Abstract
Importance
Whether the extent of coronary artery disease (CAD) is associated with the occurrence of heart failure (HF) after myocardial infarction (MI) is not known. Further, whether this association might differ by HF type according to preserved or reduced ejection fraction (EF) has yet to be determined.
Objective
To evaluate in a community cohort of patients with incident (first ever) MI, the association of angiographic CAD with subsequent HF and to examine the prognostic role of CAD according to HF subtypes: HF with reduced EF (HFrEF) and HF with preserved EF (HFpEF).
Design, Setting and Participants
Population-based cohort study of Olmsted County, Minnesota residents (n=1,922; mean age, 64 years) with incident MI diagnosed between 1990–2010 and no prior HF; followed through 2013.
Main Outcomes and Measures
The extent of angiographic CAD was defined at baseline according to the number of major epicardial coronary arteries with ≥50% lumen diameter obstruction. HF was ascertained by the Framingham criteria and classified by type according to EF (50% cutoff).
Results
During a mean (SD) follow-up of 6.7 (5.9) years, 588 patients developed HF. With death and recurrent MI modeled as competing risks, the cumulative incidence rates of post-MI HF among patients with 0–1, 2, and 3 diseased vessels were 10.7%, 14.6% and 23.0% at 30 days; and 14.7%, 20.6% and 29.8% at 5 years, respectively (p for trend<.001). After adjustment for clinical characteristics in a Cox model, the hazard ratios (95% CIs) for HF were 1.25 (0.99–1.59) and 1.75 (1.40–2.20) in patients with 2 and 3 vs 0–1 occluded vessels, respectively (p for trend<.001). The increased risk with greater number of occluded vessels was independent of the occurrence of a recurrent MI and did not differ appreciably by HF type.
Conclusions and Relevance
The extent of angiographic CAD is predictive of post-MI HF, regardless of HF type and independently of recurrent MI. These data underscore the need to further investigate the processes taking place in the transition from myocardial injury to HF.
Introduction
Several recent publications have drawn attention to the extent of coronary disease as a therapeutic target in acute myocardial infarction (MI) beyond the treatment of the culprit lesion.1–3 How preventive revascularization of non-infarct related arteries is protective against death is not fully understood and, in particular, it is not known whether this beneficial effect could be related to a reduction in heart failure (HF) after MI. This is important as HF remains frequent after MI despite widespread use of acute revascularization.4–6 The mechanisms linking acute MI to HF development may theoretically be envisioned as direct sequelaes of the MI including loss of functioning myocytes, development of myocardial fibrosis, and subsequent left ventricular (LV) remodeling adversely affecting ventricular function.7 The overall atherosclerotic burden could also be evoked as a mechanism of post MI HF. Indeed, chronic myocardial dysfunction resulting from hypoperfusion and/or hibernation may increase the risk of HF,8 particularly if superimposed on a ventricle with irreversibly damaged myocardium.9,10 While these complex putative mechanisms are challenging to explore clinically, a pragmatic approach is to study the relationship between atherosclerotic burden and HF in a cohort of patients with acute MI where comprehensive follow-up can account for recurrent MI. This is important because a clearer clinical appraisal of the determinants of HF after MI could support consideration of revascularization after MI that would extend beyond the acute treatment of the culprit lesion to prevent HF. We therefore evaluated the association of angiographic CAD with subsequent HF in a well-defined community cohort of patients with incident (first ever) MI. Further, community-based studies have shown that CAD, diagnosed based on a history of MI, revascularization, or electrocardiographic changes, is common in HFpEF, and is present in 40% to 50% of patients.11–15 To assess the clinical relevance of CAD in the genesis of HF with preserved ejection fraction (EF),11 we examined the prognostic role of CAD according to HF subtypes: HF with reduced EF (HFrEF) and HF with preserved EF (HFpEF).
Methods
Study Design and Setting
This study was conducted in Olmsted County, Minnesota (2014 population, approximately 150,287), a setting well suited for disease association research due to its relative isolation from other metropolitan centers and because complete medical records from all sources of care for the local population are indexed and linked via the Rochester Epidemiology Project.16 Since virtually all Olmsted County residents are represented in this system, this data source provides a practically complete enumeration of the source population for many decades.17 Following approval as a minimal risk study by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards, the study was carried out utilizing the above resources. All persons included in the study provided authorization for use of their medical records for research.
Cohort Identification and Validation
Residents admitted to Olmsted County hospitals with possible MI from 1990 to 2010 were identified with methods previously described.18 Briefly, all events with International Classification of Diseases, 9th revision (ICD-9) code 410 (acute MI) were reviewed. In addition, a random sample of events with code 411 (other ischemic heart disease) were reviewed (a 50% random sample through 1998, a 10% random sample from 1999 through 2002, and a 100% sample from 2003 through 2010). Additional codes were not included because of their low yield. MIs were validated using standard epidemiologic criteria. Patients diagnosed with MI prior to 1990 were excluded so that only incident (first-ever) cases were studied. The diagnosis of MI was verified based on the presence of two out of three of the following: cardiac pain, electrocardiogram (ECG) changes, and elevated biomarkers. Cases were reviewed to ensure there were no alternative causes resulting in biomarker elevation.
Primary Exposure Measure
Registries of all coronary angiography procedures, diagnostic and therapeutic, performed in Olmsted County have been maintained since 1979. Because Mayo Clinic is the sole provider of coronary angiography in the county, a complete retrieval is possible via the Mayo Clinic Coronary Care Unit database. The primary exposure variable was the extent of CAD as expressed by the number of major coronary arteries with a significant obstruction (0-, 1-, 2-, or 3-vessel disease) obtained from coronary angiograms at a median (25th–75th percentile) of 0 (0–1) day after MI. A significant obstruction was defined as angiographic evidence of 50% or more luminal stenosis of any of the epicardial coronary vessels, including side branches.19
Additional Covariates
The medical record was reviewed to determine cardiovascular risk factors, comorbid conditions, MI characteristics, and acute interventions at the time of incident MI. Cigarette use was classified as current, past, or never. Body mass index (BMI, kg/m2) was calculated using the current weight and earliest adult height. Clinical definitions were used to identify hypertension, diabetes mellitus, and hyperlipidemia. Overall comorbidity burden was assessed by the Charlson index,20 which consists of 17 serious comorbid conditions weighted according to the degree to which they predict mortality. The Modification of Diet in Renal Disease equation21 was used to estimate glomerular filtration rate. ST elevation, anterior MI and Killip class were recorded. The latter was determined within 24 hours of index MI and analyzed as a categorical variable (class >1 vs. class 1). Acute interventions included reperfusion (thrombolytic therapy or percutaneous coronary intervention (PCI)) and coronary artery bypass grafting (CABG) during the index hospitalization.
Outcome Measures
The primary endpoint was time to incident HF. Participants were followed using their complete inpatient and outpatient medical records in the community from the index MI (January 1990-December 2010) to HF incidence, death, or the most recent clinical contact (last follow-up, March 2013). Participants diagnosed with HF by ICD-9 code 428 were identified. Abstractors then reviewed records to validate HF using the Framingham criteria. These criteria require the presence of at least 2 major criteria, or 1 major criterion in addition to 2 minor criteria, to confirm HF.22 This approach has been applied previously, showing minimal missing data and excellent inter-observer agreement.23 The type of HF was defined according to echocardiographic measurement as HFrEF (EF<50%) and HFpEF (EF≥50%). EF was measured using an approach that was recently described.24 The EF measurement that was closest to the HF diagnosis (applying a predefined maximum period of 60 days) was recorded for each participant. The cutoff of 50% to define preserved/reduced EF was selected according to the guidelines.25 Death (occurrence and date) was ascertained by multiple sources including autopsy reports, death certificates filed in Olmsted County, obituary notices, and electronic death certificates obtained from the Section of Vital Statistics, Minnesota Department of Health. Recurrent MI (occurrence and date) data were obtained via the Rochester Epidemiology Project on the basis of clinical diagnoses.26
Statistical Analysis
Baseline characteristics across CAD categories are presented as mean and standard deviations for continuous variables and as frequencies for categorical variables. Cox proportional hazards regression models27 were constructed to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for HF incidence in CAD categories. Several adjustment methods were employed. A traditional multivariable adjustment was performed with age, sex, year of index MI, BMI, Charlson comorbidity index, smoking status, hypertension, hyperlipidemia, atrial fibrillation, Killip class, ST-segment elevation MI, anterior MI, CABG, reperfusion, and estimated glomerular filtration rate as covariates in the model. Secondly, a propensity score was constructed using multinomial logistic regression, through which the probability of being classified into a specific CAD category (0–1, 2, or 3 diseased vessels), conditional on observed baseline covariates, was estimated. Baseline covariates in the latter model included various socio-demographic measures, cardiovascular risk factors, MI characteristics, comorbid conditions, laboratory data, and clinical interventions. Inverse probability weighs were calculated using the propensity score28,29 and used to create a synthetic sample in which the distribution of measured baseline covariates is independent of CAD category, thus accounting for differences between the patients in the CAD categories that could influence the outcome. Because of the instability that can be induced by extreme weights, stabilized weights were used which also preserve the original sample size. Truncation was additionally applied by resetting observations with weights below the 1st percentile and above the 99th percentile to the values of the 1st and 99th percentiles, respectively.28,29 Additionally, since in the presence of competing risks, standard survival predictions might produce biased estimates,30 the Fine and Gray subdistribution hazard regression model was employed, with death and recurrent MI treated as competing events.31 Multivariable-adjusted cumulative incidence rates across CAD categories, based on the abovementioned competing risks model, were estimated using the direct adjustment method.32 Survival analyses were repeated with HFrEF and HFpEF as individual outcomes. The proportional hazards assumption was tested using different approaches and found not to be violated. Missing values did not exceed 2% in any of the variables used except for EF (18% of HF cases). When applicable, an indicator variable reflecting unknown EF was used. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
Between January 1990 and December 2010, 2,943 residents of Olmsted County, Minnesota, were hospitalized with first MI. Among these, 347 patients had a history of prior HF, 601 had no angiographic assessment available at study entry, and 73 had missing data on important variables and were therefore excluded, leaving 1,922 participants in the present study [mean (SD) age, 64 (13) years; 66% men).
Classified according to the number of occluded coronary arteries, 692 had 3-vessel CAD, 595 had 2-vessel CAD, 566 had 1-vessel CAD, and 69 showed no evidence of substantial coronary occlusion. For analytical purposes, the latter two groups were combined. On average, patients with more extensive angiographic CAD were older, presented with greater comorbidities, had a worse cardiovascular risk factor profile, and higher Killip class. They were also more likely to undergo CABG and less likely to undergo reperfusion compared with patients with fewer diseased vessels (Table 1). Stabilized weighting using the inverse propensity score of being classified into a specific CAD category resulted in achieving balance in baseline characteristics between the groups (Table 1).
Table 1.
Characteristics of Incident Myocardial Infarction Patients by Coronary Artery Disease Category, Olmsted County, Minnesota, 1990–2010
| Observed Data | Weighted by Inverse of Propensity Score | |||||||
|---|---|---|---|---|---|---|---|---|
| CAD categories |
CAD categories |
|||||||
| Characteristic | 0–1 vessel (n=635) |
2 vessels (n=595) |
3 vessels (n=692) |
p | 0–1 vessel (n=592) |
2 vessels (n=601) |
3 vessels (n=690) |
p |
| Age, mean (SD), y | 60.4 (13.4) | 64.4 (13.3) | 67.6 (12.8) | <.001 | 63.1 (13.1) | 64.5 (14.0) | 64.2 (13.8) | .18 |
| Male sex, No. (%) | 393 (62) | 395 (66) | 470 (68) | .06 | 382 (65) | 397 (66) | 442 (64) | .71 |
| Body mass index, mean (SD), kg/m2 | 29.2 (5.9) | 28.9 (5.8) | 28.7 (5.5) | .26 | 28.9 (5.6) | 29.0 (5.9) | 28.9 (5.6) | .91 |
| Smoking, No. (%) | .019 | .91 | ||||||
| None | 232 (37) | 205 (35) | 275 (40) | 215 (36) | 226 (38) | 253 (37) | ||
| Past | 212 (33) | 222 (37) | 260 (38) | 208 (35) | 215 (36) | 237 (34) | ||
| Current | 191 (30) | 168 (28) | 157 (23) | 169 (29) | 160 (27) | 200 (29) | ||
| Hypertension, No. (%) | 325 (51) | 348 (59) | 448 (65) | <.001 | 338 (57) | 351 (58) | 397 (58) | .91 |
| Diabetes mellitus, No. (%) | 67 (11) | 134 (23) | 183 (26) | <.001 | 106 (18) | 125 (21) | 137 (20) | .46 |
| Hyperlipidemia, No. (%) | 327 (52) | 319 (54) | 419 (61) | .002 | 324 (55) | 329 (55) | 385 (56) | .91 |
| Atrial fibrillation at index MI, No. (%) | 45 (7) | 52 (9) | 100 (15) | <.001 | 49 (8) | 67 (11) | 70 (10) | .21 |
| Charlson comorbidity index, No. (%) | <.001 | .47 | ||||||
| 0 | 377 (59) | 264 (44) | 278 (40) | 292 (49) | 277 (46) | 337 (49) | ||
| 1–2 | 199 (31) | 245 (41) | 262 (38) | 209 (35) | 234 (39) | 238 (35) | ||
| ≥3 | 59 (9) | 86 (15) | 152 (22) | 91 (15) | 90 (15) | 115 (17) | ||
| Killip class greater than 1, No. (%) | 108 (17) | 133 (22) | 183 (26) | <.001 | 120 (20) | 137 (23) | 145 (21) | .56 |
| ST-elevation MI, No. (%) | 227 (36) | 208 (35) | 223 (32) | .36 | 211 (36) | 208 (35) | 238 (35) | .90 |
| Anterior MI, No. (%) | 240 (38) | 199 (33) | 226 (33) | .11 | 206 (35) | 210 (35) | 235 (34) | .94 |
| Coronary artery bypass grafting, No. (%) | 11 (2) | 39 (7) | 173 (25) | <.001 | 35 (6) | 77 (13) | 81 (12) | <.00 1 |
| Reperfusion, No. (%) | 495 (78) | 499 (84) | 419 (61) | <.001 | 462 (78) | 444 (74) | 511 (74) | .16 |
| Estimated GFR, mean (SD) mL/min/1.73 m2 |
70 (20) | 66 (22) | 64 (22) | <.001 | 68 (19) | 66 (22) | 66 (22) | .36 |
CAD, coronary artery disease; GFR, glomerular filtration rate; MI, myocardial infarction; SD, standard deviation.
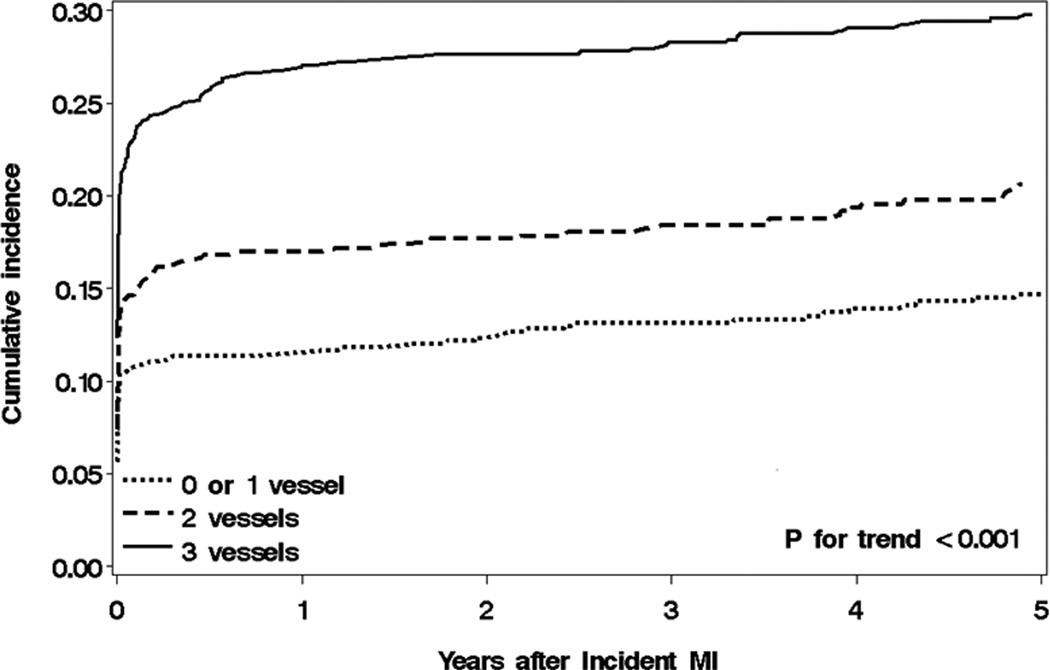
During a mean (SD) follow-up of 6.7 (5.9) years, 588 patients developed HF (78 [13%] in the outpatient setting). Of these, 295 had HFrEF, 186 had HFpEF, and 107 had no EF assessment available. The cumulative incidence rates of HF during follow-up across CAD categories, treating death and recurrent MI as competing risks, are depicted in Figure 1. The cumulative incidence rates of HF among patients with 0–1, 2, and 3 diseased vessels were 10.7%, 14.6% and 23.0% at 30 days; and 14.7%, 20.6% and 29.8% at 5 years after MI, respectively (p for trend<.001). Thus, a higher incidence rate with increasing number of diseased coronary arteries was evident in both short-term and long-term follow-up after MI.
Figure 1.
Cumulative incidence rates of HF according to CAD categories
Cumulative incidence rates of post-myocardial infarction (MI) heart failure across coronary artery disease (CAD) categories in Olmsted County, MN, 1990–2010. Death and recurrent MI are treated as competing events. Follow-up begins at the time of the index MI and is truncated at 6 years (the mean follow-up duration in this analysis).
On a relative scale, worse CAD was associated with an increased risk of subsequent HF (Table 2). A strong, dose-response relationship in the unadjusted model was attenuated, but not eliminated, upon the traditional multivariable adjustment and the inverse probability weighting using the propensity score. In general, the results of the two adjustment methods were similar. Analyzed by HF type, the associations did not differ materially between HFrEF and HFpEF for either type of adjustment method (Table 2). Further adjustment for recurrent MI as a time-dependent covariate in the multivariable Cox regression model yielded HRs (95% CIs) for HF of 1.24 (0.98–1.57) and 1.66 (1.33–2.09) in patients with 2 and 3 vs 0–1 occluded vessels, respectively (p for trend<.001); the increasing trend in HRs was observed similarly for HFrEF [1.19 (0.85–1.65) and 1.71 (1.24–2.35), p for trend=.001] and HFpEF [1.23 (0.81–1.89) and 1.67 (1.11–2.50), p for trend=.014], respectively. Thus, although recurrent MI was associated with HF risk (HR=4.01, 95% CI: 3.03–5.30), it did not substantially confound the CAD-HF association. Similarly, infarct size, as estimated by creatine kinase-MB and cardiac troponin T, has been shown to be associated with HF risk after MI ,26 but no association was observed between the biomarkers and more extensive CAD (p for trend=0.64 and 0.91 for creatine kinase-MB and cardiac troponin T, respectively). Thus infarct size did not substantially confound the CAD-HF association either. Effect modifications of year of index MI, age and sex on the association between CAD extent and HF were assessed and rejected (all p>.10).
Table 2.
Hazard Ratios (95% CIs) for Heart Failure Incidence According to Coronary Artery Disease Categories among Myocardial Infarction Patients in Olmsted County, Minnesota, 1990–2010
| Adjustment Methods | |||
|---|---|---|---|
| CAD category |
Unadjusted | Multivariable regressiona |
Inverse probability weighting using the propensity scoreb |
| Any HF | |||
| 0–1 vessel | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 2 vessels | 1.51 (1.20–1.90) | 1.25 (0.99–1.59) | 1.43 (1.15–1.80) |
| 3 vessels | 2.43 (1.97–2.99) | 1.75 (1.40–2.20) | 1.67 (1.35–2.06) |
| p for trend | <.001 | <.001 | <.001 |
| HFrEF | |||
| 0–1 vessel | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 2 vessels | 1.46 (1.06–2.01) | 1.23 (0.88–1.71) | 1.43 (1.04–1.96) |
| 3 vessels | 2.30 (1.71–3.08) | 1.81 (1.31–2.49) | 1.68 (1.25–2.27) |
| p for trend | <.001 | <.001 | <.001 |
| HFpEF | |||
| 0–1 vessel | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 2 vessels | 1.52 (1.01–2.30) | 1.24 (0.81–1.90) | 1.46 (0.99–2.16) |
| 3 vessels | 2.53 (1.74–3.68) | 1.75 (1.16–2.63) | 1.79 (1.24–2.58) |
| p for trend | <.001 | .005 | .002 |
CAD, coronary artery disease; CI, confidence interval; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Multivariable Cox regression model adjusting for age, sex, year of index MI, body mass index, Charlson comorbidity index, smoking status, hypertension, hyperlipidemia, atrial fibrillation, Killip class, ST-segment elevation MI, anterior MI, coronary artery bypass grafting, reperfusion, and estimated glomerular filtration rate.
Inverse probability weighting Cox regression model based on the propensity score for CAD category.
In ancillary analyses, results from the Fine and Gray subdistribution hazard regression models, with death and recurrent MI treated as competing events, were similar to those obtained from the multivariable adjusted models. Once again, a dose-response association was observed between CAD extent and HF [HR (95% CI) for HF of 1.24 (0.96–1.59) and 1.55 (1.21, 1.99) in patients with 2 and 3 vs 0–1 occluded vessels, respectively (p for trend<.001)]. When analyzed by type of HF, similar results were seen for HFrEF [1.26 (0.88–1.81) and 1.66 (1.17–2.34, p for trend=0.004)], while a greater attenuation was observed for HFpEF [1.06 (0.68–1.65) and 1.28 (0.83–1.98), p for trend=0.24].
In a second ancillary analysis, patients with no evidence of coronary artery occlusion (n=69) were excluded from the analysis. The results were almost identical to those presented herein.
Discussion
The present study represents the comprehensive experience of an entire community, with nearly 2,000 patients with validated first MI, no prior HF, and baseline angiographic assessment followed longitudinally for a mean duration of 7 years. During this period, over 30% of the participants developed incident HF. Patients with greater atherosclerotic burden at baseline tended to be older with higher Killip class and worse cardiovascular profiles; their HF-free survival was substantially shorter than that of patients with lower atherosclerotic burden in a clear dose-response fashion. Using comprehensive statistical adjustment methods to balance the baseline characteristics of the compared groups and accounting for competing risks during follow-up, the association between angiographic CAD extent and HF incidence remained robust, statistically significant and clinically substantial. When evaluated by HF type, the magnitude of the association did not differ materially according to preserved or reduced EF. Thus, increasing extent of CAD, as detected by angiography at the time of first MI, is predictive of HF incidence during long-term follow-up. The association manifests itself promptly after MI, is independent of recurrent MI and applies similarly to HFrEF and HFpEF.
The association between angiographically-determined CAD extent and post-MI HF was previously reported in two studies. Among 1,619 patients enrolled in the Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) trials, the number of diseased vessels was predictive of HF development, both in-hospital and during 1-year follow-up.33 In the Valsartan in Acute Myocardial Infarction Study (VALIANT), among patients with acute MI complicated by either clinical or radiologic signs of HF, Janardhanan et al.34 suggested a prognostic role for increasing extent of CAD in adverse cardiovascular outcomes, including HF exacerbation. While these findings are important, caution should be used in interpreting them. As both studies used selected populations of clinical trial participants, the generalizability of their results is uncertain.35 Moreover, these results now reflect somewhat dated cohorts which do not capture major changes in the epidemiology of MI that occurred in the last 2 decades, including increased proportion of non-ST-segment elevation MI, improved treatment, reduced short-term case fatality and recurrent MI rates, and increased proportion of morbidity and mortality from noncardiovascular causes.18,36,37 Temporal trends have also occurred in HF complicating MI, with a decline in its incidence4–6 and a change in the case mix with an increasing proportion of HFpEF.4
The clinical implications of CAD among 376 patients with HFpEF were recently reported.11 Angiographically-proven CAD, present in 68% of the subjects, was associated with increased mortality and greater deterioration in ventricular function. A worse prognosis associated with increasing extent of CAD was also noted in HFrEF.38 While these studies demonstrated the prognostic role of angiographic CAD in symptomatic patients with HFrEF and HFpEF, to the best of our knowledge no study to date has evaluated the association between angiographic CAD at the time of acute MI and subsequent risk for HF according to EF. Herein, we address this gap in knowledge and provide evidence that the number of diseased vessels, as defined angiographically at the time of the first ever MI, is a strong predictor of both HFrEF and HFpEF.
The mechanisms through which concomitant atherosclerosis in coronary vessels other than the culprit artery adversely affects HF risk post-MI need further study. Diffuse atherosclerosis may directly or indirectly exert an adverse impact on long-term prognosis of MI, either because of the extent of ischemic damage or by causing subsequent events (e.g., recurrent MI) that increase the risk of HF. Because we capture recurrent MIs during follow-up in our community cohort and accounted for recurrent MIs analytically, our results do not give credence to recurrent MI being a determinant of the CAD-HF association. Interestingly, LV dilation was shown to frequently occur after primary PCI in patients with acute MI despite sustained patency of the infarct-related artery and preservation of regional and global LV function.39 This finding, while highlighting the importance of optimal microvascular flow and tissue reperfusion, also suggests that other factors different from infarct size and culprit vessel patency may play a role in post-MI LV remodeling and subsequent HF. Patients with both epicardial and endocardial CAD may have chronic hypoperfusion which leads to increased myocardial stiffness secondary to chronic inflammation and fibrosis. This, in turn, may impair systolic and diastolic function.10 These findings resonate with reports of an association between CAD and HFpEF.11
Limitations and Strengths
Some limitations of our study should be acknowledged to aid in data interpretation. As in any observational study, we cannot rule out the effect of residual confounding due to unmeasured variables. Of the potentially eligible patients, 601 subjects (23%) did not have an angiographic assessment during the index hospitalization, which may limit the generalizability of this sample. Of the participants diagnosed with HF, 106 (18%) were missing EF data. These results emanate from a single community of mostly white race/ethnicity and the racial and ethnic composition of the population may limit the generalizability to groups not adequately represented.
The present investigation has several notable strengths. We capitalized on the comprehensive data resources of the Rochester Epidemiology Project to examine the role of angiographically-determined CAD on post-MI prognosis in the community. We report on a large, population-based inception cohort registered at the time of their first MI validated by standardized criteria.18 HF, the primary outcome measure, was rigorously ascertained and also validated using established criteria.22 Echocardiographic data allowed categorization into HFrEF and HFpEF, which is important to understand the HF syndrome. Finally, different analytical methods were used to estimate the net effect of CAD on HF risk, all yielding similar results, which attest to the robustness of our findings.
Summary and Implications.
Over the past 2 decades, major changes in the epidemiology of MI have occurred. Progress in its acute treatment improved short-term survival, but HF remains frequent after MI and leads to excess mortality. Hence, the acute treatment of MI aimed at restoring vessel patency is not sufficient to prevent HF, underscoring the importance of understanding the contemporary mechanisms leading to its development. Our study provides insight into the prognostic role of the extent of CAD at the time of first MI in the development of HF, and sheds some light on the mechanisms involved. The present findings underscore the importance of further investigations into processes taking place in the transition from the initial myocardial injury to HF. Understanding this transition is crucial to prevent HF after MI.
Acknowledgments
Dr. Roger had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Sources: This study was supported by the National Institutes of Health, Bethesda, MD, (R01 HL59205, R01 HL72435, and R01 HL120957), and made possible by the Rochester Epidemiology Project, Rochester, MN (R01 AG034676) from the National Institute on Aging, Bethesda, MD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr. Enriquez-Sarano reports grants from Edwards LLC, outside the submitted work. Dr. Roger reports grants from National Institutes of Health, grants from National Institutes on Aging, during the conduct of this study. All other authors have nothing to report.
Author Contributions: Conception or design: Gerber, Weston, Roger; Acquisition, analysis, or interpretation of data: Gerber, Weston, Jiang, Roger; Drafting of the manuscript: Gerber, Weston, Enriquez-Sarano, Manemann, Chamberlain, Roger; Critical revision of the manuscript for important intellectual content: Gerber, Weston, Enriquez-Sarano, Manemann, Chamberlain, Roger; Statistical analysis: Gerber, Weston, Jiang; Obtaining funding: Roger; Administrative, technical, or material support: Roger; Study Supervision: Gerber, Roger
References
- 1.Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115–1123. doi: 10.1056/NEJMoa1305520. [DOI] [PubMed] [Google Scholar]
- 2.Mauri L. Nonculprit lesions--or guilty by association. N Engl J Med. 2013;369(12):1166–1167. doi: 10.1056/NEJMe1309383. [DOI] [PubMed] [Google Scholar]
- 3.El-Hayek GE, Gershlick AH, Hong MK, et al. Meta-Analysis of Randomized Controlled Trials Comparing Multivessel Versus Culprit-Only Revascularization for Patients With ST-Segment Elevation Myocardial Infarction and Multivessel Disease Undergoing Primary Percutaneous Coronary Intervention. Am J Cardiol. 2015;115(11):1481–1486. doi: 10.1016/j.amjcard.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 4.Gerber Y, Weston SA, Berardi C, et al. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol. 2013;178(8):1272–1280. doi: 10.1093/aje/kwt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McManus DD, Chinali M, Saczynski JS, et al. 30-year trends in heart failure in patients hospitalized with acute myocardial infarction. Am J Cardiol. 2011;107(3):353–359. doi: 10.1016/j.amjcard.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Hsieh AF, Dharmarajan K, Masoudi FA, Krumholz HM. National trends in heart failure hospitalization after acute myocardial infarction for Medicare beneficiaries: 1998–2010. Circulation. 2013;128(24):2577–2584. doi: 10.1161/CIRCULATIONAHA.113.003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 8.Wijns W, Vatner SF, Camici PG. Hibernating myocardium. N Engl J Med. 1998;339(3):173–181. doi: 10.1056/NEJM199807163390307. [DOI] [PubMed] [Google Scholar]
- 9.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97(3):282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 10.Velagaleti RS, Vasan RS. Heart failure in the twenty-first century: is it a coronary artery disease or hypertension problem? Cardiol Clin. 2007;25(4):487–495. v. doi: 10.1016/j.ccl.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(25 Pt A):2817–2827. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 13.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(1):18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355(3):260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 15.Tribouilloy C, Rusinaru D, Mahjoub H, et al. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. 2008;29(3):339–347. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 16.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roger VL, Weston SA, Gerber Y, et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121(7):863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33(6):1756–1824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 22.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 23.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. Jama. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 24.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5(6):720–726. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith AS. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–e327. doi: 10.1161/CIR.0b013e31829e8776. A.Smith,A.Smith,A.Smith,A. 2013. [DOI] [PubMed] [Google Scholar]
- 26.Gerber Y, Jaffe AS, Weston SA, Jiang R, Roger VL. Prognostic value of cardiac troponin T after myocardial infarction: a contemporary community experience. Mayo Clin Proc. 2012;87(3):247–254. doi: 10.1016/j.mayocp.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox DR. Regression analysis and life table. J. R. Stat. Soc. (Series B) 1972;34:187–222. [Google Scholar]
- 28.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernán MA, Robins JM. Causal Inference. Boca Raton: Chapman & Hall/CRC; 2016. forthcoming. [Google Scholar]
- 30.Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: Methods and application to coronary risk prediction. Epidemiology. 2009;20(4):555–561. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 31.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 32.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101(1):87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connor CM, Hathaway WR, Bates ER, et al. Clinical characteristics and long-term outcome of patients in whom congestive heart failure develops after thrombolytic therapy for acute myocardial infarction: development of a predictive model. Am Heart J. 1997;133(6):663–673. doi: 10.1016/s0002-8703(97)70168-6. [DOI] [PubMed] [Google Scholar]
- 34.Janardhanan R, Kenchaiah S, Velazquez EJ, et al. Extent of coronary artery disease as a predictor of outcomes in acute myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. Am Heart J. 2006;152(1):183–189. doi: 10.1016/j.ahj.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Steg PG, Lopez-Sendon J, Lopez de Sa E, et al. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. 2007;167(1):68–73. doi: 10.1001/archinte.167.1.68. [DOI] [PubMed] [Google Scholar]
- 36.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 37.Gerber Y, Weston SA, Jiang R, Roger VL. The changing epidemiology of myocardial infarction in Olmsted County, Minnesota, 1995–2012. Am J Med. 2015;128(2):144–151. doi: 10.1016/j.amjmed.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39(2):210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 39.Bolognese L, Neskovic AN, Parodi G, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106(18):2351–2357. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]