Summary
Human paramyxoviruses are the etiological agents for life-threatening respiratory virus infections of infants and young children. These viruses – including respiratory syncytial virus (RSV), the human parainfluenza viruses (hPIV1-4), and human metapneumovirus (hMPV) – are responsible for millions of serious lower respiratory tract infections each year worldwide. There are currently no standard treatments and no licensed vaccines for any of these pathogens. Here we review research with which Sendai virus, a mouse parainfluenza virus type 1, is being advanced as a Jennerian vaccine for hPIV1 and as a backbone for RSV, hMPV, and other hPIV vaccines for children.
Keywords: Sendai virus, paramyxovirus, pediatric vaccine, respiratory tract, antibody, T cell, vaccine vector, intranasal
Pediatric disease caused by the human paramyxoviruses
Currently, there are approximately 130 million births worldwide each year. By the time these children reach their fifth birthday, millions will suffer a serious viral respiratory disease, which may culminate in hospitalization and possibly death [1,2]. The lay community is largely unaware of the many viruses responsible for serious pediatric respiratory tract diseases. ‘Flu’ is used as a catch-all phrase, when in fact the most frequent, serious respiratory virus infections of the <5 year old age group are paramyxovirus infections, not influenza virus infections. Other viral respiratory infections (e.g. adenovirus, rhinovirus, or coronavirus infections) are usually less threatening in pediatrics, except when children are immunocompromised [2,3].
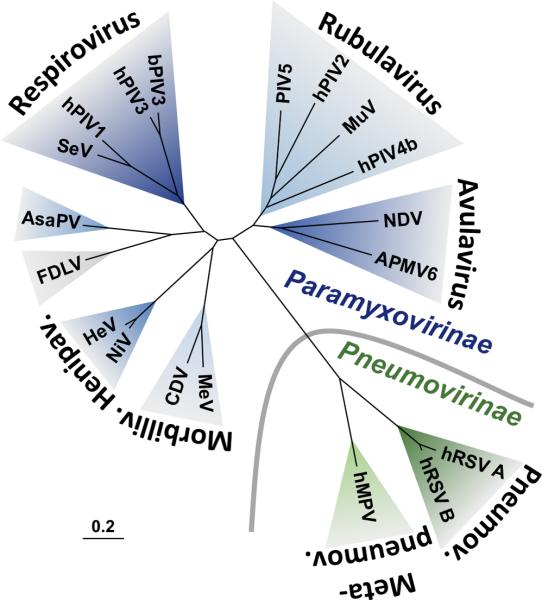
The family Paramyxoviridae consists of two subfamilies, Paramyxovirinae and Pneumovirinae, and multiple genera [4]. A phylogenetic tree is shown in Figure 1 to illustrate the relationships between representative viruses in the two subfamilies. Important human pathogens from the Paramyxovirinae subfamily include measles virus (MeV), mumps virus, Nipah virus (NiV), Hendra virus (HeV), and the human parainfluenza viruses (hPIVs). The Pneumovirinae subfamily includes human respiratory syncytial viruses A and B (hRSVA and hRSVB) and the more recently discovered human metapneumovirus (hMPV).
FIGURE 1. Phylogenetic tree based on the F protein sequences of selected paramyxoviruses.
Paramyxovirinae (blue) and Pneumovirinae (green) subfamilies are separated by a gray curved line. Genera are named in a bold, curved font. Aquaparamyxovirus and Ferlavirus genera are not shown but member species Atlantic salmon paramyxovirus (AsaPV) and Fer-de-Lance paramyxovirus (FDLV), respectively, are shown. Other virus names are abbreviated as follows: avian paramyxovirus 6 (APMV6), bovine parainfluenza virus 3 (bPIV3), canine distemper virus (CDV), Hendra virus (HeV), human metapneumovirus (hMPV), human parainfluenza virus 1 (hPIV1), human parainfluenza virus 2 (hPIV2), human parainfluenza virus 3 (hPIV3), human parainfluenza virus 4b (hPIV4b), human respiratory syncytial virus (hRSV), measles virus (MeV), mumps virus (MuV), Newcastle disease virus (NDV), Nipah virus (NiV), parainfluenza virus 5 (PIV5), Sendai virus (SeV). Virus taxonomy is shown according to the 2014 release by the International Committee on the Taxonomy of Viruses (ICTV). The phylogenetic tree was generated with CLC Main Workbench (CLC bio). The scale bar represents branch length as base substitutions per site.
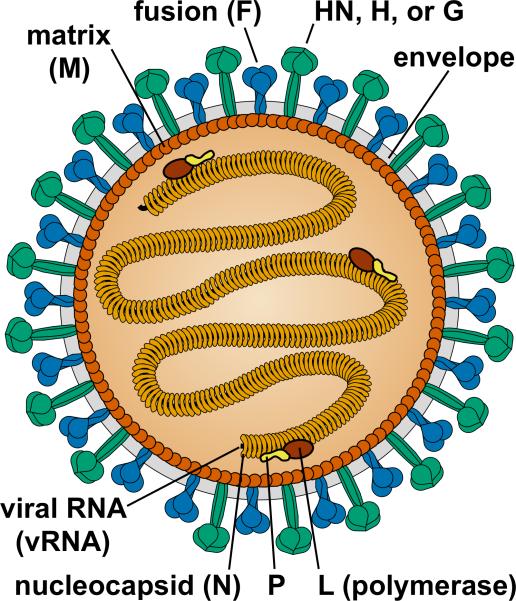
The structure of a paramyxovirus is illustrated in Figure 2. These are enveloped viruses encased by a lipid bilayer derived from the plasma membrane of their host cell. They contain a single-stranded, non-segmented, negative strand RNA genome of approximately 15 kb. The paramyxoviruses that are best known as etiological agents for serious, pediatric respiratory tract disease are RSV, hPIVs, and hMPV [1,2,4-6].
FIGURE 2. Structure of a paramyxovirus genome [4].
Viral RNA (vRNA) is encapsidated by nucleoproteins (N). The ribonucleoprotein (RNP) core consists of vRNA, N protein, phosphoprotein (P), and large (L) polymerase protein. The matrix (M) protein interacts with the RNP, plasma membrane, and envelope glycoproteins to promote virus assembly and budding. All known paramyxoviruses contain a fusion (F) protein and an attachment protein named hemagglutinin-neuraminidase (HN), hemagglutinin (H), or glycoprotein (G) depending on its functional activities or lack thereof. Only conserved paramyxovirus structural proteins are shown. Many paramyxovirus virions are spherical, 150 to 350 nm diameter, but some are pleomorphic or filamentous.
Respiratory syncytial virus (RSV)
RSV is a major cause of serious pediatric hospitalizations throughout the world due to acute lower respiratory tract infections (ALRIs)[1,7-10]. By 3 years of age, almost all children will have been exposed to RSV [7], a virus that can cause life-threatening bronchiolitis and pneumonia. In the United States alone, 70,000 to 120,000 infants under the age of 6 months are hospitalized each year due to RSV infection [11], at a cost of several hundred million dollars [12]. Worldwide, there are approximately 34 million episodes of RSV-associated ALRI in children less than 5 years of age with at least 3 million cases resulting in hospitalization [1]. A comprehensive review of RSV incidence among infants across several countries revealed a range of 10-30 cases of RSV-associated severe ALRI per 1000 children years in the age group [5]. Worldwide, there may be up to 199,000 deaths per year, 99% of which occur in developing countries [1].
Infants are highly vulnerable to RSV upon their first exposure, particularly if they are premature, immunodeficient, or suffer from congenital heart or lung disease. Maternal antibodies can be protective in the neonate and antibody titers correlate with improved outcome, but antibodies wane rapidly during the first few months of life [13]. For survivors of a first infection, an endogenous, protective immune response is usually generated so that disease, if any, caused by a second exposure to the same virus is often mild and hospitalizations are relatively rare [14]. Nonetheless, a first episode of disease can be followed by long-term sequelae including wheezing and asthma. Currently there is no vaccine and no standard treatment. For those who are most vulnerable, Palivizumab monoclonal antibody treatment is recommended to prevent RSV illness, but this is a costly form of prophylaxis that is unavailable to most individuals who need it [15]. Furthermore, a significant fraction of children progress to serious disease despite Palivizumab treatment. A new antibody was developed in recent years to replace Palivizumab (Motavizumab), but improvements in efficacy were insufficient to support advanced drug development [16,17].
Human parainfluenza viruses (hPIV)
Human parainfluenza viruses exist as four distinct serotypes. Types 1, 2, and 3 cause the most disease. In one study, hPIV1, hPIV2, and hPIV3 were respectively responsible for 6, 3, and 12 percent of pediatric hospitalizations for respiratory disease [18]. hPIV4 is identified only rarely as a cause of disease, although most children are likely infected as evidenced by seroprevalence data [9]. As a group, the parainfluenza viruses may cause as many ALRIs as RSV, but the consequences are not usually as severe [5,9,19,20]. hPIV types 1 and 2 are responsible for most cases of laryngotracheobronchitis, better known as croup, but can also cause bronchiolitis, tracheobronchitis, pneumonia, wheezing, and other forms of lower respiratory tract disease [9,21]. These viral diseases usually strike children at an age of greater than 6 months. Outbreaks with hPIV1 or hPIV2 may occur biennially, sometimes in alternating years with one another. Most children recover from hPIV1 and hPIV2 virus infections in the developing world, but significant morbidity can be experienced. There is also considerable financial burden and missed work by caregivers, and the viruses can be deadly in patients who suffer from immunodeficiencies. One United States study was conducted among children <6 years of age from September to December in the epidemic year of 1991 to monitor infections with hPIV1 and hPIV2. Results were extrapolated to suggest that there were approximately 250,000 emergency room visits and about 70,000 hospitalizations that year nationally caused by hPIV1 and hPIV2, with overall costs approaching 200 million US dollars [21]. hPIV3 is different from hPIV1 and hPIV2 in that it often strikes children less than 6 months of age, being second only to RSV as a cause of bronchiolitis and pneumonia in these young infants [9].
Human metapneumovirus (hMPV)
hMPV was discovered in 2001. Since then, the Centers for Disease Control and Prevention (CDC) have been collecting data from children who have suffered an ALRI. Results showed that among infants less than 6 months of age, hMPV was responsible for annual rates of hospitalization of approximately 3 per 1000 [6]. A similar study in Soweto revealed a rate of approximately 6 per 1000 infant years [22]. These hospital rates are less than some reported for RSV or for the combined hPIVs, but are clearly indicative of significant morbidity in pediatrics. Disease caused by hMPV includes pneumonia, bronchiolitis, and asthma [6].
Young infants and children clearly constitute a highly vulnerable population to each of the paramyxoviruses, but these are not the only victims. Elderly adults and people of any age with compromised immune, pulmonary, or cardiac systems are highly susceptible to RSV, hMPV, and the hPIVs [3,6,8,20,23-25]. As an example, there may be 11,000 elderly persons who die annually in the United States alone from diseases related to RSV infections [26]. Solutions including licensed antivirals or preventive vaccines are not yet available for any age group.
Strategies to vaccinate against respiratory paramyxoviruses
Despite the high impact of RSV, hMPV, and the hPIVs on public health, there are no standard treatments for any of the respiratory paramyxoviruses apart from supportive care. Vaccination is the single best health-care solution to infectious disease, yet no licensed vaccines are currently available [11,15,27,28].
Based on the epidemiology and impact of the paramyxoviruses, current vaccination programs target multiple populations, including immunologically naïve infants (less than 6 months of age for RSV) and the elderly. In addition, there is a current focus on young, school-aged children, because these children are often infected, and although not seriously ill, may frequently transmit RSV to high-risk populations [29]. Vaccination programs are also currently targeting women of child-bearing years and expectant mothers. By boosting maternal antibody levels, vaccines may reduce infections and disease both in the mothers and in the infants upon passive transfer of high-titered antibodies at birth [30,31].
A plethora of approaches have been and are being taken to develop vaccines against RSV, hMPV, and the hPIVs (reviewed in [25,27,28,32-37]). Inactivated whole-virus vaccine trials in the 1960s using formalin-inactivated RSV (FI-RSV) or a trivalent cocktail of FI-hPIV (that included types 1, 2, and 3) failed to protect children from infection. Notoriously, in the case of the FI-RSV vaccine, there was enhanced illness after the vaccinated individuals were naturally exposed to RSV [38,39]. Because of this unexpected outcome, development of whole-inactivated vaccines for infants has since been discouraged in the RSV field [40].
Purified protein vaccines have been pursued for decades, with much attention given to the G [41,42] or F proteins [43] of RSV. Today, the RSV F protein is used preferentially in RSV vaccine candidates due to its high conservation among circulating viral isolates, but many other proteins, both external and internal, can be and have been used (e.g. G, SH, M, NP, and P [27,42,44-48]). Protein-based vaccine candidates have included subunit (purified proteins and peptides) and particulate (virus-like particles, virosomes, and nanoparticles) preparations [49]. RSV F proteins have been presented in both post-fusion and pre-fusion conformations, both of which have shown some merit [33,50-52]. A number of neutralizing monoclonal antibody binding sites has been mapped precisely to one or both of the two forms [53-55]. A nanoparticle vaccine produced with post-fusion F proteins derived from baculovirus has advanced to clinical trials in the elderly and in women of child-bearing age, with encouraging results [50]. Another strategy for paramyxovirus vaccine development utilizes non-viral gene-based vectors, including RNA and DNA, the latter delivered in purified form or by bacteria-mediated plasmid transfer [56].
Currently, purified protein vaccines and other vaccines that are replication incompetent (as described above) are deemed most attractive for elderly populations, while replication-competent mucosal vaccines are deemed most attractive for young infants [57]. Possibly, these preferences will change with time as new vaccines are developed. To date, pre-clinical and clinical tests have been conducted using numerous replication-competent vaccines including attenuated (e.g. by cold-adaptation or site-directed mutagenesis) RSV, hPIV, and chimeric vaccines [24,28,58,59]. Replication-competent vaccine backbones used to deliver RSV, hPIV, and/or hMPV antigens have included poxvirus, alphavirus, adenovirus, adeno-associated virus (AAV), measles virus, bovine PIV3 (bPIV3), PIV5, Newcastle disease virus (NDV), and SeV, the focus of the present review [28,60-64].
SeV, the virus
SeV is a mouse parainfluenza virus type 1 that was discovered in Sendai, Japan, in the 1950s [65]. The virus was once named Hemagglutinating Virus of Japan (HVJ) by the Japanese Society for Virology, but was later termed ‘newborn virus pneumonitis (type Sendai)’ [66]. The name Sendai virus, or SeV, is currently most popular. The virus was first recovered from a mouse used to passage a human patient sample, causing confusion as to the virus’ origin [65-67]. SeV is now understood to be a pathogen of mice, not humans [25]. Fukumi et. al. first described SeV infections of mice in 1954 [67]. This infection can be subclinical, but SeV is also known as one of the leading causes of pneumonia in certain mouse strains [67,68]. SeV is uniquely sensitive to interferon-associated responses in humans, perhaps explaining, at least in part, its host-range restriction [69]. SeV grows to high titers in both chicken eggs and in FDA-approved mammalian cell lines, an advantage for vaccine production.
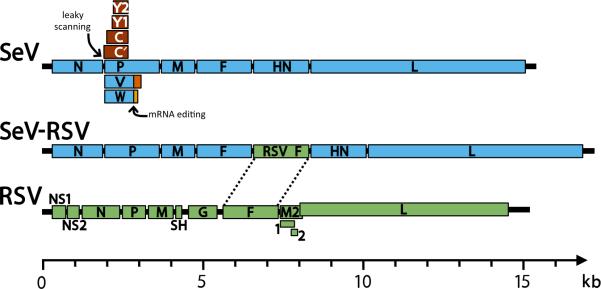
SeV is a member of the Respirovirus genus of the Paramyxovirinae subfamily. As with other paramyxoviruses, SeV is an enveloped virus with a non-segmented, negative-strand RNA genome [4]. A schematic of the SeV genome is shown at the top of Figure 3. The SeV genome includes a 3’ leader sequence, a 5’ trailer, and six structural genes, which are transcribed in the order of nucleocapsid (N), phosphoprotein (P), matrix (M), fusion (F), hemagglutinin-neuraminidase (HN), and large polymerase (L). It is noteworthy that several additional proteins are produced by leaky scanning (yielding C’, C, Y1, and Y2 proteins)[70,71] and pseudotemplated addition of nucleotides, otherwise known as mRNA editing (yielding V and W proteins)[72,73].
FIGURE 3. Schematic diagram of paramyxovirus genomes [4].
Genomes shown include Sendai virus (SeV, blue), respiratory syncytial virus (RSV, green), and a SeV-vectored vaccine that has the RSV F protein inserted between SeV F and HN genes (SeV-RSV). In each genome the 3’ leader is positioned on the left terminus and the 5’ trailer is on the right. Intergenic junctions between genes (not shown) contain transcription stop, intergenic, and transcription start sequences. In the SeV P gene, leaky scanning products include the C’, C, Y1, and Y2 proteins (brown). Also in the SeV P gene, mRNA editing produces V and W proteins that share their N-terminal portion with V (blue) and have an alternate reading frame in their C-terminal portion (orange). For simplicity, the alternate products from the P gene are omitted from the SeV-RSV schematic. In the RSV genome, the M2 gene contains two overlapping products, M2-1 and M2-2. The RSV M2 and L genes also overlap. Genomes are drawn to scale (bottom).
At the core of the virion is a helical nucleocapsid, which contains the viral RNA genome and ~2,600 N, 300 P, and 50 L proteins [74](see Figure 2). Efficient replication of the SeV genome and antigenome requires that the total number of RNA nucleotides is an even multiple of six [75-77]. Therefore, it is necessary to obey the “rule of six” when cloning a foreign antigen insert into the SeV vector. The M protein is the most abundant protein in the virion, and it functions to promote virus-particle formation by interacting with itself, the nucleocapsid, the plasma membrane of the host cell, and the cytoplasmic tails of the F and HN envelope glycoproteins [78-83]. The F and HN proteins are Type I and II membrane proteins, respectively, with ectodomains that project as spikes perpendicularly from the surface of the viral envelope [84,85]. During virus entry the HN protein binds to sialic-acid containing receptors on the plasma membrane surface, triggering the F protein at neutral pH to refold into a hairpin structure that causes fusion between the viral and host cell membranes [86-88]. During envelope glycoprotein trafficking to the cell surface, the HN protein destroys its own receptors to allow efficient release of progeny virions and to prevent virion aggregration or superinfection of previously infected cells [85,89].
The polar mechanism of SeV transcription is typical of the paramyxoviruses and other members of the order Mononegavirales, negative-strand RNA viruses that have all genes in tandem on a single genome [4](see Figure 3, top diagram). For SeV, the N gene is transcribed first and 90% of initiated N mRNA transcripts are completed [90]. Between each gene is a viral gene junction that contains a gene end region, an intergenic region (GUU), and a gene start sequence. At each gene junction, the viral RNA-dependent RNA polymerase (RdRp) is directed to terminate transcription, polyadenylate the nascent mRNA, and reinitiate transcription of the next gene. However, the frequency of reinitiating transcription is imprecise, and depends on the identity of the transcript start sequence [91]. This results in a gradient of mRNA transcripts being produced with the following abundance: N > P > M >> F > HN > L. Consequently, when a foreign reporter gene or vaccine antigen is inserted into the SeV genome, the degree to which the insert is positioned toward the initiating 3’ end of the genome determines the extent to which the foreign gene is expressed and the degree to which SeV replication is attenuated [92,93].
SeV as a Jennerian vaccine for hPIV1
Because SeV is a mouse parainfluenza virus type 1, it has been advanced as a Jennerian vaccine for hPIV1 [94]. The strategy follows that of Edward Jenner, a clinician of the late 1790s who tested a substance from cow lesions as a vaccine for the human smallpox virus. While unbeknownst to Jenner at the time, the substance (cowpox virus) shared significant protein similarity with the smallpox virus, and therefore primed cross-reactive lymphocytes including B cells, T helper (TH) cells, and cytotoxic T lymphocytes (CTL). Furthermore, this live-viral vaccine could elicit immune responses that lasted for decades after a single immunization [95,96]. Jenner's vaccine strategy was eventually advanced around the globe in a campaign sponsored by the World Health Organization, resulting in the complete eradication of smallpox from the human population . No other vaccine has yet matched this degree of success.
In the 1990s, researchers at St. Jude observed that SeV and hPIV1, like cowpox and smallpox, were well matched in their protein sequences [97]. Results encouraged the testing of B cells, TH cells, and CTL for cross-reactivity between the two viruses. Indeed, there was significant cross-reactivity for both cellular and humoral activities [98,99]. When tested in mice, SeV could elicit rapid and durable PIV-specific B cell and T cell responses systemically, and also in the respiratory mucosa [100,101]. Responses generally peaked within the first month post-vaccination, but were sustained at significant values in sera and respiratory tissues for the animal's lifetime. Due to long-lasting virus-specific cells in respiratory tracts, animals maintained a robust defense against pathogen at its point of entry.
The potential for SeV as a Jennerian vaccine was further revealed when mice were vaccinated with hPIV1 and subsequently challenged with SeV. Both adult and young mice were protected [102]. Experiments in African green monkeys were then conducted to support translation of the SeV vaccine to human clinical trials [103]. Monkeys were vaccinated and boosted with SeV intranasally. The vaccine infected the respiratory tract transiently and was cleared with no evidence of adverse events. Serum antibody responses were identified just days after the first vaccination. Monkeys also exhibited serum neutralizing activities, and antibodies were measured both in blood and in nasal passages. When vaccinated monkeys were challenged with hPIV1, all animals were completely protected against infection, whereas all control animals were infected [103].
Pre-clinical results encouraged advancement of SeV to a clinical study as a vaccine for hPIV1. With FDA oversight, SeV was tested first in adults and then in 3-6 year old children who were seropositive for hPIV1 [104,105]. In each age group, three sequential doses of SeV were tested: 5×105, 5×106 and 5×107 EID50. The vaccine was well tolerated in all participants. Boosts in preexisting PIV-specific binding and neutralizing antibody activities were observed in a subset of adults and in the majority of children, suggesting that even when an individual was already hPIV1 seropositive, he/she may have benefited from vaccination with SeV. A clinical study of SeV in 1-2 year old children is currently underway.
SeV is particularly attractive as a human vaccine, because it is a known pathogen of mice, and has never caused a confirmed disease in humans [25]. SeV is not an attenuated human virus and is therefore not burdened with the concern that it will revert to its original pathogenic phenotype [106,107]. SeV is also attractive, because it grows transiently in the mammalian cell, allowing the cell to express antigens endogenously and with post-translational modifications matching those of target antigens and neutralizing epitopes [108]. Endogenous expression of antigens also ensures robust activation of CD8+ T cells [109]. These cells provide a fail-safe mechanism; if antibodies do not completely eliminate all incoming infectious particles, the CD8+ T cells can kill infected mammalian cells to block virus amplification.
SeV as a vaccine for RSV
With the advent of reverse genetics [110-113], the possibility of using SeV as a backbone for an RSV vaccine was realized. SeV can accommodate a foreign gene or genes of large size. Specifically, as demonstrated by Sakai et. al., a gene of > 3 KB can be inserted and efficiently expressed in SeV [111].
SeV vectors were produced with either the RSV F or G gene inserted between the F and HN genes of SeV [114-116]. Figure 3 depicts the insertion of RSV F between F and HN genes of the SeV genome. Following vaccination, SeV delivers the RSV F gene into mammalian cells. The RSV F gene is transcribed by the RdRp in the cytoplasm, translated into the endoplasmic reticulum, and trafficked through the secretory pathway to the surface of the mammalian host cell. RSV proteins, like mammalian proteins, are excluded from progeny SeV virions during virus assembly and budding [78,115].
It was originally proposed that at least two vectors may be required to represent RSV subtypes A and B [18]. However, a single SeV vector expressing the full-length RSV F protein (or a secreted F protein ectodomain) proved sufficient to generate neutralizing antibodies and protection in cotton rats against a variety of RSV challenges, including primary isolates of both A and B subtypes [115,116]. This result was consistent with the finding that Palivizumab, a monoclonal antibody against RSV F, provides significant prophylaxis against most strains of RSV [15]. There was no enhanced immunopathology in vaccinated animals upon RSV challenge, unlike the situation previously experienced with the ill-fated FI-RSV vaccine [117]. In a cotton rat model, the vaccine was also efficacious when administered in the presence of maternal antibodies at titers typical of a 2 month old human infant [118]. A recombinant SeV expressing the full-length RSV F protein of the A2 strain was then tested in African green monkeys. Per FDA request, the vaccine was administered both intranasally and intratracheally. Transient vaccine infection was observed with no adverse events. Upon intranasal challenge of test and control animals with RSV, vaccinated animals, but not controls, were completely protected from RSV infection of the lower respiratory tract [119].
An SeV-RSV-F recombinant has now been manufactured for clinical testing. Meanwhile, another recombinant SeV-based vaccine has been advanced to clinical studies. The vaccine was developed by the International AIDS Vaccine Initiative and the DNAVEC Corporation to prevent HIV [120]. The clinical protocol involved a prime-boost in adult participants using a recombinant SeV expressing HIV-1 Gag and an adenovirus 35 vector expressing a fusion protein comprising HIV-1 gag, reverse transcriptase, integrase, and nef (GRIN). This study was initiated in Rwanda, Kenya, and the United Kingdom in 2013. First results suggested safety and the induction of cellular and humoral immune responses in study participants [120].
SeV as a vaccine backbone for other hPIVs and for hMPV
Following the success of the SeV-based RSV vaccine in pre-clinical studies, a number of additional recombinants were produced. Specifically, the hPIV2 and hPIV3 hemagglutinin proteins were individually inserted into the SeV backbone and each new vaccine was shown to be protective in cotton rats against challenge with the target pathogen [121,122]. A protective SeV-based hMPV vaccine was also successful when tested in the cotton rat model (unpublished data). It was additionally shown that SeV vaccines could be mixed together for a single intranasal application. As an example, three SeV recombinants were mixed in a cocktail for simultaneous vaccination of cotton rats. Vaccine components expressed the hPIV2 HN protein, the hPIV3 HN protein, and the RSV F protein, respectively. Upon challenge with either hPIV1, hPIV2, hPIV3, or RSV, vaccinated animals were protected against each of the challenge viruses [122]. Results demonstrated the strength of the SeV cocktail vaccine approach as a means to target several paramyxovirus pathogens at once. A single SeV vector may also accommodate more than one gene. Two genes representing different pathogens might be inserted in tandem or in different positions within the SeV backbone, a strategy that is currently under investigation. SeV can also be used in combination with other vaccine platforms, either administered at the same time, or in prime-boost protocols [123,124]. In Table 1 are listed some of the several pathogens that have thus far been targeted with SeV research.
TABLE 1.
Paramyxovirus and other targets in SeV vaccine studies
| Pathogen targets of SeV-based vaccines | In vivo study hosts | References |
|---|---|---|
| PIV1 | Mice, Cotton rats, African green monkey, Humans | [100-105,125] |
| PIV2 | Cotton rats | [122] |
| PIV3 | Cotton rats | [121,122] |
| RSV | Cotton rats, African green monkeys | [37,114-116,119,121,122] |
| hMPV | Cotton rats | Manuscript in preparation |
| HIV | Mice, Macaques, Humans | [120,123,124,126,127] |
| Combination Targets | Cotton rats | [121,122] |
A way forward
RSV and hPIV vaccines have been sought for over one-half century. hMPV vaccines have been sought since the discovery of the pathogen over a decade ago. As stated above, no vaccines have yet reached licensure despite the testing of numerous vaccine candidates. Nonetheless, optimism is heightened by recent advances in the field and by the >30 year success of the live-viral mumps, measles, and rubella vaccine (MMR). The history of MMR proves the feasibility of preventing paramyxovirus infections by vaccination, and demonstrates the vast benefits to human health afforded by preventive healthcare. Over ½ century ago, the number of measles cases in a single year in the United States reached 503,282 [128], but in the year 2000, measles was declared eliminated in the United States. Today, in comparison to past years, cases have dropped by >99.9%. Outbreaks do occur, but these are due to the importation of measles virus by travelers and the exposure of unvaccinated populations [129,130]. Similarly, mumps virus infections have been reduced by >99% in the United States in the modern vaccine era as compared to the pre-vaccine era [128]. The MMR vaccine success further illustrates the attributes of live viral vaccines, in that durable humoral and cellular immune responses are induced to function interactively. Antibodies bind and neutralize virus particles as a first line of defense while T cells secrete cytokines and kill virus-infected cells if/when antibodies are not sterilizing [130,131].
Possibly, SeV will one day serve as another successful live viral vaccine for the prevention of paramyxoviruses infections. The SeV vector has many attractive qualities outlined in this review and in Table 2. Advantages of the SeV vector include the following: (1) high productive capacity in FDA-approved mammalian cell lines and chicken eggs; (2) an ability to induce durable immune responses (both humoral and cellular) systemically and at the respiratory mucosal surfaces of vaccinated animals; (3) protection afforded to small animals and non-human primates by vaccination; and (4) safety and immunogenicity demonstrated in children and adults in clinical trials. In small animals, SeV cocktails target not just one pathogen but multiple pathogens at once. An SeV vaccine success against the paramyxoviruses could have an enormous global healthcare benefit by preventing millions of cases of morbidity/mortality caused by RSV, hPIVs, and hMPV each year.
TABLE 2.
Attributes of SeV as a paramyxovirus vaccine
| Major SeV Attributes | References |
|---|---|
| There has never been a disease in humans known to be caused by SeV. SeV is a pathogen of mice, not humans. | [25,132] |
| SeV is not an attenuated human virus, and is not burdened by the concern that it may revert to its wildtype phenotype. | [106,107,133] |
| Following SeV vaccination, viral antigens are expressed endogenously by the mammalian cell and will undergo natural post-translational modifications within the cell, providing a good match for antigens expressed by the target pathogen. Neutralizing antibodies often depend on these precise protein conformations. When vaccine antigens are produced synthetically or in non-mammalian cells, their structures may not be well matched with those of the pathogen target. | [108,134] |
| SeV induces humoral responses systemically and mucosally. Robust responses in the nasal mucosa may block a respiratory pathogen at its point-of entry. | [100,101] |
| Endogenous production of viral antigens in SeV-infected cells promotes robust virus-specific CD8+ T cell responses. | [101,109] |
| Both B cell and T cell immune responses are long sustained. | [100,101] |
| SeV can be amplified in hens' eggs or mammalian tissue culture lines. | [135] |
| Phase I clinical studies show that SeV is well tolerated in adults and children. | [104,105] |
| The majority of children in phase I clinical studies showed improved immune responses following SeV vaccination despite the sero-positivity of children at study entry. | [105] |
| SeV is efficacious in a maternal antibody model, designed to mimic the passively-acquired maternal antibodies typical of a 2 month old infant. | [118] |
| SeV vectors and vaccines can be designed to target multiple, different paramyxoviruses, either individually or at the same time. | [121,122] |
EXPERT COMMENTARY
SeV has thus far proven effective for the induction of B cell and T cell immune responses and the protection of small and large research animals from paramyxovirus infections including hPIV1, hPIV2, hPIV3 and RSV. SeV-based vaccines can be used either singly or in combination to target one or more than one paramyxovirus pathogen. SeV can be used alone or in combination with other vaccines in prime-boost strategies. Safety and immunogenicity of SeV are evident from phase I clinical studies in human adults and children. Data from SeV-based vaccine studies encourage rapid clinical advancement of the SeV platform.
FIVE-YEAR VIEW
Basic research in the laboratory will continue to introduce new strategies (e.g. insertion of more than one foreign gene into a single SeV backbone), new vaccine combinations, and new pathogen targets. Perhaps clinical studies conducted in the next 5 years will culminate in proof of efficacy in humans and licensure of SeV-based vaccine products for the protection of humans from respiratory virus infections.
KEY ISSUES.
Respiratory paramyxoviruses kill hundreds of thousands of individuals each year and hospitalize millions.
There are no standard treatments and no licensed vaccines for any of these viruses.
Sendai virus (SeV)-based vaccines safely protect against paramyxovirus infections in small and large research animals.
Phase I clinical studies demonstrate SeV safety and immunogenicity in adults and children.
Acknowledgments
This work was supported by NIH NIAID R01 AI088729, R01 AI083370, and P01 AI054955, NCI P30 CA21765, and the American Lebanese Syrian Associated Charities (ALSAC). C Russell and J Hurwitz are named on a provisional patent describing a SeV vaccine backbone.
Abbreviations
- SeV
Sendai virus
- RSV
Respiratory syncytial virus
- hPIV
human parainfluenza virus; hMPV-human metapneumovirus
- FI-RSV
formalin-inactivated RSV
- ALRI
acute lower respiratory tract infection
- RdRp-RNA
dependent RNA polymerase
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
REFERENCES
Reference annotations
* Of interest
** Of considerable interest
- 1.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Shi T, McLean K, Campbell H, Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: A systematic review and meta-analysis. J Glob Health. 2015;5(1):010408. doi: 10.7189/jogh.05.010408. [This paper describes serious pediatric diseases caused by respiratory viruses worldwide.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couch RB, Englund JA, Whimbey E. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med. 1997;102(3A):2–9. doi: 10.1016/S0002-9343(97)00003-X. discussion 25-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamb RA, Parks GD. Paramyxoviridae: The viruses and their replication. In: Knipe DM, Howley PM, Griffin DE, et al., editors. 5th Edition Lippincott Williams and Wilkins; Philadelphia, PA: Fields Virology. 2007. pp. 1449–1496. [Google Scholar]
- 5.Schanzer DL, Langley JM, Tam TW. Hospitalization attributable to influenza and other viral respiratory illnesses in Canadian children. Pediatr.Infect.DIs.J. 2006;25(9):795–800. doi: 10.1097/01.inf.0000232632.86800.8c. [DOI] [PubMed] [Google Scholar]
- 6.Edwards KM, Zhu Y, Griffin MR, et al. Burden of human metapneumovirus infection in young children. N Engl J Med. 2013;368(7):633–643. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am.J Dis.Child. 1986;140(6):543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 8.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N.Engl.J.Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henrickson KJ. Lower respiratory viral infections in immunocompetent children. Advances in Pediatric Infectious Diseases. 1994;9:59–96. [PubMed] [Google Scholar]
- 10.Shaw CA, Ciarlet M, Cooper BW, et al. The path to an RSV vaccine. Curr.Opin.Virol. 2013;3(3):332–342. doi: 10.1016/j.coviro.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Schickli JH, Dubovsky F, Tang RS. Challenges in developing a pediatric RSV vaccine. Hum.Vaccin. 2009;5(9):582–591. doi: 10.4161/hv.9131. [DOI] [PubMed] [Google Scholar]
- 12.Marks MI. Respiratory syncytial virus infections. Clinical pediatrics. 1992;31(11):688–691. doi: 10.1177/000992289203101109. [DOI] [PubMed] [Google Scholar]
- 13.Hacimustafaoglu M, Celebi S, Aynaci E, et al. The progression of maternal RSV antibodies in the offspring. Arch.Dis.Child. 2004;89(1):52–53. doi: 10.1136/adc.2002.017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinoff JJ, O'Brien KL, Thumar B, et al. Young infants can develop protective levels of neutralizing antibody after infection with respiratory syncytial virus. J.Infect.Dis. 2008;198(7):1007–1015. doi: 10.1086/591460. [DOI] [PubMed] [Google Scholar]
- 15.Simoes EA, Groothuis JR, Carbonell-Estrany X, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J.Pediatr. 2007;151(1):34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Wu H, Pfarr DS, Johnson S, et al. Development of Motavizumab, an Ultra-potent Antibody for the Prevention of Respiratory Syncytial Virus Infection in the Upper and Lower Respiratory Tract1. J.Mol.Biol. 2007;368(3):652–665. doi: 10.1016/j.jmb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Carbonell-Estrany X, Simoes EA, Dagan R, et al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125(1):e35–e51. doi: 10.1542/peds.2008-1036. [DOI] [PubMed] [Google Scholar]
- 18.Murphy BR, Collins PL. Live-attenuated virus vaccines for respiratory syncytial and parainfluenza viruses: applications of reverse genetics. The Journal of clinical investigation. 2002;110(1):21–27. doi: 10.1172/JCI16077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed JA, Katz MA, Auko E, et al. Epidemiology of respiratory viral infections in two long-term refugee camps in Kenya, 2007-2010. BMC infectious diseases. 2012;12:7. doi: 10.1186/1471-2334-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberg GA, Hall CB, Iwane MK, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J.Pediatr. 2009;154(5):694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Henrickson KJ, Kuhn SM, Savatski LL. Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children. Clin.Infect.Dis. 1994;18(5):770–779. doi: 10.1093/clinids/18.5.770. [DOI] [PubMed] [Google Scholar]
- 22.Groome MJ, Moyes J, Cohen C, et al. Human metapneumovirus-associated severe acute respiratory illness hospitalisation in HIV-infected and HIV-uninfected South African children and adults. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2015;69:125–132. doi: 10.1016/j.jcv.2015.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan A, Wang C, Yang J, Shenep JL, Leung WH, Hayden RT. Symptomatic parainfluenza virus infections in children undergoing hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(10):1520–1527. doi: 10.1016/j.bbmt.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol. 2013;372:259–284. doi: 10.1007/978-3-642-38919-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karron RA, Collins PL. Parainfluenza Viruses. In: Knipe DM, Howley PM, Griffin DE, et al., editors. Fields Virology. 5th Edition. Lippincott Williams and Wilkins; Philadelphia, PA: 2007. pp. 1497–1526. [Google Scholar]
- 26.Branche AR, Falsey AR. Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs Aging. 2015;32(4):261–269. doi: 10.1007/s40266-015-0258-9. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz JL. Respiratory syncytial virus vaccine development. Expert Rev.Vaccines. 2011;10(10):1415–1433. doi: 10.1586/erv.11.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudraraju R, Jones BG, Sealy R, Surman SL, Hurwitz JL. Respiratory syncytial virus: current progress in vaccine development. Viruses. 2013;5(2):577–594. doi: 10.3390/v5020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munywoki PK, Koech DC, Agoti CN, et al. The source of respiratory syncytial virus infection in infants: a household cohort study in rural Kenya. J Infect Dis. 2014;209(11):1685–1692. doi: 10.1093/infdis/jit828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31(Suppl 2):B209–B215. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambach P, Hombach J, Ortiz JR. A global perspective of maternal influenza immunization. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen SC, Williams JV. New Approaches for Immunization and Therapy against Human Metapneumovirus. Clinical and vaccine immunology. 2015;CVI22(8):858–866. doi: 10.1128/CVI.00230-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham BS, Anderson LJ. Challenges and opportunities for respiratory syncytial virus vaccines. Curr Top Microbiol Immunol. 2013;372:391–404. doi: 10.1007/978-3-642-38919-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson LJ. Respiratory syncytial virus vaccine development. Semin Immunol. 2013;25(2):160–171. doi: 10.1016/j.smim.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Haynes LM. Progress and challenges in RSV prophylaxis and vaccine development. J Infect Dis. 2013;208(Suppl 3):S177–183. doi: 10.1093/infdis/jit512. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt AC, Schaap-Nutt A, Bartlett EJ, et al. Progress in the development of human parainfluenza virus vaccines. Expert Rev Respir Med. 2011;5(4):515–526. doi: 10.1586/ers.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takimoto T, Hurwitz JL, Zhan X, et al. Recombinant Sendai virus as a novel vaccine candidate for respiratory syncytial virus. Viral Immunology. 2005;18(2):255–266. doi: 10.1089/vim.2005.18.255. [DOI] [PubMed] [Google Scholar]
- 38.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am.J Epidemiol. 1969;89(4):449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 39.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am.J.Epidemiol. 1969;89:435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 40.Beeler JA, Eichelberger MC. Influenza and respiratory syncytial virus (RSV) vaccines for infants: safety, immunogenicity, and efficacy. Microb.Pathog. 2013;55:9–15. doi: 10.1016/j.micpath.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Power UF, Nguyen TN, Rietveld E, et al. Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. J.Infect.Dis. 2001;184(11):1456–1460. doi: 10.1086/324426. [DOI] [PubMed] [Google Scholar]
- 42.Choi Y, Mason CS, Jones LP, Crabtree J, Jorquera PA, Tripp RA. Antibodies to the central conserved region of respiratory syncytial virus (RSV) G protein block RSV G protein CX3C-CX3CR1 binding and cross-neutralize RSV A and B strains. Viral Immunology. 2012;25(3):193–203. doi: 10.1089/vim.2011.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piedra PA, Cron SG, Jewell A, et al. Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: a multi-center trial in children with cystic fibrosis. Vaccine. 2003;21(19-20):2448–2460. doi: 10.1016/s0264-410x(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 44.Brideau RJ, Walters RR, Stier MA, Wathen MW. Protection of cotton rats against human respiratory syncytial virus by vaccination with a novel chimeric FG glycoprotein. J.Gen.Virol. 1989;70:2637–2644. doi: 10.1099/0022-1317-70-10-2637. [DOI] [PubMed] [Google Scholar]
- 45.Connors M, Collins PL, Firestone CY, Murphy BR. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. Journal of Virology. 1991;65(3):1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Power UF, Plotnicky-Gilquin H, Huss T, et al. Induction of protective immunity in rodents by vaccination with a prokaryotically expressed recombinant fusion protein containing a respiratory syncytial virus G protein fragment. Virology. 1997;230(2):155–166. doi: 10.1006/viro.1997.8465. [DOI] [PubMed] [Google Scholar]
- 47.Dagouassat N, Robillard V, Haeuw JF, et al. A novel bipolar mode of attachment to aluminium-containing adjuvants by BBG2Na, a recombinant subunit hRSV vaccine. Vaccine. 2001;19(30):4143–4152. doi: 10.1016/s0264-410x(01)00168-2. [DOI] [PubMed] [Google Scholar]
- 48.Schepens B, De Baets S, Sedyen K, et al. Meeting presentation: SHe's a novel target for RSV vaccination.. 8th Respiratory Syncytial Virus Symposium; Santa Fe, NM. 2012. [Google Scholar]
- 49.Morrison TG, Walsh EE. Subunit and virus-like particle vaccine approaches for respiratory syncytial virus. Curr Top Microbiol Immunol. 2013;372:285–306. doi: 10.1007/978-3-642-38919-1_14. [DOI] [PubMed] [Google Scholar]
- 50*.Glenn GM, Fries LF, Thomas DN, et al. A randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J Infect Dis. 2015 doi: 10.1093/infdis/jiv406. [This paper describes a clinical RSV vaccine success in women of child bearing years.] [DOI] [PubMed] [Google Scholar]
- 51.Smith G, Raghunandan R, Wu Y, et al. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS One. 2012;7(11):e50852. doi: 10.1371/journal.pone.0050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swanson KA, Settembre EC, Shaw CA, et al. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc.Natl.Acad.Sci.U.S.A. 2011;108(23):9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLellan JS, Chen M, Leung S, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340(6136):1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McLellan JS, Chen M, Chang JS, et al. Structure of a major antigenic site on the respiratory syncytial virus fusion glycoprotein in complex with neutralizing antibody 101F. Journal of Virology. 2010;84(23):12236–12244. doi: 10.1128/JVI.01579-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLellan JS, Yang Y, Graham BS, Kwong PD. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol. 2011;85(15):7788–7796. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loomis RJ, Johnson PR. Gene-based vaccine approaches for respiratory syncytial virus. Curr Top Microbiol Immunol. 2013;372:307–324. doi: 10.1007/978-3-642-38919-1_15. [DOI] [PubMed] [Google Scholar]
- 57.Yang K, Varga SM. Mucosal vaccines against respiratory syncytial virus. Curr Opin Virol. 2014;6:78–84. doi: 10.1016/j.coviro.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162(1-2):80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Bayon JC, Lina B, Rosa-Calatrava M, Boivin G. Recent developments with live-attenuated recombinant paramyxovirus vaccines. Rev Med Virol. 2013;23(1):15–34. doi: 10.1002/rmv.1717. [DOI] [PubMed] [Google Scholar]
- 60.Murawski MR, McGinnes LW, Finberg RW, et al. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. Journal of Virology. 2010;84(2):1110–1123. doi: 10.1128/JVI.01709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karron RA, Thumar B, Schappell E, et al. Evaluation of two chimeric bovine-human parainfluenza virus type 3 vaccines in infants and young children. Vaccine. 2012;30(26):3975–3981. doi: 10.1016/j.vaccine.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fu YH, He JS, Wang XB, et al. A prime-boost vaccination strategy using attenuated Salmonella typhimurium and a replication-deficient recombinant adenovirus vector elicits protective immunity against human respiratory syncytial virus. Biochem.Biophys.Res.Commun. 2010;395(1):87–92. doi: 10.1016/j.bbrc.2010.03.144. [DOI] [PubMed] [Google Scholar]
- 63.Mok H, Lee S, Utley TJ, et al. Venezuelan equine encephalitis virus replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. Journal of Virology. 2007;81(24):13710–13722. doi: 10.1128/JVI.01351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crowe J, Mok H, Hedgecock J, Shepherd B, Johnston R. Alphavirus-based respiratory syncytial virus vaccine induces protection mediated by both humoral and cellular responses, even in the presence of passively-acquired antibodies. (Ed.^(Eds). 7th International Respiratory Syncytial Virus Symposium; Rotterdam-The Netherlands. 2010.p. 142. [Google Scholar]
- 65.Ishida N, Homma M. Sendai virus. Advances in Virus Research. 1978;23:349–383. doi: 10.1016/s0065-3527(08)60103-7. [DOI] [PubMed] [Google Scholar]
- 66.Kuroya M, Ishida N. Newborn virus pneumonitis (type Sendai). II. The isolation of a new virus possessing hemagglutinin activity. Yokohama medical bulletin. 1953;4(4):217–233. [PubMed] [Google Scholar]
- 67.Fukumi H, Nishikawa F, Kitayama T. A pneumotropic virus from mice causing hemagglutination. Japanese journal of medical science & biology. 1954;7(4):345–363. doi: 10.7883/yoken1952.7.345. [DOI] [PubMed] [Google Scholar]
- 68.Parker JC, Whiteman MD, Richter CB. Susceptibility of inbred and outbred mouse strains to Sendai virus and prevalence of infection in laboratory rodents. Infect.Immun. 1978;19(1):123–130. doi: 10.1128/iai.19.1.123-130.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bousse T, Chambers RL, Scroggs RA, Portner A, Takimoto T. Human parainfluenza virus type 1 but not Sendai virus replicates in human respiratory cells despite IFN treatment. Virus Res. 2006;121(1):23–32. doi: 10.1016/j.virusres.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 70.Giorgi C, Blumberg BM, Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- 71.Curran J, Kolakofsky D. Ribosomal initiation from an ACG codon in the Sendai virus P/C mRNA. The EMBO journal. 1988;7(1):245–251. doi: 10.1002/j.1460-2075.1988.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vidal S, Curran J, Kolakofsky D. Editing of the Sendai virus P/C mRNA by G insertion occurs during mRNA synthesis via a virus-encoded activity. J Virol. 1990;64(1):239–246. doi: 10.1128/jvi.64.1.239-246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hausmann S, Garcin D, Morel AS, Kolakofsky D. Two nucleotides immediately upstream of the essential A6G3 slippery sequence modulate the pattern of G insertions during Sendai virus mRNA editing. J Virol. 1999;73(1):343–351. doi: 10.1128/jvi.73.1.343-351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamb RA, Mahy BW, Choppin PW. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- 75.Samal SK, Collins PL. RNA replication by a respiratory syncytial virus RNA analog does not obey the rule of six and retains a nonviral trinucleotide extension at the leader end. Journal of Virology. 1996;70(8):5075–5082. doi: 10.1128/jvi.70.8.5075-5082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calain P, Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J Virol. 1993;67(8):4822–4830. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kolakofsky D, Pelet T, Garcin D, Hausmann S, Curran J, Roux L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72(2):891–899. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takimoto T, Bousse T, Coronel EC, Scroggs RA, Portner A. Cytoplasmic domain of Sendai virus HN protein contains a specific sequence required for its incorporation into virions. Journal of Virology. 1998;72(12):9747–9754. doi: 10.1128/jvi.72.12.9747-9754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lamb RA, Choppin PW. The synthesis of Sendai virus polypeptides in infected cells. II. Intracellular distribution of polypeptides. Virology. 1977;81(2):371–381. doi: 10.1016/0042-6822(77)90153-2. [DOI] [PubMed] [Google Scholar]
- 80.Sanderson CM, McQueen NL, Nayak DP. Sendai virus assembly: M protein binds to viral glycoproteins in transit through the secretory pathway. J Virol. 1993;67(2):651–663. doi: 10.1128/jvi.67.2.651-663.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanderson CM, Wu HH, Nayak DP. Sendai virus M protein binds independently to either the F or the HN glycoprotein in vivo. J Virol. 1994;68(1):69–76. doi: 10.1128/jvi.68.1.69-76.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heggeness MH, Smith PR, Choppin PW. In vitro assembly of the nonglycosylated membrane protein (M) of Sendai virus. Proc Natl Acad Sci U S A. 1982;79(20):6232–6236. doi: 10.1073/pnas.79.20.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ali A, Nayak DP. Assembly of Sendai virus: M protein interacts with F and HN proteins and with the cytoplasmic tail and transmembrane domain of F protein. Virology. 2000;276(2):289–303. doi: 10.1006/viro.2000.0556. [DOI] [PubMed] [Google Scholar]
- 84.Scheid A, Choppin PW. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- 85.Scheid A, Choppin PW. The hemagglutinating and neuraminidase protein of a paramyxovirus: interaction with neuraminic acid in affinity chromatography. Virology. 1974;62(1):125–133. doi: 10.1016/0042-6822(74)90308-0. [DOI] [PubMed] [Google Scholar]
- 86.Russell CJ, Luque LE. The structural basis of paramyxovirus invasion. Trends Microbiol. 2006;14(6):243–246. doi: 10.1016/j.tim.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Russell CJ, Jardetzky TS, Lamb RA. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. The EMBO journal. 2001;20(15):4024–4034. doi: 10.1093/emboj/20.15.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lamb RA, Paterson RG, Jardetzky TS. Paramyxovirus membrane fusion: lessons from the F and HN atomic structures. Virology. 2006;344(1):30–37. doi: 10.1016/j.virol.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang RT, Rott R, Wahn K, Klenk HD, Kohama T. The function of the neuraminidase in membrane fusion induced by myxoviruses. Virology. 1980;107(2):313–319. doi: 10.1016/0042-6822(80)90299-8. [DOI] [PubMed] [Google Scholar]
- 90.Vidal S, Kolakofsky D. Modified model for the switch from Sendai virus transcription to replication. J Virol. 1989;63(5):1951–1958. doi: 10.1128/jvi.63.5.1951-1958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kato A, Kiyotani K, Hasan MK, et al. Sendai virus gene start signals are not equivalent in reinitiation capacity: moderation at the fusion protein gene. Journal of Virology. 1999;73(11):9237–9246. doi: 10.1128/jvi.73.11.9237-9246.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tokusumi T, Iida A, Hirata T, Kato A, Nagai Y, Hasegawa M. Recombinant Sendai viruses expressing different levels of a foreign reporter gene. Virus Res. 2002;86(1-2):33–38. doi: 10.1016/s0168-1702(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 93.Burke CW, Mason JN, Surman SL, et al. Illumination of parainfluenza virus infection and transmission in living animals reveals a tissue-specific dichotomy. PLoS.Pathog. 2011;7(7):e1002134. doi: 10.1371/journal.ppat.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hurwitz JL. Development of recombinant Sendai virus vaccines for prevention of human parainfluenza and respiratory syncytial virus infections. Pediatr.Infect.DIs.J. 2008;27(10 Suppl):S126–S128. doi: 10.1097/INF.0b013e318168b780. [DOI] [PubMed] [Google Scholar]
- 95.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(10):4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 96.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol.Rev. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 97.Lyn D, Gill DS, Scroggs RA, Portner A. The nucleoproteins of human parainfluenza virus type 1 and Sendai virus share amino acid sequences and antigenic and structural determinants. Journal of General Virology. 1991;72:983–987. doi: 10.1099/0022-1317-72-4-983. [DOI] [PubMed] [Google Scholar]
- 98.Dave VP, Allan JE, Slobod KS, et al. Viral cross-reactivity and antigenic determinants recognized by human parainfluenza virus type 1-specific cytotoxic T-cells. Virology. 1994;199:376–383. doi: 10.1006/viro.1994.1135. [DOI] [PubMed] [Google Scholar]
- 99.Smith FS, Portner A, Leggiadro RJ, Turner EV, Hurwitz JL. Age-related development of human memory T-helper and B-cell responses toward parainfluenza virus type-1. Virology. 1994;205(2):453–461. doi: 10.1006/viro.1994.1665. [DOI] [PubMed] [Google Scholar]
- 100*.Sealy R, Jones BG, Surman SL, Hurwitz JL. Robust IgA and IgG-producing antibody forming cells in the diffuse-NALT and lungs of Sendai virus-vaccinated cotton rats associate with rapid protection against human parainfluenza virus-type 1. Vaccine. 2010;28(41):6749–6756. doi: 10.1016/j.vaccine.2010.07.068. [This paper describes the long-term establishment of virus-specific IgA and IgG responses in respiratory tract tissues.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101**.Rudraraju R, Surman S, Jones B, Sealy R, Woodland DL, Hurwitz JL. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology. 2011;410(2):429–436. doi: 10.1016/j.virol.2010.12.017. [This paper demonstrates the robust and long term immune responses induced by SeV vaccinations] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sangster M, Smith FS, Coleclough C, Hurwitz JL. Human parainfluenza virus-type 1 immunization of infant mice protects from subsequent Sendai virus infection. Virology. 1995;212:13–19. doi: 10.1006/viro.1995.1448. [DOI] [PubMed] [Google Scholar]
- 103.Hurwitz JL, Soike KF, Sangster MY, et al. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine. 1997;15:533–540. doi: 10.1016/s0264-410x(97)00217-x. [DOI] [PubMed] [Google Scholar]
- 104.Slobod KS, Shenep JL, Lujan-Zilbermann J, et al. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine. 2004;22(23-24):3182–3186. doi: 10.1016/j.vaccine.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 105**.Adderson E, Branum K, Sealy RE, et al. Safety and immunogenicity of an intranasal Sendai virus-based parainfluenza virus type 1 vaccine in 3-6 year old children. Clin.Vaccine Immunol. 2014 doi: 10.1128/CVI.00618-14. [This manuscript demonstrates the safety and immunogenicity of SeV in 3-6 year old children] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luongo C, Yang L, Winter CC, et al. Codon stabilization analysis of the “248” temperature sensitive mutation for increased phenotypic stability of respiratory syncytial virus vaccine candidates. Vaccine. 2009;27(41):5667–5676. doi: 10.1016/j.vaccine.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schickli JH, Kaur J, Tang RS. Nonclinical phenotypic and genotypic analyses of a Phase 1 pediatric respiratory syncytial virus vaccine candidate MEDI-559 (rA2cp248/404/1030DeltaSH) at permissive and non-permissive temperatures. Virus Res. 2012;169(1):38–47. doi: 10.1016/j.virusres.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 108.Henrickson KJ, Kingsbury DW, van Wyke Coelingh KL, Naeve CW, Portner A. Neutralizing epitopes of human parainfluenza virus type 3 are conformational and cannot be imitated by synthetic peptides. Vaccine. 1991;9:243–249. doi: 10.1016/0264-410x(91)90107-h. [DOI] [PubMed] [Google Scholar]
- 109.York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu.Rev.Immunol. 1996;14:369–396. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]
- 110.Nagai Y, Kato A. Paramyxovirus reverse genetics is coming of age. Microbiol.Immunol. 1999;43(7):613–624. doi: 10.1111/j.1348-0421.1999.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 111.Sakai Y, Kiyotani K, Fukumura M, et al. Accommodation of foreign genes into the Sendai virus genome: sizes of inserted genes and viral replication. FEBS Lett. 1999;456(2):221–226. doi: 10.1016/s0014-5793(99)00960-6. [DOI] [PubMed] [Google Scholar]
- 112.Kato A, Sakai Y, Shioda T, Kondo T, Nakanishi M, Nagai Y. Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative or positive sense. Genes Cells. 1996;1(6):569–579. doi: 10.1046/j.1365-2443.1996.d01-261.x. [DOI] [PubMed] [Google Scholar]
- 113.Garcin D, Pelet T, Calain P, Roux L, Curran J, Kolakofsky D. A highly recombinogenic system for the recovery of infectious Sendai paramyxovirus from cDNA: generation of a novel copy-back nondefective interfering virus. The EMBO journal. 1995;14(24):6087–6094. doi: 10.1002/j.1460-2075.1995.tb00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takimoto T, Hurwitz JL, Coleclough C, et al. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J Virol. 2004;78(11):6043–6047. doi: 10.1128/JVI.78.11.6043-6047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhan X, Hurwitz JL, Krishnamurthy S, et al. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine. 2007;25(52):8782–8793. doi: 10.1016/j.vaccine.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhan X, Slobod KS, Jones BG, et al. Sendai virus recombinant vaccine expressing a secreted, unconstrained respiratory syncytial virus fusion protein protects against RSV in cotton rats. Int Immunol. 2015;27(5):229–236. doi: 10.1093/intimm/dxu107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Prince GA, Jenson AB, Hemming VG, et al. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. Journal of Virology. 1986;57(3):721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jones BG, Sealy RE, Surman SL, et al. Sendai virus-based RSV vaccine protects against RSV challenge in an in vivo maternal antibody model. Vaccine. 2014;32(26):3264–3273. doi: 10.1016/j.vaccine.2014.03.088. [This manuscript demonstrates that SeV is protective in the presence of passively acquired antibodies, modeling the maternal antibodies of a 2 month old infant] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119**.Jones BG, Sealy RE, Rudraraju R, et al. Sendai virus-based RSV vaccine protects African green monkeys from RSV infection. Vaccine. 2012;30(5):959–968. doi: 10.1016/j.vaccine.2011.11.046. [This manuscript demonstrates the safety and efficacy of a SeV-based RSV vaccine in primates] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ishii H, Matano T. Development of an AIDS vaccine using Sendai virus vectors. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.06.114. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 121.Zhan X, Slobod KS, Krishnamurthy S, et al. Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine. 2008;26(27-28):3480–3488. doi: 10.1016/j.vaccine.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122**.Jones B, Zhan X, Mishin V, et al. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine. 2009;27(12):1848–1857. doi: 10.1016/j.vaccine.2009.01.041. [This manuscript demonstrates that cocktails of SeV can be used to protect against multiple paramyxoviruses at once.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brown SA, Surman SL, Sealy R, et al. Heterologous Prime-Boost HIV-1 Vaccination Regimens in Pre-Clinical and Clinical Trials. Viruses. 2010;2(2):435–467. doi: 10.3390/v2020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yu S, Feng X, Shu T, et al. Potent specific immune responses induced by prime-boost-boost strategies based on DNA, adenovirus, and Sendai virus vectors expressing gag gene of Chinese HIV-1 subtype B. Vaccine. 2008;26(48):6124–6131. doi: 10.1016/j.vaccine.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 125.Skiadopoulos MH, Surman SR, Riggs JM, et al. Sendai virus, a murine parainfluenza virus type 1, replicates to a level similar to human PIV1 in the upper and lower respiratory tract of African green monkeys and chimpanzees. Virology. 2002;297(1):153–160. doi: 10.1006/viro.2002.1416. [DOI] [PubMed] [Google Scholar]
- 126.Matano T, Kano M, Nakamura H, Takeda A, Nagai Y. Rapid appearance of secondary immune responses and protection from acute CD4 depletion after a highly pathogenic immunodeficiency virus challenge in macaques vaccinated with a DNA prime/Sendai virus vector boost regimen. J Virol. 2001;75(23):11891–11896. doi: 10.1128/JVI.75.23.11891-11896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Matano T, Kobayashi M, Igarashi H, et al. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. The Journal of experimental medicine. 2004;199(12):1709–1718. doi: 10.1084/jem.20040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Graham BS, Crowe Jr JE. Immunization against Viral Diseases. In: Knipe DM, Howley PM, Griffin DE, et al., editors. Fields Virology. 5th Edition. Lippincott Williams and Wilkins; Philadelphia, PA: 2007. pp. 487–538. [Google Scholar]
- 129.Lievano F, Galea SA, Thornton M, et al. Measles, mumps, and rubella virus vaccine (M-M-RII): a review of 32 years of clinical and postmarketing experience. Vaccine. 2012;30(48):6918–6926. doi: 10.1016/j.vaccine.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 130.Zachariah P, Stockwell MS. Measles vaccine- past, present and future. J Clin Pharmacol. 2015 doi: 10.1002/jcph.606. [DOI] [PubMed] [Google Scholar]
- 131.Jaye A, Magnusen AF, Sadiq AD, Corrah T, Whittle HC. Ex vivo analysis of cytotoxic T lymphocytes to measles antigens during infection and after vaccination in Gambian children. The Journal of clinical investigation. 1998;102(11):1969–1977. doi: 10.1172/JCI3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chanock RM, Murphy BR, Collins PL. Parainfluenza viruses. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams and Wilkins; Philadelphia PA: 2001. pp. 1341–1379. [Google Scholar]
- 133.Elliott MB, Pryharski KS, Yu Q, et al. Recombinant respiratory syncytial viruses lacking the C-terminal third of the attachment (G) protein are immunogenic and attenuated in vivo and in vitro. Journal of Virology. 2004;78(11):5773–5783. doi: 10.1128/JVI.78.11.5773-5783.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Javaherian K, Langlois AJ, Montefiori DC, et al. Studies of the conformation-dependent neutralizing epitopes of simian immunodeficiency virus envelope protein. Journal of Virology. 1994;68(4):2624–2631. doi: 10.1128/jvi.68.4.2624-2631.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Muramatsu M, Homma M. Trypsin action on the growth of Sendai virus in tissue culture cells. V. An activating enzyme for Sendai virus in the chorioallantoic fluid of the embryonated chicken egg. Microbiol Immunol. 1980;24(2):113–122. doi: 10.1111/j.1348-0421.1980.tb00569.x. [DOI] [PubMed] [Google Scholar]