Abstract
Amphetamine withdrawal increases anxiety and stress sensitivity related to blunted ventral hippocampus (vHipp) and enhances the central nucleus of the amygdala (CeA) serotonin responses. Extracellular serotonin levels are regulated by the serotonin transporter (SERT) and organic cation transporter 3 (OCT3), and vHipp OCT3 expression is enhanced during 24 hours of amphetamine withdrawal, while SERT expression is unaltered. Here, we tested whether OCT3 and SERT expression in the CeA is also affected during acute withdrawal to explain opposing regional alterations in limbic serotonergic neurotransmission and if respective changes continued with two weeks of withdrawal. We also determined whether changes in transporter expression were confined to these regions. Male rats received amphetamine or saline for two weeks followed by 24 hours or two weeks of withdrawal, with transporter expression measured using Western immunoblot. OCT3 and SERT expression increased in the CeA at both withdrawal timepoints. In the vHipp, OCT3 expression increased only at 24 hours of withdrawal, with an equivalent pattern seen in the dorsomedial hypothalamus. No changes were evident in any other regions sampled. These regionally specific changes in limbic OCT3 and SERT expression may partially contribute to the serotonergic imbalance and negative affect during amphetamine withdrawal.
Keywords: psychostimulant, anxiety, ventral hippocampus, central amygdala, dorsomedial hypothalamus, serotonin
Introduction
Chronic amphetamine and subsequent withdrawal induce greater anxiety and heightened sensitivity to stress in both humans and animal models.1–5 These negative emotional states contribute to relapse after both acute and prolonged withdrawal periods.6–9
In experimental animals, acute and extended withdrawal from chronic amphetamine leads to dysregulation of stress-induced extracellular serotonin in the limbic system.10–13 Specifically, rats undergoing either acute (24 hours) or prolonged (two weeks) amphetamine withdrawal show markedly attenuated stress- or corticosterone-induced extracellular serotonin levels in the ventral hippocampus (vHipp).13,14 In contrast, amphetamine withdrawal at both time points is characterized by augmented extracellular serotonin in the central nucleus of the amygdala (CeA) in response to either restraint stress or corticotrophin-releasing factor (CRF) infusion into the dorsal raphe.10,13 These differential changes in vHipp and CeA serotonergic function may contribute to the increased anxiety and stress sensitivity exhibited during withdrawal. For example, serotonergic lesions in the vHipp of drug-naïve rats increase anxiety-like behavior on the elevated plus maze, while increasing serotonin in the vHipp alleviates heightened anxiety in rats undergoing amphetamine withdrawal.3 Conversely, increased extracellular serotonin in CeA in drug-naïve rats is associated with increased fear behavior.15 Given the importance of serotonergic transmission in these brain regions for regulating emotion, the underlying mechanisms for serotonin dysregulation in the limbic system following chronic amphetamine need to be fully elucidated.
Extracellular serotonin levels are regulated, in part, by reuptake via the serotonin transporter (SERT). Alterations in SERT expression or function have been associated with anxiety,16–18 fear-associated learning,19 psychostimulant administration,20 and depression.21 While the reduction in extracellular serotonin observed in the vHipp during acute withdrawal from chronic amphetamine does not appear to be mediated by changes in SERT expression or function,12 this does not rule out the possibility that SERT expression in the vHipp or CeA may be altered beyond the 24-hour period to explain the changes in stress-induced extracellular serotonin seen during protracted withdrawal.13 Therefore, we measured the expression of SERT in the CeA and vHipp following both acute and protracted withdrawal from chronic amphetamine.
In contrast to SERT, an increased organic cation transporter 3 (OCT3) expression and function is observed in the vHipp at 24 hours of withdrawal following chronic amphetamine treatment.12 Such an increase in vHipp OCT3 function may result in greater clearance of extracellular serotonin levels during stress to promote heightened anxiety and stress sensitivity throughout withdrawal.3,13 This is because OCT3 acts in the brain as a high-capacity, low-specificity transporter to provide clearance of cations, including monoamine neurotransmitters,22–25 particularly when extracellular levels exceed the capacity of dedicated monoamine transporters such as SERT, such as during stress.26,27 Furthermore, OCT3 is sensitive to inhibition by corticosterone,22,23,26 and, as such, has been recently implicated in modulating the aminergic function in stress-sensitive psychiatric disorders, including substance abuse, depression, and anxiety.28–32 Pharmacological blockade of OCT3s also increases extracellular 5-HT in brain regions that mediate stress responses, such as the hippocampus (including the vHipp) and the dorsomedial hypothalamus (DMH).12,25,33,34 Altogether, this suggests that alterations in OCT3 expression may also be induced by amphetamine treatment and protracted amphetamine withdrawal, leading to changes in serotonin transporter function to explain the persistent down- and upregulation of serotonergic activity in the vHipp and CeA, respectively. We therefore determined whether changes in OCT3 expression were evident in the vHipp and CeA at both 24 hours and two weeks of withdrawal from amphetamine.
In addition to the vHipp and CeA, OCT3 expression is high in other brain regions that either receive dense mono-aminergic projections or mediate interactions with stress-responsive peripheral organs and systems.35 These regions have been demonstrated to have an important role in regulating stress and/or anxiety and include, but are not limited to, the dorsomedial hypothalamus (DMH),36–40 lateral septum (LS),41–43 bed nucleus of stria terminalis (BNST),44,45 and dorsal hippocampus (dHipp).46–48 Therefore, we also assessed the expression of OCT3 in these regions to determine whether the effects of amphetamine treatment on this transporter at 24 hours of withdrawal are specific to the vHipp12 or are evident across the distributed limbic system that modulates stress and anxiety.
Materials and Methods
Animals
Male Sprague-Dawley rats (Animal Resource Center, University of South Dakota, Vermillion, SD) were housed in pairs in a room maintained at 60% relative humidity and 22°C temperature with a 12-hour reverse light-dark cycle (lights off at 10:00 am) and free access to food and water. At eight weeks of age, each rat in a given pair was randomly assigned to receive once daily intraperitoneal injections of either d-amphetamine (2.5 mg/kg) or saline for two weeks. Following injections, rats underwent either a 24-hour or two-week withdrawal period (n = 11–12 per treatment and withdrawal period). This treatment regimen and withdrawal period in our hands reliably increased behavioral stress sensitivity and anxiety-like behavior at both withdrawal time points, along with dampened corticosterone- and stress-induced serotonin in the vHipp and enhanced stress-induced serotonin in the CeA.2,3,13,14 At the end of the withdrawal period, rats were decapitated and brains were rapidly removed and stored at −80°C until sectioned. Collection of tissue was conducted blind to treatment. All experiments were approved by the University of South Dakota IACUC and carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Brain and tissue processing
Frozen whole brains were sectioned at a thickness of 300 microns in a cryostat (Leica Jung CM 1800; North Central Instruments, Plymouth, MN, USA) and stored at −80°C. Tissue from the vHipp, dHipp, CeA, DMH, LS, and BNST was located using the rat brain atlas of Paxinos and Watson,49 microdissected on a frozen stage (Physitemp Instrument Inc, NJ, USA) and collected in 40 µL HEPES buffer (1.19%, pH 7.5) containing 14 µL/mL of protease inhibitor (Roche Diagnostics, IN, USA). Tissue was then sonicated (Fisher scientific, PA, ISA), with protein concentration determined using the Bradford method;50 (BioRad Laboratories, Hercules, CA, USA) and read on microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
Western immunoblot
Procedures were followed as described by Barr et al12 The samples were normalized for protein content and mixed with 1.5 M Tris-loading buffer containing β-mercaptoethanol and boiled for 3 min. Samples were vortexed and loaded (50 µg for both hippocampal regions and BNST; 40 µg for CeA; 35 µg for the LS and DMH) on 10% sodium dodecyl sulfate polyacrylamide gels for electrophoresis (BioRad Laboratories) at 90 V for 1.5–2 hrs. Proteins were then electroblotted onto a polyvinylidene difluoride (PVDF) membrane (0.2 µm, BioRad Laboratories) using a semi-dry blotting apparatus (BioRad laboratories) for 90 min. The PVDF membrane was blocked in a cocktail of 5% nonfat dry milk (NFDM) and 1% bovine serum albumin (BSA; 1:1 ratio) overnight at 4°C followed by incubation with OCT3 primary antibody (1:1000 dilution; OCT31-A, Alpha Diagnostic International, TX, USA) for 24 hours at 4°C. Membranes were washed 3 times (10 min each) in Tris-buffered saline containing tween-20 (TBST), then incubated with secondary anti-rabbit IgG antibody (1:5000 dilution; 611-132-122, Rockland, PA) followed by three washes with TBST. Membranes were scanned at 800 nm using an infrared LiCor imaging system (Odyssey CLx, LiCor Biosciences, NE, USA) to visualize protein bands. The membranes were then washed in TBST in light and reprobed for SERT protein using ST (C-20) primary antibody (1:200 dilution; sc-1458, Santa-Cruz biotechnology Inc, CA, USA) and anti-goat IgG secondary antibody (1:5000 dilution; 305-155-003; Jackson Immuno-research, PA, USA), and reprobed with actin primary antibody (1:2000 dilution; MAB1501R; Millipore, CA, USA) overnight at 4°C in 5% NFDM and 1% BSA cocktail and anti-mouse secondary IgG antibody (1:5000 dilution; 610-132-121; Rockland, PA, USA). All protein bands were quantified and normalized against actin as the loading control.
Data collation and statistics
Optical density of OCT3 and SERT bands obtained from amphetamine-treated rats were normalized to saline-treated rats within the same membrane. Grubb’s test was used to identify statistical outliers, resulting in the removal of a total of three data points from a possible total of 336 across the entire experiment. One membrane from the DMH was lost due to technical reasons, resulting in loss of one to two rats per treatment group for that analysis. Separate two-way ANOVAs were used to assess the effects of treatment or withdrawal period on OCT3 and SERT protein optical density in both the CeA and vHipp, with significant interactions followed up by Holm-Sidak tests for multiple pairwise comparisons. Previously, we had reported an increased OCT3 expression in the vHipp after acute (24 hours) withdrawal from amphetamine. In these cases where OCT3 expression was assessed only at 24 hours of withdrawal, separate one-way ANOVAs were used to assess the differences between treatment groups. All analyses were performed using SigmaStat v.3.5, with significance set at P < 0.05, with at least one investigator responsible for analysis blind to treatment.
Results
Transporter expression in the ventral hippocampus (vHipp)
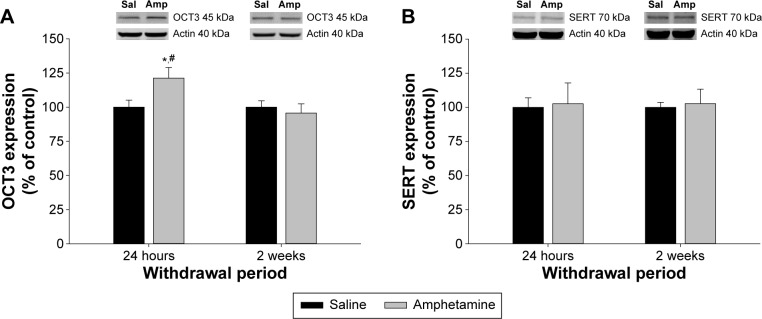
In the vHipp, there was a significant interaction between treatment and withdrawal period for OCT3 expression (F(1,40) = 4.127, P = 0.049; Fig. 1A). Expression of OCT3 was increased in the amphetamine-treated group at 24 hours of withdrawal compared to saline-treated rats at the same withdrawal time point, and compared to amphetamine-treated rats at two weeks of withdrawal (P < 0.05; Fig. 1A). However, OCT3 expression in the vHipp was similar between amphetamine- and saline-treated rats at two weeks of withdrawal (P > 0.05; Fig. 1A). With regard to SERT expression in the vHipp (Fig. 1B), there was no significant effect of treatment (F(1,41) = 0.074, P = 0.792), withdrawal period (F(1,41) = 0.000, P = 0.996), nor a significant interaction between the two factors (F(1,41) = 0.000, P = 0.966).
Figure 1.
The effect of chronic amphetamine treatment on (A) organic cation transporter 3 (OCT3) expression and (B) serotonin transporter expression (SERT) in the ventral hippocampus (vHipp) at 24 hours and two weeks of withdrawal. All means ± SEM are expressed as percentage of control, n = 10–12 per treatment group. *P < 0.05 between saline- and amphetamine-treated groups within the same withdrawal period; #P < 0.05 between amphetamine groups across withdrawal periods.
Transporter expression in the central nucleus of the amygdala (CeA)
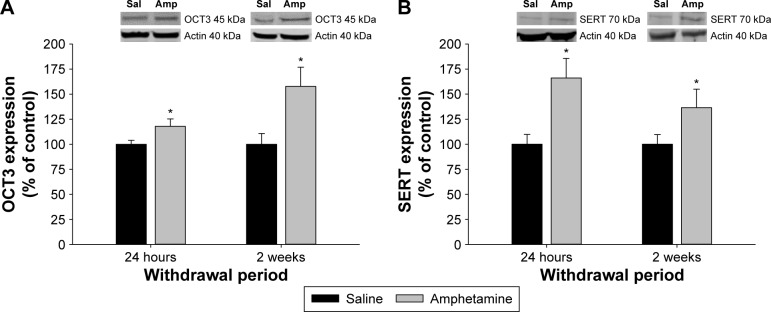
In contrast to the vHipp, there was only a significant main effect of treatment on OCT3 expression in the CeA (F(1,41) = 9.289, P = 0.004; Fig. 2A), and no significant main effect of withdrawal (F(1,41) = 2.797, P = 0.102) or an interaction between treatment and withdrawal (F(1,41) = 2.516, P = 0.120). Thus, OCT3 expression remained elevated in amphetamine-treated rats over the two withdrawal periods as compared to the saline-treated group (Fig. 2A). Similar to OCT3 expression, there was a significant main effect of treatment on SERT expression in the CeA (F(1,41) = 10.777, P = 0.002; Fig. 2B) but no significant main effect of withdrawal (F(1,41) = 0.772, P = 0.385) or an interaction between treatment and withdrawal (F(1,41) = 1.126, P = 0.295). Again, SERT expression remained higher than saline-treated controls over the entire amphetamine withdrawal period (Fig. 2B).
Figure 2.
The effect of chronic amphetamine treatment on (A) organic cation transporter 3 (OCT3) expression and (B) serotonin transporter expression (SERT) in the central nucleus of the amygdala (CeA) at 24 hours and two weeks of withdrawal. All means ± SEM are expressed as percentage of control, n = 10–12 per treatment group. *P < 0.05 between saline- and amphetamine-treated groups within the same withdrawal period.
Transporter expression in the dorsomedial hypothalamus (DMH)
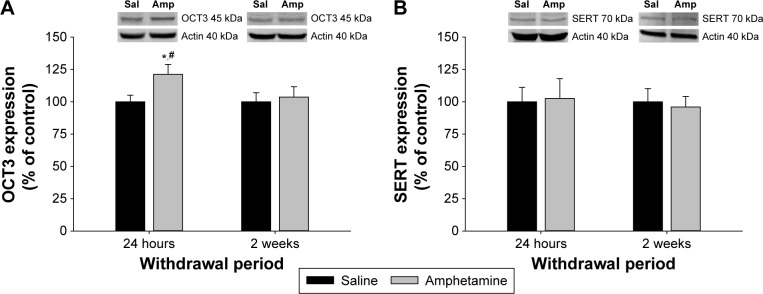
Similar to the vHipp, there was a significant interaction between treatment and withdrawal period for OCT3 expression in the DMH (F(1,37) = 4.308, P = 0.043; Fig. 3A). At 24 hours of withdrawal, OCT3 expression was increased in amphetamine-treated rats compared to saline-treated rats, and also compared to amphetamine-treated rats at two weeks of withdrawal (P < 0.05; Fig. 3A). However, at two weeks of withdrawal, OCT3 expression in the DMH was similar between amphetamine- and saline-treated rats (P > 0.05; Fig. 3A). Also similar to the vHipp, SERT expression in the DMH was unaffected (Fig. 3B), with no significant effect of treatment (F(1,37) = 0.424, P = 0.519), withdrawal period (F(1,37) = 0.244, P = 0.624), nor a significant interaction between the two factors (F(1,37) = 0.244, P = 0.624).
Figure 3.
The effect of chronic amphetamine treatment on (A) organic cation transporter 3 (OCT3) expression and (B) serotonin transporter expression (SERT) in the dorsomedial hypothalamus (DMH) at 24 hours and two weeks withdrawal. All means ± SEM are expressed as percentage of control, n = 9–11 per treatment group. *P < 0.05 between saline- and amphetamine-treated groups within the same withdrawal period; #P < 0.05 between amphetamine groups across withdrawal periods.
OCT3 expression in dorsal hippocampus (dHipp), bed nucleus of the stria terminalis (BNST), and lateral septum (LS)
The expression of OCT3 in the dHipp (F(1,19) = 0.982, P = 0.333), BNST (F(1,20) = 0.285, P = 0.599), and LS (F(1,19) = 0.665, P = 0.425) at 24 hours of withdrawal following chronic amphetamine was not significantly different when compared to saline-treated rats (Table 1), suggesting that changes in OCT3 expression with acute withdrawal from amphetamine are limited to the vHipp, CeA, and DMH. Therefore, no further analysis of these regions was undertaken.
Table 1.
OCT3 expression remained unchanged in dorsal hippocampus (dHipp), bed nucleus of the stria terminalis (BNST), and lateral septum (LS) at 24 hours of withdrawal following chronic amphetamine. All means ± SEM are expressed as percentage of control.
| BRAIN REGION | SALINE | AMPHETAMINE | F | P |
|---|---|---|---|---|
| MEAN ± SEM | MEAN ± SEM | |||
| dHipp | 100.0 ± 5.2 | 109.0 ± 7.4 | 0.982 | 0.333 |
| BNST | 100.0 ± 7.7 | 94.4 ± 6.9 | 0.285 | 0.599 |
| LS | 100.0 ± 1.9 | 92.0 ± 9.5 | 0.665 | 0.425 |
Discussion
Both OCT3 and SERT expression were increased in the CeA during acute and chronic withdrawal from amphetamine. Increases of similar magnitude in OCT3 expression in the vHipp of amphetamine-withdrawn rats, as measured by Western blot, result in enhanced serotonin clearance in vivo.12 Together, these results suggest that the capacity for clearing extracellular serotonin should be similarly increased in the CeA following amphetamine withdrawal. However, this contradicts the results of our earlier work showing that amphetamine-withdrawn rats exhibit augmented serotonin levels in the CeA in response to intra-dorsal raphe infusions of CRF at 24 hours of withdrawal,10 and in response to restraint stress after two weeks of withdrawal.13 Hence, transporter expression in the CeA during withdrawal would be expected to decrease, rather than increase, to explain the maintenance of heightened extracellular serotonin. Instead, we posit that the increased expression of OCT3 and SERT in the CeA may represent a compensatory mechanism for persistently increased amygdalar extracellular serotonin in response to amphetamine treatment.51,52 This is supported by findings that patients with social anxiety disorder show an increased SERT expression in the same brain regions in which serotonergic overactivity is observed, which includes the amygdala.53 Therefore, we suggest that an increased SERT and OCT3 expression in the CeA during amphetamine withdrawal may be induced by amphetamine-elicited enhancements of serotonin neurotransmission in this region10,13 rather than directly contributing to serotonergic dysfunction during withdrawal.
While alterations in SERT and OCT3 expression in the CeA may not explain the enhanced stress-induced serotonin neurotransmission in this region during amphetamine withdrawal, a possible alternative mechanism may involve enhanced stress-induced release by CRF2 receptors in the dorsal raphe, which supplies serotonergic innervation to the CeA.54 An increased expression of CRF2 (but not CRF1) receptors is observed in the dorsal raphe nucleus up to six weeks of withdrawal from chronic amphetamine.55 Activation of dorsal raphe CRF2 receptors can disinhibit serotonergic neurons,56 and CRF2 receptor antagonism in the dorsal raphe prevents augmented CRF-induced increases in CeA serotonin release during amphetamine withdrawal.10 Furthermore, pharmacological blockade of CRF2 receptors in the dorsal raphe reverses the heightened anxiety seen during both acute and protracted amphetamine withdrawal.2 Future work should assess whether stress-induced enhancements in serotonin neurotransmission within the CeA during withdrawal are due to CRF2 receptor activation in the dorsal raphe, and whether preventing this causes a corresponding normalization of OCT3 and SERT expression.
Consistent with our previous findings,12 OCT3 expression in the vHipp was increased by ~20% at 24 hours of withdrawal from chronic amphetamine, whereas SERT expression was not altered. The Barr et al12 study also showed that an increase in OCT3 expression of this magnitude significantly increased serotonin uptake in this region. Therefore, we hypothesized that enhanced serotonin clearance, as mediated by an increased expression of OCT3 in the vHipp, accounted for the profound deficit in extracellular serotonin accumulation observed during either acute or protracted amphetamine withdrawal in response to KCl stimulation, corticosterone, or restraint stress.12–14 However, in the current study, OCT3 expression in the vHipp returned to control levels by two weeks of withdrawal from amphetamine, and SERT expression also remained unchanged at this time point. Thus, neither changes in OCT3 nor changes in SERT expression can explain the markedly reduced extracellular serotonin in the vHipp observed in response to stress at two weeks of amphetamine withdrawal.13 Our previous work has correlated a lack of change in total serotonin transporter expression such as measured here with no change in in vivo serotonin clearance.12 However, it should be noted that lack of change in total protein as measured here does not preclude the possibility that altered trafficking of serotonin transporters occurred at two weeks of withdrawal to result in changes in serotonin activity at this time point.
Alternatively, deficient serotonergic function in response to stress during protracted withdrawal may be due to reductions in glucocorticoid receptors (GRs). Corticosterone applied locally to the vHipp of anesthetized rats increases extracellular serotonin through the activation of GRs,14 but vHipp GR expression is reduced by acute withdrawal from amphetamine, which correspondingly dampens GR-mediated serotonin release.14 Moreover, stress-induced serotonin release in the vHipp in control animals, which is not evident in amphetamine-withdrawn rats, was blocked by the GR antagonist mifepristone perfused in to the vHipp, suggesting the involvement of local GRs in stress-induced serotonin release in this brain region.13 Serotonin in the vHipp is believed to promote the ability to cope with stressors and reduce anxiety15,57,58 and various conditions associated with increased anxiety are characterized by reductions in vHipp GRs, including chronic stress59–63 and chronic psychostimulant administration.64,65 Therefore, reductions in extracellular vHipp serotonin during protracted withdrawal from amphetamine may primarily be driven by reduced function and expression of GR receptors13,14 rather than augmented serotonin clearance mechanisms.
Similar to the vHipp, OCT3 but not SERT expression was increased in DMH at 24 hours of withdrawal from amphetamine and returned to control levels by two weeks of withdrawal. The DMH functions to integrate the neuroendocrine, autonomic, and behavioral responses to stressors (reviewed in),36 and activation of the DMH cause increased anxiety and panic.66–68 Corticosterone-sensitive organic cation transporters in the DMH are believed to contribute to rapid changes in extracellular serotonin (and possibly other cationic neurotransmitters) in response to stress.34 While the effects of amphetamine withdrawal on serotonin release or accumulation in the DMH have not been explored to date, the current findings suggest that increased OCT3-mediated clearance would reduce serotonin availability during acute withdrawal, similar to the function of the vHipp. In addition, GR expression in the DMH is decreased following cocaine self-administration,69 similar to the effects of repeated psychostimulants in the vHipp,64,65 which could also promote reduced stress-induced serotonin function in this region during protracted stimulant withdrawal. Additional studies will be required to determine if this is the case.
In contrast to the vHipp, CeA, and DMH, OCT3 expression remained unchanged in the dHipp, BNST, and LS at 24 hours of withdrawal following chronic amphetamine treatment. This suggests that chronic amphetamine exposure induces region-specific differential regulation of OCT3 protein expression within brain regions associated with anxiety, rather than having global limbic effects. There are several plausible mechanisms by which chronic amphetamine could selectively alter OCT3 or SERT expression in the CeA, DMH, and vHipp seen here. Amphetamine increases corticosterone levels in both mice and rats,70–72 and GR agonists have been shown to increase OCT1 expression and OCT2 mRNA in human primary hepatocytes and in Medin-Derby Canine kidney cells, respectively.73,74 Repeated activation of GRs by amphetamine-induced corticosterone during chronic treatment may therefore upregulate OCT3 expression in GR-rich regions such as the vHipp, DMH, and CeA. This hypothesis is partially supported by recent findings that acute or chronic stress (presumably activating glucocorticoid release) can upregulate OCT3 mRNA in the hippocampus of rats.75 In addition, SERT expression has been shown to be regulated in an allele-dependent manner by GRs.76 Thus, glucocorticoids have the potential to alter OCT3 and SERT expression in stress-sensitive limbic regions, with important implications for future work in deciphering the mechanisms by which amphetamine withdrawal alters transporter expression and subsequent modulation of serotonergic activity to affect anxiety-like behavior.
In summary, our findings demonstrate that chronic amphetamine treatment and withdrawal differentially affect OCT3 or SERT expression within the limbic system, leading to a unique regional and temporal pattern of transporter upregulation. These changes in transporter expression, particularly in the vHipp, may contribute to the onset of dysphoric states associated with amphetamine withdrawal. Future work should focus on the mechanisms by which amphetamine increases OCT3 and SERT expression in a regionally differential manner and on the effects of chronic amphetamine on DMH serotonin as a further potential mediator of stress and anxiety states during drug withdrawal.
Acknowledgments
We thank Drs. Khosrow Rezvani and Erin Terpstra for help with Western blot experiments. We also thank Dr. Christopher Lowry for helpful guidance of these experiments.
Abbreviations
- BNST
bed nucleus of stria terminalis
- BSA
bovine serum albumin
- CRF
corticotrophin releasing factor
- CeA
central nucleus of the amygdala
- dHipp
dorsal hippocampus
- DMH
dorsomedial hypothalamus
- GR
glucocorticoid receptor
- LS
lateral septum
- NFDM
nonfat dry milk
- OCT3
organic cation transporter
- PVDF
polyvinylidene difluoride
- SERT
serotonin transporter
- TBST
Tris-buffered saline containing tween-20
- vHipp
ventral hippocampus
Footnotes
ACADEMIC EDITOR: Lora Talley Watts, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 515 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by NIH RO1 DA019921 and a USD graduate research grant to Rajeshwari Solanki. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: RS, KR, GF. Analyzed the data: RS, JS, GF. Wrote the first draft of the manuscript: RS, GF. Contributed to the writing of the manuscript: RS, JS, KR, MW, GF. Agree with manuscript results and conclusions: RS, JS, KR, MW, GF. Jointly developed the structure and arguments for the paper: RS, GF. Made critical revisions and approved final version: RS, JS, KR, MW, GF. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Srisurapanont M, Jarusaraisin N, Kittirattanapaiboon P. Treatment for amphetamine dependence and abuse. Cochrane Database Syst Rev. 2001;2:CD003021. doi: 10.1002/14651858.CD003022. [DOI] [PubMed] [Google Scholar]
- 2.Vuong SM, Oliver HA, Scholl JL, Oliver KM, Forster GL. Increased anxiety-like behavior of rats during amphetamine withdrawal is reversed by CRF2 receptor antagonism. Behav Brain Res. 2010;208:278–281. doi: 10.1016/j.bbr.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tu W, Cook A, Scholl JL, et al. Serotonin in the ventral hippocampus modulates anxiety-like behavior during amphetamine withdrawal. Neuroscience. 2014;281:35–43. doi: 10.1016/j.neuroscience.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liakoni E, Dolder PC, Rentsch K, Liechti ME. Acute health problems due to recreational drug use in patients presenting to an urban emergency department in Switzerland. Swiss Med Wkly. 2015;145:w14166. doi: 10.4414/smw.2015.14166. [DOI] [PubMed] [Google Scholar]
- 5.Vorspan F, Mehtelli W, Dupuy G, Bloch V, Lepine J. Anxiety and substance use disorders: co-occurrence and clinical issues. Curr Psychiatry Rep. 2015;17(2):4. doi: 10.1007/s11920-014-0544-y. [DOI] [PubMed] [Google Scholar]
- 6.Koob GF. Negative reinforcement in drug addiction. Curr Opin Neurobiol. 2013;23:559–563. doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Koob GF, Buck CL, Cohen A, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in neurobiology of addiction. Front Neuroendocrinol. 2014;35:234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuloaga DG, Jacobskind JS, Raber J. Methamphetamine and the hypothalamic-pituitary-adrenal axis. Front Neurosci. 2015;9:178. doi: 10.3389/fnins.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholl JL, Vuong SM, Forster GL. Chronic amphetamine treatment enhances corticotropin-releasing factor-induced serotonin release in the amygdala. Eur J Pharmacol. 2010;644:80–87. doi: 10.1016/j.ejphar.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu VM, Schenk JO. Mechanism of action of methamphetamine within the catecholamine and serotonin areas of the central nervous system. Curr Drug Abuse Rev. 2012;5(3):227–242. doi: 10.2174/1874473711205030227. [DOI] [PubMed] [Google Scholar]
- 12.Barr JL, Scholl JL, Solanki RR, et al. Influence of chronic amphetamine treatment and acute withdrawal on serotonin synthesis and clearance mechanisms in the rat ventral hippocampus. Eur J Neurosci. 2013;37:479–490. doi: 10.1111/ejn.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Scholl JL, Tu W, et al. Serotonergic responses to stress are enhanced in the central amygdala and inhibited in the ventral hippocampus during amphetamine withdrawal. Eur J Neurosci. 2014;40:3684–3692. doi: 10.1111/ejn.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr JL, Forster GL. Serotonergic neurotransmission in the ventral hippocampus is enhanced by corticosterone and altered by chronic amphetamine treatment. Neuroscience. 2011;19:105–114. doi: 10.1016/j.neuroscience.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forster GL, Feng N, Watt MJ, et al. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Bordukalo-Niksic T, Mokrovic G, Stefulj J, Zivin M, Jernej B, Cicin-Sain L. 5-HT1A receptors and anxiety like behaviors: studies in rats with constitutionally upregulated/downregulated serotonin transporter. Behav Brain Res. 2010;213:238–245. doi: 10.1016/j.bbr.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Pluess M, Velders FP, Belsky J, et al. Serotonin transporter polymorphism moderates effects of prenatal maternal anxiety on infant negative emotionality. Biol Psychiatry. 2011;69:520–525. doi: 10.1016/j.biopsych.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Verheij M, Karel P, Cools AR, Homberg JR. Reduced cocaine-induced serotonin, but not dopamine and noradrenaline, release in rats with a genetic deletion of serotonin transporter. Neuropsychopharmacology. 2014;24:1850–1854. doi: 10.1016/j.euroneuro.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Bijlsma EY, Hendriksen H, Baas JM, Millan MJ, Groenink L. Lifelong disturbance of serotonin transporter functioning results in fear learning deficits: reversal by blockage of CRF1 receptors. Eur Neuropsychopharmacol. 2015;25:1733–1743. doi: 10.1016/j.euroneuro.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Steinkellner T, Montgomery TR, Hofmaier T, et al. Amphetamine action at the cocaine and amphetamine sensitive serotonin transporter is modulated by alpha CaMKII. J Neurosci. 2015;35:8258–8271. doi: 10.1523/JNEUROSCI.4034-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talati A, Guffanti G, Odgerel Z, et al. Genetic variants within the serotonin transporter associated with familial risk for major depression. Psychiatry Res. 2015;228:170–173. doi: 10.1016/j.psychres.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998;1(5):349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Kekuda R, Huang W, et al. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. J Biol Chem. 1998;273(49):32776–32786. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- 24.Amphoux A, Vialou V, Drescher E, et al. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Baganz NL, Horton RE, Calderon AS, et al. Organic cation transporter 3: keeping the brake on extracellular serotonin in the serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A. 2008;105(48):18976–18981. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasser PJ, Lowry CA, Orchinik M. Corticosterone sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress induced modulation of monoaminergic neurotransmission. J Neurosci. 2006;26:8758–8766. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baganz N, Horton R, Martin K, Holmes A, Daws LC. Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun. J Neurosci. 2010;30:15185–15195. doi: 10.1523/JNEUROSCI.2740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graf EN, Wheeler RA, Baker DA, et al. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci. 2013;33:11800–11810. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitaichi K, Fukuda M, Nakayama H, et al. Behavioral changes following anti-sense oligonucleotide-induced reduction of organic cation transporter-3 in mice. Neurosci Lett. 2005;382:195–200. doi: 10.1016/j.neulet.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Marcinkiewcz CA, Devine DP. Modulation of OCT3 expression by stress, and antidepressant-like activity of decynium-22 in an animal model of depression. Pharmacol Biochem Behav. 2015;131:33–41. doi: 10.1016/j.pbb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Horton RE, Apple DM, Owens WA, et al. Decynium-22 enhances SSRI induced antidepressant-like effects in mice; uncovering novel targets to treat depression. J Neurosci. 2013;33:10534–10543. doi: 10.1523/JNEUROSCI.5687-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daws LC, Koek W, Mitchell NC. Revisiting serotonin reuptake inhibitors and the therapeutic potential of “uptake-2” in psychiatric disorders. ACS Chem Neurosci. 2014;4(1):16–21. doi: 10.1021/cn3001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng N, Mo B, Johnson PL, Orchinik M, Lowry CA, Renner KJ. Local inhibition of organic cation transporters increases extracellular serotonin in the medial hypothalamus. Brain Res. 2005;1063:69–76. doi: 10.1016/j.brainres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Feng N, Lowry CA, Lukkes JL, Orchinik M, Forster GL, Renner KJ. Organic cation transporter inhibition increases medial hypothalamic serotonin under basal conditions and during mild restraint. Brain Res. 2010;1326:105–113. doi: 10.1016/j.brainres.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3: a corticosterone sensitive monoamine transporter, in the rat brain. J Comp Neurol. 2009;512:529–555. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- 36.DiMicco JA, Samuels BC, Zaretskaia MV, Zaretskv DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71(3):469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 37.Shaw D, Norwood K, Leslie JC. Chlordiazepoxide and lavender oil alter unconditioned anxiety-induced c-fos expression in the rat brain. Behav Brain Res. 2011;224:1–7. doi: 10.1016/j.bbr.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Sevoz-Couche C, Brouillard C, Camus F, et al. Involvement of the dorsomedial hypothalamus and the nucleus tractus solitarii in chronic cardiovascular changes associated with anxiety in rats. J Physiol. 2013;591:1871–1887. doi: 10.1113/jphysiol.2012.247791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nascimento JO, Kikuchi LS, De Bortoli VC, Zangrossi H, Jr, Viana MB. Dorsomedial hypothalamus serotonin 1A receptors mediate a panic related response in the elevated T-maze. Brain Res Bull. 2014;109:39–45. doi: 10.1016/j.brainresbull.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Silva MS, Pereira BA, Cespedes IC, Nascimento JO, Bittencourt JC, Viana MB. Dorsomedial hypothalamus CRF type 1 receptors selectively modulate inhibitory avoidance response in the elevated T-maze. Behav Brain Res. 2014;271:249–257. doi: 10.1016/j.bbr.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Chee SS, Menard JL, Dringenberg HC. Behavioral anxiolysis without reduction of hippocampal theta frequency after histamine application in the lateral septum of rats. Hippocampus. 2014;24:615–627. doi: 10.1002/hipo.22244. [DOI] [PubMed] [Google Scholar]
- 42.Stamatakis A, Kalpachidou T, Raftogianni A, et al. Rat dams exposed repeatedly to a daily brief separation from the pups exhibit increased maternal behavior, decreased anxiety and altered levels of receptors for estrogens (ER-alpha, ER-beta), oxytocin and serotonin (5-HT1A) in their brain. Psychoneuroendocrinology. 2015;52:212–228. doi: 10.1016/j.psyneuen.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 43.Zoicas I, Slattery DA, Neumann ID. Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology. 2014;39:3027–3035. doi: 10.1038/npp.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luyten L, Hendrickx S, Raymaekers S, Gabriels L, Nuttin B. Electrical stimulation in the bed nucleus of stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.124. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Nagaya N, Acca GM, Maren S. Allopregnanolone in the bed nucleus of stria terminalis modulates contextual fear in rats. Front Behav Neurosci. 2015;9:205. doi: 10.3389/fnbeh.2015.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canto-de-souza L, Garcao DC, Romaguera F, Mattioli R. Dorsal hippocampus microinjection of chlorpheniramine reverses the anxiolytic-like effects of l-histidine and impairs emotional memory in mice. Neurosci Lett. 2015;587:11–16. doi: 10.1016/j.neulet.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 47.Deusser J, Schmidt S, Ettle B, et al. Serotonergic dysfunction in the A53T alpha-synuclein mouse model of Parkinson’s disease. J Neurochem. 2015;135:589–597. doi: 10.1111/jnc.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lisboa SF, Borges AA, Nejo P, Fassini A, Guimaraes FS, Resstel LB. Cannabinoid CB1 receptors in the dorsal hippocampus and prelimbic medial prefrontal cortex modulate anxiety-like behavior in rats: additional evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2015;59:76–83. doi: 10.1016/j.pnpbp.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. San Diego, CA: Academic Press; 1998. Imprint of Elsevier. [Google Scholar]
- 50.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 51.Hilber B, Scholze P, Dorostkar MM, et al. Serotonin-transporter mediated efflux: a pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacology. 2005;49:811–819. doi: 10.1016/j.neuropharm.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Seidel S, Singer EA, Just H, et al. Amphetamines take two to tango: an oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol Pharmacol. 2005;67:140–151. doi: 10.1124/mol.67.1.. [DOI] [PubMed] [Google Scholar]
- 53.Frick A, Ahs F, Engman J, et al. Serotonin synthesis and reuptake in social anxiety disorder: positron emission tomography study. JAMA Psychiatry. 2015;72:794–802. doi: 10.1001/jamapsychiatry.2015.0125. [DOI] [PubMed] [Google Scholar]
- 54.Petrov T, Krukoff TL, Jhamandas JH. Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res. 1994;277:289–295. doi: 10.1007/BF00327776. [DOI] [PubMed] [Google Scholar]
- 55.Pringle RB, Mouw NJ, Lukkes JL, Forster GL. Amphetamine treatment increases corticotropin-releasing factor receptors in the dorsal raphe nucleus. Neurosci Res. 2008;62(1):62–65. doi: 10.1016/j.neures.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24(6):1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 58.Joca SR, Padovan CM, Guimaraes FS. Activation of post-synaptic 5-HT(1A) receptors in the dorsal hippocampus prevents learned helplessness development. Brain Res. 2003;978:177–184. doi: 10.1016/s0006-8993(03)02943-3. [DOI] [PubMed] [Google Scholar]
- 59.Eraslan E, Akyazi I, Erqul-Ekiz E, Matur E. Noise stress induced changes in mRNA levels of corticotropin releasing hormone family molecules and glucocorticoid receptors in the rat brain. Folia Biol (Praha) 2015;61:66–73. [PubMed] [Google Scholar]
- 60.Papadopoulou A, Siamatras T, Delgado-Morales R, et al. Acute and chronic stress differentially regulate cyclin-depending kinase 5 in mouse brain: implications to glucocorticoid actions and major depression. Transl Psychiatry. 2015;5:e578. doi: 10.1038/tp.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nikolopoulou E, Mytillinaios D, Calogero AE, et al. Modulation of central glucocorticoid receptors in short and long term experimental hyperthyroidism. Endocrine. 2015;49:828–841. doi: 10.1007/s12020-015-0528-7. [DOI] [PubMed] [Google Scholar]
- 62.Panagiotakopoulos L, Kelly S, Neigh GN. HIV-1 proteins accelerate HPA axis habituation in female rats. Physiol Behav. 2015;150:8–15. doi: 10.1016/j.physbeh.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pawluski JL, Csaszar E, Savage E, Martinez-claros M, Steinbusch HW, Van den Hove D. Effects of stress early in gestation on hippocampal neurogenesis and glucocorticoid receptor density in pregnant rats. Neuroscience. 2015;290:379–388. doi: 10.1016/j.neuroscience.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 64.Budziszewska B, Leskiewicz M, Jaworska-Feil L, Lason W. Repeated cocaine administration down regulates glucocorticoid receptors in the rat brain cortex and hippocampus. Pol J Pharmacol. 1996;48:575–581. [PubMed] [Google Scholar]
- 65.Numachi Y, Yoshida S, Toda S, Matsuoka H, Sato M. Two inbred strains of rats, Fischer 344 and Lewis, showed differential behavior and brain expression of corticosterone receptor mRNA induced by methamphetamine. Ann N Y Acad Sci. 2000;914:33–45. doi: 10.1111/j.1749-6632.2000.tb05181.x. [DOI] [PubMed] [Google Scholar]
- 66.Shekhar A, Katner JS. Dorsomedial hypothalamic GABA regulates anxiety in the social interaction test. Pharmacol Biochem Behav. 1995;50:253–258. doi: 10.1016/0091-3057(94)00307-5. [DOI] [PubMed] [Google Scholar]
- 67.Nascimento JO, Zangrossi H, Jr, Vianna MB. Effects of reversible inactivation of the dorsomedial hypothalamus on panic and anxiety related responses in rats. Braz J Med Biol Res. 2010;43:869–873. doi: 10.1590/s0100-879x2010007500075. [DOI] [PubMed] [Google Scholar]
- 68.Johnson PL, Shekhar A. An animal model of panic vulnerability with chronic disinhibition of the dorsomedial/perifornical hypothalamus. Physiol Behav. 2012;107:686–698. doi: 10.1016/j.physbeh.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mantsch JR, Cullinan WE, Tang LC, et al. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2008;1167:101–111. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knych ET, Eisenberg RM. Effect of amphetamine on plasma corticosterone in the conscious rat. Neuroendocrinology. 1979;29:110–118. doi: 10.1159/000122912. [DOI] [PubMed] [Google Scholar]
- 71.Swerdlow NR, Koob GF, Cador M, Lorang M, Hauger RL. Pituitary adrenal axis responses to acute amphetamine in the rat. Pharmacol Biochem Behav. 1993;45:629–637. doi: 10.1016/0091-3057(93)90518-x. [DOI] [PubMed] [Google Scholar]
- 72.Gomez-Roman A, Ortega-Sanchez JA, Rotllant D, et al. The neuroendocrine response to stress under the effect of drugs: negative synergy between amphetamine and stressors. Psychoneuroendocrinology. 2015;63:94–101. doi: 10.1016/j.psyneuen.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 73.Shu Y, Bello CL, Mangravite LM, Feng B, Giacomini KM. Functional characteristics and steroid hormone-mediated regulation of an organic cation transporter in Madin-Darby canine kidney cells. J Pharmacol Exp Ther. 2011;299:392–398. [PubMed] [Google Scholar]
- 74.Rulcova A, Krausova L, Smutny T, et al. Glucocorticoid receptor regulates organic cation transporter 1 (OCT1, SLC22A1) expression via HNF4alpha upregulation in primary human hepatocytes. Pharmacol Rep. 2013;65:1322–1335. doi: 10.1016/s1734-1140(13)71491-9. [DOI] [PubMed] [Google Scholar]
- 75.Marcinkiewcz CA, Devine DP. Modulation of OCT3 expression by stress and antidepressant-like activity of decynium-22 in an animal model of depression. Pharmacol Biochem Behav. 2015;131:33–41. doi: 10.1016/j.pbb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 76.Glatz K, Mossner R, Heils A, Lesch KP. Glucocorticoid-regulated human serotonin transporter (5-HTT) expression is modulated by the 5-HTT gene-promotor-linked polymorphic region. J Neurochem. 2003;86:1072–1078. doi: 10.1046/j.1471-4159.2003.01944.x. [DOI] [PubMed] [Google Scholar]