The activation of the spindle assembly checkpoint
The Spindle Assembly Checkpoint (SAC) prevents anaphase onset in the absence of kinetochore-microtubule attachment, ensuring that cells with unattached kinetochores do not proceed into anaphase. This is accomplished via a signaling pathway that inhibits the Anaphase Promoting Complex/Cyclosome (APC/C), an E3 ligase that targets the anaphase inhibitor securin for degradation. The highly conserved SAC consists of Mad1, Mad2, BubR1/Mad3, Bub1, Bub3, and Mps1 kinase. In the absence of microtubule attachment, these proteins are recruited to the kinetochore scaffolding protein Knl1/Spc105, a process that is dependent on Mps1 kinase. Mps1 phosphorylates 6 repetitive MELT motifs of Spc105 to recruit Bub1/Bub3 complex to the kinetochore. Furthermore Mps1 has also been shown to phosphorylate Bub1, promoting Mad1 recruitment to the kinetochore.1 Mad1 forms a complex with Mad2, which promotes the conformational change of Mad2 from "open" to "closed" form. This closed form of Mad2 sequesters Cdc20, the activator of the APC/C, thus inhibiting the APC/C from targeting the anaphase inhibitor securin for degradation. In addition to the SAC activation process, the mechanism by which the SAC is silenced has also received a great deal of attention in recent years.
The regulation of Mps1 activity and SAC silencing
Because Mps1 kinase is essential for SAC activation, its down-regulation could be one mechanism for SAC silencing. Previous works show that Ndc80, one of the kinetochore-microtubule interface proteins, is the docking site for Mps1 kinase. Recent data indicate that the binding of Ndc80 with microtubule prevents the accessibility of Mps1 to its substrate Spc105 in budding yeast.2 In mammalian cells, Ndc80-microtubule binding has been shown to competitively abolish Mps1-Ndc80 interaction.3,4 Therefore, kinetochore-microtubule attachment likely weakens Mps1 activity at the kinetochore, which contributes to SAC silencing. However, it is unclear whether kinetochore-microtubule interaction also activates protein phosphatase at the kinetochore to reverse Mps1-imposed protein phosphorylation for SAC silencing. Moreover, this model cannot explain the role of chromosome bipolar attachment and the subsequent tension generation in SAC silencing.
Kinetochore-associated phosphatase protein 1 (PP1) and SAC silencing
The kinetochore protein Spc105 recruits PP1 in addition to the Bub1/Bub3 complex, and mutation of the PP1 binding site in yeast cells leads to metaphase arrest with intact kinetochore attachment, indicating the essential role of Spc105-bound PP1 in SAC silencing.5 Consistently, PP1 overexpression accelerates SAC silencing, further supporting the possibility that kinetochore-localized PP1 antagonizes checkpoint kinases Mps1 (and Ipl1?) to promote anaphase onset. Which substrates are dephosphorylated specifically by Spc105-associated PP1 to induce SAC silencing is a remaining question in the field, but key potential SAC substrates include Bub1, Mad1, Ndc80, and Dam1. Dephosphorylation of Bub1 is a likely a critical step in SAC silencing, as phospho-deficient Bub1 fails to recruit Mad1 to the kinetochore and cells with this mutant Bub1 show a SAC defect. Therefore, a major function of Spc105-associated PP1 is likely to promote the removal of Mad1/Mad2 from kinetochores through the dephosphorylation of Bub1.
What tips the balance of kinase/PP1 at the kinetochore to trigger SAC silencing? Recent work from our lab suggests that the tension dependent reversal of Ipl1 kinase-mediated phosphorylation is key to SAC silencing.6 The microtubule-associated Dam1 kinetochore complex strengthens Ndc80-microtubule interaction, and the reversal of Ipl1-imposed Dam1 phosphorylation allows SAC silencing in the presence of tensionless kinetochore attachment. In contrast, the phosphomimetic Dam1 mutation prevents SAC silencing as indicated by the persistent phosphorylation of SAC proteins Mad1 and Bub1. However, it remains unclear what initiates Dam1 dephosphorylation and how this dephosphorylation alters the kinase/phosphatase balance at the kinetochore.
SAC disassembly after anaphase onset
Interestingly, the kinetochore localization of PP1 is further regulated after anaphase onset through its regulatory subunit, Fin1. Our recent data along with others have shown that Fin1 is sequestered until anaphase onset by a yeast 14-3-3 homolog, Bmh1.7 Upon anaphase entry, activated Cdc14 Early Anaphase Release (FEAR) pathway dephosphorylates S-phase Cdk1 substrates, including Fin1. This dephosphorylation frees Fin1-PP1 from inhibition by Bmh1, allowing Fin1-PP1 to robustly localize to the kinetochore following anaphase onset. We observed that untimely localization of Fin1-PP1 to the kinetochore, either through BMH1 deletion or through hyperactive FEAR, resulted in premature SAC silencing. Surprisingly, we found that in the absence of Fin1, cells enter anaphase without any obvious delay but the SAC protein Bub1 was retained on the kinetochore in anaphase. This observation indicates the function of Fin1-PP1 in the removal of Bub1 from kinetochores after anaphase onset. Moreover it highlights the fact that Bub1 removal from the kinetochore is not a prerequisite for anaphase entry. Therefore, unlike the Spc105-associated PP1 that is essential for SAC silencing, Fin1-associated PP1 binds to kinetochores after anaphase onset to clear the SAC components. The two-step model for SAC regulation is illustrated in Figure 1.
Figure 1.
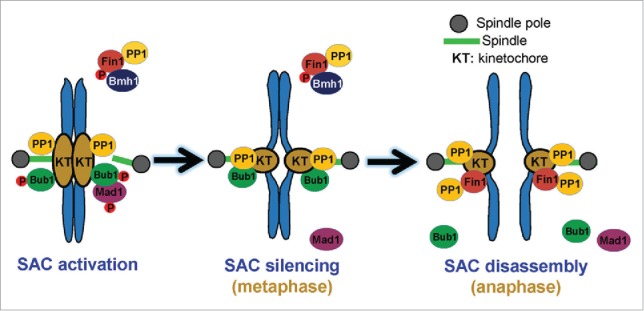
The 2-step model for the negative regulation of SAC. First, the balance of kinase (Mps1) and phosphatase (Spc105-associated PP1) likely triggers the dephosphorylation of SAC proteins like Mad1 and Bub1, resulting in SAC silencing. After anaphase onset, the dephosphorylation of Fin1 by the FEAR leads to robust kinetochore localization of Fin1-PP1, which may further dephosphorylates kinetochore proteins (Spc105), resulting in SAC removal from the kinetochore.
An open question is why cells need Fin1-PP1 to disassemble SAC after anaphase onset. One possibility is that this mechanism enables SAC reactivation by kinetochore detachment before SAC disassembly. In C. elegans, re-localization of Bub1 from the kinetochore to the spindle midzone during early anaphase is required for spindle midzone initiation. Thus, another possibility is that the kinetochore-localized Bub1 during anaphase facilitates the relocation of Bub1 to spindle midzone. However, more work is needed to understand the biological significance of the SAC removal from the kinetochore during anaphase. Also, it is necessary to clarify if higher eukaryotes share the same mechanism for kinetochore removal of SAC components.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by RO1GM102115 from NIH/NIGMS to Y.W.
References
- [1].London N, Biggins S. Mad1 kinetochore recruitment by Mps1-mediated phosphorylation of Bub1 signals the spindle checkpoint. Genes Dev 2014; 28:140-52; PMID:24402315; http://dx.doi.org/ 10.1101/gad.233700.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aravamudhan P, Goldfarb AA, Joglekar AP. RThe kinetochore encodes a mechanical switch to disrupt spindle assembly checkpoint signalling. Nat Cell Biol 2015; 17:868-79; PMID:26053220; http://dx.doi.org/ 10.1038/ncb3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ji Z, Gao H, Yu H. RCELL DIVISION CYCLE. Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science 2015; 348:1260-4; PMID:26068854; http://dx.doi.org/ 10.1126/science.aaa4029. [DOI] [PubMed] [Google Scholar]
- [4].Hiruma Y, Sacristan C, Pachis ST, Adamopoulos A, Kuijt T, Ubbink M, von Castelmur E, Perrakis A, Kops GJ. RCELL DIVISION CYCLE. Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling. Science 2015; 348:1264-7; PMID:26068855; http://dx.doi.org/ 10.1126/science.aaa4055. [DOI] [PubMed] [Google Scholar]
- [5].Rosenberg JS, Cross FR, Funabiki H. RKNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr Biol 2011; 21:942-7; PMID:21640906; http://dx.doi.org/ 10.1016/j.cub.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jin F, Wang Y. RThe signaling network that silences the spindle assembly checkpoint upon the establishment of chromosome bipolar attachment. Proc Natl Acad Sci U S A 2013; 110:21036-41; PMID:24324173; http://dx.doi.org/ 10.1073/pnas.1307595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bokros M, Gravenmier C, Jin F, Richmond D, Wang Y. RFin1-PP1 Helps Clear Spindle Assembly Checkpoint Protein Bub1 from Kinetochores in Anaphase. Cell Rep 2016; 14:1074-85; PMID:26832405; http://dx.doi.org/ 10.1016/j.celrep.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


