ABSTRACT
The accumulation of senescent disc cells in degenerative intervertebral disc (IVD) suggests the detrimental roles of cell senescence in the pathogenesis of intervertebral disc degeneration (IDD). Disc cell senescence decreased the number of functional cells in IVD. Moreover, the senescent disc cells were supposed to accelerate the process of IDD via their aberrant paracrine effects by which senescent cells cause the senescence of neighboring cells and enhance the matrix catabolism and inflammation in IVD. Thus, anti-senescence has been proposed as a novel therapeutic target for IDD. However, the development of anti-senescence therapy is based on our understanding of the molecular mechanism of disc cell senescence. In this review, we focused on the molecular mechanism of disc cell senescence, including the causes and various molecular pathways. We found that, during the process of IDD, age-related damages together with degenerative external stimuli activated both p53-p21-Rb and p16-Rb pathways to induce disc cell senescence. Meanwhile, disc cell senescence was regulated by multiple signaling pathways, suggesting the complex regulating network of disc cell senescence. To understand the mechanism of disc cell senescence better contributes to developing the anti-senescence-based therapies for IDD.
KEYWORDS: anti-senescence therapy, cause-dependent molecular pathways, disc cell senescence, Intervertebral disc degeneration, senescence-associated secreted phenotype
Introduction
Nowadays, low back pain (LBP) is prevalent worldwide and causes huge socio-economic burdens.1,2 Intervertebral disc degeneration (IDD) is a widely accepted cause of LBP. IDD is associated with the severity of LBP.3,4 Thus, elucidating the pathogenesis of IDD in detail benefits the development of effective therapies for LBP. The pathogenesis of IDD is very complicated. It has been demonstrated to involve cell apoptosis, autophagy, pro-inflammatory cytokine storm and increased matrix catabolism.5,6 Nevertheless, the mechanism of IDD is still not well established. More and more researchers devote themselves to elucidating the pathogenesis of IDD.
Since disc cell senescence was determined in degenerative discs, the number of studies discussing disc cell senescence increases every year. Cell senescence is classically defined as an irreversible cell-cycle arrest caused by telomere uncapping or various external stimuli. However, to date, the knowledge of characteristics of senescent cells has been expanded.7-10 Senescent cells tend to aggregate in cell clusters, and show increased cell size and flattened and vacuolized morphology in vitro. In vivo, the morphology of senescent cells depends on tissue structure. They are unresponsive to mitogenic stimulation and fail to replicate. Consequently, their cell cycle is halted at the G1 phase. Although losing the replicative capability, senescent cells aberrantly secretes pro-inflammatory cytokines, matrix degradation proteases, growth factors and chemokines.11,12 This pro-inflammatory phenotype of senescent cells is defined as senescence-associated secretory phenotype (SASP). The inflammatory microenvironment created by SASP via autocrine and paracrine has been identified as a key step in the occurrence and development of age-related diseases.7-10
With respect to the biomarkers of cell senescence, the first mentioned is senescence-associated β-galactosidase (SA-β-Gal). The activity of SA-β-Gal detected at pH 6.0 is related to the senescence-associated lysosomal activity. However, SA-β-Gal is not a specific biomarker of senescence because its activity is affected by the state of lysosome. Thus, other molecular biomarkers, such as p53 (tumor suppressor gene), cell-cycle kinase dependent (CDK) inhibitors (p16 and p21), cell cycle regulator (retinoblastoma protein, Rb), p38 and telomere length, are used to detect cell senescence. These molecular biomarkers are prominent cell-cycle regulators, and suggest the molecular signaling pathways of cell senescence. Based on the markers above mentioned, disc cell senescence in degenerative discs has been investigated in many researches. This phenomenon has been reviewed comprehensively.13 However, cell senescence is regulated by various molecular signaling pathways. The molecular mechanism of cell senescence is cause-dependent and cell type-dependent.7,14 Therefore, in the following sections, we will discuss the molecular mechanism of disc cell senescence in detail. The well-established molecular mechanism contributes to understanding the roles of disc cell senescence in the pathogenesis of IDD. More importantly, antagonizing disc cell senescence by regulating molecular signaling pathways helps to develop new potential therapies for IDD.
In this review, we briefly discussed the significance of disc cell senescence in the pathogenesis of IDD. Elucidating the effects of disc cell senescence on IDD helps to understand the pathogenesis of IDD better. Then, our review focused on the molecular mechanism of disc cell senescence. Because the mechanism is cause-dependent, we discussed the causes and signaling pathways of disc cell senescence respectively. In summary, regulating disc cell senescence on molecular level suggests a new potential therapeutic target of IDD.
The significance of disc cell senescence in the pathogenesis of IDD
The SA-β-Gal positive disc cells existed in both nucleus pulposus (NP) and annulus fibrosus (AF) specimens harvested from patients with IDD. They preferred to aggregate in cluster.15 The number of SA-β-Gal positive disc cells was positively correlated to the Thompson grade and Pfirrmann Grade of discs. It also was negatively related to the number of Ki67 positive cells (proliferated cells). Meanwhile, mean telomere length of disc cells shortened progressively with the degenerative grade of IDD increasing. NP cells isolated from degenerative discs proliferated slower and presented accelerated cell senescence than those from non-degenerative discs.16-19 Moreover, various signaling pathways were activated in senescent disc cells, which we will discuss in a separate section.18-22 All results above mentioned suggest that senescent disc cells accumulate in discs with IDD progressing. Enhanced disc cell senescence is a new hallmark of IDD.
The accumulation of senescent disc cells provides a novel insight into the pathogenesis of IDD. On the one hand, senescent disc cells are unable to generate new disc cells, thus the number of functional cells in discs decreases gradually due to cell death. On the other hand, the SASP of disc cells was characterized by a catabolic and pro-inflammatory phenotype.19,23, 24 Senescent disc cells may alter the pattern of secretion to change the microenvironment of discs.8,14 They decreased extracellular matrix (ECM) production and enhanced ECM degradation in IVD. Moreover, pro-inflammatory cytokines secreted by senescent disc cells, including TNF-α, IL-1β, IL-17, IL-6, COX-2 and chemokines may promote the senescence of neighboring disc cells and the infiltration of immune cells, then reinforce inflammation in the microenvironment of degenerative discs.6-10 In conclusion, senescent disc cells undergo a phenotypic shift and disrupt the balance between ECM anabolism and catabolism in discs. As a result, disc degeneration is accelerated (Fig. 1). However, we still need more evidence to elucidate the roles of disc cell senescence in the process of IDD. Further studies should investigate the precise phenotype of senescent disc cells to reveal the significance of disc cell senescence in IDD. Preventing disc cell senescence indicates a new therapeutic strategy for IDD.
Figure 1.
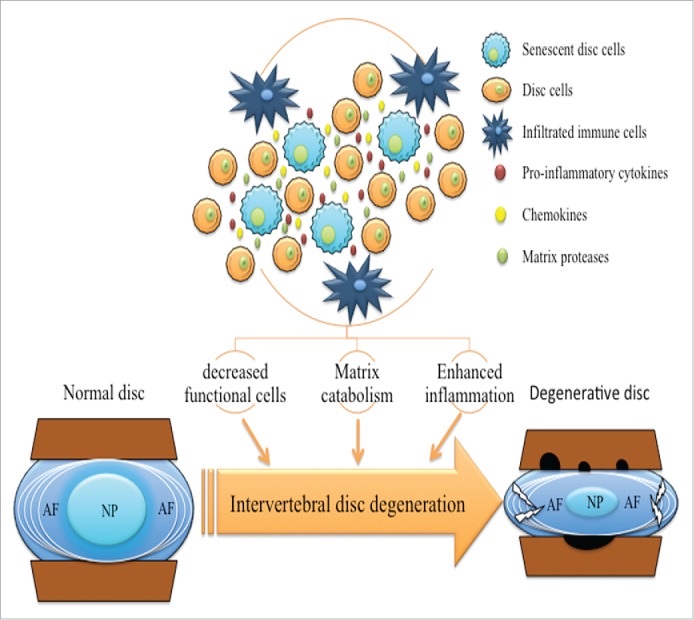
The roles of disc cell senescence in the pathogenesis of intervertebral disc degeneration. The senescent disc cells are unable to replicate, thus, the loss of functional cells occurs in discs. Furthermore, the senescence-associated secreted phenotype (SASP) of disc cells is characterized by a catabolic and pro-inflammatory phenotype. Senescent disc cells secrete matrix proteases to enhance extracellular matrix (ECM) catabolism in intervertebral disc. Meanwhile, pro-inflammatory cytokines secreted by senescent disc cells promote the senescence of surrounding disc cells and the infiltration of immune cells in discs, reinforcing the inflammation in the microenvironment of degenerative discs. As a result, disc degeneration is accelerated. NP, nucleus pulposus. AF, annulus fibrosus.
The molecular mechanism of disc cell senescence
Triggers of disc cell senescence
In this section, we focused on the molecular signaling pathways of disc cell senescence. As generally known, the activation of senescent molecular pathways is cause-dependent.7,8,14 Hence, we reviewed different causes of disc cell senescence first. Various causes of cell senescence have been identified, including telomere shortening, DNA damage, oxidative stress, oncogene activation and development cues.7-9,14,25 Herein, we just discussed the causes associated with disc cell senescence and IDD based on currently available evidence.
Telomere erosion, DNA damage and aging
During the serial replication, the telomere length becomes shorter and shorter due to the incomplete replication of the ends of DNA, which is the first identified cause of cell senescence. It explains the limited replicative capacity of eukaryotic cells. It mainly activates the p53-p21-Rb signaling pathway (discussed in the next section) to induce replicative senescence.26,27 In degenerative discs, decreased telomere length, declined telomerase activity and disc cell senescence were observed simultaneously. Telomere length decreased progressively with IDD advancing.18,19 The senescent signaling pathways were activated by telomere shortening,21 suggesting that telomere shortening triggers the replicative senescence of disc cells in the process of IDD (Fig. 2).
Figure 2.
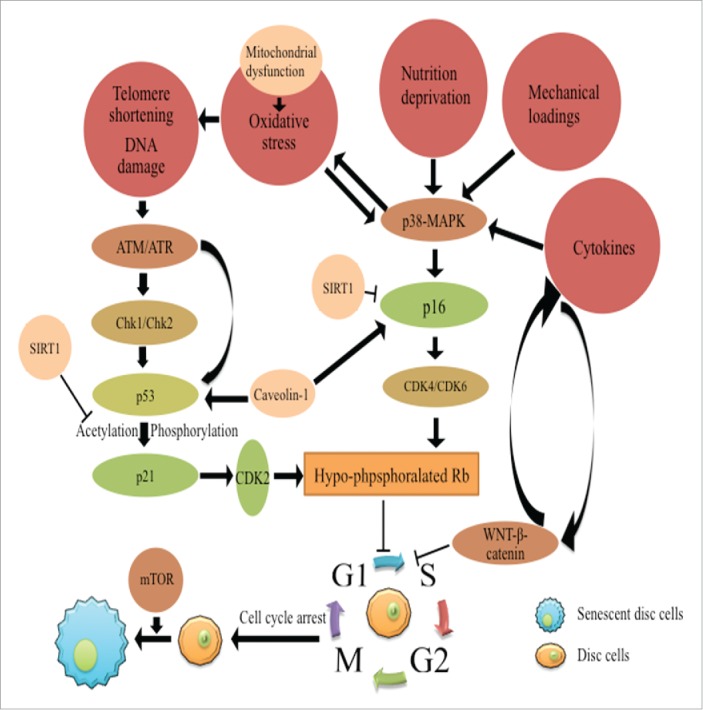
The molecular mechanism of disc cell senescence. The molecular mechanism underlying disc cell senescence includes 2 aspects, the arrest of cell cycle and the development of the senescent phenotype of disc cells. The p53-p21-Rb pathway and the p16-Rb pathway play major roles in the cell cycle arrest. Both pathways are activated by telomere shortening, DNA damage response or various stressful stimuli in the microenvironment of degenerative discs respectively. SIRT1 plays a protective role in disc cell senescence by suppressing p53 and p16. Caveoline-1 synergizes with p53 and p16 to accelerate disc cell senescence. The p38-MAPK pathway responds to various stimuli to activate the p53-p21-Rb and p16-Rb pathways. WNT-β-catenin pathway induces disc cell senescence. A positive-feedback loop of WNT signaling and cytokines enhances the pro-senescence effects of WNT-β-catenin pathway. Moreover, the mTOR pathway is required for cells to acquire the senescent phenotype.
Cells activate DNA damage response (DDR) in respond to telomere shortening. Thus, in a larger sense, DNA damage is an intrinsic trigger of cell senescence.28,29 DNA damage agents, both ionization radiation (IR) and mechlorethamine (MEC), have been found to increase the number of p16-positive cells in the NP of wild-type mouse. Deficiency of DNA repair gene, ERCC1, enhanced disc cell senescence in ERCC1-deficient mice. These deficient mice exposed to IR or MEC had more senescent disc cells.30,31 In addition, the DDR pathway was activated by oxidative stress to induce disc cell senescence.32 These findings were consistent with previous studies on other cell types. Inactivation of DNA repair genes, including Brca1, Xrcc4 and DNA ligase IV, also induced cell senescence.33-36 DNA damage was able to activate both the p53-p21-Rb and p16-Rb pathways (discussed in the next section) to induce cell senescence.37,38
Interestingly, the wild-type mice exposed to IR or MEC showed IDD changes, including reduced disc height, decreased ECM synthesis and enhanced ECM degradation, along with the up-regulated expression of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-5 in discs. Meanwhile, ERCC1-deficient mice also showed the same IDD changes reinforced by IR or MEC,30,31 suggesting that DNA damage perturbs the disc homeostasis to cause IDD. Notably, disc cell senescence occurred in response to DNA damage. Senescent disc cells increased the production of matrix proteases.19, 24 Thus, the loss of ECM homeostasis caused by DNA damage probably resulted from disc cell senescence. In other word, disc cell senescence mediated disc degeneration caused by DNA damage. These findings could explain why tobacco smoking is major risk factor for IDD.39 It has been reported that rats exposed to tobacco smoke showed reduced collagen content, disorganized AF and upregulated expression of IL-1β in IVD.40 However, the underlying mechanism remains unknown. Some studies suggested that tobacco smoke induced vasoconstriction to reduce the nutrient supply of IVD, promoting disc degeneration.41,42 A recent study reported that the mice chronically exposed to tobacco smoke exhibited enhanced disc cell senescence along with decreased proteoglycan (PG) content and reduced matrix synthesis in discs, suggesting that smoke-induced disc degeneration probably results from disc cell senescence. Many DNA damage agents in tobacco may induce disc cell senescence, leading to disc degeneration.43 Future studies should investigate the incidence of DNA damage and cell senescence in smoker's discs to confirm this viewpoint.
Considering that telomere shortening and DNA damage are the most important aging-related molecular events, senescent disc cells accumulate in discs with aging can be speculated. Contrary to our expectations, current evidence are contradictory. On the one hand, the telomere length of disc cells decreased with patient's age.18,19 The disc cells from older patients reached to senescence in vitro earlier than those from younger patients.21 On the other hand, many studies did not find the relationship between disc cell senescence and patient's age,15,17,19 except for the study done by Kim et al in 2009.18 However, in this exceptional study, the age of specimen donors was positively correlated with the Pfirrmann Grade of disc specimens. Based on this bias of case selection, this fake positive correlation maybe just reflected the positive correlation between dis cell senescence and disc degeneration. Meanwhile, various external stimuli, including oxidative stress, high glucose, serum starvation and pro-inflammatory cytokines,44-47 have been suggested as triggers of cell senescence. Therefore, except natural aging, there must be some environmental stimuli in degenerative discs causing disc cell senescence. Moreover, the diversity of the causes of disc cell senescence provides a support for the diversity of the risk factors of IDD. Aging-dependent disc cell senescence mediates age-related disc degeneration,48 and premature IDD caused by acute fractures or abnormal mechanical loading is mediated by age-independent disc cell senescence.49,50
Oxidative stress
The harsh microenvironment of degenerative discs is characterized by low nutrition,51,52 high levels of cytokines53,54 and oxidative stress.55,56 These microenvironmental stimuli cause the stress-induced premature senescence (SIPS).7,9,14 Oxidative stress is a major contributor to cellular senescence.57,58 NP cells were a source of reactive oxygen species (ROS).18 The levels of ROS in discs increased with IDD advancing.55 Notably, hydrogen peroxide (H2O2) inhibited the proliferation of NP cells. It significantly enhanced the formation of H2A.X foci (a marker of DNA damage) in NP cells, and increased the number of SA-β-Gal positive disc cells.32,59-61 Furthermore, H2O2 activated the senescent signal pathways to induce the cycle arrest of NP cells at the G0/G1 phase,32 indicating that oxidative stress in the microenvironment of degenerative discs triggers disc cell senescence (Fig. 2).
Nutrition deprivation
Reduced nutrient supply in degenerative discs affects the behavior of disc cells.51 Serum starvation inhibited the proliferation of disc cells along with enhancing the senescence of disc cells. High concentration serum increased the proliferation rate of NP cells. Conversely, glucose deprivation with serum presence showed little effect on disc cell senescence.62,63 High glucose enhanced oxidative stress through mitochondrial damage to induce disc cell senescence.61,64 These results suggest that various nutrient components have different effects on disc cell senescence. The effects of glucose on disc cell senescence depend on the glucose concentration. Normal glucose level protects disc cells from cell senescence. On the contrary, uncontrolled high glucose promotes disc cell senescence. Moreover, serum components are crucial to the regulation of disc cell cycle. Numerous serum-derived growth factors have been demonstrated to enhance the proliferation of disc cells, including insulin-like growth factor (IGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF).65,66 IGF-1 prevented disc cell senescence induced by oxidative stress.59 However, the availability of these growth factors to disc cells depends on the diffusion rate of these proteins from blood vessels to the center of discs through cartilage endplate (CEP). The permeability of CEP decreased due to the endplate calcification or the microangiopathy induced by diabetes.67,68 As a result, the major route of nutrient molecules to the center of discs was blocked, and the reduced availability of growth factors occurred in IVD, which accelerates the disc cell senescence (Fig. 2).
Pro-inflammatory cytokines
The levels of pro-inflammatory cytokines in degenerative discs increased with IDD progressing.6 These cytokines, including TNF-α, IL-1α, IL-1β, IL-6, IL-17, and various chemokines, are widely accepted mediators of IDD,53,54, 69,70 which enhance the ECM catabolism and inflammation in discs, leading to the structural and functional deterioration of discs. Recently, the effects of cytokines on disc cell senescence have been investigated. TNF-α increased the number of SA-β-Gal positive cells in organ cultured bovine discs, and induced the ECM metabolism shift from anabolism to catabolism.71 Significantly increased SA-β-Gal positive cells were also observed in rat NP cells treated with TNF-α and IL-1β,23 indicating that the cytokines in degenerative discs are powerful pro-senescence factors (Fig. 2).
Abnormal mechanical loading
Abnormal mechanical loading is a risk factor of IDD.72 Our spine resists multidirectional mechanical loadings, including compressive, tensile and shear loads from axial, radial and circumferential directions. Forelimb amputation was used to induce the upright posture of rats, which simulates the upright posture of human beings. Prolonged upright posture caused abnormal mechanical loadings in all directions. As a result, IDD changes were observed, including disorganized collagen structure, AF fissure and decreased disc height. The matrix anabolism decreased and the catabolism increased.73 Interestingly, the prolonged upright posture also accelerated disc cell senescence in rats. The abnormal loadings increased the number of SA-β-Gal positive cells, and up-regulated the expression of senescence-associated genes, including p16, p27, RB, PTEN, p27KIP, p19ARF, and RAGE, in discs,74 suggesting that abnormal mechanical loading promotes disc cell senescence (Fig. 2). Mechanical stress increased the production of ROS to accelerate the SIPS.75 These results provide further evidence of the contribution of disc cell senescence to the pathogenesis of IDD. The disc cell senescence mediates the process of load-induced disc degeneration.
To sum up, there are many stressful stimuli in the harsh microenvironment of degenerative discs, which are the triggers of disc cell senescence. Establishing some measures to ameliorate the harsh microenvironment, such as antioxidants application,32,60 growth factors supplementation,76,77 keeping healthy posture and blood glucose control, could retard disc cell senescence. Interestingly, these measures also have been suggested as promising therapeutic measures for IDD. It is consistent with our viewpoint above mentioned. Disc cell senescence promotes IDD. These potential therapeutic measures could retard IDD through suppressing disc cell senescence. Furthermore, except these known pro-senescence stimuli, further studies should identify anti-senescence factors in the microenvironment of degenerative discs. The balance between the pro-senescence stimuli and anti-senescence factors is crucial to retarding disc cell senescence. To investigate this issue will help us to understand the pathogenesis of IDD better. Moreover, maintaining this balance suggests some new therapeutic targets for IDD.
The signaling pathways of disc cell senescence
The explorations on the signaling network underlying disc cell senescence are still at the infant stage. Herein, we discussed several major and well-defined signaling pathways.
Two central pathways mediating disc cell senescence (the p53-p21-Rb and p16-Rb pathways)
The p53-p21-Rb pathway is activated in response to telomere erosion or DDR, which induces the replicative senescence.7,14 The tumor suppressor gene, p53, is involved in many aspects of cell biology, including cell proliferation, senescence and death. With regard to cell senescence, p53 responds to telomere shortening or DDR to initiate the first step to the irreversible cell-cycle arrest. The mechanism by which telomere erosion or DDR activate p53 is complicated and remains to be investigated further. So far, the widely accepted mechanism resulted from the studies of autosomal recessive disorder ataxia–telangiectasia (AT). At the sites of DNA damage, the histone H2AX is characteristically phosphorylated to γ-H2AX. γ-H2AX is involved in the changes of chromatin structure that assemble the cell-cycle checkpoint proteins, Chk1 and Chk2, and DNA repair proteins, ATM and ATR. ATM directly phosphorylates p53 to activate it. Furthermore, ATM and ATR phosphorylate Chk1 and Chk2 to activate p53 indirectly. On the downstream of p53, p21 inhibits CDK2, which suppresses the phosphorylation of Rb. Because the cell-cycle progression from G1 to S phase relies on the hyper-phosphorylation of Rb, this hypo-phosphorylated Rb is unable to activate the E2F factor to promote the expression of the genes necessary for G1 to S progression, eventually, it causes the cell-cycle arrest.78,79 The senescent NP cells in degenerative NP specimens expressed p53, p21 and Rb, along with telomere shortening and decreased telomerase activity.18 With culture passage increasing, the p53-p21-Rb pathway was activated in human NP cells to induce the replicative senescence of disc cells.21 Meanwhile, the senescent AF cells in degenerative discs showed a significant up-regulation of p53.20 A recent study demonstrated that the ATM-Chk2-p53-p21-Rb pathway was activated by oxidative stress in human NP cells. Hydrogen peroxide leaded to the formation of γ-H2AX foci in the nuclei of NP cells, then, activated the ATM-Chk2-p53-p21-Rb pathway to induce disc cell senescence (Fig. 2).
Other than the p53-dependent pathway, the p53-independent pathway, the p16-Rb pathway is activated by different stimuli, especially oxidative stress, to induce the SIPS (Fig. 2). The p16 is the inhibitor of CDK4 and CDK6. When p16 is activated by oxidative stress, the inhibition of CDK4 and CDK6 results in the hypo-phosphorylation of Rb and the halt of cell-cycle progression.80,81 Previous studies have found the expression of p16 by the senescent disc cells in degenerative disc specimens.18,19,82 The number of p16-positive cells in disc tissues positively correlated with the histological grade of disc degeneration.19 In consistent with the p53-p21-Rb pathway, the p16-Rb pathway in disc cells was also activated with culture passage increasing.21 Recently, several studies found that the p16-Rb pathway mediated the high glucose-induced disc cell senescence. High glucose concentration enhanced the generation of excessive ROS in disc cells by causing mitochondrial damage. Consequently, the p16-Rb pathway was activated to induce the SIPS of disc cells.61,64,83 In summary, the disc cell senescence is mainly mediated by both p53-p21-Rb and p16-Rb pathways. These pathways converge on RB to regulate the cell-cycle progression of disc cells. During the process of disc degeneration, both pathways are activated by DNA damage and various stimuli simultaneously to induce the cell-cycle arrest of senescent disc cells.
Silent information regulator 2 ortholog 1 (SIRT1) suppresses disc cell senescence and protects discs from degeneration
SIRT1 is a highly conserved nicotinamide (NAD+)-dependent deacetylases and has been found to be associated with age-related diseases, cancer and degenerative disorders.84 It plays key roles in lifespan extension.85,86 Previous studies have investigated the roles of SIRT1 in cell senescence. In addition to be regulated at mRNA and protein levels, p53 is regulated by post-translational modifications, such as acetylation and phosphorylation. The promyelocytic leukemia protein (PML) has been reported to activate p53 by posttranslational acetylation. SIRT1 deacetylated p53 to retard the PML-induced cell senescence.87 The overexpression of SIRT1 attenuated the H2O2-induced senescence of human endothelial cells.88 It is noteworthy that SIRT1 was expressed in human NP cells. The mRNA levels of SIRT1 in NP cells increased with patients age increasing. SIRT1 also deacetylated p53 in NP cells.89 Furthermore, the activation of SIRT1 down-regulated the expression of p16 by disc cells in mouse coccygeal discs, and relived the IDD changes caused by disc puncture. Compared to the wild-type mice, the genetic ablated mice (SIRT1+/− ) had more p16-positive cells in discs, and severer IDD changes.90 In conclusion, SIRT1 plays a protective role in the process of disc degeneration, which probably depends on suppressing the p16-dependent senescence of disc cells (Fig. 2). In the future studies, the roles of SIRT1 in the p53-dependent senescent pathway should be investigated in detail.
Caveolin-1 is associated with disc cell senescence
Caveolae are vesicular invaginations in cell membrane. Caveolin-1 is a 21-24 kDa integral membrane protein and crucial to the structure and function of caveolae.91 Many signaling proteins are regulated by caveolins, including epidermal growth factors (EGF) receptors, lipid transport, integrin and protein kinase C. The expression caveolin-1 was reported in degenerative human NP specimens. The expression of caveolin-1 was positive correlated with the expression of p16.82 Notably, caveolin-1 was suggested to be associated with the senescence of different cell types, because its key roles in maintaining the morphology and unresponsive character of senescent cells.92,93 Thus, the positive correlation between p16 and caveolin-1 suggests the roles of caveolin-1 in the SIPS of NP cells (Fig. 2). Moreover, the expression of caveolin-1 was associated with the upregulation of p53 and p21, suggesting the roles of caveolin-1 in the p53-dependent pathway. Caveolin-1 and p53 probably synergistically induced the replicative senescence of disc cells (Fig. 2).94 Future studies will be needed to investigate the mechanism by which caveolin-1 regulates the 2 central senescent pathways of disc cells.
The p38-MAPK pathway mediates the premature senescence of disc cells
The SIPS is induced by p38-MAPK pathway.95,96 The pathway is activated by various external stimuli, including cytokines, oxidative stress, growth factors and oncogenes. Particularly, p38 is activated by MAP kinase kinases (MKKs, MKK3 and MKK6) in respond to oxidative stress, followed by the activation of p16 and hypo-phosphorylation of Rb. As a result, the cell-cycle progression is halted.96 On the other hand, the p38-MAPK-mediated oxidative stress activates the DDR to induce cell senescence. The p38 up-regulates the expression of NADPH oxidase to generate excessive ROS causing DNA damage. Subsequently, the p53-p21-Rb pathway is activated.97 The general support for the roles of p38-MAPK pathway in disc cell senescence was provided by the in vivo observations that the expression of p38 was upregulated in the senescent AF cells selectively harvested from paraffin-embedded sections of human AF tissue using laser capture microdissection (LCM). Moreover, H2O2 was found to increase intracellular ROS levels and activate all 3 MAPK pathways (p38-MAPK, ERK and JNK) to induce the senescence of human NP cells. The results suggest that the p38-MAPK pathway mediates the SIPS of disc cells (Fig. 2).
Mitochondrial dysfunction accelerates the senescence of disc cells
ROS result from oxygen metabolism or exogenous stimuli.98,99 The generation of ROS involves various metabolic enzymes, such as lipoxygenase, cyclooxygenase and flavin oxidases. But in non-immune cells, the major generator of ROS is mitochondria.100 With aging, the mitochondrial DNA damage leads to mitochondrial dysfunction and abnormal electron leakage, enhancing the reduction of oxygen and increasing the production of ROS. The mitochondria-derived ROS directly initiates cell senescence through DDR.101 The enhanced mitochondrial function by pyruvate increased the production of ROS, and then activated both the p53-p21-Rb and p16-Rb pathways to induce cell senescence. On the contrary, mitochondrial respiration uncoupling decreased the production of H2O2 to delay the replicative senescence of cells.101 Mitochondrial dysfunction has been determined in the senescent AF cells. The expression of genes associated with mitochondrial function, including substrate dehydrogenases, cytochromes and substrates carriers, was up-regulated significantly in the senescent AF cells, indicating mitochondrial dysfunction in the senescent AF cells.20 More importantly, the mitochondrial-derived ROS not only accelerated the SIPS of disc cells, but also were involved in IDD (Fig. 2).61,83,100 Elucidating the mechanism of mitochondrial dysfunction in disc cells contributes to revealing the underlying mechanism of disc cell senescence and the pathogenesis of IDD.
The WNT-β-catenin signaling pathway accelerates disc cell senescence
The WNT-β-catenin signaling is crucial to the homeostasis of bone and cartilage tissues. Its roles in the development of osteoarthritis have been widely investigated.102,103 However, its roles in the development of IDD are not understood well. A previous study found that LiCl, an activator of WNT pathways, inhibited the disc cell proliferation and enhanced the senescence of disc cells, suggesting that the WNT/β-catenin signaling triggers disc cell senescence. Meanwhile, this signaling also upregulated the expression of matrix proteases in NP cells, including MMP-9 and MMP-10, indicating that this signaling pathway is involved in the matrix degradation of IDD. 24 WNTs bind to various receptors to activate different signaling pathways together with downstream transcriptional effectors. It regulates cellular proliferation, differentiation and apoptosis. 104,105 Thus, Following studies investigated the mechanism by which the WNT-β-catenin pathway regulates disc cell senescence. Both c-myc and cyclin-D1 are essential for the cell-cycle progression of NP cells. WNT signaling suppressed the expression of c-myc and cyclin-D1 in NP cells. 106,107 Klotho, an anti-aging gene on the downstream of β-catenin, was reported to induce the cell cycle arrest of NP cells. Furthermore, a positive-feedback loop of WNT signaling and TNF-α was observed in NP cells (Fig. 2). The activation of WNT pathway may stimulate the expression of TNF-α to induce disc cell senescence and disc degeneration (Fig. 2).108 The inhibition of WNT signaling probably prevents the senescence of disc cells and retard disc degeneration.
The mammalian target of rapamycin (mTOR) signaling pathway contributes to the development of the senescent phenotype of disc cells
Paradoxically, the senescent disc cells were unable to replicate but secreted more specific proteins and increased in cell size and mass, indicating that the arrest of cell cycle and the formation of senescent phenotype are uncoupled. 7 The mTOR pathway, a member of the phosphatidyl-inositol 3-kinase (PI3K) pathway family, is required for cells to acquire the senescent phenotype.109 When cells are stuck in the cell cycle, this pathway determines whether these cells get the senescent phenotype or just become quiescent (reversible cell cycle arrest). Cell cycle arrest together with mTOR pathway activation cause cellular senescence, however, it just results in cell quiescence without mTOR pathway activation.109 Noteworthy, the mTOR pathway was activated in the senescent NP cells. Glucosamine retarded the senescence of NP cells by inhibiting the mTOR pathway activation.60 The roles of mTOR pathway in the development of the senescent phenotype of disc cells will be a new valuable issue to discuss. Uncoupling the cell-cycle arrest and the development of the senescent phenotype of disc cells is a potential approach to reduce the detrimental effects of disc cell senescence on IDD (Fig. 2).
Conclusion
The accumulation of senescent disc cells in degenerative discs suggests crucial roles of cell senescence in the initiation and development of IDD. More studies will be needed to elucidate the cause-effect relationship between disc cell senescence and IDD. The molecular mechanism underlying disc cell senescence can be subdivided into 2 aspects, the halt of cell cycle and the development of the senescent phenotype of disc cells (Fig. 2). Regarding cell cycle arrest, the p53-p21-Rb pathway and the p16-Rb pathway play major roles. Both pathways are activated by DDR or various stressful stimuli in the microenvironment of degenerative discs respectively, and converge on Rb to retard the cell-cycle progression from the G1 to S phase. The two pathways are regulated by different signals. SIRT1 deacetylates p53 to suppress the p53-depenent senescent pathway. It also inhibits the activation of p16, then, plays a protective role in disc cell senescence. On the other hand, caveoline-1 synergizes with p53 and p16 to mediate disc cell senescence. In addition to these negative regulators, the p38-MAPK pathway responds to external stimuli to activate the p53-p21-Rb and p16-Rb pathways in disc cells indirectly or directly. Mitochondrial dysfunction increases the generation of ROS to enhance disc cell senescence. Furthermore, the enhanced disc cell senescence by WNT-β-catenin pathway has been reported, suggesting a complex cell-cycle regulating network in disc cells.
Referring to the development of the senescent phenotype in disc cells, the mechanism remains to be elucidated. The pro-inflammatory cytokines, matrix catabolism enzymes and chemokines secreted by senescent disc cells have detrimental effects on the process of IDD. However, the development of the senescent phenotype is suggested to be uncoupled with the cell-cycle arrest. There are several regulatory pathways for the formation of the senescent phenotype of disc cells.7 Among them, the widely known is the mTOR pathway. The cell cycle arrested cells can't develop into the senescent phenotype without the activation of mTOR pathway. Understanding the mechanism of the development of the senescent phenotype of disc cells well helps us to elucidate the cause-effect relationship between disc cell senescence and disc degeneration.
Due to the relationship between disc cell senescence and disc degeneration, several anti-senescence biological therapies targeting at different molecular pathways have been proposed to retard disc degeneration. Telomerase transduction fixed shortening the telomere and blocked the activation of senescent pathways, thus, prevented disc cell senescence.110,111 But the high risk of tumorigenesis should be considered. Regulating p53 activity via SIRT1 or caveoline-1 is possible approaches to attenuating the p53-dependent disc cell senescence. The therapeutic strategy aiming at the p38-MAPK pathway and mitochondrial dysfunction may alleviate the SIPS of disc cells. In light of that the development of the senescent phenotype and the cell-cycle arrest are uncoupled, manipulating the mTOR pathway probably ameliorates the aberrant paracrine signaling of senescent disc cells to relieve the harsh microenvironment of degenerative discs. Of course, more in vivo studies will be needed in the future to demonstrate the validity of these therapeutic strategies in preventing disc cell senescence and retarding IDD.
Abbreviations
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- AF
annulus fibrosus
- CEP
cartilage endplate
- DDR
DNA damage response
- ECM
extracellular matrix
- FGF
fibroblast growth factor
- IDD
intervertebral disc degeneration
- IGF
insulin-like growth factor
- IR
ionization radiation
- IVD
intervertebral disc
- LBP
low back pain
- LCM
laser capture microdissection
- MEC
mechlorethamine
- MMP
matrix metalloproteinase
- mTOR
the mammalian target of rapamycin
- NP
nucleus pulposus
- OA
osteoarthritis
- PDGF
platelet derived growth factor
- PG
proteoglycan
- PML
promyelocytic leukemia protein
- Rb
retinoblastoma protein
- ROS
reactive oxygen species
- SA-β-Gal
senescence-associated β-galactosidase
- SASP
senescence-associated secreted phenotype
- SIPS
stress-induced premature senescence
- SIRT1
silent information regulator two ortholog 1
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81271982, No. 81472076 and No. 81572186).
References
- [1].Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, et al.. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England) 2012; 380:2163-96; PMID:23245607; http://dx.doi.org/ 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hong J, Reed C, Novick D, Happich M. Costs associated with treatment of chronic low back pain: an analysis of the UK General Practice Research Database. Spine 2013; 38:75-82; PMID:23038621; http://dx.doi.org/ 10.1097/BRS.0b013e318276450f [DOI] [PubMed] [Google Scholar]
- [3].Cheung KM, Karppinen J, Chan D, Ho DW, Song YQ, Sham P, Cheah KS, Leong JC, Luk KD. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009; 34:934-40; PMID:19532001; http://dx.doi.org/ 10.1097/BRS.0b013e3181a01b3f [DOI] [PubMed] [Google Scholar]
- [4].Takatalo J, Karppinen J, Niinimaki J, Taimela S, Nayha S, Mutanen P, Sequeiros RB, Kyllonen E, Tervonen O. Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young Finnish adults?. Spine 2011; 36:2180-9; PMID:21358475; http://dx.doi.org/ 10.1097/BRS.0b013e3182077122 [DOI] [PubMed] [Google Scholar]
- [5].Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, Anderson DG. The molecular basis of intervertebral disc degeneration. Spine J 2013; 13:318-30; PMID:23537454; http://dx.doi.org/ 10.1016/j.spinee.2012.12.003 [DOI] [PubMed] [Google Scholar]
- [6].Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nature reviews Rheumatology 2014; 10:44-56; PMID:24166242; http://dx.doi.org/ 10.1038/nrrheum.2013.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Muller M. Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. Antioxidants & redox signaling 2009; 11:59-98; PMID:18976161; http://dx.doi.org/ 10.1089/ars.2008.2104 [DOI] [PubMed] [Google Scholar]
- [8].van Deursen JM. The role of senescent cells in ageing. Nature 2014; 509:439-46; PMID:24848057; http://dx.doi.org/ 10.1038/nature13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 2014; 15:482-96; PMID:24954210; http://dx.doi.org/ 10.1038/nrm3823 [DOI] [PubMed] [Google Scholar]
- [10].Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer 2015; 15:397-408; PMID:26105537; http://dx.doi.org/ 10.1038/nrc3960 [DOI] [PubMed] [Google Scholar]
- [11].Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al.. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008; 133:1006-18; PMID:18555777; http://dx.doi.org/ 10.1016/j.cell.2008.03.038 [DOI] [PubMed] [Google Scholar]
- [12].Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, et al.. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 2013; 15:978-90; PMID:23770676; http://dx.doi.org/ 10.1038/ncb2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang F, Cai F, Shi R, Wang XH, Wu XT Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2016; 24:398-408. [DOI] [PubMed] [Google Scholar]
- [14].Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. The international journal of biochemistry & cell biology 2005; 37:961-76; PMID:15743671; http://dx.doi.org/ 10.1016/j.biocel.2004.10.013 [DOI] [PubMed] [Google Scholar]
- [15].Roberts S, Evans EH, Kletsas D, Jaffray DC, Eisenstein SM. Senescence in human intervertebral discs. Eur Spine J 2006; 15 Suppl 3:S312-6; PMID:16773379; http://dx.doi.org/ 10.1007/s00586-006-0126-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gruber HE, Ingram JA, Norton HJ, Hanley EN Jr. Senescence in cells of the aging and degenerating intervertebral disc: immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine 2007; 32:321-7; PMID:17268263; http://dx.doi.org/ 10.1097/01.brs.0000253960.57051.de [DOI] [PubMed] [Google Scholar]
- [17].Gruber HE, Ingram JA, Davis DE, Hanley EN Jr. Increased cell senescence is associated with decreased cell proliferation in vivo in the degenerating human annulus. Spine J 2009; 9:210-5; PMID:18440281; http://dx.doi.org/ 10.1016/j.spinee.2008.01.012 [DOI] [PubMed] [Google Scholar]
- [18].Kim KW, Chung HN, Ha KY, Lee JS, Kim YY. Senescence mechanisms of nucleus pulposus chondrocytes in human intervertebral discs. Spine J 2009; 9:658-66; PMID:19540815; http://dx.doi.org/ 10.1016/j.spinee.2009.04.018 [DOI] [PubMed] [Google Scholar]
- [19].Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther 2007; 9:R45; PMID:17498290; http://dx.doi.org/ 10.1186/ar2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gruber HE, Watts JA, Hoelscher GL, Bethea SF, Ingram JA, Zinchenko NS, Hanley EN Jr. Mitochondrial gene expression in the human annulus: in vivo data from annulus cells and selectively harvested senescent annulus cells. Spine J 2011; 11:782-91; PMID:21784712; http://dx.doi.org/ 10.1016/j.spinee.2011.06.012 [DOI] [PubMed] [Google Scholar]
- [21].Jeong SW, Lee JS, Kim KW. In vitro lifespan and senescence mechanisms of human nucleus pulposus chondrocytes. Spine J 2014; 14:499-504; PMID:24345469; http://dx.doi.org/ 10.1016/j.spinee.2013.06.099 [DOI] [PubMed] [Google Scholar]
- [22].Gruber HE, Mougeot JL, Hoelscher G, Ingram JA, Hanley EN Jr. Microarray analysis of laser capture microdissected-anulus cells from the human intervertebral disc. Spine 2007; 32:1181-7; PMID:17495774; http://dx.doi.org/ 10.1097/BRS.0b013e318053ec89 [DOI] [PubMed] [Google Scholar]
- [23].Markova DZ, Kepler CK, Addya S, Murray HB, Vaccaro AR, Shapiro IM, Anderson DG, Albert TJ, Risbud MV. An organ culture system to model early degenerative changes of the intervertebral disc II: profiling global gene expression changes. Arthritis Res Ther 2013; 15:R121; PMID:24171898; http://dx.doi.org/ 10.1186/ar4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hiyama A, Sakai D, Risbud MV, Tanaka M, Arai F, Abe K, Mochida J. Enhancement of intervertebral disc cell senescence by WNT/beta-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum 2010; 62:3036-47; PMID:20533544; http://dx.doi.org/ 10.1002/art.27599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, et al.. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 2013; 155:1119-30; PMID:24238961; http://dx.doi.org/ 10.1016/j.cell.2013.10.041 [DOI] [PubMed] [Google Scholar]
- [26].Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theoretical Biol 1973; 41:181-90; PMID:4754905; http://dx.doi.org/ 10.1016/0022-5193(73)90198-7 [DOI] [PubMed] [Google Scholar]
- [27].Stein GH, Beeson M, Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science 1990; 249:666-9; http://dx.doi.org/ 10.1126/science.2166342 [DOI] [PubMed] [Google Scholar]
- [28].d'Adda di Fagagna F, Teo SH, Jackson SP. Functional links between telomeres and proteins of the DNA-damage response. Gen Dev 2004; 18:1781-99; PMID:15289453; http://dx.doi.org/ 10.1101/gad.1214504 [DOI] [PubMed] [Google Scholar]
- [29].von Zglinicki T, Saretzki G, Ladhoff J, d'Adda di Fagagna F, Jackson SP. Human cell senescence as a DNA damage response. Mech Ageing Dev 2005; 126:111-7; PMID:15610769; http://dx.doi.org/ 10.1016/j.mad.2004.09.034 [DOI] [PubMed] [Google Scholar]
- [30].Vo N, Seo HY, Robinson A, Sowa G, Bentley D, Taylor L, Studer R, Usas A, Huard J, Alber S, et al.. Accelerated aging of intervertebral discs in a mouse model of progeria. J Orthop Res 2010; 28:1600-7; PMID:20973062; http://dx.doi.org/ 10.1002/jor.21153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nasto LA, Wang D, Robinson AR, Clauson CL, Ngo K, Dong Q, Roughley P, Epperly M, Huq SM, Pola E, et al.. Genotoxic stress accelerates age-associated degenerative changes in intervertebral discs. Mech Ageing Dev 2013; 134:35-42; PMID:23262094; http://dx.doi.org/ 10.1016/j.mad.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Euro Cell Mater 2015; 30:89-102; discussion 3; PMID:26337541 [DOI] [PubMed] [Google Scholar]
- [33].Wahl GM, Carr AM. The evolution of diverse biological responses to DNA damage: insights from yeast and p53. Nat Cell Biol 2001; 3:E277-86; PMID:11781586; http://dx.doi.org/ 10.1038/ncb1201-e277 [DOI] [PubMed] [Google Scholar]
- [34].Frank KM, Sharpless NE, Gao Y, Sekiguchi JM, Ferguson DO, Zhu C, Manis JP, Horner J, DePinho RA, Alt FW. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Molecular cell 2000; 5:993-1002; PMID:10911993; http://dx.doi.org/ 10.1016/S1097-2765(00)80264-6 [DOI] [PubMed] [Google Scholar]
- [35].Gao Y, Ferguson DO, Xie W, Manis JP, Sekiguchi J, Frank KM, Chaudhuri J, Horner J, DePinho RA, Alt FW. Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development. Nature 2000; 404:897-900; PMID:10786799; http://dx.doi.org/ 10.1038/35009138 [DOI] [PubMed] [Google Scholar]
- [36].Ongusaha PP, Ouchi T, Kim KT, Nytko E, Kwak JC, Duda RB, Deng CX, Lee SW. BRCA1 shifts p53-mediated cellular outcomes towards irreversible growth arrest. Oncogene 2003; 22:3749-58; PMID:12802282; http://dx.doi.org/ 10.1038/sj.onc.1206439 [DOI] [PubMed] [Google Scholar]
- [37].Webley K, Bond JA, Jones CJ, Blaydes JP, Craig A, Hupp T, Wynford-Thomas D. Posttranslational modifications of p53 in replicative senescence overlapping but distinct from those induced by DNA damage. Mol Cell Biol 2000; 20:2803-8; PMID:10733583; http://dx.doi.org/ 10.1128/MCB.20.8.2803-2808.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, Lowe SW. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 2002; 109:335-46; PMID:12015983; http://dx.doi.org/ 10.1016/S0092-8674(02)00734-1 [DOI] [PubMed] [Google Scholar]
- [39].Mattila VM, Saarni L, Parkkari J, Koivusilta L, Rimpela A. Early risk factors for lumbar discectomy: an 11-year follow-up of 57,408 adolescents. Eur Spine J 2008; 17:1317-23; PMID:18682991; http://dx.doi.org/ 10.1007/s00586-008-0738-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ogawa T, Matsuzaki H, Uei H, Nakajima S, Tokuhashi Y, Esumi M. Alteration of gene expression in intervertebral disc degeneration of passive cigarette- smoking rats: separate quantitation in separated nucleus pulposus and annulus fibrosus. Pathobiology 2005; 72:146-51; PMID:15860932; http://dx.doi.org/ 10.1159/000084118 [DOI] [PubMed] [Google Scholar]
- [41].Holm S, Nachemson A. Nutrition of the intervertebral disc: acute effects of cigarette smoking. An experimental animal study. Ups J Med Sci 1988; 93:91-9; PMID:3376356; http://dx.doi.org/ 10.1517/03009734000000042 [DOI] [PubMed] [Google Scholar]
- [42].Uematsu Y, Matuzaki H, Iwahashi M. Effects of nicotine on the intervertebral disc: an experimental study in rabbits. J Orthop Sci 2001; 6:177-82; PMID:11484105; http://dx.doi.org/ 10.1007/s007760100067 [DOI] [PubMed] [Google Scholar]
- [43].Wang D, Nasto LA, Roughley P, Leme AS, Houghton AM, Usas A, Sowa G, Lee J, Niedernhofer L, Shapiro S, et al.. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthritis Cartilage 2012; 20:896-905; PMID:22531458; http://dx.doi.org/ 10.1016/j.joca.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res 1995; 55:2284-92; PMID:7757977 [PubMed] [Google Scholar]
- [45].Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J 1998; 332(Pt 1):43-50; PMID:9576849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Blazer S, Khankin E, Segev Y, Ofir R, Yalon-Hacohen M, Kra-Oz Z, Gottfried Y, Larisch S, Skorecki KL. High glucose-induced replicative senescence: point of no return and effect of telomerase. Biochem Biophys Res Commun 2002; 296:93-101; PMID:12147232; http://dx.doi.org/ 10.1016/S0006-291X(02)00818-5 [DOI] [PubMed] [Google Scholar]
- [47].Hubackova S, Krejcikova K, Bartek J, Hodny Z. IL1- and TGFbeta-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine ‘bystander senescence’. Aging 2012; 4:932-51; PMID:23385065; http://dx.doi.org/ 10.18632/aging.100520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].An HS, Anderson PA, Haughton VM, Iatridis JC, Kang JD, Lotz JC, Natarajan RN, Oegema TR Jr., Roughley P, Setton LA, et al.. Introduction: disc degeneration: summary. Spine 2004; 29:2677-8; PMID:15564916; http://dx.doi.org/ 10.1097/01.brs.0000147573.88916.c6 [DOI] [PubMed] [Google Scholar]
- [49].Kerttula LI, Serlo WS, Tervonen OA, Paakko EL, Vanharanta HV. Post-traumatic findings of the spine after earlier vertebral fracture in young patients: clinical and MRI study. Spine 2000; 25:1104-8; PMID:10788855; http://dx.doi.org/ 10.1097/00007632-200005010-00011 [DOI] [PubMed] [Google Scholar]
- [50].Haschtmann D, Stoyanov JV, Gedet P, Ferguson SJ. Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur Spine J 2008; 17:289-99; PMID:17929064; http://dx.doi.org/ 10.1007/s00586-007-0509-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine 2004; 29:2700-9; PMID:15564919; http://dx.doi.org/ 10.1097/01.brs.0000146499.97948.52 [DOI] [PubMed] [Google Scholar]
- [52].Grunhagen T, Wilde G, Soukane DM, Shirazi-Adl SA, Urban JP. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am 2006; 88 Suppl 2:30-5; PMID:16595440; http://dx.doi.org/ 10.2106/JBJS.E.01290 [DOI] [PubMed] [Google Scholar]
- [53].Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther 2007; 9:R77; PMID:17688691; http://dx.doi.org/ 10.1186/ar2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shamji MF, Setton LA, Jarvis W, So S, Chen J, Jing L, Bullock R, Isaacs RE, Brown C, Richardson WJ. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum 2010; 62:1974-82; PMID:20222111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nerlich AG, Bachmeier BE, Schleicher E, Rohrbach H, Paesold G, Boos N. Immunomorphological analysis of RAGE receptor expression and NF-kappaB activation in tissue samples from normal and degenerated intervertebral discs of various ages. Ann N Y Acad Sci 2007; 1096:239-48; PMID:17405935; http://dx.doi.org/ 10.1196/annals.1397.090 [DOI] [PubMed] [Google Scholar]
- [56].Hou G, Lu H, Chen M, Yao H, Zhao H. Oxidative stress participates in age-related changes in rat lumbar intervertebral discs. Arch Gerontol Geriatr 2014; 59:665-9; PMID:25081833; http://dx.doi.org/ 10.1016/j.archger.2014.07.002 [DOI] [PubMed] [Google Scholar]
- [57].Yudoh K, Nguyen T, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther 2005; 7:R380-91; PMID:15743486; http://dx.doi.org/ 10.1186/ar1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Brandl A, Hartmann A, Bechmann V, Graf B, Nerlich M, Angele P. Oxidative stress induces senescence in chondrocytes. J Orthop Res 2011; 29:1114-20; PMID:21284033; http://dx.doi.org/ 10.1002/jor.21348 [DOI] [PubMed] [Google Scholar]
- [59].Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Hanley EN. IGF-1 rescues human intervertebral annulus cells from in vitro stress-induced premature senescence. Growth factors (Chur, Switzerland) 2008; 26:220-5; PMID:19021034; http://dx.doi.org/ 10.1080/08977190802273814 [DOI] [PubMed] [Google Scholar]
- [60].Jiang L, Jin Y, Wang H, Jiang Y, Dong J. Glucosamine protects nucleus pulposus cells and induces autophagy via the mTOR-dependent pathway. J Orthop Res 2014; 32:1532-42; PMID:25087910; http://dx.doi.org/ 10.1002/jor.22699 [DOI] [PubMed] [Google Scholar]
- [61].Park JS, Park JB, Park IJ, Park EY. Accelerated premature stress-induced senescence of young annulus fibrosus cells of rats by high glucose-induced oxidative stress. Int Orthop 2014; 38:1311-20; PMID:24535573; http://dx.doi.org/ 10.1007/s00264-014-2296-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Johnson WE, Stephan S, Roberts S. The influence of serum, glucose and oxygen on intervertebral disc cell growth in vitro: implications for degenerative disc disease. Arthritis Res Ther 2008; 10:R46; PMID:18433481; http://dx.doi.org/ 10.1186/ar2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Stephan S, Johnson WE, Roberts S. The influence of nutrient supply and cell density on the growth and survival of intervertebral disc cells in 3D culture. Euro Cell Mater 2011; 22:97-108; PMID:21892804 [DOI] [PubMed] [Google Scholar]
- [64].Kong JG, Park JB, Lee D, Park EY. Effect of high glucose on stress-induced senescence of nucleus pulposus cells of adult rats. Asian Spine J 2015; 9:155-61; PMID:25901224; http://dx.doi.org/ 10.4184/asj.2015.9.2.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gruber HE, Norton HJ, Hanley EN Jr. Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine 2000; 25:2153-7; PMID:10973395; http://dx.doi.org/ 10.1097/00007632-200009010-00002 [DOI] [PubMed] [Google Scholar]
- [66].Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J 2007; 16:1858-66; PMID:17763874; http://dx.doi.org/ 10.1007/s00586-007-0408-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J 2004; 13:695-701; PMID:15048560; http://dx.doi.org/ 10.1007/s00586-003-0616-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jiang L, Zhang X, Zheng X, Ru A, Ni X, Wu Y, Tian N, Huang Y, Xue E, Wang X, et al.. Apoptosis, senescence, and autophagy in rat nucleus pulposus cells: Implications for diabetic intervertebral disc degeneration. J Orthop Res 2013; 31:692-702; PMID:23238821; http://dx.doi.org/ 10.1002/jor.22289 [DOI] [PubMed] [Google Scholar]
- [69].Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther 2005; 7:R732-45; PMID:15987475; http://dx.doi.org/ 10.1186/ar1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Seguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine 2005; 30:1940-8; PMID:16135983; http://dx.doi.org/ 10.1097/01.brs.0000176188.40263.f9 [DOI] [PubMed] [Google Scholar]
- [71].Purmessur D, Walter BA, Roughley PJ, Laudier DM, Hecht AC, Iatridis J. A role for TNFalpha in intervertebral disc degeneration: a non-recoverable catabolic shift. Biochem Biophys Res Commun 2013; 433:151-6; PMID:23438440; http://dx.doi.org/ 10.1016/j.bbrc.2013.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it?. Spine 2006; 31:2151-61; PMID:16915105; http://dx.doi.org/ 10.1097/01.brs.0000231761.73859.2c [DOI] [PubMed] [Google Scholar]
- [73].Liang QQ, Zhou Q, Zhang M, Hou W, Cui XJ, Li CG, Li TF, Shi Q, Wang YJ. Prolonged upright posture induces degenerative changes in intervertebral discs in rat lumbar spine. Spine 2008; 33:2052-8; PMID:18758360; http://dx.doi.org/ 10.1097/BRS.0b013e318183f949 [DOI] [PubMed] [Google Scholar]
- [74].Xing QJ, Liang QQ, Bian Q, Ding DF, Cui XJ, Shi Q, Wang YJ. Leg amputation accelerates senescence of rat lumbar intervertebral discs. Spine 2010; 35:E1253-61; PMID:20938386; http://dx.doi.org/ 10.1097/BRS.0b013e3181e7d087 [DOI] [PubMed] [Google Scholar]
- [75].Martin JA, Brown TD, Heiner AD, Buckwalter JA Chondrocyte senescence, joint loading and osteoarthritis. Clinical orthopaedics and related research 2004; 427 Suppl:S96-103. [DOI] [PubMed] [Google Scholar]
- [76].Masuda K, Oegema TR Jr., An HS. Growth factors and treatment of intervertebral disc degeneration. Spine 2004; 29:2757-69; PMID:15564925; http://dx.doi.org/ 10.1097/01.brs.0000146048.14946.af [DOI] [PubMed] [Google Scholar]
- [77].Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J 2006; 15 Suppl 3:S422-32; PMID:16865380; http://dx.doi.org/ 10.1007/s00586-006-0149-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gire V, Roux P, Wynford-Thomas D, Brondello JM, Dulic V. DNA damage checkpoint kinase Chk2 triggers replicative senescence. EMBO J 2004; 23:2554-63; PMID:15192702; http://dx.doi.org/ 10.1038/sj.emboj.7600259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 2004; 14:501-13; PMID:15149599; http://dx.doi.org/ 10.1016/S1097-2765(04)00256-4 [DOI] [PubMed] [Google Scholar]
- [80].Itahana K, Zou Y, Itahana Y, Martinez JL, Beausejour C, Jacobs JJ, Van Lohuizen M, Band V, Campisi J, Dimri GP. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol 2003; 23:389-401; PMID:12482990; http://dx.doi.org/ 10.1128/MCB.23.1.389-401.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Durr P, Wlaschek M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 2006; 5:379-89; PMID:16911562; http://dx.doi.org/ 10.1111/j.1474-9726.2006.00231.x [DOI] [PubMed] [Google Scholar]
- [82].Heathfield SK, Le Maitre CL, Hoyland JA. Caveolin-1 expression and stress-induced premature senescence in human intervertebral disc degeneration. Arthritis Res Ther 2008; 10:R87; PMID:18681962; http://dx.doi.org/ 10.1186/ar2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Park JB, Byun CH, Park EY. Rat Notochordal Cells Undergo Premature Stress-Induced Senescence by High Glucose. Asian Spine J 2015; 9:495-502; PMID:26240705; http://dx.doi.org/ 10.4184/asj.2015.9.4.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Carafa V, Nebbioso A, Altucci L. Sirtuins and disease: the road ahead. Front Pharmacol 2012; 3:4; PMID:22319497; http://dx.doi.org/ 10.3389/fphar.2012.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 2000; 289:2126-8; http://dx.doi.org/ 10.1126/science.289.5487.2126 [DOI] [PubMed] [Google Scholar]
- [86].Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001; 410:227-30; PMID:11242085; http://dx.doi.org/ 10.1038/35065638 [DOI] [PubMed] [Google Scholar]
- [87].Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J 2002; 21:2383-96; PMID:12006491; http://dx.doi.org/ 10.1093/emboj/21.10.2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol 2007; 43:571-9; PMID:17916362; http://dx.doi.org/ 10.1016/j.yjmcc.2007.08.008 [DOI] [PubMed] [Google Scholar]
- [89].Zhang Z, Kakutani K, Maeno K, Takada T, Yurube T, Doita M, Kurosaka M, Nishida K. Expression of silent mating type information regulator 2 homolog 1 and its role in human intervertebral disc cell homeostasis. Arthritis Res Ther 2011; 13:R200; PMID:22152608; http://dx.doi.org/ 10.1186/ar3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Xia X, Guo J, Lu F, Jiang J. SIRT1 Plays a Protective Role in Intervertebral Disc Degeneration in a Puncture-induced Rodent Model. Spine 2015; 40:E515-24; PMID:25646749; http://dx.doi.org/ 10.1097/BRS.0000000000000817 [DOI] [PubMed] [Google Scholar]
- [91].Razani B, Schlegel A, Lisanti MP. Caveolin proteins in signaling, oncogenic transformation and muscular dystrophy. J Cell Sci 2000; 113(Pt 12):2103-9; PMID:10825283 [DOI] [PubMed] [Google Scholar]
- [92].Park WY, Park JS, Cho KA, Kim DI, Ko YG, Seo JS, Park SC. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J Biol Chem 2000; 275:20847-52; PMID:10781609; http://dx.doi.org/ 10.1074/jbc.M908162199 [DOI] [PubMed] [Google Scholar]
- [93].Cho KA, Ryu SJ, Oh YS, Park JH, Lee JW, Kim HP, Kim KT, Jang IS, Park SC. Morphological adjustment of senescent cells by modulating caveolin-1 status. J Biol Chem 2004; 279:42270-8; PMID:15263006; http://dx.doi.org/ 10.1074/jbc.M402352200 [DOI] [PubMed] [Google Scholar]
- [94].Razani B, Altschuler Y, Zhu L, Pestell RG, Mostov KE, Lisanti MP. Caveolin-1 expression is down-regulated in cells transformed by the human papilloma virus in a p53-dependent manner. Replacement of caveolin-1 expression suppresses HPV-mediated cell transformation. Biochemistry 2000; 39:13916-24; PMID:11076533; http://dx.doi.org/ 10.1021/bi001489b [DOI] [PubMed] [Google Scholar]
- [95].Wang W, Chen JX, Liao R, Deng Q, Zhou JJ, Huang S, Sun P. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol 2002; 22:3389-403; PMID:11971971; http://dx.doi.org/ 10.1128/MCB.22.10.3389-3403.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Gen Cells 2003; 8:131-44; http://dx.doi.org/ 10.1046/j.1365-2443.2003.00620.x [DOI] [PubMed] [Google Scholar]
- [97].Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am 2003; 85-A Suppl 2:106-10; PMID:12721352 [DOI] [PubMed] [Google Scholar]
- [98].Tiku ML, Liesch JB, Robertson FM. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J Immunol 1990; 145:690-6; PMID:2114447 [PubMed] [Google Scholar]
- [99].McMillan TJ, Leatherman E, Ridley A, Shorrocks J, Tobi SE, Whiteside JR. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J Pharmacy Pharmacol 2008; 60:969-76; PMID:18644190; http://dx.doi.org/ 10.1211/jpp.60.8.0004 [DOI] [PubMed] [Google Scholar]
- [100].Nasto LA, Robinson AR, Ngo K, Clauson CL, Dong Q, St Croix C, Sowa G, Pola E, Robbins PD, Kang J, et al.. Mitochondrial-derived reactive oxygen species (ROS) play a causal role in aging-related intervertebral disc degeneration. J Orthop Res 2013; 31:1150-7; PMID:23389888; http://dx.doi.org/ 10.1002/jor.22320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birket MJ, Harold G, Schaeuble K, et al.. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS biology 2007; 5:e110; PMID:17472436; http://dx.doi.org/ 10.1371/journal.pbio.0050110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhu M, Chen M, Zuscik M, Wu Q, Wang YJ, Rosier RN, O'Keefe RJ, Chen D. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum 2008; 58:2053-64; PMID:18576323; http://dx.doi.org/ 10.1002/art.23614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Weng LH, Wang CJ, Ko JY, Sun YC, Wang FS. Control of Dkk-1 ameliorates chondrocyte apoptosis, cartilage destruction, and subchondral bone deterioration in osteoarthritic knees. Arthritis Rheum 2010; 62:1393-402; PMID:20131282; http://dx.doi.org/ 10.1002/art.27357 [DOI] [PubMed] [Google Scholar]
- [104].Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development (Cambridge, England) 2000; 127:3141-59; PMID:10862751 [DOI] [PubMed] [Google Scholar]
- [105].Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006; 127:469-80; PMID:17081971; http://dx.doi.org/ 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- [106].Hiyama A, Sakai D, Arai F, Nakajima D, Yokoyama K, Mochida J. Effects of a glycogen synthase kinase-3beta inhibitor (LiCl) on c-myc protein in intervertebral disc cells. J Cell Biochem 2011; 112:2974-86; PMID:21678465; http://dx.doi.org/ 10.1002/jcb.23217 [DOI] [PubMed] [Google Scholar]
- [107].Hiyama A, Sakai D, Tanaka M, Arai F, Nakajima D, Abe K, Mochida J. The relationship between the Wnt/beta-catenin and TGF-beta/BMP signals in the intervertebral disc cell. J Cell Physiol 2011; 226:1139-48; PMID:20945354; http://dx.doi.org/ 10.1002/jcp.22438 [DOI] [PubMed] [Google Scholar]
- [108].Hiyama A, Yokoyama K, Nukaga T, Sakai D, Mochida J. A complex interaction between Wnt signaling and TNF-alpha in nucleus pulposus cells. Arthritis Res Ther 2013; 15:R189; PMID:24286133; http://dx.doi.org/ 10.1186/ar4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell cycle (Georgetown, Tex) 2008; 7:3355-61; PMID:18948731; http://dx.doi.org/ 10.4161/cc.7.21.6919 [DOI] [PubMed] [Google Scholar]
- [110].Chung SA, Wei AQ, Connor DE, Webb GC, Molloy T, Pajic M, Diwan AD. Nucleus pulposus cellular longevity by telomerase gene therapy. Spine 2007; 32:1188-96; PMID:17495775; http://dx.doi.org/ 10.1097/BRS.0b013e31805471a3 [DOI] [PubMed] [Google Scholar]
- [111].Wu J, Wang D, Ruan D, He Q, Zhang Y, Wang C, Xin H, Xu C, Liu Y. Prolonged expansion of human nucleus pulposus cells expressing human telomerase reverse transcriptase mediated by lentiviral vector. J Orthop Res 2014; 32:159-66; PMID:23983186; http://dx.doi.org/ 10.1002/jor.22474 [DOI] [PubMed] [Google Scholar]


