Abstract
Background
Aging is associated with decline in executive function (EF), upper-level cognitive abilities such as planning, problem solving, and working memory (WM). This decline is associated with age-related volume loss and reduced functional connectivity in the frontal lobes. Cognitive training interventions aim to counter these losses but often fail to elicit benefits beyond improvements on trained tasks. Recent interventions pairing WM training with transcranial direct current stimulation (tDCS) have improved WM and elicited transfer to untrained EF tasks. Limitations in previous work include exclusive use of laboratory-based computer training and testing and poor characterization of the mechanism(s) of durable tDCS-linked change.
Objective/Hypothesis
To determine if tDCS-linked WM training improves performance on ecologically valid transfer measures administered in participants’ homes. To explore intervention-based changes using neuroimaging (fNIRS) and genotyping (COMT val158met).
Methods
90 healthy older adult participants completed 5 sessions of WM training paired with tDCS (Sham, 1 mA tDCS, 2 mA tDCS; 15 min). At follow-up, we assessed performance change on laboratory-based and ecologically valid tasks.
Results
All participants showed improvement on trained tasks. Importantly, 2 mA of tDCS induced significantly greater far transfer gains after 1 month without contact. Gains were observed on standard far transfer tasks along with ecologically valid far transfer tasks, and stimulation was well tolerated by all participants. FNIRS and genotyping results were less conclusive but provide promising avenues for future research initiatives.
Conclusion
These findings highlight the translational value for tDCS-based interventions in healthy older adults interested in maintaining cognitive function.
Keywords: Working Memory, TDCS, Aging, Cognition, COMT
Introduction
Anyone struggling to straighten age-stiffened knees can attest to physical consequences of aging. More distressing are cognitive changes, especially those related to executive functions (EF) such as problem solving, decision-making, working memory (WM), and planning [1]. These higher functions are needed for completing everyday activities and for maintaining independence with age. Neurally, EF tasks rely on frontal lobe structures that are particularly susceptible to age-related gray matter volume loss and reduced functional connectivity [2–4]. Consequently, EF decline can impair older adults’ ability to participate in daily activities. For example, age-related decline can impair older adults’ ability to navigate, problem solve, and brake while driving [5–7].
Given the growing aging population, there is great interest in developing effective approaches to counter age-related cognitive decline. Working memory (WM) is targeted in cognitive interventions because it is an EF with strong links to cognitive abilities like fluid intelligence [8]. Ideally, WM training should improve performance on trained WM tasks, and these benefits should extend to other WM tasks and to EF in general [9, 10]. Commercial interventions involving working memory (WM) training are well-advertised, but outcomes are not compelling [11]. Furthermore, although WM training studies consistently improve performance on trained tasks, there is inconsistent improvement on untrained, or transfer, tasks [12], particularly in older adults [13]. Two forms of transfer, near and far, reflect the transfer task’s similarity to training tasks. Far transfer gains represent global cognitive improvement and are tremendously elusive. Few researchers test for far transfer or report small effects [14]. Finally, most testing occurs in laboratories using tasks with little everyday relevance [15].
One experimental technique with growing translational promise is transcranial direct current stimulation (tDCS). Acutely, tDCS modulates resting potentials in underlying neural populations with anodal (positive) current associated with improved learning, for review: [16]. Longitudinally, tDCS may enhance cognition by improving cortical efficiency [17], and/or by encouraging neuroplasticity in activated networks [16], and/or by modulating dopamine (DA) signaling [18, 19]. Genetics play a role with single nucleotide polymorphisms predicting differential responses to tDCS. For example, the COMT val158met genotypes differentially modulate DA signaling in prefrontal cortex (PFC), and demonstrate dissimilar responses to tDCS. Specifically, Met/Met carriers exhibit worse cognitive flexibility following anodal tDCS [19]. An enhanced understanding of tDCS mechanism in PFC will improve tDCS protocol development.
Pairing WM training with tDCS can extend and expand training gains [20–22]. Previously, we reported that healthy older adults benefited from ten sessions of WM training + tDCS. Anodal tDCS to frontoparietal sites including the right PFC induced sustained gains in trained tasks after one month without contact. Importantly, anodal tDCS significantly improved performance on near transfer tasks [20]. However, we exclusively relied on computer-based tasks with low ecological validity. Other challenges to optimizing tDCS-linked cognitive interventions include substantial variability across tDCS protocols [23]. For example, in determining the ideal tDCS intensity we are only aware of work in young adults showing that 1mA elicited greater cognitive improvement than higher intensities [24]. Conversely, in some patient populations, higher tDCS intensity (i.e. 2mA) appears to be more beneficial [25–27]. Presently, we are unaware of research clarifying optimal tDCS intensity for healthy older adults. Other factors, such as education level, also influence their response to tDCS [28] and should be considered.
For translational purposes, the underlying mechanism(s) of tDCS induced cognitive improvements in older adults must be clarified. This project begins to address these gaps. First, we paired WM training with parametric anodal tDCS to the PFC (low (1 mA), high (2 mA) intensity) and evaluated far transfer to ecologically valid tasks. We investigated PFC changes using functional near infrared spectroscopy (fNIRS). Neuroimaging has identified cortical changes after cognitive training in healthy older adults [29], and we evaluated whether WM training + tDCS training elicited comparable changes. FNIRS is less expensive, with fewer exclusionary criteria making it appropriate for measuring effects of WM training + tDCS from most participants. Finally, we assessed the interaction of tDCS with a polymorphism in the COMT gene that dictates dopamine signaling in the PFC. Behaviorally, we expected parametric improvements on trained and transfer tasks with increased tDCS intensity and gains on everyday far transfer tasks performed at home. Mechanistically, we predicted that fNIRS would show different PFC engagement as a function of training group. Finally, we hypothesized that COMT genotype may predict differential effects depending on the task demands. COMT genotype predicts different behavioral profiles during EF tasks; individuals carrying a met allele have greater cognitive stability while individuals carrying a Val allele COMT genotype possess greater cognitive flexibility [30].
Materials & Methods
Participants
Ninety healthy older adults were randomly assigned membership into 3 gender-, age-, and education- matched groups of 30 (Sham: 16 females, age: 69.9 years, education: 15.23 years; Active1: 16 females, age: 68.6, education: 15.78 years; Active2: 17 females, age: 68.6, education: 15.73 years). Screening excluded anyone with a history of neurological or psychiatric diseases, seizure disorders, pacemakers, pregnancy, and current prescriptions for anti-psychotic, hypnotic, or sedative medications. We also used a brief assessment of cognitive status, the Mini Mental Status Exam (MMSE; [31]) and included individuals who scored >22. The groups were matched for health status (assessed by SF–36; [32]) and demographics (all p’s > 0.287). TDCS intensity was manipulated between groups (Sham: placebo, Active1: 1 mA, Active2: 2 mA). Following each session, participants completed a questionnaire that assessed subjective experience of side effects; none of the participants reported any significant tDCS side effects. Participants signed informed consent documents approved by the University Institutional Review Board. Participants received financial reimbursement ($15/hr). No participants left the study or missed any sessions.
Study Design
Participants’ 1st session occurred at home, followed by a 5-day lab-based intervention, a month of no contact, a post-intervention lab visit, and a post-intervention home visit; see Figure 1. At the 1st home visit participants completed standard far transfer tasks (processing speed, arithmetic, cognitive flexibility). At the post-intervention home visit participants repeated these tasks and completed new ecologically valid far transfer tasks that did not have test/re-test options (Weekly Calendar Planning Activity (WCPA; [33]) and Road Law & Road Craft Test, (OT-DORA; [34]). The WCPA assessed participants’ ability to follow rules while scheduling appointments amid distractions. The OT-DORA assessed participants’ driving knowledge (e.g. what steps are involved in changing lanes?), safety awareness (e.g. what can affect a person’s driving ability?) and route planning ability. We characterize these two measures as ecologically valid because the tasks mirror activities individuals complete in everday life (e.g. scheduling an appointment) in their natural environment (e.g. at the kitchen table).
Figure 1. Study Design.
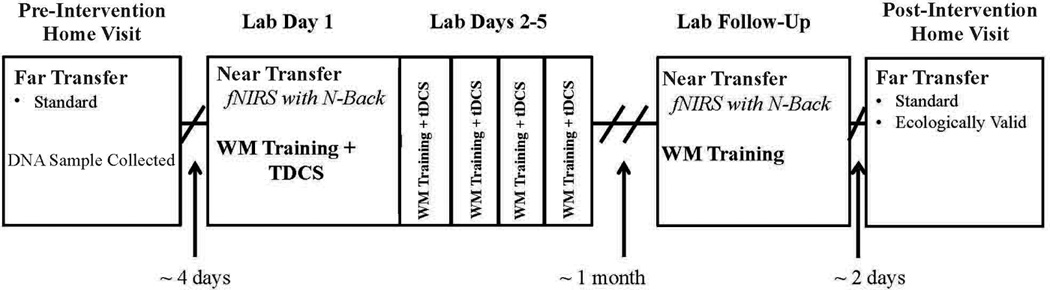
Participants completed standard and ecologically valid far-transfer tasks during preliminary and follow-up home visits. DNA samples were collected during the preliminary home visit. Participants completed near transfer tasks on the first lab visit and follow-up lab visit; the n-Back task was completed during functional near infrared spectroscopy (fNIRS) recording. Participants received active or sham transcranial direct current stimulation (tDCS) during each lab visit, except the final lab visit. At all lab visits, participants completed working memory (WM) training tasks. Events are presented in the figure from top to bottom in the order they were administered.
During the 1st lab and post-intervention lab visits, participants completed 2 near transfer tasks: a Letter Span verbal WM task and the n-Back visual WM task during fNIRS. In the Letter Span task, participants heard a series of letters and were instructed to increment each presented letter (e.g. “A” becomes “B”) and repeat the manipulated series in order. We used a block design version of the n-Back visual WM task appropriate for fNIRS, modifying our original study [20]. Participants completed 16 pseudo-randomized blocks of 0-back (control task – participants pressed a button for centrally located targets) and 2-Back (WM task – participants identified targets matching items presented 2 trials prior). Each block consisted of 14 trials and lasted 21 seconds. There were 9 seconds of rest between blocks.
Participants received tDCS during lab visits 1–5 and performed the WM training tasks. Participants also completed training tasks on the follow-up lab visit without tDCS. The WM training tasks included a subtract 2 span [35], automated Operation Span (O-SPAN; [36]), and a spatial and visual WM task designed in our lab and used in previous work [20]. In the spatial recall task, 5 items were presented (3° visual angle, 200 ms.) and after a delay (4000 ms.), 12 images appeared. Participants clicked on the locations occupied during stimulus encoding. In the visual recall task, 5 items were presented on a computer monitor (3° visual angle, 2000 ms.) and after a delay (500 ms.), 16 items appeared. Participants selected the 5 items in the stimulus array. Both tasks had infinite time for participant response. During training sessions (lasting approximately 45 minutes) participants completed 2 blocks of each task types with 25 trials per block; blocks were presented in a counterbalanced order.
One challenge with these data was that there were a number of tasks included in each condition (trained, near and far transfer). To provide a measure of performance change for each condition, we made use of separate indices to reflect the overall change in performance. This allowed us to collapse across performance for the tasks in each condition, thereby reducing the multiple comparisons problem, and leveraging our limited power to the question of primary interest: would there be training and transfer effects after the intervention? Behavioral performance for ecologically valid tasks was represented as a total score to represent aggregate performance across the measure. For all other measures, performance was represented as a composite benefit index for each task type (training tasks, near transfer task, and standard far transfer tasks)1. These indexes represent performance change from baseline performance (pre-intervention) to follow-up (post-intervention) performance. This calculation reduces baseline differences (although none existed; see Supplemental Tables 1–3) and is used in other training studies to capture collective behavioral changes [37–39].
FNIRS Protocol
FNIRS (TechEn CW6 fNIRS System, Milford, MA) recorded bilateral PFC (F3 and F4; [40]. Data were collected during n-Back blocks (5 s ramp up, 30 s task, 50 Hz sampling rate). Off-line data pre-processing (HomER 2; [41]) included low pass filtering (.5 Hz) to eliminate biological noise. Trial blocks were excluded if significant motion artifacts were detected (Δ in signal of +/− 50+ standard deviations from baseline) within 2 seconds of stimulus onset. We focused on oxygenated hemoglobin (HbO), instead of deoxygenated hemoglobin, as HbO has a better signal-to-noise ratio and greater sensitivity to blood flow changes [42–45]. For each participant, we collected a baseline normalized peak amplitude HbO value during n-Back performance for each channel. We compared pre- and post- activations in each channel of left and right PFC using a formula representing change over time: (Peak Amp. Post – Peak Amp. Pre)/(Peak Amp. Post + Peak Amp. Pre). This formula produced either a positive or negative value for each channel, representing increases (+ values) or decreases (− values) in regional recruitment over time. For each participant, we calculated the percentage of channels with decreased activation over time. We used a percentage of channels for each participant because we were unable to collect data from all 14 channels in every participant due to occasional individual channel signal loss (e.g. due to thick hair). Data were included from participants (N = 67) where more than half of the fNIRS channels per hemisphere were available (i.e. 4+ channels), and the number of unused channels was not different between the tDCS groups.
TDCS Protocol & Current Flow Modeling
TDCS timing (i.e. administered during practice) and electrode placement (i.e. right PFC) emulated our previous study in this population [20]. Participants received sham or anodal tDCS to the right PFC (F4; [40]) for 15 minutes (1 mA, 2 mA) using a constant current stimulator (Eldith MagSteim, GmbH, Ilmenau, Germany) while completing practice trials of the WM training tasks. Current was delivered through two 5 × 7 cm2 electrodes encased in saline-soaked sponges, and we secured the reference electrode on the contralateral cheek with elastic straps [28, 46–51]. Participants were informed that they were receiving tDCS but were blind to their stimulation group. All participants reported experiencing the subjective sensation of stimulation (e.g. tingling, itching), as sham supplies brief (20 s) stimulation to preserve condition masking. We elected not to include a tDCS-only control group, as tDCS is effective when administered during a task (Filmer et al., 2014). We modeled current flow using HD-Explore™/tDCS-Explore™ software (Soterix Medical Inc., New York, New York). Using a generic adult head model, we provided tDCS intensity (1, 2mA) and the anodal and cathodal electrode sites. The software determined the field intensity (V/m) across the brain.
Genotyping Procedure
89 participants (1 sample was insufficient) provided DNA for COMT val158met (rs4680) genotyping, which was completed off site (GenoTek Labs, UT) using standard procedures (http://www.dnagenotek.com/US/genomicservices/genofind.html). Consistent with the Hardy-Weinberg equilibrium (χ2 = .371; p = .543), there were: 20 Val/Val; 45 Met/Val; 24 Met/Met participants. As we learned participants’ genotype after training, COMT genotype was unequally distributed across tDCS groups. Two tasks were analyzed with the factor of COMT genotype: a cognitive stability task, the 2-Back [52], and a cognitive flexibility task, the Go/No-Go task [53].
Data Analysis
Analyses were conducted in SPSS. Degrees of freedom were modified as needed using Greenhouse-Geisser corrections and post-hoc pairwise comparisons were Bonferroni corrected for multiple comparisons.
Results
Baseline Performance
All three groups performed similarly on all tasks during the initial baseline tests. Performance at baseline for each task was subjected to a one-way repeated measures ANOVA and training tasks (all p’s > .29), near transfer (both p’s > .18), or far transfer tasks (all p’s > .12) were similar across groups; see Supplemental Tables 1–3.
TDCS-Linked Improvement
To test whether tDCS group (Sham, Active1, Active2) affected improvement on training (verbal, visual WM tasks), near transfer (n-Back visual WM task, Letter Span verbal WM task), and far transfer tasks (standard tests: processing speed, cognitive flexibility, arithmetic; ecologically valid tests: WCPA, OT-DORA), the composite benefit indexes for trained, near transfer, and far transfer tasks were subjected to separate one-way ANOVAs. There were no group differences in the WM training (F(2, 89) = .201, p = .819, partial η2= .047), or near transfer tasks (F(2, 89) = 1.645, p = .199, partial η2= .037). There was a numerical trend showing greater benefits for the Active2 tDCS group on near transfer tasks; see Supplemental Table 2.
Importantly, for standard (represented as a composite benefit index) and ecologically valid (represented as a total score for each task) far transfer tasks there were significant group differences such that stronger tDCS during training induced transfer; see Figure 2. The Active2 group significantly outperformed the Sham group on standard far transfer tasks (F(2, 87) = 3.33, p = .041, partial η2= .073); see Figure 2A. The Active2 group showed significantly greater far transfer compared to the Sham group; see Supplemental Table 3. The Active1 group was intermediate with numerically smaller gains than the Active2 group and numerically greater gains than the Sham group. Next, we explored the effects of tDCS and training on ecologically valid far transfer tasks. For the WCPA, significant group differences (F(2, 89) = 5.512, p = .006, partial η2= .116) reflected dose-dependent tDCS effects in which the Active2 (M = 13.036, SE = 0.444) group performed significantly better than the Sham group (M = 10.207, SE = 0.718, p = .004), but neither Active2 nor Sham was significantly different from the Active1 group (M = 11.900, SE = 0.601, vs. Active2: p =. 558: vs. Sham: p =. 144); see Figure 2B. For the OT-DORA task there was also a significant main effect of group (F(2,89) = 6.120, p =.003, partial η2= .123), reflecting significant differences between active tDCS groups (Active1: M = 32.433, SE = 0.406; Active2: M = 32.000, SE = 0.450) and Sham (M = 29.833, SE = 0.764; p =.005. and p =.024, respectively), but no difference between the Active2 and Active1 groups, (p =1.000); see Figure 2C. Generally, stronger tDCS during WM training resulted in superior performance on standard and ecologically valid far transfer tasks at follow up.
Figure 2. TDCS-Linked Far Transfer Benefits.
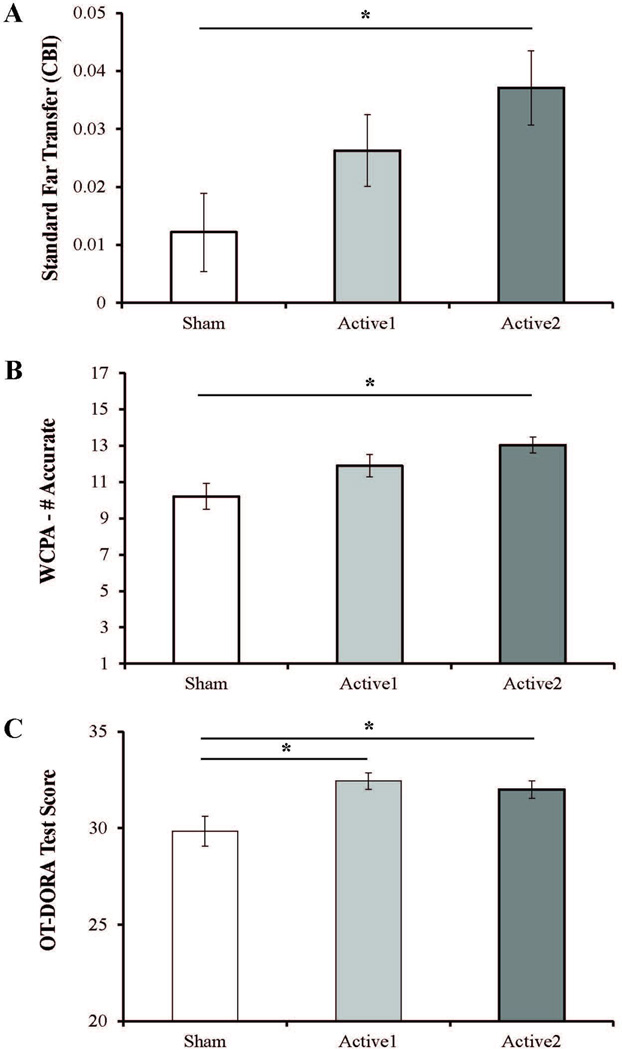
Standard Far Transfer Tasks (A) The CBI (composite benefit index) represents a collective change in performance averaged across 3 standard far transfer tasks with re-test option (Go/No-Go, Functional Math, WAIS Coding). Significant group differences were observed. Active2 had a significantly higher CBI than Sham. No significant differences were observed between Active2 and Active1 or between Active1 and Sham. Ecologically Valid Far Transfer Tasks. (B–C) (B) Significant group differences were observed on the WCPA (max score = 17). Active2 had significantly higher accuracy than Sham. No significant differences were observed between Active2 and Active1 or between Active1 and Sham. (C). Significant group differences were observed on the OT-DORA (max score = 37). Both Active1 and Active2 significantly out-performed Sham. No significant differences were observed between Active1 and Active2. (A–C) Positive values indicate improved performance Error bars represent standard error of the mean; * indicates p<0.05
Intensity-Dependent Effects of tDCS in Older Adults
To better understand dose-dependent tDCS effects, we modeled current flow for the Active1 and Active2 groups; see Figure 3. The model indicates that the Active1 group experienced lower field intensity in the right PFC with little global change; see Figure 3A. Conversely, the Active2 group experienced higher field intensity in right PFC accompanied by broad current reaching left PFC regions and posterior right hemisphere regions; see Figure 3B. These models provide plausible depictions of tDCS current flow across stimulation intensities. Individual head models per participant would strengthen interpretive power. The Active2 group likely experienced higher field intensity and flow density in right PFC regions potentially leading to superior far transfer in an older adult population compared to 1 mA or sham stimulation.
Figure 3. Current Flow Modeling.
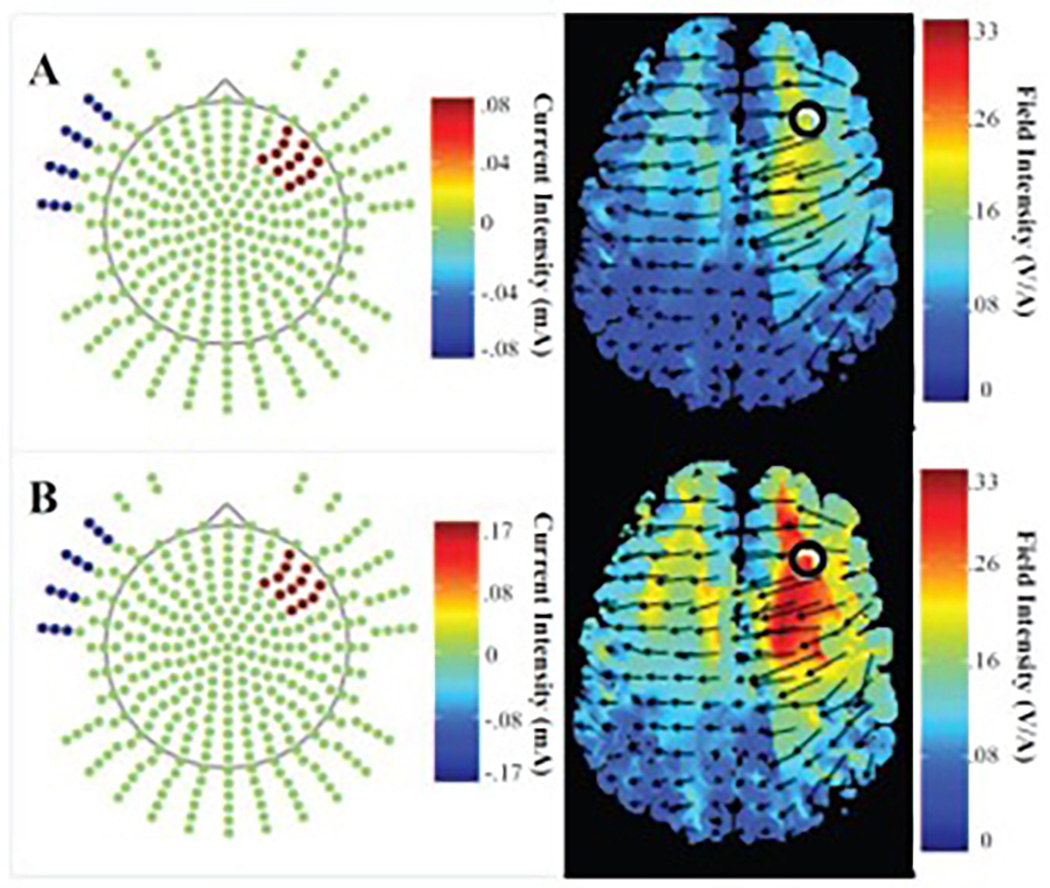
Field intensity (V/m) is lower in right PFC after 1mA of tDCS (A) compared to 2 mA of tDCS (B). 2 mA of tDCS also results in a greater spread of current flow to left hemisphere and posterior right hemisphere regions.
TDCS Mechanism: fNIRS in bilateral PFC
To investigate if WM training + tDCS prompted lasting changes in PFC activity, we examined the relationship between hemodynamic response (HbO) and behavioral performance of 2-Back visual WM task. There was a significant positive relationship between percentage of channels with decreased activation in right PFC and good performance on the 2-Back task (r (64) = .279, p = .026); see Figure 4. However, this did not interact with tDCS status (F (2,64) = .658, p =.521, partial η2= .021)
Figure 4. 2-Back Performance & R. PFC Activation.
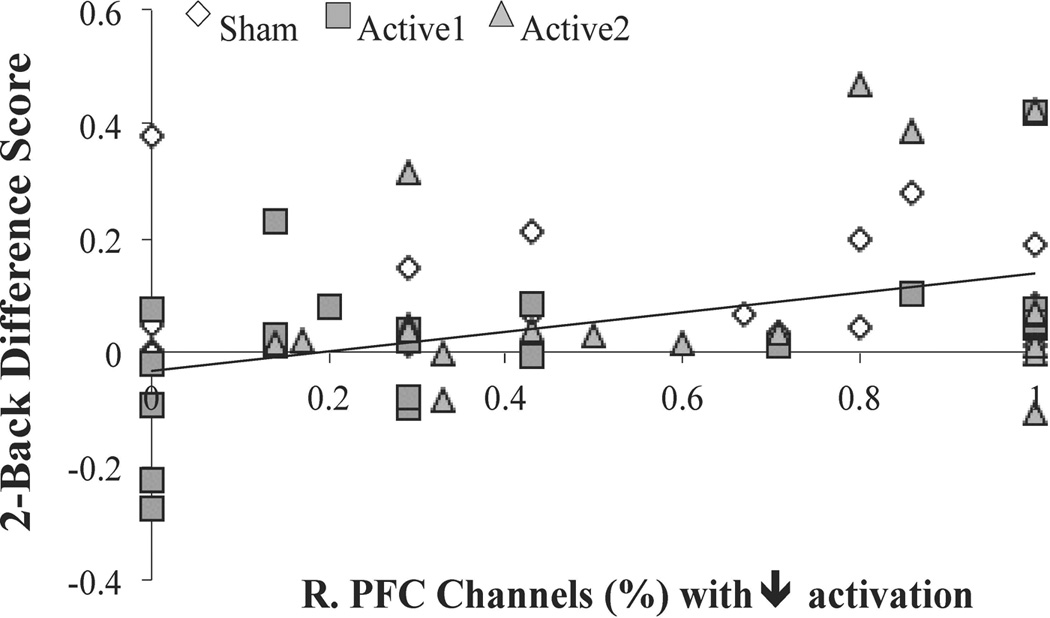
We observed a significant positive correlation between 2-Back performance (represented as change in performance over time) and the percentage of channels in R. PFC with decreased activations. This relationship was observed irrespective of tDCS condition.
TDCS Mechanism: Genetic Influences
To capture how tDCS interacts with the common COMT val158met single point mutation we examined change (post – pre training performance) on tasks measuring cognitive flexibility and stability in conjunction with COMT genotype. Due to small N per cell, we used non-parametric Kruskal-Wallis H tests (tDCS group × COMT genotype; smallest cell N = 5). The measure of cognitive stability was the 2-Back task and the measure of cognitive flexibility was the Go/No-Go task. Baseline performance on neither task was significantly different between tDCS × COMT groups, and no significant group differences were observed following WM training + tDCS (p values >.05).
Discussion
Older adults are cognitively vulnerable. Interventions improving everyday cognition are in short supply, and those currently available show little wide-reaching lasting benefit [11]. We used WM training + tDCS in a population of healthy older adults and found that everyone improved on trained tasks, regardless of tDCS group. This finding confirms an effective WM training protocol. TDCS induced greater benefits when the transfer tasks were examined. The near transfer task revealed a behavioral trend indicating that participants who received 2 mA of tDCS had better performance on the near transfer tasks. We suspect that changing the 2-Back from our previously successful design [20], to a blocked design to accommodate fNIRS, may have meaningfully altered the task. In contrast to our previous work, a substantial number of participants (47%) performed at ceiling (> 90% accuracy) or floor (below chance) on the 2-Back block design during their 1st laboratory session; this limited our ability to measure performance change. Of notable interest, participants in the Active2 group performed significantly better on standard and ecologically valid far transfer tasks performed at their homes. As noted previously [20], far transfer benefits were identified after one month of no contact or stimulation. This indicates that far transfer can be elicited by pairing WM training with tDCS. It also clarifies that stronger intensity stimulation led to greater effects, in contrast to some work in younger adults showing some protocols elicit strongest benefits with lower intensity tDCS (e.g., 1 mA; [24]), but consistent with studies demonstrating WM gains following longer stimulation duration and larger current density [54]. Additionally, higher intensity tDCS at PFC may be essential for older adults, as frontal lobes are particularly susceptible to gray-matter atrophy [3].
Our protocol was so well tolerated by our participants that we had complete participant compliance (zero dropouts in 720 sessions). This novel and successful experimental design supports the use of WM training + 2 mA tDCS in older adults to promote sustained everyday cognitive ability. The far transfer benefits extends our previous observation of long lasting near transfer benefits after WM training paired with 1.5 mA tDCS [20]. In other words, WM training + sufficient tDCS can elicit generalizable improvements in tasks relevant to everyday life.
To better tailor tDCS protocols the underlying mechanism requires further clarification. Here, we examined the possible influence of a common polymorphism in the COMT gene (val158met) that determines tonic DA signaling in the PFC [55–60] and moreover predicts WM performance [30]. We did not observe significant interactions between COMT val158met genotype and tDCS on behavioral performance, as the combination of tDCS group + COMT status rendered very small groups. Larger study groups would likely elucidate an interaction, as previous research has demonstrated that individual differences may predict WM improvement after tDCS. Specifically, we hypothesize that tDCS may further enhance predisposed WM abilities – improving cognitive flexibility in Val carriers and improving cognitive stability in Met carriers. This hypothesis is consistent with our previous findings that tDCS benefited older adults with greater education [28] and young adults with greater WM capacity [28]. In essence, tDCS seems to strengthen existing skills, making the rich richer. However, this is not incontrovertible. Low WM capacity young adults benefited from tDCS when additional financial incentive was supplied [66]. Therefore, it may be possible to enhance cognition across heterogeneous groups of participants when group-appropriate ‘tweaks’ are provided. This knowledge will be needed for a full range of cognitive skills, and age groups before tDCS can serve a wide translational role in cognitive maintenance.
We acknowledge limitations that could limit the utility of tDCS for translational practice. We were unable to replicate our previous findings where tDCS elicited both greater training and transfer gains [20]. As noted above, the modification to the 2-Back, may have clouded the near transfer effects, but the lack of training effects is curious. We shortened the training duration by 5 days and used difficult WM training tasks. Perhaps, with more training days (e.g. 10), we would have observed training benefits in the Active2 group. In the present study, we achieved our objective of eliciting far transfer effects with 2mA of tDCS. However, in studies where training gains are sought, researchers may consider longer training. We also anticipated seeing differential change, via fNIRS, associated with 2mA of tDCS, but fNIRS results were not different between tDCS groups. Full brain coverage from fMRI or HD-EEG measurements would have been more informative, but older adults are not always able or willing. Future work will permit observation of temporal components in cortical activations or even changes in network connectivity specific to the 2mA group. Neuroimaging data would also enhance the understanding of anatomic specificity of tDCS effects. Our study was somewhat limited in using only one stimulation site, although in previous work, we had consistent behavioral findings with multiple stimulation sites [20]. We encourage future research to incorporate numerous neuroimaging methods to better clarify the causal effects of neuromodulation and cognitive training.
Conclusions
Aging brings cognitive changes most of us wish to delay. WM training combined with tDCS is an effective intervention that improves cognitive performance across a range of EF tasks and is not associated with substantial unpleasant side effects. This approach may be useful to address the long-term goal of maintaining cognitive performance as we age. This research has implications for both basic and applied science. Pairing tDCS with cognitive training propagated improvement on meaningful far transfer tasks. We suggest that tDCS and training interventions could be employed when healthy older adults are seeking to improve or maintain functional WM ability, e.g., beginning to notice cognitive changes. This project also adds to the growing field of tDCS-based cognitive enhancement. Given the current findings and our previous research, an effective tDCS design for healthy older adults should include 2 mA of tDCS to the PFC combined with appropriate training tasks (e.g., WM). Furthermore, the interaction we expected, but did not observe, between COMT and tDCS-linked behavioral gains may explain certain tDCS-linked training benefits. Future research should continue to explore individual differences and cortical changes following WM training + tDCS interventions. Combined approaches could facilitate individually tailored paradigms for individuals with specific cognitive concerns. The questions arising now are to determine how far far transfer can reach, for how long it can last, and what parameters provide the greatest benefit on an individual basis.
Supplementary Material
Highlights for Review.
We used tDCS & working memory (WM) training in a healthy older adult population.
We tested for training gains and everyday task improvement in participants’ homes.
Everyday task improvement was only observed in adults who received 2mA of tDCS.
These improvements were apparent 1 month after tDCS & working memory training.
Acknowledgments
This work was funded by NEI R15EY022775 (M.E.B), and NIGMS IDeA Grant 1P20GM103650 (Project 1 Leader M.E.B.) and by support from the Bilinski Foundation (J.A.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data from all participants were included in these calculations, with the following exception. The processing speed task was completed by hand. 2 participants had outlier performance due to fine motor deficits (e.g. arthritis). When we removed these data from the far transfer composite benefit index, our results were not altered.
References
- 1.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44(2):259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 4.Verwer RW, Baker RE, Boiten EF, Dubelaar EJ, van Ginkel CJ, Sluiter AA, et al. Post-mortem brain tissue cultures from elderly control subjects and patients with a neurodegenerative disease. Exp Gerontol. 2003;38(1–2):167–172. doi: 10.1016/s0531-5565(02)00154-7. [DOI] [PubMed] [Google Scholar]
- 5.Anstey KJ, Horswill MS, Wood JM, Hatherly C. The role of cognitive and visual abilities as predictors in the Multifactorial Model of Driving Safety. Accident; analysis and prevention. 2012;45:766–774. doi: 10.1016/j.aap.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Anstey KJ, Wood J, Lord S, Walker JG. Cognitive, sensory and physical factors enabling driving safety in older adults. Clinical psychology review. 2005;25(1):45–65. doi: 10.1016/j.cpr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Engstrom J, Aust ML, Vistrom M. Effects of working memory load and repeated scenario exposure on emergency braking performance. Human factors. 2010;52(5):551–559. doi: 10.1177/0018720810381072. [DOI] [PubMed] [Google Scholar]
- 8.Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. Journal of experimental psychology. General. 1999;128(3):309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- 9.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(19):6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klingberg T. Training and plasticity of working memory. Trends in cognitive sciences. 2010;14(7):317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS, et al. Putting brain training to the test. Nature. 2010;465(7299):775–778. doi: 10.1038/nature09042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shipstead Z, Redick TS, Engle RW. Is working memory training effective? Psychological bulletin. 2012;138(4):628–654. doi: 10.1037/a0027473. [DOI] [PubMed] [Google Scholar]
- 13.von Bastian CC, Langer N, Jancke L, Oberauer K. Effects of working memory training in young and old adults. Mem Cognit. 2013;41(4):611–624. doi: 10.3758/s13421-012-0280-7. [DOI] [PubMed] [Google Scholar]
- 14.Karbach J, Verhaeghen P. Making working memory work: a meta-analysis of executive-control and working memory training in older adults. Psychol Sci. 2014;25(11):2027–2037. doi: 10.1177/0956797614548725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison AB, Chein JM. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychonomic bulletin & review. 2011;18(1):46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- 16.Filmer HL, Dux PE, Mattingley JB. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 2014;37(12):742–753. doi: 10.1016/j.tins.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Meinzer M, Lindenberg R, Sieg MM, Nachtigall L, Ulm L, Floel A. Transcranial direct current stimulation of the primary motor cortex improves word-retrieval in older adults. Front Aging Neurosci. 2014;6:253. doi: 10.3389/fnagi.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitsche MA, Kuo MF, Grosch J, Bergner C, Monte-Silva K, Paulus W. D1-receptor impact on neuroplasticity in humans. J Neurosci. 2009;29(8):2648–2653. doi: 10.1523/JNEUROSCI.5366-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plewnia C, Zwissler B, Langst I, Maurer B, Giel K, Kruger R. Effects of transcranial direct current stimulation (tDCS) on executive functions: influence of COMT Val/Met polymorphism. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49(7):1801–1807. doi: 10.1016/j.cortex.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Jones KT, Stephens JA, Alam M, Bikson M, Berryhill ME. Longitudinal neurostimulation in older adults improves working memory. PLoS One. 2015;10(4):e0121904. doi: 10.1371/journal.pone.0121904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SH, Seo JH, Kim YH, Ko MH. Long-term effects of transcranial direct current stimulation combined with computer-assisted cognitive training in healthy older adults. Neuroreport. 2014;25(2):122–126. doi: 10.1097/WNR.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 22.Richmond LL, Wolk D, Chein J, Olson IR. Transcranial direct current stimulation enhances verbal working memory training performance over time and near transfer outcomes. J Cogn Neurosci. 2014;26(11):2443–2454. doi: 10.1162/jocn_a_00657. [DOI] [PubMed] [Google Scholar]
- 23.Berryhill ME, Peterson DJ, Jones KT, Stephens JA. Hits and misses: leveraging tDCS to advance cognitive research. Front Psychol. 2014;5:800. doi: 10.3389/fpsyg.2014.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoy KE, Emonson MR, Arnold SL, Thomson RH, Daskalakis ZJ, Fitzgerald PB. Testing the limits: Investigating the effect of tDCS dose on working memory enhancement in healthy controls. Neuropsychologia. 2013;51(9):1777–1784. doi: 10.1016/j.neuropsychologia.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson's disease. Journal of the neurological sciences. 2006;249(1):31–38. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 26.Brunoni AR, Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Boggio PS, et al. Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Progress in neuropsychopharmacology & biological psychiatry. 2011;35(1):96–101. doi: 10.1016/j.pnpbp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, et al. Improved naming after transcranial direct current stimulation in aphasia. Journal of neurology, neurosurgery, and psychiatry. 2008;79(4):451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- 28.Berryhill ME, Jones KT. tDCS selectively improves working memory in older adults with more education. Neuroscience letters. 2012;521(2):148–151. doi: 10.1016/j.neulet.2012.05.074. [DOI] [PubMed] [Google Scholar]
- 29.Brehmer Y, Rieckmann A, Bellander M, Westerberg H, Fischer H, Backman L. Neural correlates of training-related working-memory gains in old age. Neuroimage. 2011;58(4):1110–1120. doi: 10.1016/j.neuroimage.2011.06.079. [DOI] [PubMed] [Google Scholar]
- 30.Versace M, Zorzi M. The role of dopamine in the maintenance of working memory in prefrontal cortex neurons: input-driven versus internally-driven networks. International journal of neural systems. 2010;20(4):249–265. doi: 10.1142/S0129065710002401. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 33.Toglia J. Weekly Calendar Planning Activity: A Performance Test of Executive Function. AOTA Press; 2015. p. 176. [Google Scholar]
- 34.Unsworth CA, Pallant JF, Russell KJ, Odell M. Driver Off-Road Assessment Battery. American Occupational Therapy Association (AOTA) Press; 2011. [Google Scholar]
- 35.Kausler DH, Salthouse TA, Saults JS. Temporal memory over the adult lifespan. Am J Psychol. 1988;101(2):207–215. [PubMed] [Google Scholar]
- 36.Unsworth N, Heitz RP, Schrock JC, Engle RW. An automated version of the operation span task. Behav Res Methods. 2005;37(3):498–505. doi: 10.3758/bf03192720. [DOI] [PubMed] [Google Scholar]
- 37.Borella E, Carretti B, Cantarella A, Riboldi F, Zavagnin M, De Beni R. Benefits of training visuospatial working memory in young-old and old-old. Dev Psychol. 2014;50(3):714–727. doi: 10.1037/a0034293. [DOI] [PubMed] [Google Scholar]
- 38.Carretti B, Borella E, Fostinelli S, Zavagnin M. Benefits of training working memory in amnestic mild cognitive impairment: specific and transfer effects. Int Psychogeriatr. 2013;25(4):617–626. doi: 10.1017/S1041610212002177. [DOI] [PubMed] [Google Scholar]
- 39.Noack H, Lovden M, Schmiedek F, Lindenberger U. Cognitive plasticity in adulthood and old age: gauging the generality of cognitive intervention effects. Restorative neurology and neuroscience. 2009;27(5):435–453. doi: 10.3233/RNN-2009-0496. [DOI] [PubMed] [Google Scholar]
- 40.Jasper H. Report of the committee on methods of clinical examination in electroencephalography: 1957. Electroencephalography and clinical neurophysiology. 1958;10(2):370–375. [Google Scholar]
- 41.Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt. 2009;48(10):D280–D298. doi: 10.1364/ao.48.00d280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoshi Y. Functional near-infrared spectroscopy: current status and future prospects. J Biomed Opt. 2007;12(6):062106. doi: 10.1117/1.2804911. [DOI] [PubMed] [Google Scholar]
- 43.Hoshi Y, Kobayashi N, Tamura M. Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol. 2001;90(5):1657–1662. doi: 10.1152/jappl.2001.90.5.1657. (1985) [DOI] [PubMed] [Google Scholar]
- 44.Plichta MM, Heinzel S, Ehlis AC, Pauli P, Fallgatter AJ. Model-based analysis of rapid event-related functional near-infrared spectroscopy (NIRS) data: a parametric validation study. Neuroimage. 2007;35(2):625–634. doi: 10.1016/j.neuroimage.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto T, Kato T. Paradoxical correlation between signal in functional magnetic resonance imaging and deoxygenated haemoglobin content in capillaries: a new theoretical explanation. Phys Med Biol. 2002;47(7):1121–1141. doi: 10.1088/0031-9155/47/7/309. [DOI] [PubMed] [Google Scholar]
- 46.Berryhill ME, Wencil EB, Branch Coslett H, Olson IR. A selective working memory impairment after transcranial direct current stimulation to the right parietal lobe. Neurosci Lett. 2010;479(3):312–316. doi: 10.1016/j.neulet.2010.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elmer S, Burkard M, Renz B, Meyer M, Jancke L. Direct current induced short-term modulation of the left dorsolateral prefrontal cortex while learning auditory presented nouns. Behav Brain Funct. 2009;5:29. doi: 10.1186/1744-9081-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu TY, Tseng LY, Yu JX, Kuo WJ, Hung DL, Tzeng OJ, et al. Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. Neuroimage. 2011;56(4):2249–2257. doi: 10.1016/j.neuroimage.2011.03.059. [DOI] [PubMed] [Google Scholar]
- 49.Jones KT, Berryhill ME. Parietal contributions to visual working memory depend on task difficulty. Front Psychiatry. 2012;3:81. doi: 10.3389/fpsyt.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tseng P, Hsu TY, Chang CF, Tzeng OJ, Hung DL, Muggleton NG, et al. Unleashing potential: transcranial direct current stimulation over the right posterior parietal cortex improves change detection in low-performing individuals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(31):10554–10561. doi: 10.1523/JNEUROSCI.0362-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaehle T, Sandmann P, Thorne JD, Jancke L, Herrmann CS. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: combined behavioural and electrophysiological evidence. BMC Neurosci. 2011;12:2. doi: 10.1186/1471-2202-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berryhill ME, Wiener M, Stephens JA, Lohoff FW, Coslett HB. COMT and ANKK1-Taq-Ia genetic polymorphisms influence visual working memory. PloS one. 2013;8(1):e55862. doi: 10.1371/journal.pone.0055862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nieratschker V, Kiefer C, Giel K, Kruger R, Plewnia C. The COMT Val/Met Polymorphism Modulates Effects of tDCS on Response Inhibition. Brain Stimul. 2015;8(2):283–288. doi: 10.1016/j.brs.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Hill AT, Fitzgerald PB, Hoy KE. Effects of Anodal Transcranial Direct Current Stimulation on Working Memory: A Systematic Review and Meta-Analysis of Findings From Healthy and Neuropsychiatric Populations. Brain Stimul. 2015 doi: 10.1016/j.brs.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Backman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neuroscience and biobehavioral reviews. 2010;34(5):670–677. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 56.Harris SE, Deary IJ. The genetics of cognitive ability and cognitive ageing in healthy older people. Trends in cognitive sciences. 2011;15(9):388–394. doi: 10.1016/j.tics.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Laukka EJ, Lovden M, Herlitz A, Karlsson S, Ferencz B, Pantzar A, et al. Genetic effects on old-age cognitive functioning: a population-based study. Psychology and aging. 2013;28(1):262–274. doi: 10.1037/a0030829. [DOI] [PubMed] [Google Scholar]
- 58.Nyberg L, Andersson M, Kauppi K, Lundquist A, Persson J, Pudas S, et al. Age-related and genetic modulation of frontal cortex efficiency. Journal of cognitive neuroscience. 2014;26(4):746–754. doi: 10.1162/jocn_a_00521. [DOI] [PubMed] [Google Scholar]
- 59.Raz N, Rodrigue KM, Kennedy KM, Land S. Genetics and vascular modifiers of age-sensitive cognitive skills: Effects of COMT, BDNF, ApoE, and Hypertension. Neuropsychology. 2009;23(1):105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stormer VS, Passow S, Biesenack J, Li SC. Dopaminergic and cholinergic modulations of visual-spatial attention and working memory: insights from molecular genetic research and implications for adult cognitive development. Developmental psychology. 2012;48(3):875–889. doi: 10.1037/a0026198. [DOI] [PubMed] [Google Scholar]
- 61.Jones KT, Gozenman F, Berryhill ME. The strategy and motivational influences on the beneficial effect of neurostimulation: a tDCS and fNIRS study. Neuroimage. 2015;105:238–247. doi: 10.1016/j.neuroimage.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


