Abstract
Objectives
To provide a comprehensive simultaneous relation of various semiquantitative knee OA MRI features as well as the presence of baseline radiographic OA to quantitative longitudinal cartilage loss.
Methods
We studied Multicenter OA Study (MOST) participants from a longitudinal observational study that included quantitative MRI measurement of cartilage thickness. These subjects also had Whole Organ MRI Score (WORMS) scoring of cartilage damage, bone marrow lesions (BMLs), meniscal pathology, and synovitis, as well as baseline radiographic evaluation for Kellgren and Lawrence (KL) grading. Knee compartments were classified as progressors when exceeding thresholds of measurement variability in normal knees. All potential risk factors of cartilage loss were dichotomized into “present” (score ≥2 for cartilage, ≥1 for others) or “absent”. Differences in baseline scores of ipsi-compartmental risk factors were compared between progressor and non-progressor knees by multivariable logistic regression, adjusting for age, sex, body mass index, alignment axis (degrees) and baseline KL grade. Odds ratios (OR) and 95% CIs were calculated for medial (MFTC) and lateral (LFTC) cartilage loss. Cartilage loss across both compartments was studied using Generalized Estimating Equations.
Results
196 knees of 196 participants were included (age 59.8±6.3 years [mean±SD], BMI 29.5±4.6, 62% women). For combined analyses of MFTC and LFTC, baseline factors related to cartilage loss were radiographic OA (KL grade ≥2: aOR 4.8 [2.4-9.5], cartilage damage (aOR 2.3 [1.2-4.4]), meniscal damage (aOR 3.9 [2.1-7.4]) and extrusion (aOR 2.9 [1.6-5.3]), all in the ipsilateral compartment, but not BMLs or synovitis.
Conclusion
Baseline radiographic OA and semiquantitatively assessed MRI-detected cartilage damage, meniscal damage and extrusion, but not BMLs or synovitis is related to quantitatively measured ipsicompartmental cartilage thinning over 30 months.
Keywords: meniscus, effusion, synovitis, cartilage, semiquantitative, quantitative
Introduction
Several studies have shown that structural features of knee OA that can be graded semiquantitatively (SQ) with MRI, are associated with subsequent SQ determined structural progression. These include meniscal pathology [1,2], bone marrow lesions [3,4], and cartilage damage [5,6]. In addition Hoffa-synovitis and effusion-synovitis are commonly assessed using SQ scoring methods, but the relation of synovitis and subsequent cartilage loss is debated [5,7,8,9,10].
Evaluating both cartilage loss, the outcome, and structural features, i.e. factors that relate to this outcome, in the same images at the same time, may theoretically introduce bias. Quantitatively measured cartilage loss is commonly used as an outcome measure in longitudinal studies of structural change in knee OA and the process is done by readers who are not involved in semiquantitative assessment of baseline features, enabling evaluation of outcome measures totally independent of baseline readings without the risk of biasing the outcome assessment.
The purpose of this study was to provide a comprehensive simultaneous relation of various SQ knee OA MRI features as well as the presence of baseline radiographic OA to quantitative longitudinal cartilage loss, either in the medial or lateral compartments, or in the whole tibiofemoral knee joint.
PATIENTS AND METHODS
Study Design and Subjects
Subjects were participants in the Multicenter Osteoarthritis Study (MOST), a prospective study of 3,026 persons aged 50-79 years with a goal of identifying risk factors for incident and progressive knee OA in a sample either with OA or at high risk of developing disease. Participants from two US communities, Birmingham, Alabama and Iowa City, Iowa were enrolled in the study over a 22 month period. Details of subject inclusion, exclusion and recruitment have been described previously [7,11]. The study protocol was approved by the institutional review boards at the University of Iowa, University of Alabama, Birmingham, University of California, San Francisco and Boston University Medical Campus, and written informed consent was obtained from all participants.
At baseline, all participants without contraindications for 1.0T extremity MRI and whose knees were not too large for the extremity scanner had 1.0 T MR images acquired on both knees. At the baseline and 30 month clinic visits serial 1.5 T large bore MRI scans were also acquired on a subset of participants to obtain quantitative measures of cartilage loss. During a 12 month period during the baseline visit every third person at the Alabama site and every fourth person at the Iowa site was asked to participate, and those who agreed and did not have knee MRI contraindications had 1.5T scans of both knees. Baseline 1.5T MRIs were obtained in 426 subjects and 30 month 1.5T scans in 300 subjects. Of these subjects, 196 knees (one knee per subject) had longitudinal measurements of quantitative cartilage loss as well as semiquantitative WORMS assessment at baseline and at 30 months. The dominant or the right (if dominance was unknown) knee was measured. If images for this knee had poor orientation, only tibial cartilage thickness was measured. If images for this knee had poor quality (e.g. due to fat saturation failure, or motion artifact), then the contralateral knee was measured. These 196 knees were included in our study (Figure 1).
Figure 1.
Flowchart summarizing the subject inclusion/exclusion process.
Radiographs
At baseline, all subjects underwent weight-bearing posteroanterior (PA) fixed flexion knee radiographs using a plexiglass positioning frame (SynaFlexer™). Radiographs were read by a team of three readers including one author (DTF), blinded to clinical data, who graded radiographs according to Kellgren-Lawrence (KL) grade, followed by an adjudication process [7,11]. KL grade 2 or above was considered to have radiographic OA. The weighted kappa coefficient of inter-observer reliability for the KL readings was 0.79.
Full-limb radiographs of both legs were obtained at baseline using a 14-in × 51-in cassette. The mechanical axis was defined as the angle formed by the intersection of a line from the center of the head of the femur to the center of the tibial spines and a line from the center of the talus to the center of the tibial spines. The interobserver intraclass correlation coefficient for the mechanical axis was 0.99 (P < 0.0001). Varus alignment was defined as a hip-knee-ankle (HKA) angle <179°; 179-181° was considered neutral and valgus alignment was defined as an HKA angle >181°.
MRI Acquisition
In the MOST parent study, MR imaging was performed using a 1.0 T extremity-based OrthOne scanner (Oni MSK Extreme, GE Healthcare, Waukesha, WI). Images were acquired using a circumferential extremity coil using fat-suppressed, fast spin echo, proton density-weighted sequence in two planes, sagittal (TR=4800 ms, TE=35 ms, 3.0mm slice thickness, 0mm interslice gap, FOV 14×14cm, matrix 288×192, NEX2); and axial (TR=4700 ms, TE=13.2 ms, 3.0mm slice thickness, 0mm interslice gap, FOV 14cm, matrix 288×192, NEX2) and a short tau inversion recovery sequence (STIR) in the coronal plane (TR=7820 ms, TE=14 ms, TI=100 ms, 3.0mm slice thickness, 0mm interslice gap, FOV 14cm, matrix 256×256, NEX2).
Coronal T1-weighted fast low-angle shot (FLASH) MRI with water excitation (TR=17 or 18.6 ms, TE=4.2 – 9.3 ms, 1.5 mm slice thickness, 0 mm interslice gap, FOV 0.3125×0.3125 mm in-plane resolution) was obtained at baseline and 30-month follow-up using a 1.5 T MRI (Siemens, Erlangen, Germany) in the participants, who volunteered for the longitudinal substudy, in which MRI measurement of cartilage thickness and volume were performed.
MRI Interpretation
MRI readings were performed independently by two musculoskeletal radiologists (AG, FWR), with 14 and 12 years of experience respectively in semiquantitative MR assessment of knee OA using the WORMS grading scheme. These readers were blinded to all other data [12]. Cartilage signal intensity and morphology were scored according to WORMS from 0 to 6 (depending upon depth and extent of cartilage loss) in five subregions each in the medial and lateral tibiofemoral compartments, for a total of 10 tibiofemoral subregions. Meniscal status was graded from 0 to 4 in the anterior horn, body, and posterior horn of each meniscus, defining tear as a WORMS score ≥1 in one or more segment. In addition, extrusion of each meniscal body was scored on the coronal image from 0 to 2, defining the presence of extrusion as a score ≥1 [2]. MR images were assessed using eFilmTM software (Version 2.0.0, Merge Healthcare, Milwaukee, WI). In addition, bone marrow lesions (BMLs) and meniscal damage were assessed according to the WORMS system at baseline. BML size was scored from 0–3 based on the extent of regional involvement.
Signal alterations in the infrapatellar and intercondylar regions of Hoffa’s fat pad were scored from 0 to 3 as a surrogate for synovial thickening according to the literature as this feature is not part of the original WORMS system [8,9,13]. We will refer to these scores as ‘Hoffa-synovitis’ in the following sections, although acknowledging that these signal changes also include non-specific alterations not necessarily related to synovitis [10,14]. WORMS uses a combined measure of joint effusion and synovitis based on the amount of intraarticular fluid-equivalent signal. This composite score is graded from 0 to 3 according to the estimated maximal distention of the synovial cavity. and was applied in addition to mentioned signal changes in Hoffa’s fat pad [15].We will refer to this scoring measure as ‘effusion-synovitis’ in the following sections to acknowledge both constituents of the composite score [10]. All semiquantitative MR assessments were dichotomized into “present” (score ≥2 for cartilage since score of 1 represents a hyperintensity of the cartilage of unknown significance, ≥1 for others) or “absent” for the purpose of statistical analysis.
Quantification of Cartilage Thickness Loss on MRI
Segmentation of the tibial and femoral cartilage involved manual tracing of the total subchondral bone area (tAB) and the cartilage surface area (AC) of the medial tibia, lateral tibia, central (weight-bearing) medial femoral condyle, and central (weight-bearing) lateral femoral condyle using custom software (Chondrometrics GmbH, Ainring, Germany). Segmentation was performed by trained readers with several years of experience in cartilage segmentation. Baseline and follow-up images were displayed simultaneously but with blinding to the acquisition order or date, to allow a consistent selection of the number of slices and peripheral edges. Quality control of all segmentations was performed by one expert (F.E.). The cartilage thickness was computed from the cartilage surfaces (tAB and AC) as described previously [16]. The reliability of the technique has been published before [17].
The mean cartilage thickness (considering denuded areas as “0”) in the medial femorotibial compartment (MFTC) was obtained by adding the cartilage thickness measured in the medial tibia and the central, weight-bearing part of the medial femoral condyle. The cartilage thickness in the LFTC was similarly computed as the sum of the cartilage thickness observed in the lateral tibia and the central, weight-bearing part of the lateral femoral condyle.
Outcome Definition
The classification of knees as progressors (defined as loss above a certain threshold in cartilage thickness – see below) and non-progressors (loss below the threshold or increase in cartilage thickness) was based on one-year measurement variability observed in the medial (MFTC) and lateral (LFTC) femorotibial compartment of participants from the healthy reference cohort of the OA Initiative [18] [http://oai.epi-ucsf.org/datarelease/]. These were not expected to show a change in cartilage thickness other than measurement variability, biological variability, and aging, given the absence of radiographic or symptomatic OA and the non-exposure to risk factors for the onset of OA. The change observed in that cohort in the MFTC and the LFTC using a coronal FLASH 3D MRI sequence had a mean value close to zero (MFTC: 2μm, LFTC: 7μm) [18]. The thresholds of progression / non-progression were chosen so that 95% of the knees analyzed in the OAI healthy reference cohort would be classified as non-progressors, with 2.5% of these knees at each end of the range showing cartilage thinning or thickening, respectively. Hence, knees from the MOST cohort were classified as progressors (cartilage thinning) when exceeding a thresholds of -162μm in the MFTC, and/or -145μm in the LFTC.
Statistical Analysis
Differences in baseline scores of ipsi-compartmental independent variables were compared between progressor and non-progressor knees by multivariable logistic regression, adjusting for age, sex, body mass index, mechanical alignment axis (degrees) and baseline KL grade. Given the literature evidence that BMLs and effusion-synovitis/Hoffa-synovitis can fluctuate over time, to evaluate the effect of transient vs. persistent BMLs and synovitis, we performed additional analyses by stratifying subjects based on the following criteria for the analysis using these three baseline MRI features: score ≥1 at baseline and disappears (score 0) at follow up vs. score ≥1 at baseline and stays ≥1 at follow up. Odds ratios (OR) and 95% CIs were calculated for MFTC and LFTC cartilage loss, respectively. We further combined MFTC and LFTC to calculate an OR of ipsi-compartmental cartilage loss across compartments, using Generalized Estimating Equations. As a secondary analysis, we did logistic regression model of step-wise selection, including MRI features (meniscal damage, meniscal extrusion, cartilage damage, BMLs, effusion synovitis, and Hoffa synovitis) and KL grade, with entry level =0.2 and stay level =0.1. The aforementioned baseline demographic characteristics were forced in the model. All statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC, USA).
RESULTS
196 knees from 196 participants were included (Table 1) and their mean age was 59.8±6.3 years, mean BMI was 29.5±4.6, and 62% were women. 46 knees had radiographic knee OA (KL grade 2 or above) at baseline.
Table 1.
Demographic characteristics (Knee-based data: N=196 knees)
| Baseline data | |||
|---|---|---|---|
| Age (years): Mean (standard deviation) | 59.8 (6.3) | ||
|
| |||
| BMI (kg/m-2): Mean (standard deviation) | 29.5 (4.6) | ||
|
| |||
| Sex | Female: n (%) | 122 (62.2) | |
| Male: n (%) | 74 (37.8) | ||
|
| |||
| Clinical site | Alabama: n (%) | 108 (55.1) | |
| Iowa: n (%) | 88 (44.9) | ||
|
| |||
| Malalignment | Varus (<179°): n (%) | 86 (43.9) | |
| Neutral (179-181°): n (%) | 73 (37.2) | ||
| Valgus (>181°): n (%) | 37 (18.9) | ||
|
| |||
| Kellgren and Lawrence grade | 0 | 108 (55.1) | |
| 1 | 42 (21.4) | ||
| 2 | 25 (12.8) | ||
| 3 | 18 (9.2) | ||
| 4 | 3 (1.5) | ||
|
| |||
| Longitudinal data | |||
|
| |||
| Change in lateral tibial and femoral mean cartilage thickness (μm) | -25.10 (140.54) | ||
|
| |||
| Change in medial tibial and femoral mean cartilage thickness (μm) | -63.01 (184.11) | ||
In the MFTC (Table 2), there were 35 progressors and 161 non-progressors. The only baseline factor related to cartilage thickness loss was baseline radiographic OA (aOR 2.51, [95%CI 1.03-6.09]). None of the MRI-based OA features in the MFTC is related to subsequent cartilage thickness loss in the same compartment.
Table 2.
Prognostic value of semiquantitative MRI-based risk factors in the medial TF compartment
| Risk factor at baseline | Non-progressors (N=161) n (%) | Progressors: cartilage thickness decrease >162μm (N=35) n (%) | aOR (95% CI) | P-value |
|---|---|---|---|---|
| Meniscal damage WORMS score ≥1 * | 42 26.58 | 17 50.00 | 2.07*** (0.87, 4.90) | 0.0978 |
| Meniscal extrusion (present)** | 49 31.21 | 21 61.76 | 2.13*** (0.90, 5.03) | 0.0839 |
| Cartilage WORMS score ≥2* | 109 68.99 | 27 79.41 | 1.56*** (0.56, 4.30) | 0.3947 |
| BML WORMS score ≥1 * | 45 28.48 | 16 47.06 | 1.51*** (0.64, 3.56) | 0.3463 |
| Effusion synovitis WORMS score ≥1 * | 108 68.35 | 27 79.41 | 2.29*** (0.85, 6.16) | 0.1013 |
| Hoffa synovitis WORMS score ≥1 * | 92 58.23 | 19 55.88 | 0.84*** (0.36, 1.93) | 0.6731 |
| Radiographic OA (KL grade ≥2) | 40 24.84 | 16 45.71 | 2.51**** (1.03, 6.09) | 0.0418 |
4 subjects had missing WORMS reading for this feature and were excluded from analysis
5 subjects had missing WORMS reading for this feature and were excluded from analysis
Adjusted for age, BMI, gender, clinic site, alignment and KL grade
Adjusted for age, BMI, gender, clinic site, and alignment
In the LFTC (Table 3), there were 29 progressors and 167 non-progressors. Baseline factors related to cartilage thickness loss were baseline radiographic OA (aOR 8.50 [1.97-36.63], prevalent lateral cartilage damage (aOR 3.08 [1.14-8.29]) and lateral meniscal damage (aOR 12.16 [2.64-56.00]).
Table 3.
Prognostic value of semiquantitative MRI-based risk factors in the lateral TF compartment
| Risk factor at baseline | Non-progressors (N=167) n (%) | Progressors: cartilage thickness decrease >147 μm (N=29) n (%) | aOR (95% CI) | P-value |
|---|---|---|---|---|
| Meniscal damage WORMS score ≥1 * | 7 4.29 | 8 27.59 | 12.16*** (2.64, 56.00) | 0.0013 |
| Meniscal extrusion (present)** | 10 6.17 | 7 24.14 | 1.43*** (0.52, 3.92) | 0.4889 |
| Cartilage WORMS score ≥2* | 74 45.40 | 21 72.41 | 3.08*** (1.14, 8.29) | 0.0259 |
| BML WORMS score ≥1 * | 40 24.54 | 10 34.48 | 1.46*** (0.50, 4.21) | 0.4865 |
| Effusion synovitis WORMS score ≥1 * | 116 71.17 | 19 65.52 | 1.43*** (0.52, 3.92) | 0.4889 |
| Hoffa synovitis WORMS score ≥1 * | 95 58.28 | 16 55.17 | 0.74*** (0.29, 1.86) | 0.5198 |
| Radiographic OA (KL grade ≥2) | 5 2.99 | 7 24.14 | 8.50**** (1.97, 36.63) | 0.0041 |
4 subjects had missing WORMS reading for this feature and were excluded from analysis
5 subjects had missing WORMS reading for this feature and were excluded from analysis
Adjusted for age, BMI, gender, clinic site, alignment and KL grade
Adjusted for age, BMI, gender, clinic site, and alignment
For analysis combining MFTC and LFTC, baseline factors related to cartilage thickness loss in the ipsilateral compartment were (Table 4) baseline radiographic OA (aOR 4.79 [2.41-9.53], cartilage damage (aOR 2.27 [1.18-4.37]), meniscal damage (aOR 3.94 [2.09-7.43]) and meniscal extrusion (aOR 2.92 [1.62-5.26]).
Table 4.
Odds ratios of having ipsi-compartmental cartilage loss due to risk factors in the same compartment (i.e. medial cartilage thinning with medial risk factors, and lateral cartilage thinning with lateral risk factors)
| Risk factor at baseline | No. of compartments without cartilage loss (medial + lateral) (N=321) n (%) | No. of compartments with cartilage loss (medial+lateral): (N=64) n (%) | aOR (95% CI) | p-value ** |
|---|---|---|---|---|
| Meniscal damage WORMS score ≥1 * | 49 15.26 | 25 39.68 | 3.94*** (2.09, 7.43) | <0.0001 |
| Meniscal extrusion (present)* | 59 18.50 | 28 44.44 | 2.92*** (1.62, 5.26) | 0.0004 |
| Cartilage WORMS score ≥2* | 183 57.01 | 48 76.19 | 2.27*** (1.18, 4.37) | 0.0136 |
| BML WORMS score ≥1 * | 85 26.48 | 26 41.27 | 1.53*** (0.85, 2.74) | 0.1523 |
| Effusion synovitis WORMS score ≥1 * | 224 69.78 | 46 73.02 | 1.71*** (0.82, 3.53) | 0.1499 |
| Hoffa synovitis WORMS score ≥1 * | 187 58.26 | 35 55.56 | 0.79*** (0.43, 1.47) | 0.4640 |
| Radiographic OA (KL grade ≥2) | 45 13.72 | 23 35.94 | 4.79**** (2.41, 9.53) | <0.0001 |
4 subjects had missing WORMS reading for this feature and were excluded from analysis
p<0.023 is considered statistically significant, after Bonferroni correction for multiple comparisons
Adjusted for age, BMI, gender, clinic site, alignment and KL grade
Adjusted for age, BMI, gender, clinic site, and alignment
In all analyses (MFTC, LFTC and combined), stratification of subjects according transient vs. persistent BMLs and synovitis did not alter our results (i.e. BMLs and effusion-synovitis/Hoffa-synovitis were not associated with subsequent cartilage thickness loss regardless of whether they were transient or persistent).
DISCUSSION
The aim of our study was to determine, which SQ MRI-detected OA features are related to quantitative cartilage thinning over a 30-month period. We demonstrated that the baseline factors related to cartilage thinning were baseline radiographic OA, prevalent SQ cartilage damage, meniscal damage and extrusion in the same femorotibial compartment. Of these features, meniscal damage and extrusion were most strongly associated with cartilage thinning. We did not find a statistically significant association of baseline SQ BMLs, effusion-synovitis or Hoffa-synovitis with subsequent quantitative cartilage loss. Lack of statistically significant results for these semiquantitative MRI features may be related to the fact that most of our study knees (150 of 196 knees) did not have radiographic OA. It has been shown previously that prevalent SQ cartilage damage is related to further SQ cartilage loss over time [5,7]. Recent studies based on data from the Joints on Glucosamine Study [5] and the MOST study [7] showed that SQ cartilage damage at baseline was associated with SQ cartilage loss (i.e. worsening of SQ cartilage scores) over 6 months [5] and 30 months [7], respectively. Moreover, prevalent SQ cartilage damage has also been shown to be related to quantitative cartilage volume loss over a longer than 2-year period [19,20] in Tasmanian Older Adult Cohort Study. It is interesting to note that cartilage damage was not statistically significant associated with cartilage thinning in the analysis of MFTC, while it was in the analyses of LFTC and the combined analysis. The reason for this result is unclear. However, perhaps it would be more important to focus on the results of combined analysis rather than individual medial and lateral compartmental analyses, since the knee OA pathologic process involves the whole FT joint. An important implication of our findings for the future knee OA clinical trials is that investigators may wish to preferentially include persons with baseline SQ cartilage damage to assess efficacy of a new therapy targeting articular cartilage, outcome of which is measured quantitatively.
In our study, the presence of BMLs at the baseline or its fluctuation was not associated with cartilage thickness loss over time. Our finding is discordant with the available literature evidence showing BMLs related to SQ cartilage loss [5, 21-24] as well as quantitatively measured cartilage volume loss [21-24]. However, to the best of our knowledge, our study is the first to examine baseline BMLs as well as it changes as potential risk factors for quantitative cartilage thickness loss. When calculating aORs for BMLs, statistical significance was lost when we adjusted our model for baseline KL grade. This implies that the presence or fluctuation of BMLs may be closely related to the severity of radiographic OA, which is an indirect marker for cartilage thinning (i.e. higher KL grade means more joint space narrowing). BMLs and cartilage thinning may be closely related to each other in knee OA pathogenesis, and one may cause the other or vice versa. It remains difficult to determine ‘which comes first’, however.
SQ meniscal damage and extrusion were the two strongest factors related to quantitative cartilage loss in our study. Several studies have reported associations between baseline meniscal damage and cartilage loss over time. Chang et al. showed SQ medial meniscal body tear was associated with quantitatively measured thickness loss of meniscus-covered portion of femorotibial cartilage over two years [25]. Berthiaume et al. showed SQ medial meniscal tear or extrusion are strongly related to medial compartment cartilage volume loss over two years [26]. In a study by Crema et al, the risk of quantitative medial femorotibial cartilage thickness loss over two years increased significantly in knees with SQ medial meniscal tears or macerations [27]. Specifically, cartilage loss in the external medial tibia was associated with tears of the posterior horn of the medial meniscus. Hunter et al demonstrated baseline SQ medial meniscal damage and malpositioning were associated with increased risk of SQ cartilage score worsening within the medial femorotibial compartment over 30 months based on the WORMS [2]. Findings of our study further support the strong relationship between SQ meniscal pathology and quantitative cartilage loss over time.
Our study showed effusion-synovitis or Hoffa synovitis were not associated with quantitative cartilage thinning over time. Studies examining this issue using semiquantitative cartilage loss data have not been consistent in their findings [5,7,8,9,10]. A study based on the MOST study showed longitudinal fluctuation in synovitis has borderline association with SQ cartilage loss [9]. In that study, MRI signal changes within Hoffa fat pad, suprapatellar and intercondylar regions on a non-enhanced sequence were used as a surrogate for synovitis, similar to the way we assessed Hoffa synovitis. Another study using the MOST data showed effusion-synovitis had a borderline association with SQ cartilage loss over 30 months in the femorotibial joint [7]. Data from the Joints on Glucosamine study demonstrated that baseline effusion (= “effusion synovitis” in the current study) was a strong risk factor for patellofemoral cartilage loss over a 6-month period [5]. Moreover, one arthroscopic study showed synovitis was related to progression of cartilage damage over one year assessed by repeat arthroscopy, although adjustment was not performed to take into account other structural features that might have caused both the synovitis and cartilage loss [28]. Possible reasons for these discrepancies between our study and previous publications are unclear. Even though synovitis may be a prominent component of disease in some knees with OA, unlike meniscal factors and cartilage defects, it appeared to have only mild association with cartilage loss. However, it should be noted that no studies have been reported to show if synovitis as detected by contrast-enhanced MRI is associated with cartilage loss over time. Considering that synovitis is more accurately assessed with contrast-enhanced MRI, further studies are needed to determine the relationship between CE-MRI-assessed baseline synovitis and future cartilage loss. A strength of our study is the fact that the odds ratio for cartilage loss over time remained statistically significant after adjustment for alignment as well as the demographic characteristics, implying our findings hold true regardless of the alignment status. Another notable strength of our study is that we examined quantitative cartilage thickness loss, while all MRI-based assessments were semiquantitatively assessed by readers who were not involved in quantitative outcome analysis. This avoided potential bias in reading. Since readers are usually blinded to the research questions asked at the time of reading, and in large scale multicenter epidemiological studies, analyses are often designed after the reading is completed, reader bias due to simultaneous assessment may be unlikely. However, if there were bias in examination of the progression of cartilage lesions, for example, our data is not vulnerable to the bias associated with grading independent and outcome variables together in the same session by the same readers. The semiquantative MR measurements and OA status were not included in one model since the order of MRI features and the causal relationship among them are not clearly understood yet. If some baseline factors are potential confounders to the association of a specific factor and the outcome, the results we observed would be biased. On the other hand, if some baseline factors are on the path way from a specific factor to the outcome, i.e., mediators, adjusting them in the model is not appropriate. As we did not have enough knowledge to separate the potential confounders and mediators, we chose to assess the relation of each baseline factor without controlling for other factors.
Limitations of our study include the fact that synovitis, in the form of effusion synovitis and Hoffa synovitis, was assessed using non-contrast enhanced MRI. MRI assessment of synovitis in knee OA should ideally be performed using contrast-enhanced sequence [29]. However, the cohort of patients included in our study did not undergo contrast-enhanced MRI examination and such data could not be collected.
In conclusion, baseline radiographic OA and the baseline presence of MRI-detected cartilage damage, meniscal damage and extrusion in the ipsilateral FTC were associated with quantitatively assessed cartilage thickness loss over 30-months, but not BMLs, effusion-synovitis or Hoffa-synovitis.
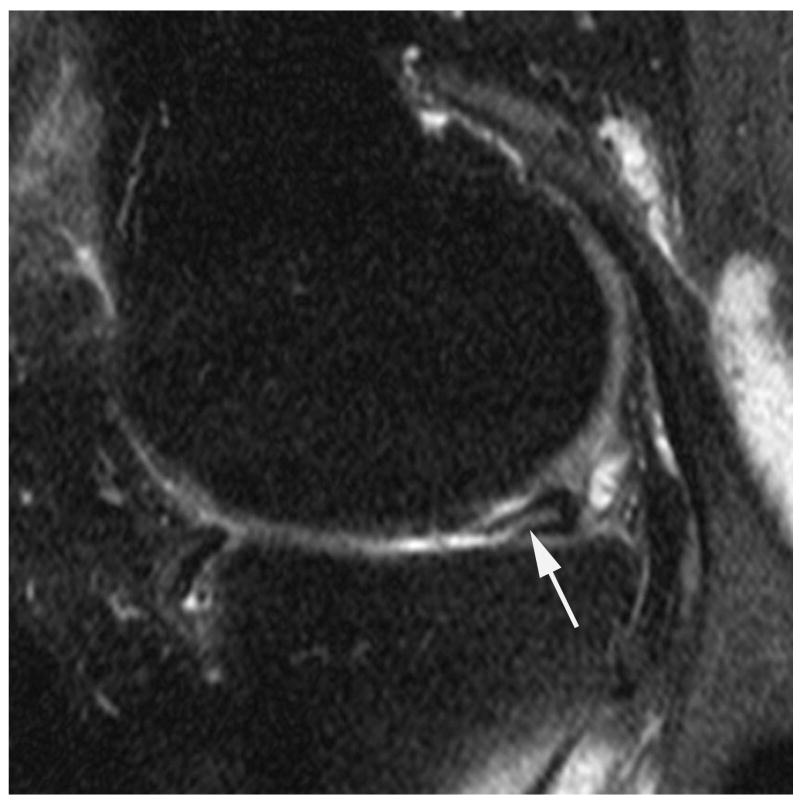
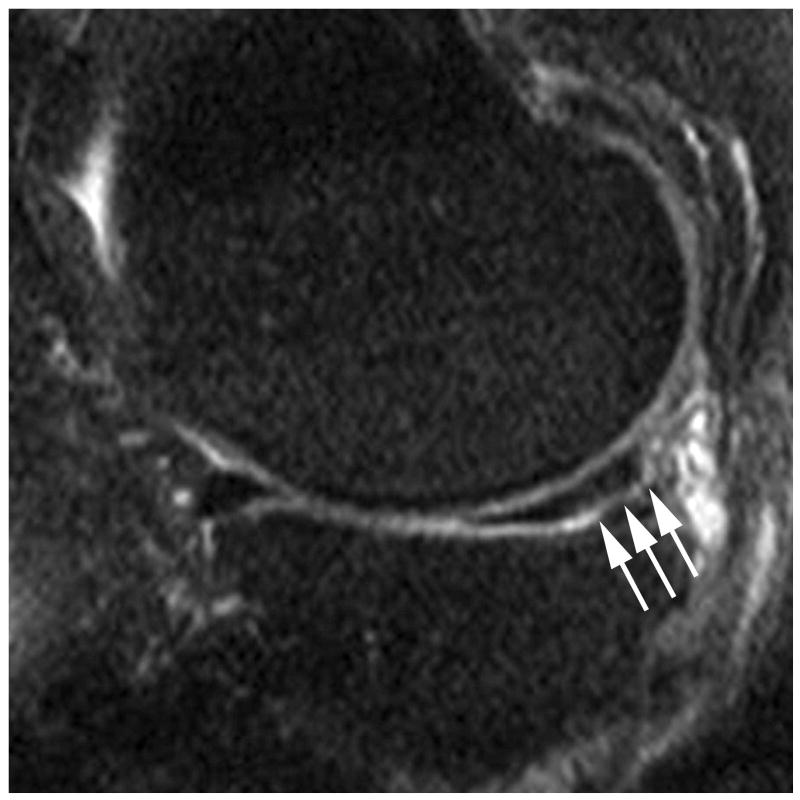
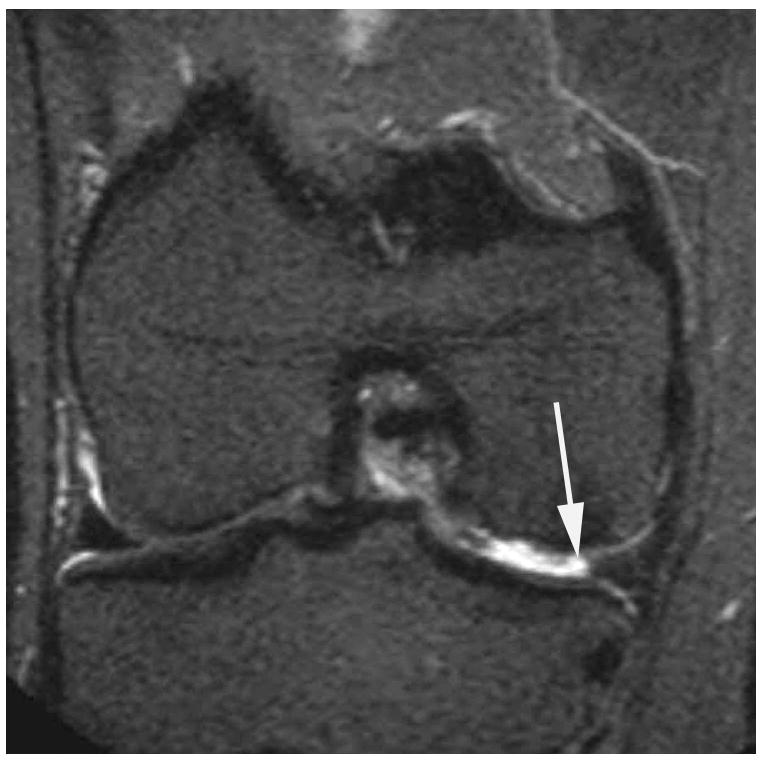
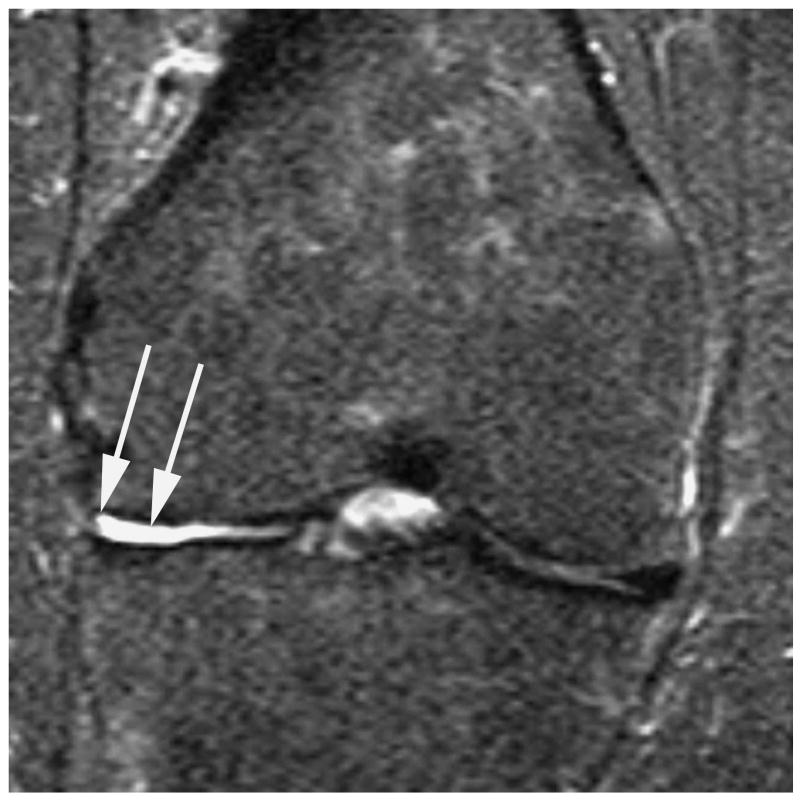
Figure 2.
Sagittal proton density-weighted fat suppressed MRI shows (a) a parrot-beak tear of the posterior horn of the medial meniscus reaching the inferior surface of the meniscus (arrow). This lesion would be scored as a grade 1 lesion in WORMS; (b) a non-displaced horizontal-oblique tear of the posterior horn of the medial meniscus reaching both, the superior and inferior surfaces of the meniscus (arrows). This tear type would be assessed as a grade 2 lesion in WORMS. Coronal STIR MRI shows (c) partial maceration (i.e. substance loss) of the meniscal body with an amputated triangular appearance (arrow). This finding represents a grade 3 lesion in the WORMS system; (d) complete maceration or substance loss of the lateral meniscal body. No meniscus is seen in the weight-bearing central subregions of the lateral femorotibial compartment (arrows). This finding represents a grade 4 lesion in WORMS.
Acknowledgments
Funding
The MOST study is supported by National Institutes of Health (NIH) grants from the National Institute on Aging to Drs Torner (U01-AG-18832), Nevitt (U01-AG-19069), and Felson (U01-AG-18820). NIH grants AR053161 and K23AR053855.
Footnotes
Contributors
1. Guarantor of integrity of the entire study: AG, FE, DTF
2. Study concepts and design: All authors
3. Literature research: AG, FE, DH, FWR, WW, DTF
4. Data collection and interpretation: AG, FE, DH, FWR, WW, DTF
5. Statistical analysis: TY, JN, DTF
6. Manuscript preparation: AG, FE, DH, FWR, WW, DTF
7. Manuscript editing: All authors
8. Final approval of manuscript: All authors
Competing Interests
Ali Guermazi is the president of Boston Imaging Core Lab, LLC (BICL), Boston, MA, a company providing radiological image assessment services. He is a consultant to MerckSerono, TissueGene and Ortho-Trophix. Frank Roemer is a shareholder and CMO of BICL. Felix Eckstein is CEO and co-owner of Chondrometrics GmbH and provides consulting services to Merck and Synarc. Wolfgang Wirth is a co-owner of Chondrometrics GmbH. None of the other authors have declared any possible conflict of interest.
References
- 1.Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58:1716–26. doi: 10.1002/art.23462. [DOI] [PubMed] [Google Scholar]
- 2.Hunter DJ, Zhang YQ, Niu JB, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 3.Hunter DJ, Zhang Y, Niu J, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54:1529–35. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 4.Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–6. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 5.Roemer FW, Kwoh K, Hannon MJ, et al. Risk factors for magnetic resonance imaging-detected patellofemoral and tibiofemoral cartilage loss during a six-month period: the joints on glucosamine study. Arthritis Rheum. 2012;64:1888–1898. doi: 10.1002/art.34353. [DOI] [PubMed] [Google Scholar]
- 6.Ding C, Cicuttini F, Scott F, et al. Association of prevalent and incident knee cartilage defects with loss of tibial and patellar cartilage: a longitudinal study. Arthritis Rheum. 2005;52:3918–3927. doi: 10.1002/art.21474. [DOI] [PubMed] [Google Scholar]
- 7.Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252:772–780. doi: 10.1148/radiol.2523082197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill CL, Gale DG, Chaisson CE, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28:1330–1337. [PubMed] [Google Scholar]
- 9.Hill CL, Hunter DJ, Niu J, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roemer FW, Guermazi A, Felson DT, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70:1804–1809. doi: 10.1136/ard.2011.150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–2992. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 12.Felson DT, Nevitt MC. Blinding images to sequence in osteoarthritis: evidence from other diseases (editorial) Osteoarthritis Cartilage. 2009;17:281–283. doi: 10.1016/j.joca.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Madrid F, Karvonen RL, Teitge RA, et al. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging. 1995;13:177–183. doi: 10.1016/0730-725x(94)00119-n. [DOI] [PubMed] [Google Scholar]
- 14.Roemer FW, Guermazi A, Zhang Y, et al. Hoffa’s Fat Pad: Evaluation on unenhanced MR images as a measure of patellofemoral synovitis in osteoarthritis. AJR Am J Roentgenol. 2009;192:1696–1700. doi: 10.2214/AJR.08.2038. [DOI] [PubMed] [Google Scholar]
- 15.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27:737–44. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 17.Eckstein F, Charles HC, Buck RJ, et al. Accuracy and precision of quantitative assessment of cartilage morphology by magnetic resonance imaging at 3.0T. Arthritis Rheum. 2005;52:3132–3136. doi: 10.1002/art.21348. [DOI] [PubMed] [Google Scholar]
- 18.Eckstein F, Nevitt M, Gimona A, Picha K, Lee JH, Davies RY, et al. Rates of change and sensitivity to change in cartilage morphology in healthy knees and in knees with mild, moderate, and end-stage radiographic osteoarthritis: Results from 831 participants from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2011;63:311–319. doi: 10.1002/acr.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding C, Martel-Pelletier J, Pelletier JP, et al. Two-year prospective longitudinal study exploring the factors associated with change in femoral cartilage volume in a cohort largely without knee radiographic osteoarthritis. Osteoarthritis Cartilage. 2008;16:443–9. doi: 10.1016/j.joca.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Carnes J, Stannus O, Cicuttini F, et al. Knee cartilage defects in a sample of older adults: natural history, clinical significance and factors influencing change over 2.9 years. Osteoarthritis Cartilage. 2012;20:1541–7. doi: 10.1016/j.joca.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Wluka AE, Wang Y, Davies-Tuck M, et al. Bone marrow lesions predict progression of cartilage defects and loss of cartilage volume in healthy middle-aged adults without knee pain over 2 yrs. Rheumatology (Oxford) 2008;47:1392–1396. doi: 10.1093/rheumatology/ken237. [DOI] [PubMed] [Google Scholar]
- 22.Wluka AE, Hanna F, Davies-Tuck M, et al. Bone marrow lesions predict increase in knee cartilage defects and loss of cartilage volume in middle-aged women without knee pain over 2 years. Ann Rheum Dis. 2009;68:850–855. doi: 10.1136/ard.2008.092221. [DOI] [PubMed] [Google Scholar]
- 23.Tanamas SK, Wluka AE, Pelletier JP, et al. Bone marrow lesions in people with knee osteoarthritis predict progression of disease and joint replacement: a longitudinal study. Rheumatology (Oxford) 2010;49:2413–2419. doi: 10.1093/rheumatology/keq286. [DOI] [PubMed] [Google Scholar]
- 24.Dore D, Martens A, Quinn S, et al. Bone marrow lesions predict site-specific cartilage defect development and volume loss: a prospective study in older adults. Arthritis Res Ther. 2010;12:R222. doi: 10.1186/ar3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang A, Moisio K, Chmiel JS, et al. Subregional effects of meniscal tears on cartilage loss over 2 years in knee osteoarthritis. Ann Rheum Dis. 2011;70:74–79. doi: 10.1136/ard.2010.130278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berthiaume MJ, Raynauld JP, Martel-Pelletier J, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556–563. doi: 10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crema MD, Guermazi A, Li L, et al. The association of prevalent medial meniscal pathology with cartilage loss in the medial tibiofemoral compartment over a 2-year period. Osteoarthritis Cartilage. 2010;18:336–343. doi: 10.1016/j.joca.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Ayral X, Pickering EH, Woodworth TG, et al. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis -- results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi D, Roemer FW, Katur A, et al. Imaging of synovitis in osteoarthritis: current status and outlook. Semin Arthritis Rheum. 2011;41:116–130. doi: 10.1016/j.semarthrit.2010.12.003. [DOI] [PubMed] [Google Scholar]