Abstract
Purpose of review
Vitiligo and alopecia areata are common, disfiguring skin diseases. Treatment options are limited and include non-targeted approaches such as corticosteroids, topical calcineurin inhibitors, narrow band UVB phototherapy, and other immune-modifying agents. The purpose of this article is to review shared, novel mechanisms between vitiligo and alopecia areata, as well as discuss how they inform the development of future targeted treatments.
Recent findings
Vitiligo and alopecia areata are both autoimmune diseases, and striking similarities in pathogenesis have been identified at the level of both the innate and adaptive immune system. Increased reactive oxygen species and high cellular stress level have been suggested as the initiating trigger of the innate immune system in both diseases, and genome-wide association studies have implicated risk alleles that influence both innate and adaptive immunity. Most importantly, mechanistic studies in mouse models of vitiligo and alopecia areata have specifically implicated an IFN-γ-driven immune response, including IFN-γ, IFN-γ-induced chemokines, and cytotoxic CD8+ T cells as the main drivers of disease pathogenesis. These recent discoveries may reveal an effective strategy to develop new treatments, and several proof-of-concept clinical studies support this hypothesis.
Summary
The identification of IFN-γ-driven immune signaling pathways has enabled discoveries of potential new treatments for vitiligo and alopecia areata, and supports initiation of larger clinical trials.
Keywords: vitiligo, alopecia areata, autoimmune, IFN-γ, Janus kinase (JAK)
Introduction
Vitiligo and alopecia areata are common, disfiguring autoimmune diseases of the skin, however their outward appearances greatly differ: vitiligo manifests as white patches on the skin while alopecia areata exhibits patchy hair loss. Until recently, these clinical differences made mechanistic similarities seem unlikely.
Autoimmune diseases have been traditionally categorized by medical subspecialty and target tissue. While this is useful for clinical purposes, it often leads to oversimplification of disease pathology and implementation of general, non-targeted treatments. Over the last three decades, researchers have made significant progress understanding the immune system, which has allowed us to transition from categorizing by target tissue to immune pathogenesis. This paradigm shift has allowed for the development of targeted treatments. This is nowhere more evident than with the use of TNF-alpha blockers in psoriasis, rheumatoid arthritis, and inflammatory bowel disease – three conditions affecting different target tissues brought together by a shared dependence on a single cytokine.
In this article, we will explore recently recognized similarities between vitiligo and alopecia areata, specifically regarding their dependence on inflammatory pathways for pathogenesis. We will focus on the IFN-γ-driven immune response, including IFN-γ and IFN-γ-induced chemokines, and the important role of cytotoxic CD8+ T cells. A better understanding of these mechanisms has already translated into targeted treatments, providing great promise for much needed therapies.
Clinical Features and Current Management of Vitiligo and Alopecia Areata
Before discussing the mechanistic intricacies of autoimmunity, an understanding of clinical features and the limited management options for vitiligo and alopecia areata allows for appreciation of recent discoveries.
Vitiligo is an acquired, chronic depigmenting disorder of the skin caused by destruction of epidermal melanocytes. Estimated prevalence varies, but is often cited as 0.5–1%. Vitiligo is a condition not unfamiliar to pediatricians: of those affected by vitiligo, about a third present before age 12 years, and half before the age of 20 [1*]. Vitiligo can be divided into two major forms: the common form of vitiligo and its segmental variant (Figure 1A–C). The common form of vitiligo is characterized by bilateral and symmetric patches of depigmentation and can progress unpredictably. In contrast, segmental vitiligo has a unilateral distribution, progresses rapidly over 6 to 12 months, and then stabilizes for the life of the patient.
Figure 1. Clinical presentations of vitiligo and alopecia areata.
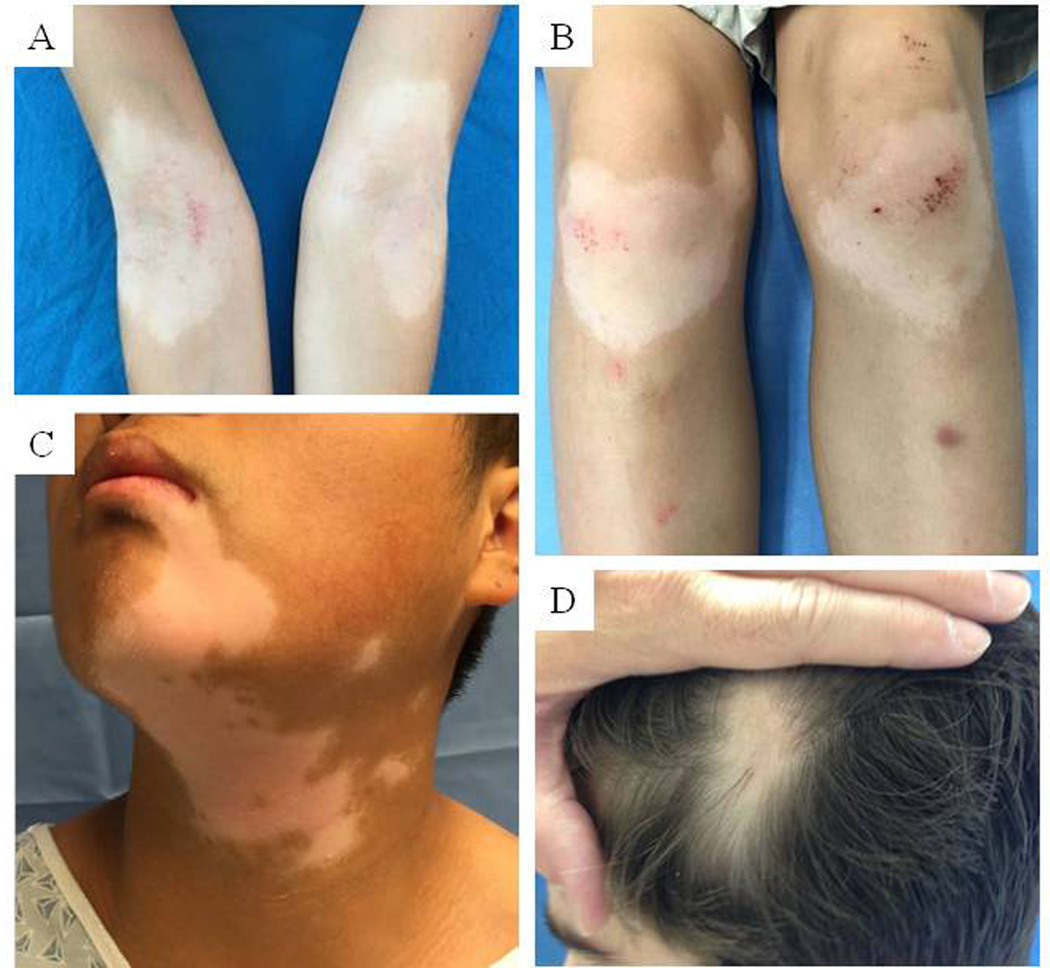
A-B) Common vitiligo on the anticubital fossa and extensor knees of a 9-year-old male; C) Segmental vitiligo in a 15-year-old male; D) Alopecia areata on the scalp of a 12-year-old male.
Mainstay vitiligo treatments include topical corticosteroids, topical calcineurin inhibitors, and narrow band UVB phototherapy. These are non-targeted approaches to treatment, and are used off-label. In fact, the only Food and Drug Administration approved treatment is monobenzone cream (Benoquin), which permanently depigments, rather than repigments, the skin. In rapidly progressing cases, systemic treatments such as corticosteroids can also be considered. According to a recent Cochrane systematic review, evidence for treatment efficacy is limited due to significant variations in study design and outcome measures [2]. Anecdotally, efficacy is moderate, and more sophisticated vitiligo treatments are long overdue.
In contrast, alopecia areata is an acquired form of non-scarring hair loss. For many patients, disease onset is before age 30 and it is thus also a familiar condition to pediatricians [3]. Disease severity ranges from small patches of alopecia (Figure 1D), to complete loss of scalp hair (alopecia totalis), and/or remaining body hair (alopecia universalis). Approximately 50 percent of patients experience spontaneous hair regrowth within one year, although most will relapse[4, 5]. The unpredictable course of the disease causes significant psychological distress.
Much like vitiligo, available therapies are non-targeted, which likely explains their underwhelming results. Potential treatments include topical corticosteroids, intralesional corticosteroids, contact dermatitis with chemicals such as diphenylcyclopropenone (DPCP) or squaric acid, or systemic immunosupressants such as corticosteroids or methotrexate. Response to treatment varies widely and there are few well-designed clinical trials to properly evaluate efficacy[5].
Despite these clinical differences, vitiligo and alopecia areata share clinical commonalities. Both have a patchy distribution and are minimally symptomatic, unlike more inflammatory skin diseases like psoriasis or atopic dermatitis. Both are associated with other autoimmune conditions, specifically thyroid autoimmunity, with an estimated prevalence as high as 19.4% with vitiligo and 28% with alopecia areata [1*, 4]. Both conditions also appear to occur more commonly together than expected by chance, with a reported 3 to 8% prevalence of vitiligo in alopecia areata patients [4]. Exploring these similarities have allowed for a better understanding of their pathogenesis.
Immune System Basics
In an attempt to make this topic more accessible to readers, we provide a basic primer on the human immune system below. To begin, the immune system is typically divided into innate and adaptive arms. The innate immune system can be thought of as the “front line,” composed of antigen non-specific responses driven by neutrophils, eosinophils, natural killer cells, mast cells, antimicrobial peptides, complement, and cytokines. These innate immune responses can activate antigen-presenting cells, which carry and present antigens to T cells, the effectors of the antigen-specific adaptive immune system. Antigen-presenting cells thus link the innate and the adaptive immune system. Examples of antigen-presenting cells in the skin include a variety of dendritic cells within the dermis and Langerhans cells within the epidermis.
The adaptive immune system is composed of T cells and B cells. T cells can be further divided into CD4+ and CD8+ T cells. CD4+ T cells are regarded as T helper (Th) cells and CD8+ T cells are cytotoxic (Tc) cells. CD4+ Th cells recognize foreign antigen peptides bound to MHC class II and support the activity of other cells of the immune response, including B cells and CD8+ T cells, predominantly through cytokines. CD8+ Tc cells recognize foreign antigen peptides bound to MHC class I molecules and can directly destroy their targets.
Crucial to our discussion will be the Th1 mediated immune response: CD4+ cells may stimulate CD8+ cell division and cytotoxicity, thereby providing ‘help’ for CD8+ cells[6]. The predominant Th1 cytokine is IFN-γ. Remembering the importance of CD8+ T cells and IFN-γ will help facilitate a mechanistic understanding of vitiligo and alopecia areata autoimmunity.
Autoimmunity in Vitiligo
Various factors have been proposed to contribute to the pathophysiology of vitiligo including those that are genetic, environmental, and autoimmune. As with most mechanisms, there is interaction among these factors, which culminate in autoimmunity as a central player in disease pathogenesis, specifically an IFN-γ-driven immune response [1*]. To begin, a shared underlying genetic susceptibility among a number of autoimmune diseases is becoming clear. We observe this clinically, since vitiligo patients and their family members have a higher risk of other autoimmune diseases, including thyroiditis, type 1 diabetes, pernicious anemia, and Addison’s disease [7]. Genome-wide association studies have identified numerous susceptibility loci encoding for immune system components. These loci involve both the innate immune system (NLRP1, IFIH1[MDA5], CASP7, C1QTNF6, TRIF) and adaptive immune system (FOXP3, BACH2, CD80, CCR6, PTPN22, IL2R, alpha GZMB, HLA class I and II) [1, 8, 9].
The initial triggering events that lead to the activation of the immune system in vitiligo are not fully understood; however, multiple studies suggest a combination of melanocyte intrinsic defects and exposure to specific environmental factors. This is especially well-described in the context of depigmentation in a subset of patients who develop vitiligo after exposure to certain chemicals. These include phenolic and catecholic chemicals found in hair dyes, other dyes, resins/adhesives, leather, and other substances [10*, 11]. Recent in vitro studies reveal that chemicals with similar structures as the amino acid tyrosine act as tyrosine analogs within the melanocyte, and precipitate higher cellular stress levels, increase production of reactive oxygen species (ROS), and trigger an abnormal unfolded protein response within the cells [12–14]. These events lead to activation, production, and release of proteins and cytokines involved in innate immunity (HSP70, IL-1β, IL6, and IL-8). Importantly, IL-1β production is shown to be increased in subjects homozygous for the NLRP1 risk allele, and serum and skin IL-6 levels are reported to be elevated even in vitiligo patients with no clear chemical exposure. This suggests the same mechanisms may contribute to disease pathogenesis in non-chemically induced vitiligo [10*].
These innate immune responses activate dendritic cells, which carry and present melanocyte-specific antigens to T cells [10*, 15]. In addition, cytokines secreted by innate immune cells likely act as the initial signal to help T cells locate stressed melanocytes [10*, 16, 17]. This represents the beginning of the autoimmune response in vitiligo, ultimately leading to targeted cell killing by CD8+ cytotoxic T cells (Figure 2).
Figure 2. IFN-γ is required for recruitment of CD8+ cytotoxic T cells to sites of inflammation in both vitiligo and alopecia areata.
Melanocytes are the target of destructive immune responses in vitiligo, which reside in basal (lower) epidermis. IFN-γ expression recruits a superficial T cell infiltration at the dermal-epidermal junction, which is accessible by topical treatments and narrow band ultraviolet B (nbUVB) phototherapy. The bulk of T cell infiltration in alopecia areata, however, occurs in and around the hair bulb, which is deep within the dermis. Treatment modalities for alopecia areata must therefore be able to effectively penetrate the skin. Systemic treatment could be similarly effective for both diseases. nbUVB, narrowband ultraviolet B; UVA, ultraviolet A.
As melanocytes reside far from the blood vessels in the epidermis, additional chemokine signaling mechanisms exist to promote circulating CD8+ T cell recruitment to peripheral tissue. Chemokines are small, secreted proteins that act as chemoattractants to guide T cell migration. Recently, using gene expression profiling on lesional skin from patients and a mouse model of vitiligo, we discovered that IFN-γ and IFN-γ-induced chemokines (CXCL9 and CXCL10) were highly expressed in the skin and blood. Mechanistic experiments in our mouse model further indicated that IFN-γ and CXCL10 were functionally required for both disease progression and maintenance [18*, 19]. More recently, a separate study demonstrated that serum CXCL10 was not only higher in patients with vitiligo compared to healthy controls, but its level was associated with disease activity and significantly decreased after successful treatment, suggesting it may be used as a biomarker to monitor the disease activity and treatment response [20*].
Despite advances in understanding vitiligo pathogenesis, there are few studies on the differential role of immune system in variants of the disease. For example, segmental vitiligo is a subtype of vitiligo that presents with rapid development of unilateral lesions and subsequent clinical stability. Recent studies have suggested that segmental vitiligo may be secondary to a postzygotic, somatic mutation that leads to development of a vulnerable subpopulation of melanocytes[21]. It appears that this local vulnerability, combined with autoimmunity, results in depigmentation limited to one area of the body. Its resistance to treatment may in part be due to the melanocyte abnormalities, and it is unclear if the targeting the IFN-γ-driven immune response will be more effective than current treatments.
Autoimmunity in Alopecia areata
To better understand autoimmunity in alopecia areata, we should appreciate that hair follicles enjoy relative immune privilege. This may be accomplished through multiple mechanisms, including down regulation of MHC class I and II molecules, decreased frequency of Langerhans cells, and expression of immunosuppressive cytokines (α-MSH, TGF-β, IGF-1); however, the key changes that mediate this privilege are not entirely clear. Immune privilege seems to be critical for continued hair cycling without triggering the skin immune system to damage the follicles, and its collapse is thought to be central to the pathogenesis of alopecia areata [3, 22–24]. The initiating events that lead to this breakdown are not fully understood, but physical/emotional stress, infections, and hormones have been suggested to play a role in genetically predisposed patients [3].
Similar to vitiligo, genome-wide association studies have implicated both innate and adaptive immunity in the pathogenesis of alopecia areata [25**, 26]. Mechanistic experiments in mouse models also indicate a predominantly Th1 mediated immune response, specifically CD8+ NKG2D+ T cells, as the main effectors both necessary and sufficient to induce hair loss [27**]. Gene expression profiling of lesional skin reveals high expression of IFN-γ and IFN-γ-induced chemokines (CXCL9, CXCL10) [28]. IFN-γ has been shown to expose hair follicle autoantigen to autoreactive T cells through ectopic expression of MHC class I molecule and also induces the expression of chemokines, which thus amplifies the adaptive immune response by recruiting more T cells. Subsequently, cytotoxic CD8+ T cells accumulate in and around the hair bulb and possibly target specific autoantigens, which may include the melanogenesis-associated peptides expressed by anagen hair follicles (Figure 2) [3, 22–24].
Consistent with this model, a recent study of global transcriptional profiling of mouse and human alopecia areata lesional skin revealed gene expression signatures indicative of cytotoxic T cell infiltration, IFN-γ response, and upregulation of several γ-chain cytokines involved in activation of CD8+ NKG2D+ T cells [27**]. Treating mice with IFN-γ, IL-2, or IL-15 Rβ blocking antibodies prevented alopecia areata by reducing the accumulation of CD8+ NKG2D+ T cells in skin, confirming the pathogenic role of these cells in the disease pathogenesis. Interestingly, systemic inhibition of Janus kinase (JAK) proteins, downstream participants in IFN-γ signaling, prevented alopecia areata development in mice, and topical application of these inhibitors reversed the disease in mice with established hair loss [27**]. More recently, a clinical study associated alopecia areata with a Th2-mediated immune response and IL-23, and suggested targeting Th2 cytokines and IL-23 as an alternative treatment strategy [29]; however, functional data confirming the pathogenic contribution of these cytokines is not yet available.
Mechanistic Similarities and Differences
Vitiligo and alopecia areata share many similarities in pathogenesis [30]. Increased ROS and high cellular stress level have been suggested as the initiating trigger of the innate immune system in both diseases [10*, 13, 16, 17, 31–34], genome-wide association studies have demonstrated both innate and adaptive immunity [8, 9, 25**, 26], and mechanistic studies in mouse models have specifically implicated an IFN-γ-driven immune response, including IFN-γ, IFN-γ induced chemokines, and cytotoxic CD8+ T cells as the main pathogenic factors [18*, 19, 27**]. There exist, however, notable differences between the two conditions. In vitiligo, epidermal melanocytes have been recognized as the autoimmune cellular targets, and melanocyte specific peptides (gp-100, MART-1, tyrosinase) have been identified as T-cell autoantigens. Therefore, it is not surprising that immune infiltration occurs superficially within the skin’s dermal-epidermal junction where melanocytes reside [30].
In contrast, it is not clear whether the immune response in alopecia areata is against a specific cell-type, and the disease autoantigens are currently unknown. While melanogenesis-associated peptides have been suggested as top candidates, this is mainly based on the observation that alopecia areata often targets pigmented hairs and disease recovery is frequently associated with white, unpigmented hair regrowth [24]. The majority of immune infiltration in alopecia areata also occurs deeper within the skin, in and around the hair follicle bulb, which differs from the more superficial inflammation in vitiligo.
Future Management Strategies: Proofs of Concept
The recent discoveries that an IFN-γ-driven immune response is critical for disease pathogenesis in both diseases suggests that targeting IFN-γ or its downstream effectors (IFN-γ receptor, JAKs, STAT1, CXCR3, or CXCL10) may be an effective strategy to develop new treatments. Importantly, different antibodies and small-molecule inhibitors have already been developed to block many of these molecules and tested in early phase clinical trials for treatment of other autoimmune diseases, including psoriasis, rheumatoid arthritis, and Crohn’s disease[35*]. Unlike vitiligo and alopecia areata, these diseases are primarily driven by cytokines other than IFN-γ and therefore, the results of these trials have been largely disappointing.
There has been notable recent momentum behind chemical inhibitors of the JAKs, a family of tyrosine kinase proteins essential for cytokine signaling, including IFN-γ. Oral tofacitinib and ruxolitinib, two JAK inhibitors with different selectivity, have been recently reported to be effective in reversing the disease in several separate cases of vitiligo and alopecia areata [27**, 36*, 37*–40*]. As systemic administration of JAK inhibitors may have unwanted effects due to generalized immunosuppression, a recent study tested topical ruxolitinib in a patient with alopecia universalis that was treatment-resistant and reported significant hair regrowth in the applied areas[41].
STAT1 is another protein required for IFN-γ signal transduction. A previous in vitro study showed that statins, which are HMG-CoA reductase inhibitors, could block STAT1 function [42], and a vitiligo patient was reported to improve after taking oral simvastatin [43]. To investigate whether statins have the potential to be used as a treatment for vitiligo, we tested systemic simvastatin in our mouse model, and found it to be effective in both preventing and reversing the disease [44]. We are currently analyzing the results of a small clinical trial to determine the efficacy of high-dose oral simvastatin in patients with generalized vitiligo [45, 46]. A separate study is recruiting patients to evaluate the benefits of combining atorvastatin and UVB for the treatment of active vitiligo [46]. Interestingly, a recent study tested the efficacy of simvastatin/ezetimibe in a series of patients with alopecia areata and concluded it was effective in both reversing the hair loss and inducing a stable remission [47].
More recently, a small study tested ustekinumab, an IL-12/IL-23p40 neutralizing antibody approved by the FDA for the treatment of psoriasis, in three patients with alopecia areata and reported hair regrowth in all patients. The authors concluded that treatment efficacy was due to blocking of IL-23 signaling [48], however ustekinumab also inhibits IL-12, which may stimulate IFN-γ production. It is therefore possible the treatment response was primarily due to interfering with the IFN-γ signaling. As with most medical treatments, these targeted therapies are seemingly not curative and recurrence is likely after cessation.
Conclusion
Substantial progress in understanding the key immune signaling pathways involved in the pathogenesis of vitiligo and alopecia areata, specifically an IFN-γ-driven immune response that includes IFN-γ, IFN-γ induced chemokines, and cytotoxic CD8+ T cells, has enabled researchers to pursue new treatment approaches for these disfiguring autoimmune dermatologic conditions. Recent reports of successful application of novel targeted immunotherapies in patients are promising, and support initiation of larger controlled clinical trials.
Key points.
Vitiligo and alopecia areata are common disfiguring autoimmune skin diseases with limited treatment options
The identification of IFN-γ as the primary contributing signaling pathway has enabled us to strategize new treatment approaches for vitiligo and alopecia areata
Proof of concept case studies and small clinical trials have shown promise for inhibitors of IFN-γ, including JAK inhibitors, STAT1 inhibitors, and IL-12.
Acknowledgments
None
Financial support and sponsorship. The authors would like to acknowledge the support of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the NIH, under Award Numbers AR061437 and AR069114, and research grants from the Kawaja Vitiligo Research Initiative, Vitiligo Research Foundation, and Dermatology Foundation Stiefel Scholar Award (to J.E.H.).
Footnotes
Conflicts of interest. None
References
- 1. Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386:74–84. doi: 10.1016/S0140-6736(14)60763-7. This is a comprehensive review of current vitiligo literature.
- 2.Whitton M, Pinart M, Batchelor JM, et al. Evidence-Based Management of vitiligo: summary of a Cochrane systematic review. Br J Dermatol. 2015 doi: 10.1111/bjd.14356. [DOI] [PubMed] [Google Scholar]
- 3.Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366:1515–1525. doi: 10.1056/NEJMra1103442. [DOI] [PubMed] [Google Scholar]
- 4.Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177–188. doi: 10.1016/j.jaad.2009.10.032. quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 5.Hordinsky M, Donati A. Alopecia areata: an evidence-based treatment update. Am J Clin Dermatol. 2014;15:231–246. doi: 10.1007/s40257-014-0086-4. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill L, Zarbo A, Isedeh P, et al. Comorbid autoimmune diseases in patients with vitiligo: A cross-sectional study. J Am Acad Dermatol. 2016;74:295–302. doi: 10.1016/j.jaad.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 8.Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676–680. doi: 10.1038/ng.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spritz RA. Six decades of vitiligo genetics: genome-wide studies provide insights into autoimmune pathogenesis. J Invest Dermatol. 2012;132:268–273. doi: 10.1038/jid.2011.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris JE. Cellular stress and innate inflammation in organ-specific autoimmunity: lessons learned from vitiligo. Immunol Rev. 2016;269:11–25. doi: 10.1111/imr.12369. This is a review of the existing evidence on the role of cell stress and innate immunity in vitiligo pathogenesis.
- 11.Wu S, Li WQ, Cho E, et al. Use of permanent hair dyes and risk of vitiligo in women. Pigment Cell Melanoma Res. 2015;28:744–746. doi: 10.1111/pcmr.12402. [DOI] [PubMed] [Google Scholar]
- 12.Mosenson JA, Flood K, Klarquist J, et al. Preferential secretion of inducible HSP70 by vitiligo melanocytes under stress. Pigment Cell Melanoma Res. 2014;27:209–220. doi: 10.1111/pcmr.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toosi S, Orlow SJ, Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol. 2012;132:2601–2609. doi: 10.1038/jid.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Boorn JG, Picavet DI, van Swieten PF, et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. J Invest Dermatol. 2011;131:1240–1251. doi: 10.1038/jid.2011.16. [DOI] [PubMed] [Google Scholar]
- 15.Mosenson JA, Zloza A, Nieland JD, et al. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005127. 174ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie H, Zhou F, Liu L, et al. Vitiligo: How do oxidative stress-induced autoantigens trigger autoimmunity? J Dermatol Sci. 2016;81:3–9. doi: 10.1016/j.jdermsci.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Zhu G, Yang Y, et al. Oxidative Stress-Induced Chemokine Production Mediates CD8(+) T Cell Skin Trafficking in Vitiligo. J Investig Dermatol Symp Proc. 2015;17:32–33. doi: 10.1038/jidsymp.2015.8. [DOI] [PubMed] [Google Scholar]
- 18. Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3007811. 223ra223. The authors propose a pathogenic role of CXCL10 in vitiligo pathogenesis and its potential to be targeted therapeutically.
- 19.Harris JE, Harris TH, Weninger W, et al. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869–1876. doi: 10.1038/jid.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Wang Q, Wu J, et al. Increased Expression of CXCR3 and its Ligands in Vitiligo Patients and CXCL10 as a Potential Clinical Marker for Vitiligo. Br J Dermatol. 2016 doi: 10.1111/bjd.14416. The authors demonstrate a correlation between serum CXCL10 level and vitiligo activity/response to treatment.
- 21.van Geel N, Speeckaert R, Melsens E, et al. The distribution pattern of segmental vitiligo: clues for somatic mosaicism. Br J Dermatol. 2013;168:56–64. doi: 10.1111/bjd.12013. [DOI] [PubMed] [Google Scholar]
- 22.Islam N, Leung PS, Huntley AC, Gershwin ME. The autoimmune basis of alopecia areata: a comprehensive review. Autoimmun Rev. 2015;14:81–89. doi: 10.1016/j.autrev.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Ito T, Tokura Y. The role of cytokines and chemokines in the T-cell-mediated autoimmune process in alopecia areata. Exp Dermatol. 2014;23:787–791. doi: 10.1111/exd.12489. [DOI] [PubMed] [Google Scholar]
- 24.McElwee KJ, Gilhar A, Tobin DJ, et al. What causes alopecia areata? Exp Dermatol. 2013;22:609–626. doi: 10.1111/exd.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Betz RC, Petukhova L, Ripke S, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun. 2015;6:5966. doi: 10.1038/ncomms6966. The authors report results of the first genome-wide meta-analysis to identify the susceptibility loci in alopecia areata.
- 26.Petukhova L, Christiano AM. Functional Interpretation of Genome-Wide Association Study Evidence in Alopecia Areata. J Invest Dermatol. 2016;136:314–317. doi: 10.1038/JID.2015.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–1049. doi: 10.1038/nm.3645. The authors reveal the pathogenic role of NKG2D+ cytotoxic T cells in alopecia areata pathogenesis, and the potential of reversing hair loss in human by inhibition of JAK pathway.
- 28.McPhee CG, Duncan FJ, Silva KA, et al. Increased expression of Cxcr3 and its ligands, Cxcl9 and Cxcl10, during the development of alopecia areata in the mouse. J Invest Dermatol. 2012;132:1736–1738. doi: 10.1038/jid.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suarez-Farinas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277–1287. doi: 10.1016/j.jaci.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 30.Harris JE. Vitiligo and alopecia areata: apples and oranges? Exp Dermatol. 2013;22:785–789. doi: 10.1111/exd.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakry OA, Elshazly RM, Shoeib MA, Gooda A. Oxidative stress in alopecia areata: a case-control study. Am J Clin Dermatol. 2014;15:57–64. doi: 10.1007/s40257-013-0036-6. [DOI] [PubMed] [Google Scholar]
- 32.Prie BE, Voiculescu VM, Ionescu-Bozdog OB, et al. Oxidative stress and alopecia areata. J Med Life. 2015;8(Spec Issue):43–46. [PMC free article] [PubMed] [Google Scholar]
- 33.Richmond JM, Frisoli ML, Harris JE. Innate immune mechanisms in vitiligo: danger from within. Curr Opin Immunol. 2013;25:676–682. doi: 10.1016/j.coi.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yenin JZ, Serarslan G, Yonden Z, Ulutas KT. Investigation of oxidative stress in patients with alopecia areata and its relationship with disease severity, duration, recurrence and pattern. Clin Exp Dermatol. 2015;40:617–621. doi: 10.1111/ced.12556. [DOI] [PubMed] [Google Scholar]
- 35. Rashighi M, Harris JE. Interfering with the IFN-gamma/CXCL10 pathway to develop new targeted treatments for vitiligo. Ann Transl Med. 2015;3:343. doi: 10.3978/j.issn.2305-5839.2015.11.36. This is a review of existing investigational anitbodies and small-molecule inhibitors with the potential to be tested in trial for vitiligo treatment.
- 36. Craiglow BG, King BA. Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy. JAMA Dermatol. 2015;151:1110–1112. doi: 10.1001/jamadermatol.2015.1520. This is the first case report of a patient with vitiligo improving on an oral JAK inhibitor.
- 37. Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA) J Am Acad Dermatol. 2016;74:370–371. doi: 10.1016/j.jaad.2015.09.073. This case report described a patient with vitiligo and alopecia areata who improved on ruxolitinib and his response to treatment was associated with a decrease in serum CXCL10 level.
- 38.Jabbari A, Dai Z, Xing L, et al. Reversal of Alopecia Areata Following Treatment With the JAK1/2 Inhibitor Baricitinib. EBioMedicine. 2015;2:351–355. doi: 10.1016/j.ebiom.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82–83. doi: 10.1002/ajh.23871. [DOI] [PubMed] [Google Scholar]
- 40. Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988–2990. doi: 10.1038/jid.2014.260. This is the first case report of a patient with alopecia areata improving on an oral JAK inhibitor.
- 41.Craiglow BG, Tavares D, King BA. Topical Ruxolitinib for the Treatment of Alopecia Universalis. JAMA Dermatol. 2015 doi: 10.1001/jamadermatol.2015.4445. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y, Gartner U, Smith FJ, McLean WH. Statins downregulate K6a promoter activity: a possible therapeutic avenue for pachyonychia congenita. J Invest Dermatol. 2011;131:1045–1052. doi: 10.1038/jid.2011.41. [DOI] [PubMed] [Google Scholar]
- 43.Noel M, Gagne C, Bergeron J, et al. Positive pleiotropic effects of HMG-CoA reductase inhibitor on vitiligo. Lipids Health Dis. 2004;3:7. doi: 10.1186/1476-511X-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal P, Rashighi M, Essien KI, et al. Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J Invest Dermatol. 2015;135:1080–1088. doi: 10.1038/jid.2014.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.University of Massachusetts. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2015 Nov 19]. Clinical Trial of Simvastatin to Treat Generalized Vitiligo. Available from: https://clinicaltrials.gov/ct2/show/NCT01517893 NLM Identifier: NCT01517893. [Google Scholar]
- 46.Centre Hospitalier Universitaire de Nice. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2016 Jan 19]. Atorvastatin in Active Vitiligo. Available from: https://clinicaltrials.gov/ct2/show/NCT02432534 NLM Identifier: NCT02432534. [Google Scholar]
- 47.Lattouf C, Jimenez JJ, Tosti A, et al. Treatment of alopecia areata with simvastatin/ezetimibe. J Am Acad Dermatol. 2015;72:359–361. doi: 10.1016/j.jaad.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Guttman-Yassky E, Ungar B, Noda S, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol. 2016;137:301–304. doi: 10.1016/j.jaci.2015.11.001. [DOI] [PubMed] [Google Scholar]



