mucosa-free/microfluidic models may be ideal for dissecting the role of microbe-microbe interactions. Some questions can be addressed using the existing models alone or in combination, whereas others will require model advancement and refinement. We can envision advancing the current in vitro model systems to include physiologically relevant immune cells that are both spatially and temporally incorporated to faithfully mimic host responses of the female genital tract. An optimal BV model system must allow for the controlled evaluation of host genetics and host proteomic and metabolomic contributions, as well as controlled introduction of external factors (eg, diet, douching, and sexual contact), that are reported to impact the vaginal microbiome. Such a model system must also consistently support the development of reproducible bacterial communities that represent normobiotic and dysbiotic states in different host genetic backgrounds.
Continued experimentation with simplified microbial communities is needed to better understand the interactions among bacterial species and strains in the context of a shifting vaginal environment. Further standardization of growth conditions, identification of the relevant strains/strain-types for BV and its sequelae, and development of genetic tools will advance our ability to interrogate vaginal bacterial communities in mucosa-free models. Use of microfluidics technology to model the low shear flow of fluid (vaginal secretions mixed with mucus) at the mucosal surface, will improve the physiological relevance of multi-species vaginal biofilm models. Ideally, microfluidic or flow cell devices will one day be used to study the formation of adherent biofilms in a host-free context, as well as on single-cell and/or multiple-cell-layer models of the cervicovaginal mucosa. However, several technological advancements, including fluorescent labeling and genetic modification of bacterial strains, are needed before these experiments would be optimally informative and cost-effective.
In vivo models have had limited success in modeling the complex clinical entity of BV. Most attempts have relied upon single bacterial species (generally G. vaginalis) inoculation of hosts with vaginal microbiota that do not resemble those of humans. Characterization of the specific vaginal microbial communities of model hosts such as mice is a technically feasible next step that would help deepen our understanding of such colonization models and potentially identify naturally occurring dysbioses with similarity to BV. The availability of gnotobiotic mice and the potential to associate such animals with complex bacterial communities corresponding to BV or non-BV states is an exciting frontier in this field. Combining the genetic tractability of the mouse with the potential to humanize the vaginal microbiota may finally provide a system to address lingering questions of BV pathogenesis. Work with new in vivo models should strive to recapitulate complex vaginal microbiota community states observed in humans, in addition to single microbial agents, to advance our understanding of these complex interactions. Effectively modeling the polymicrobial biofilm in vitro will aid in development of new and effective biofilm disruptors and antimicrobials or combinations. Likewise, modeling polymicrobial biofilms in vivo will allow for preclinical evaluation of new biofilm disruptors, antimicrobials, or combinations of the two to allow for translation to clinical trials.
CONCLUSIONS
As described herein, each of the systems currently available to model the vaginal microbiota has both strengths and limitations. The most powerful approach to studying the pathogenesis of BV may require use of multiple models, as has been necessary with other complex diseases. The appropriate model will vary depending on the clinical question being addressed: in vitro models may work well to study the effect of new topical antimicrobials or probiotics on bacterial colonization, but animal models may be better suited to evaluation of the pathogenesis of adverse reproductive health outcomes. In addition, combining these in vitro and in vivo models or using advanced versions of these model systems to tackle the difficult remaining questions may be required to advance the BV field and translational impact of new interventions.
Notes
Supplement sponsorship. This article appears as part of the supplement “Proceedings of the 2015 NIH/NIAID Bacterial Vaginosis Expert Consultation,” sponsored by the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases in partnership with the University of Alabama at Birmingham Sexually Transmitted Infections Clinical Trials Group; contract HHSN272201300012I.
Acknowledgments. We thank the National Institutes of Health (NIH) representatives and organizers of the Sexually Transmitted Infections Clinical Trials Group BV Technical Consultation Team for coordinating this meeting and promoting cross-collaboration on this manuscript.
Potential conflicts of interest. A. J. R. reports that a patent application has been filed by Columbia University for the use of DNase as an antibiofilm therapy in bacterial vaginosis, and he is listed as an inventor on that application. C. M. is a member of the scientific advisory board for Perrigo Pharmaceuticals. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 2005; 353:1899–911. [DOI] [PubMed] [Google Scholar]
- 2.Donders GG, Van Calsteren K, Bellen G et al. . Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG 2009; 116:1315–24. [DOI] [PubMed] [Google Scholar]
- 3.Hillier SL, Nugent RP, Eschenbach DA et al. . Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 1995; 333:1737–42. [DOI] [PubMed] [Google Scholar]
- 4.Martin HL, Richardson BA, Nyange PM et al. . Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999; 180:1863–8. [DOI] [PubMed] [Google Scholar]
- 5.Bradshaw CS, Morton AN, Hocking J et al. . High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006; 193:1478–86. [DOI] [PubMed] [Google Scholar]
- 6.Fettweis JM, Brooks JP, Serrano MG et al. . Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 2014; 160:2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anahtar MN, Byrne EH, Doherty KE et al. . Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015; 42:965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swidsinski A, Mendling W, Loening-Baucke V et al. . An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol 2008; 198:97 e1–6. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan S, Hoffman NG, Morgan MT et al. . Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 2012; 7:e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod 2005; 73:1253–63. [DOI] [PubMed] [Google Scholar]
- 11.Hill DR, Brunner ME, Schmitz DC et al. . In vivo assessment of human vaginal oxygen and carbon dioxide levels during and post menses. J Appl Physiol (1985) 2005; 99:1582–91. [DOI] [PubMed] [Google Scholar]
- 12.Jones NM, Holzman C, Friderici KH et al. . Interplay of cytokine polymorphisms and bacterial vaginosis in the etiology of preterm delivery. J Reprod Immunol 2010; 87:82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chappell CA, Rohan LC, Moncla BJ et al. . The effects of reproductive hormones on the physical properties of cervicovaginal fluid. Am J Obstet Gynecol 2014; 211:226 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod 1997; 57:847–55. [DOI] [PubMed] [Google Scholar]
- 15.Herbst-Kralovetz MM, Quayle AJ, Ficarra M et al. . Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol 2008; 59:212–24. [DOI] [PubMed] [Google Scholar]
- 16.Eade CR, Diaz C, Wood MP et al. . Identification and characterization of bacterial vaginosis-associated pathogens using a comprehensive cervical-vaginal epithelial coculture assay. PLoS One 2012; 7:e50106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fichorova RN, Yamamoto HS, Delaney ML, Onderdonk AB, Doncel GF. Novel vaginal microflora colonization model providing new insight into microbicide mechanism of action. MBio 2011; 2:e00168–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machado A, Salgueiro D, Harwich M, Jefferson KK, Cerca N. Quantitative analysis of initial adhesion of bacterial vaginosis-associated anaerobes to ME-180 cells. Anaerobe 2013; 23:1–4. [DOI] [PubMed] [Google Scholar]
- 19.Ayehunie S, Cannon C, Lamore S et al. . Organotypic human vaginal-ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine-care products. Toxicol In Vitro 2006; 20:689–98. [DOI] [PubMed] [Google Scholar]
- 20.McGowin CL, Popov VL, Pyles RB. Intracellular Mycoplasma genitalium infection of human vaginal and cervical epithelial cells elicits distinct patterns of inflammatory cytokine secretion and provides a possible survival niche against macrophage-mediated killing. BMC Microbiol 2009; 9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose WA 2nd, McGowin CL, Spagnuolo RA, Eaves-Pyles TD, Popov VL, Pyles RB. Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PLoS One 2012; 7:e32728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyles RB, Vincent KL, Baum MM et al. . Cultivated vaginal microbiomes alter HIV-1 infection and antiretroviral efficacy in colonized epithelial multilayer cultures. PLoS One 2014; 9:e93419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunawardana M, Mullen M, Moss JA et al. . Global expression of molecular transporters in the human vaginal tract: implications for HIV chemoprophylaxis. PLoS One 2013; 8:e77340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hjelm BE, Berta AN, Nickerson CA, Arntzen CJ, Herbst-Kralovetz MM. Development and characterization of a three-dimensional organotypic human vaginal epithelial cell model. Biol Reprod 2010; 82:617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radtke AL, Herbst-Kralovetz MM. Culturing and applications of rotating wall vessel bioreactor derived 3D epithelial cell models. J Vis Exp 2012; 62:3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radtke AL, Quayle AJ, Herbst-Kralovetz MM. Microbial products alter the expression of membrane-associated mucin and antimicrobial peptides in a three-dimensional human endocervical epithelial cell model. Biol Reprod 2012; 87:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doerflinger SY, Throop AL, Herbst-Kralovetz MM. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J Infect Dis 2014; 209:1989–99. [DOI] [PubMed] [Google Scholar]
- 28.Barrila J, Radtke AL, Crabbe A et al. . Organotypic 3D cell culture models: using the rotating wall vessel to study host-pathogen interactions. Nat Rev Microbiol 2010; 8:791–801. [DOI] [PubMed] [Google Scholar]
- 29.Turovskiy Y, Sutyak Noll K, Chikindas ML. The aetiology of bacterial vaginosis. J Appl Microbiol 2011; 110:1105–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner HL, Dukes CD. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified non-specific vaginitis. Am J Obstet Gynecol 1955; 69:962–76. [PubMed] [Google Scholar]
- 31.Johnson AP, Ison CA, Hetherington CM et al. . A study of the susceptibility of three species of primate to vaginal colonization with Gardnerella vaginalis. Br J Exp Pathol 1984; 65:389–96. [PMC free article] [PubMed] [Google Scholar]
- 32.Mardh PA, Holst E, Moller BR. The grivet monkey as a model for study of vaginitis. Challenge with anaerobic curved rods and Gardnerella vaginalis. Scand J Urol Nephrol Suppl 1984; 86:201–5. [PubMed] [Google Scholar]
- 33.Yildirim S, Yeoman CJ, Janga SC et al. . Primate vaginal microbiomes exhibit species specificity without universal Lactobacillus dominance. ISME J 2014; 8:2431–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dukes CD, Gardner HL. Identification of Haemophilus vaginalis. J Bacteriol 1961; 81:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDuffie RS Jr, Kunze M, Barr J et al. . Chronic intrauterine and fetal infection with Gardnerella vaginalis. Am J Obstet Gynecol 2002; 187:1263–6. [DOI] [PubMed] [Google Scholar]
- 36.Joo HM, Hyun YJ, Myoung KS et al. . Lactobacillus johnsonii HY7042 ameliorates Gardnerella vaginalis-induced vaginosis by killing Gardnerella vaginalis and inhibiting NF-kappaB activation. Int Immunopharmacol 2011; 11:1758–65. [DOI] [PubMed] [Google Scholar]
- 37.Hymes SR, Randis TM, Sun TY, Ratner AJ. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J Infect Dis 2013; 207:1491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert NM, Lewis WG, Lewis AL. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One 2013; 8:e59539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teixeira GS, Carvalho FP, Arantes RM et al. . Characteristics of Lactobacillus and Gardnerella vaginalis from women with or without bacterial vaginosis and their relationships in gnotobiotic mice. J Med Microbiol 2012; 61:1074–81. [DOI] [PubMed] [Google Scholar]
- 40.Matu MN, Orinda GO, Njagi EN, Cohen CR, Bukusi EA. In vitro inhibitory activity of human vaginal lactobacilli against pathogenic bacteria associated with bacterial vaginosis in Kenyan women. Anaerobe 2010; 16:210–5. [DOI] [PubMed] [Google Scholar]
- 41.Pybus V, Onderdonk AB. Evidence for a commensal, symbiotic relationship between Gardnerella vaginalis and Prevotella bivia involving ammonia: potential significance for bacterial vaginosis. J Infect Dis 1997; 175:406–13. [DOI] [PubMed] [Google Scholar]
- 42.Geshnizgani AM, Onderdonk AB. Defined medium simulating genital tract secretions for growth of vaginal microflora. J Clin Microbiol 1992; 30:1323–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian Y, He X, Torralba M et al. . Using DGGE profiling to develop a novel culture medium suitable for oral microbial communities. Mol Oral Microbiol 2010; 25:357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kieweg SL, Katz DF. Squeezing flows of vaginal gel formulations relevant to microbicide drug delivery. J Biomech Eng 2006; 128:540–53. [DOI] [PubMed] [Google Scholar]
- 45.Alves P, Castro J, Sousa C, Cereija TB, Cerca N. Gardnerella vaginalis outcompetes 29 other bacterial species isolated from patients with bacterial vaginosis, using in an in vitro biofilm formation model. J Infect Dis 2014; 210:593–6. [DOI] [PubMed] [Google Scholar]