Abstract
Stereotactic body radiation therapy (SBRT) utilizing a small number of high dose radiation therapy fractions continues to expand in clinical application. Though many approaches have been proposed to radiosensitize tumors with conventional fractionation, how these radiosensitizers will translate to SBRT remains largely unknown. Here, we review our current understanding of how SBRT eradicates tumors, including the potential contributions of endothelial cell death and immune system activation. In addition, we identify several new opportunities for radiosensitization generated by the move toward high dose per fraction radiation therapy.
Keywords: Stereotactic body radiation therapy, radiosensitizers, endothelial cells, immune therapy, DNA repair, hypoxia
Introduction
With improvements in radiation treatment planning and delivery, it has become possible to deliver radiation more precisely and accurately to tumors while limiting dose to surrounding normal tissues. These advancements have given rise to treatment approaches that aim to deliver a small number (5 or fewer) of highly precise, large doses of radiation to a target in certain anatomic sites1. These techniques, which have been termed stereotactic body radiation therapy (SBRT) or stereotactic ablative radiation therapy (SABR) for extracranial treatment and stereotactic radiosurgery (SRS) for intracranial treatment, are being increasingly utilized in different clinical settings to improve the local control of cancer2. SBRT represents a significant change from conventional fractionation, which takes advantage of several radiobiological principles by delivering smaller daily doses over the course of several weeks. This new era in radiation therapy brings an opportunity to incorporate radiosensitizers to counteract potential radiobiological limitations from a shortened treatment course to harness the full potential of large dose per fraction SBRT.
To date, most proposed radiosensitizers have been designed for use in combination with conventionally fractionated radiotherapy. Though the routine clinical application of radiosensitizers and radioprotectors has been limited, our knowledge of the cellular mechanisms that regulate tumor and normal tissue response to radiation has continued to grow, offering new insights into potential targets that may increase the clinical efficacy and safety of radiation therapy3. Although many of these targets are likely conserved with SBRT, it is possible that ablative radiation therapy techniques induce different mechanisms of tumor eradication. Conventional fractionation optimizes the therapeutic ratio based on the “5 R’s” of classic radiation biology4. The smaller number of high dose fractions delivered with SBRT may lead to radiobiological trade-offs, but could also provide new opportunities when combined with targeted drugs. Applying the knowledge that we have already accumulated as we move forward in the SBRT era will require a better understanding of the mechanisms underlying tumor eradication by SBRT and how these processes potentially differ from the effects of conventionally fractionated radiation therapy.
SBRT mechanisms of action
Clinical outcomes with SBRT have been impressive for many tumor types, and these results are often significantly better than what has been achieved historically with conventional fractionation2. Although this improvement may be attributed at least in part to the improved precision and accuracy of SBRT enabling higher doses of radiation to be delivered to tumors, some have proposed that there are fundamental differences in how tumors and normal tissues respond to ultra-high dose per fraction radiotherapy given over a small number of fractions (i.e. oligofractionated radiotherapy). For example, based on results from preclinical studies, some have proposed that the enhanced tumor response to SBRT results from endothelial cell death within tumors5. Others have postulated that SBRT activates the immune system not only to ablate irradiated tumors, but also to attack distant tumors via an abscopal effect6.
Elevated biologically effective dose
Although dose fractionation is unequivocally better for sparing late-responding normal tissues compared with early-responding tumors7, shortening a radiation therapy course to five or fewer fractions delivered over less than two weeks dramatically increases the ability of a given radiation dose to eradicate a tumor. The advent of stereotactic and image-guided radiation delivery has enabled a shift towards oligofractionation with large doses per fraction (i.e. hypofractionation) by confining the high dose region to a restricted planning target volume, thereby limiting the radiation dose to normal tissues. As a result, tumors cells may accumulate more DNA damage and may have less opportunity to repopulate during the overall treatment course. When accounting for this shift in fractionation using the classic linear quadratic model or other models (e.g. universal survival curve) that may be more accurate for large radiation doses per fraction, the biologically effective dose (BED) delivered with SBRT is much higher than with conventional fractionation8, 9.
This biologically effective dose escalation alone may be sufficient to account for the improved clinical outcome with SBRT10. In non-small cell lung cancer, BED calculated using the linear quadratic model correlates strongly with tumor control probability regardless of the fractionation scheme9. Similar results have been observed in patients treated for brain metastases11. Because the linear quadratic model does not take into account distinct biological mechanisms at higher radiation doses, these correlative studies do not support the idea that there are fundamental differences in tumor response to radiation therapy at the high doses per fraction used for SBRT4. Instead, it appears that SBRT may simply enable larger doses to be delivered to tumors than can be achieved with conventional radiation techniques.
Endothelial cell death and revascularization
The initial success of SBRT was paralleled by the reported observation in transplanted mouse tumor models that radiation sensitivity of tumors to high single dose radiation treatment is regulated by the death of endothelial cells within tumors via membrane damage-triggered apoptosis12. Using transplanted fibrosarcomas and melanomas, the authors reported a threshold of 8-10 Gy, above which death of CD34-expressing cells was triggered within 4 to 6 hours after irradiation. Furthermore, decreased growth delay and reduced probability of tumor cure were observed in acid sphingomyelinase-deficient or Bax-deficient mice, which lacked this rapid radiation-induced cell death in stromal cells12, 13. While this was an attractive and timely explanation for the excellent clinical results being reported with the high dose per fraction used in SBRT14, these conclusions were initially challenged15, 16, and alternative interpretations of these data have been proposed4. Indeed, other experiments with transplanted mouse tumor models suggested that stromal cells are not key targets of radiation therapy17-19. However, other groups have also utilized transplanted tumor models to propose that vascular damage is critical for the response of tumors to SBRT5.
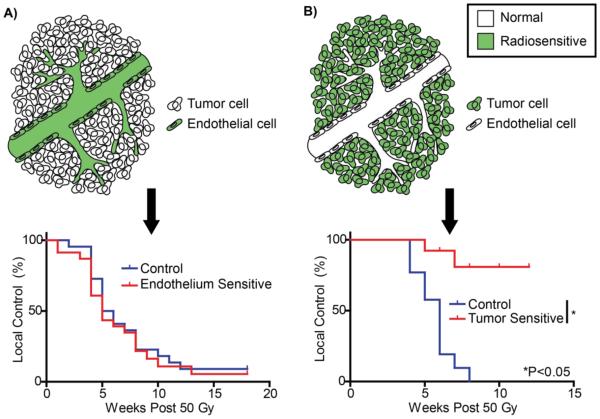
To dissect the role of endothelial cells in primary tumor response to SBRT, we recently developed a novel genetically engineered mouse model (GEMM) of soft tissue sarcoma (Figure 1)20, 21. When these primary sarcomas were treated with a single 20 Gy dose, we observed increased extravasation of dextran out of the vasculature into the tumor.22 Using a genetic approach called dual recombinase technology (for Flp and Cre recombinases), we utilized Flp recombinase to initiate primary sarcomas and Cre recombinase to delete Atm or Bax selectively in the endothelial cells of mice with autochthonous tumors with the goal of either sensitizing the vasculature to radiation-induced cell death or protecting the vasculature from the proposed membrane damage-triggered apoptosis, respectively. Interestingly, we did not observe a rapid wave of endothelial cell apoptosis in our primary sarcoma model, and deleting Bax from the vasculature did not affect radiation-induced endothelial cell death or the response of tumors to doses of radiation commonly used in SBRT. In contrast, deletion of Atm in endothelial cells successfully increased endothelial cell death at 24 hours after radiation treatment and prolonged tumor regrowth with non-curative single and fractionated doses of radiation therapy20. However, sensitizing the tumor vasculature to radiotherapy did not improve the probability of tumor eradication with curative single or fractionated doses of radiation21. Importantly, we also deleted Atm specifically within tumor cells in complementary experiments to demonstrate that sensitizing tumor cells substantially increases the probability of tumor eradication by radiation therapy. These results in a primary cancer model system clearly suggest that the increased tumor control observed with SBRT for many tumors is not due to increased endothelial cell death.
Figure 1. Tumor cells, not endothelial cells, mediate tumor eradication by SBRT.
A) Using a novel mouse model of primary soft tissue sarcoma, endothelial cells were selectively sensitized to radiation via deletion of Atm. However, there was no effect on local control following high, single dose radiation therapy (50 Gy × 1). Similar results were obtained with 20 Gy × 4, which is similar to dose schedules typically used for SBRT. B) A reciprocal experiment deleted Atm specifically from the tumor cells in primary sarcomas. In contrast to endothelial cells, radiosensitizing tumor cells significantly improved tumor eradication. Adapted with permission21.
Our findings do not exclude the vasculature as a possible target for radiosensitizers used in combination with SBRT. Although endothelial cell death does not appear to contribute to local control following SBRT, establishing and maintaining a blood supply are critical hallmarks of cancer23. In fact, the ability of tumors to re-establish their vasculature following SBRT may explain why endothelial cell death is not a critical target to cure cancers with radiotherapy. Although numerous studies have shown tumor vasculature damage and dysfunction following typical SBRT doses5, 24, tumors have also been reported to employ local angiogenesis that may be supported by the recruitment of bone marrow derived cells, vasculogenesis through the recruitment of endothelial progenitor cells, and vascular mimicry to maintain adequate blood flow25, 26. The relative contribution of these mechanisms in human tumors following radiation treatment requires further investigation24; however, targeting any of these processes alone or in combination could potentially improve local tumor control with SBRT. Furthermore, as we will describe later, tumor hypoxia can represent a significant hurdle for SBRT that could potentially be overcome with vascular normalization strategies.
Priming the immune system
As tumors develop, they must evolve to evade surveillance by the immune system27, and through this process, they often develop an immunosuppressive microenvironment28. Numerous studies have demonstrated that irradiation can effectively activate the immune system by triggering the release of pro-inflammatory molecules, exposing new tumor antigens through cross-presentation, and upregulating MHC-1 and death receptor expression on tumor cells29, 30. Accordingly, tumor-specific immune responses have been shown to develop during the course of radiation therapy in both animal models and humans31-33. Furthermore, there have been case reports of local irradiation leading to tumor regression outside of the radiation field34-36, and animal studies have demonstrated that T cells mediate this abscopal effect37. These observations have led to enthusiasm that focal radiation therapy could act as an in situ vaccine leading to systemic immune responses38. Preclinical observations have typically concluded that hypofractionation and higher radiation doses are more effective than conventional fractionation at triggering immune responses39. However, there remains debate over whether a large single dose of radiation or fractionated radiotherapy is more effective33, 40-42.
The ability of the immune system to detect cancer cells as “non-self” relies on the recognition of tumor antigens. Tumor antigens can be highly tumor-specific if they arise from mutant proteins that are only expressed in tumor cells or if proteins that are normally confined to germline cells are expressed in cancer cells. Alternatively, tumor antigens can be less tumor-specific, such as tumor expression of tissue-specific proteins or overexpression of endogenous genes important for tumor growth43. Specific T cell responses to tumors could potentially arise from overexpression of endogenous (i.e. wild-type) genes if tumor cells exceed a threshold level of antigen expression required for T cell recognition. Many preclinical studies examining the impact of radiotherapy on the immune system have relied on the overexpression of artificial exogenous antigens such as ovalbumin to monitor tumor-specific immune responses44. How these results will translate to patients where antigens are generated from mutations or overexpression of endogenous genes remains to be elucidated. Evidence to date indicates great variability in tumor immunogenicity45, and the effectiveness with which radiation triggers immune responses will likely depend on the tumor type. Although preclinical studies with transplanted tumors in mice suggest that the immune system helps to clear tumors following SBRT41, the impact of immune response to the success of SBRT in primary tumors remains to be defined relative to the contribution of increased tumor cell kill from dose escalation.
Although SBRT alone may be unable to overcome tumor-induced immunosuppression and may even enhance the immunosuppressive microenvironment in some cases46, there is tremendous excitement that SBRT could act synergistically with immunotherapies (Figure 2). A variety of clinical approaches to activate the immune system have previously been employed to treat tumors, including administration of cytokines or bacteria and the generation of tumor vaccines47, 48. The clinical response rate with these approaches has typically been low, but patients who do respond often have dramatic and durable disease regression. More recently, evidence has mounted that blocking inhibitory immune checkpoints using antibodies against cytotoxic T-lymphocyte associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1)/PD-L1 may be a more potent approach to trigger anti-tumor immunity49. The combination of immune checkpoint blockade and radiation treatment has been shown to be synergistic in preclinical models50-54. As a result, several clinical trials are underway combining SBRT with a variety of immunotherapies55. Early results appear promising39, and the abscopal effect on distant disease outside of the radiation field has been observed clinically in patients treated with a combination of immune checkpoint inhibition and SBRT52, 56-58. However, additional preclinical and clinical trials are necessary to optimize the timing of immunotherapies relative to radiation treatment and to determine the ideal fractionation schemes to activate the immune system.
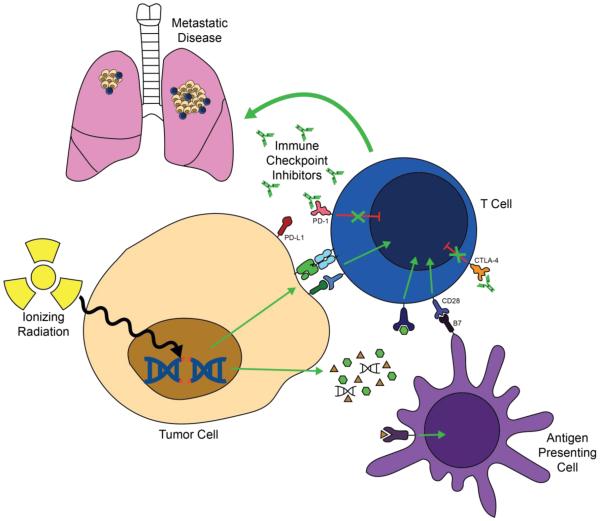
Figure 2. Combining SBRT with immune checkpoint inhibitors.
Ionizing radiation increases the release of cytokines and pro-inflammatory molecules from tumor cells that recruit antigen presenting cells and immune effector cells to the tumor microenvironment. In addition, ionizing radiation increases the release and presentation of tumor antigens that can trigger tumor-specific immune responses. Immune checkpoint inhibitors such as anti-PD-1 and anti-CTLA-4 antibodies synergize with radiation by blocking T cell inhibitory signals. Immune cells can help to kill tumors at the site of radiation, and radiation may trigger systemic tumor-specific immune responses that could lead to regression of metastatic disease outside of the radiation field.
Harnessing the 5 R’s with SBRT
Over the past century, radiobiologists have identified several factors that govern the radiation response of tumors and normal tissues to fractionated radiotherapy. The most critical factors have classically been called the five R’s of radiation therapy, which include repair of sublethal cellular damage, redistribution of cells within the cell cycle, reoxygenation of surviving cells, repopulation of cells after irradiation, and the radiosensitivity intrinsic to the cells59. These factors can work in favor or against tumor eradication depending on context and whether they are applied to tumor cells or normal tissues. Classic approaches to radiosensitize tumors have generally targeted these factors to widen the therapeutic window. Below we consider how the 5 R’s can influence local control with SBRT and how targeting these factors could further increase the efficacy of SBRT (Figure 3).
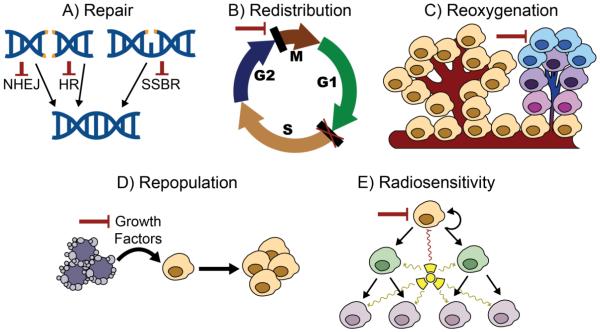
Figure 3. Targeting the 5 R’s of radiation biology.
A) Non-homologous end joining (NHEJ), homologous recombination (HR), and single-strand break repair (SSBR) are critical for repairing double-strand and single-strand DNA breaks caused by SBRT. Targeting the proteins involved in each of these responses alone or in combination leads to radiosensitization. B) Blocking cell cycle checkpoints such as the G2/M transition may lead to mitotic catastrophe following SBRT. These effects are likely increased in tumor cells deficient in other checkpoints due to mutations or altered gene expression. C) Hypoxic cells are resistant to radiation. Targeting these cells could increase local control with SBRT. D) Dying cells may secrete growth factors that lead to the proliferation of surviving tumor cells. Blocking the formation of these factors or their interaction with surviving cells may prevent repopulation of tumor cells following SBRT. E) The radiosensitivity of tumor cells and their ability to regenerate following radiation therapy vary within a tumor. Targeting so-called cancer stem cells, which may be particularly radioresistant, could increase tumor eradication with SBRT.
Repair of sublethal cellular damage
Though some groups have suggested that high dose radiation therapy may cause cell death via damage to the cell membrane or other cellular compartments13, the high dose per fraction of SBRT can kill cells by DNA damage similar to conventional fractionation60. Larger radiation dose fractions delivered over a shorter time period will increase the amount of DNA damage and may lead to more complex alterations that are more difficult to repair. The response of cells to DNA damage varies depending on cell type, type of damage, and cell cycle phase, but targeting the many arms of the DNA damage response has been shown to potently sensitize cells to irradiation61, 62. Given the significant redundancy in the DNA damage response, synergistic radiosensitization may be achieved by targeting multiple DNA damage response proteins simultaneously.
A promising approach may be to target the PI3K-like kinase (PI3KK) family. This family includes several serine/threonine kinases with catalytic domains that have homology to PI3K and play a role in DNA damage response. These proteins include mTOR, which is critical for regulating cellular growth, proliferation, and survival, along with ATM, DNA-PKcs, and ATR63. Due to the homology of these proteins, one drug can inhibit multiple or all members of the PI3KK family, and many PI3K inhibitors are currently in clinical trials without concurrent radiation treatment64. One such drug, NVP-BEZ235, has shown efficacy as a radiosensitizer in preclinical models65-67. Though DNA repair is critical for both tumors and normal tissues, we have recently shown that targeting ATM preferentially radiosensitizes rapidly dividing endothelial cells in mice following doses typically used in SBRT20. Furthermore, we demonstrated that NVP-BEZ235 preferentially radiosensitizes primary mouse sarcomas lacking p53, but not cardiac tissue21, suggesting that targeting the PI3KK family may improve the therapeutic ratio for tumors that arise from slowly proliferating normal tissues such as sarcomas or central nervous system tumors.
Redistribution of cells within the cell cycle
The sensitivity of cells to radiation treatment varies with their position in the cell cycle68. During conventional fractionation, tumor cells progress through the cell cycle between fractions, increasing the probability that they will be in a sensitive phase during delivery of one or more of the fractions. With oligofractionation or single doses, this effect is less pronounced, and some tumor cells may be in resistant phases of the cell cycle during radiation delivery. Interestingly, the high doses used for SBRT may lead to prolonged engagement of cell cycle checkpoints and interphase death69. This suggests that SBRT may be more effective at killing tumor cells in non-radiation sensitive cell cycle phases compared to conventional fractionation, but further investigation is necessary.
Numerous targeted therapies have been developed that can either trigger or halt progression through the cell cycle70, 71. It may be beneficial to manipulate the cell cycle phase of tumor cells or their ability to trigger cell cycle checkpoints to increase radiosensitivity. For example, cells are most sensitive to irradiation during mitosis, and blocking the critical G2/M checkpoint by inhibiting the WEE1 G2 checkpoint kinase may radiosensitize cells by triggering mitotic catastrophe72, 73. Inhibiting more than one cell cycle checkpoint is likely critical, since blocking either the G1/S or G2/M cell cycle checkpoint alone was not shown to increase cellular radiosensitivity in vitro74, 75. However, many cancer cells have mutations in the p53 pathway causing altered G1/S cell cycle arrest. Targeting the G2/M checkpoint in these cells may therefore be sufficient to trigger radiosensitization76. In addition, targeting cell cycle checkpoints may lead to increased radiosensitization in the setting of deficient DNA repair given that the radiosensitizing effect of targeting ATM appears to rely on cell cycle progression20.
Reoxygenation of surviving cells
Tumor hypoxia has long been believed to decrease the efficacy of radiation therapy77. Hypoxia can be transient due to fluctuations in tumor blood flow or chronic due to increased demand for oxygen within tumors and the irregularity of tumor vasculature78. Transient hypoxia during radiation therapy can be offset by radiation fractionation, which allows surviving cells to be reoxygenated between fractions. As a result, oligofractionation has been predicted to lead to reduced cell killing in hypoxic tumors compared with conventional fractionation79. It has been theorized that the benefits of fractionation for reoxygenation are maximized after approximately 7 fractions80, so in some tumors hypoxic cells may prove to be critical targets for radiosensitizers during SBRT. Many approaches have been utilized to account for and lessen the impact of tumor hypoxia on radiation therapy, including increasing oxygen delivery, selectively sensitizing hypoxic cells to radiation, and killing hypoxic cells directly with cytotoxins81. In addition, the application of vascular normalization strategies with antiangiogenic drugs warrants further investigation82. Despite many years of investigation in this area, the clinical benefit of modifying tumor hypoxia is small when applied to all radiation oncology patients, and no clinical approach targeting tumor hypoxia has consistently improved outcomes after conventional radiotherapy. Aside from its influence on radiation therapy efficacy, tumor hypoxia could also adversely impact oncologic outcomes through a direct effect that renders tumor cells more likely to become metastatic or through a stromal effect that stimulates angiogenesis, indirectly leading to tumor regrowth. Regardless, the move to oligofractionation warrants revisiting both the potential challenges of tumor hypoxia and opportunities for new therapeutic combinations in the SBRT era with a fresh perspective. For example, doranidazole, a hypoxic cell radiosensitizer, improved 3-year survival for pancreatic cancer treated with intraoperative radiation therapy using a single 25 Gy fraction83, which is a dose used for SBRT.
Repopulation of cells after radiation
As overall treatment time decreases, tumor cells have less time to divide during the course of therapy, suggesting the role of repopulation could be negligible in single fraction or oligofraction SBRT. However, recent studies have suggested that cell death could trigger tumor cell repopulation following radiation therapy. In this “Phoenix Rising” pathway, executioner caspases activated by the apoptotic machinery in dying cancer cells trigger growth signals such as prostaglandin E2 that stimulate repopulation by surviving tumor cells84. Furthermore, signaling through caspase 3 in dying tumor cells may also stimulate angiogenesis to re-establish tumor vasculature following radiation85. Given the extensive cell death triggered by SBRT, blocking these mechanisms of tumor repopulation has the potential to increase tumor control. Indeed, caspase inhibitors have demonstrated an ability to augment radiation therapy in preclinical tumor models86, 87.
Intrinsic radiosensitivity
Although the intrinsic radiosensitivity of tumor cells is defined as a fixed characteristic of a given tumor, vast variation in tumor cell radiosensitivity exists across different tumor types and from patient to patient88, 89. Additionally, single-cell sequencing has identified multiple genetically unique populations within a given tumor90, and these distinct clones likely vary in radiation sensitivity. Recent studies have suggested that certain populations of cells within tumors are critical for tumor propagation and regrowth following therapy91-93. These tumor propagating cells or cancer stem cells may be particularly resistant to radiation due to their location in hypoxic regions of tumors, enhanced DNA repair, and/or quiescence94. These factors may lead to resistance to SBRT alone. Because the probability of tumor cure relies upon the eradication of tumor clonogens, finding novel approaches to disrupt the stem cell niche or target cancer stem cells directly may improve the efficacy of SBRT95.
Some intrinsic tumor factors may be amenable to selective targeting via synthetic lethality96. For example, inhibiting poly ADP ribose polymerase (PARP), a protein involved in DNA base excision repair, causes synthetic lethality in BRCA-mutant tumors97, 98 and appears to selectively radiosensitize cells with defects in DNA double-strand break repair99. Similarly, the idea of synthetic lethality can be applied to cell cycle checkpoint inhibitors in p53-mutant cancers or cytotoxins that target hypoxic cancer cells as described above. Just as certain mutations can determine the radiosensitivity of cells and their susceptibility to synthetic lethality, they also likely affect how cells respond to altered fractionation. A better understanding of the molecular underpinnings that determine the radiation dose-response relationship in tumor cells will enable us to understand how tumor genotype impacts local control following SBRT and may lead to new targets for radiosensitization.
Summary
The remarkable early clinical success of SBRT for numerous cancers has opened the door for an increasing number of clinical applications, and the move to 1 to 5 treatments with high dose per fraction represents more than a passing trend in radiation oncology. This approach heralds a change from radiobiological sparing of normal tissues via fractionation to harnessing advances in radiation treatment planning and delivery to shrink target volumes and selectively irradiate tumors. The dose escalation afforded by SBRT comes with inherent difficulties in killing hypoxic tumors and cells in resistant phases of the cell cycle due to reduced fraction number. While some targets for radiosensitization from conventional radiotherapy like DNA repair, hypoxia, and cell cycle manipulation remain promising with SBRT, it is likely that new approaches such as immunomodulation will play an increasing role in combination with SBRT.
Acknowledgements
The authors thank the U.S. National Institutes of Health, the Duke Department of Radiation Oncology, and the Duke Cancer Institute for long-term financial support.
Footnotes
Conflicts of Interest
DGK serves on the scientific advisory board and owns stock in Lumicell Inc., which is commercializing intraoperative imaging technology. DGK has received research funding from Lumicell Inc., GlaxoSmithKline, and Janssen.
References
- 1.Lo SS, Fakiris AJ, Chang EL, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7:44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol. 2014;32:2847–54. doi: 10.1200/JCO.2014.55.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat Rev Drug Discov. 2013;12:526–42. doi: 10.1038/nrd4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88:254–62. doi: 10.1016/j.ijrobp.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park HJ, Griffin RJ, Hui S, et al. Radiation-Induced Vascular Damage in Tumors: Implications of Vascular Damage in Ablative Hypofractionated Radiotherapy (SBRT and SRS) Radiat Res. 2012 doi: 10.1667/rr2773.1. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein SE, Timmerman R, McBride WH, et al. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin Dev Immunol. 2011;2011:439752. doi: 10.1155/2011/439752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83:554–68. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–52. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 9.Mehta N, King CR, Agazaryan N, et al. Stereotactic body radiation therapy and 3-dimensional conformal radiotherapy for stage I non-small cell lung cancer: A pooled analysis of biological equivalent dose and local control. Pract Radiat Oncol. 2012;2:288–95. doi: 10.1016/j.prro.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Brown JM, Brenner DJ, Carlson DJ. Dose escalation, not "new biology," can account for the efficacy of stereotactic body radiation therapy with non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85:1159–60. doi: 10.1016/j.ijrobp.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shuryak I, Carlson DJ, Brown JM, et al. High-dose and fractionation effects in stereotactic radiation therapy: Analysis of tumor control data from 2965 patients. Radiother Oncol. 2015;115:327–34. doi: 10.1016/j.radonc.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 13.Truman JP, Garcia-Barros M, Kaag M, et al. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS One. 2010;5:e12310. doi: 10.1371/journal.pone.0012310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Brown M, Bristow R, Glazer P, et al. Comment on "Tumor response to radiotherapy regulated by endothelial cell apoptosis" (II) Science. 2003;302:1894. doi: 10.1126/science.1089517. author reply 1894. [DOI] [PubMed] [Google Scholar]
- 16.Suit HD, Willers H. Comment on "Tumor response to radiotherapy regulated by endothelial cell apoptosis" (I) Science. 2003;302:1894. doi: 10.1126/science.1089918. author reply 1894. [DOI] [PubMed] [Google Scholar]
- 17.Budach W, Taghian A, Freeman J, et al. Impact of stromal sensitivity on radiation response of tumors. J Natl Cancer Inst. 1993;85:988–93. doi: 10.1093/jnci/85.12.988. [DOI] [PubMed] [Google Scholar]
- 18.Gerweck LE, Vijayappa S, Kurimasa A, et al. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Res. 2006;66:8352–5. doi: 10.1158/0008-5472.CAN-06-0533. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa K, Boucher Y, Kashiwagi S, et al. Influence of tumor cell and stroma sensitivity on tumor response to radiation. Cancer Res. 2007;67:4016–21. doi: 10.1158/0008-5472.CAN-06-4498. [DOI] [PubMed] [Google Scholar]
- 20.Moding EJ, Lee CL, Castle KD, et al. Atm deletion with dual recombinase technology preferentially radiosensitizes tumor endothelium. J Clin Invest. 2014;124:3325–38. doi: 10.1172/JCI73932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moding EJ, Castle KD, Perez BA, et al. Tumor cells, but not endothelial cells, mediate eradication of primary sarcomas by stereotactic body radiation therapy. Sci Transl Med. 2015;7:278ra34. doi: 10.1126/scitranslmed.aaa4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moding EJ, Clark DP, Qi Y, et al. Dual-energy micro-computed tomography imaging of radiation-induced vascular changes in primary mouse sarcomas. Int J Radiat Oncol Biol Phys. 2013;85:1353–9. doi: 10.1016/j.ijrobp.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Kozin SV, Duda DG, Munn LL, et al. Neovascularization after irradiation: what is the source of newly formed vessels in recurring tumors? J Natl Cancer Inst. 2012;104:899–905. doi: 10.1093/jnci/djs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn GO, Brown JM. Role of endothelial progenitors and other bone marrow-derived cells in the development of the tumor vasculature. Angiogenesis. 2009;12:159–64. doi: 10.1007/s10456-009-9135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–8. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 27.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 28.Gajewski TF, Woo SR, Zha YY, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Current Opinion in Immunology. 2013;25:268–276. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Schaue D, McBride WH. Opportunities and challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol. 2015;12:527–40. doi: 10.1038/nrclinonc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalbasi A, June CH, Haas N, et al. Radiation and immunotherapy: a synergistic combination. J Clin Invest. 2013;123:2756–63. doi: 10.1172/JCI69219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nesslinger NJ, Sahota RA, Stone B, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13:1493–502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 32.Schaue D, Comin-Anduix B, Ribas A, et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clinical Cancer Research. 2008;14:4883–4890. doi: 10.1158/1078-0432.CCR-07-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaue D, Ratikan JA, Iwamoto KS, et al. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306–10. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehlers G, Fridman M. Abscopal effect of radiation in papillary adenocarcinoma. Br J Radiol. 1973;46:220–2. doi: 10.1259/0007-1285-46-543-220. [DOI] [PubMed] [Google Scholar]
- 35.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol. 1975;48:863–6. doi: 10.1259/0007-1285-48-574-863. [DOI] [PubMed] [Google Scholar]
- 36.Nobler MP. The abscopal effect in malignant lymphoma and its relationship to lymphocyte circulation. Radiology. 1969;93:410–2. doi: 10.1148/93.2.410. [DOI] [PubMed] [Google Scholar]
- 37.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Formenti SC, Demaria S. Radiation Therapy to Convert the Tumor Into an In Situ Vaccine. International Journal of Radiation Oncology Biology Physics. 2012;84:879–880. doi: 10.1016/j.ijrobp.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gandhi SJ, Minn AJ, Vonderheide RH, et al. Awakening the immune system with radiation: Optimal dose and fractionation. Cancer Letters. 2015;368:185–190. doi: 10.1016/j.canlet.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 40.Filatenkov A, Baker J, Mueller AMS, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clinical Cancer Research. 2015;21:3727–3739. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coulie PG, Van den Eynde BJ, van der Bruggen P, et al. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135–46. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 44.Dranoff G. Experimental mouse tumour models: what can be learnt about human cancer immunology? Nature Reviews Immunology. 2012;12:61–66. doi: 10.1038/nri3129. [DOI] [PubMed] [Google Scholar]
- 45.Blankenstein T, Coulie PG, Gilboa E, et al. The determinants of tumour immunogenicity. Nature Reviews Cancer. 2012;12:307–313. doi: 10.1038/nrc3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaue D, Xie MW, Ratikan JA, et al. Regulatory T cells in radiotherapeutic responses. Front Oncol. 2012;2:90. doi: 10.3389/fonc.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patyar S, Joshi R, Byrav DS, et al. Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci. 2010;17:21. doi: 10.1186/1423-0127-17-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 51.Deng LF, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. Journal of Clinical Investigation. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–9. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3:345–55. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crittenden M, Kohrt H, Levy R, et al. Current Clinical Trials Testing Combinations of Immunotherapy and Radiation. Seminars in Radiation Oncology. 2015;25:54–64. doi: 10.1016/j.semradonc.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hiniker SM, Chen DS, Reddy S, et al. A Systemic Complete Response of Metastatic Melanoma to Local Radiation and Immunotherapy. Translational Oncology. 2012;5:404–407. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golden EB, Demaria S, Schiff PB, et al. An Abscopal Response to Radiation and Ipilimumab in a Patient with Metastatic Non-Small Cell Lung Cancer. Cancer Immunology Research. 2013;1:365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steel GG, McMillan TJ, Peacock JH. The 5Rs of radiobiology. Int J Radiat Biol. 1989;56:1045–8. doi: 10.1080/09553008914552491. [DOI] [PubMed] [Google Scholar]
- 60.Núñez M, McMillan T, Valenzuela M, et al. Relationship between DNA damage, rejoining and cell killing by radiation in mammalian cells. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1996;39:155–220. doi: 10.1016/0167-8140(96)01732-x. [DOI] [PubMed] [Google Scholar]
- 61.Helleday T, Petermann E, Lundin C, et al. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 62.Jorgensen TJ. Enhancing radiosensitivity: targeting the DNA repair pathways. Cancer Biol Ther. 2009;8:665–70. doi: 10.4161/cbt.8.8.8304. [DOI] [PubMed] [Google Scholar]
- 63.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–68. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 64.Liu P, Cheng H, Roberts TM, et al. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gil Del Alcazar CR, Hardebeck MC, Mukherjee B, et al. Inhibition of DNA Double-Strand Break Repair by the Dual PI3K/mTOR Inhibitor NVP-BEZ235 as a Strategy for Radiosensitization of Glioblastoma. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fokas E, Yoshimura M, Prevo R, et al. NVP-BEZ235 and NVP-BGT226, dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitors, enhance tumor and endothelial cell radiosensitivity. Radiat Oncol. 2012;7:48. doi: 10.1186/1748-717X-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Konstantinidou G, Bey EA, Rabellino A, et al. Dual phosphoinositide 3-kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Cancer Res. 2009;69:7644–52. doi: 10.1158/0008-5472.CAN-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sinclair WK, Morton RA. X-Ray and Ultraviolet Sensitivity of Synchronized Chinese Hamster Cells at Various Stages of Cell Cycle. Biophysical Journal. 1965;5:1. doi: 10.1016/s0006-3495(65)86700-5. -&. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park HJ, Lyons JC, Ohtsubo T, et al. Cell cycle progression and apoptosis after irradiation in an acidic environment. Cell Death and Differentiation. 2000;7:729–738. doi: 10.1038/sj.cdd.4400702. [DOI] [PubMed] [Google Scholar]
- 70.Asghar U, Witkiewicz AK, Turner NC, et al. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nature Reviews Drug Discovery. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Medema RH, Macurek L. Checkpoint control and cancer. Oncogene. 2012;31:2601–2613. doi: 10.1038/onc.2011.451. [DOI] [PubMed] [Google Scholar]
- 72.Caretti V, Hiddingh L, Lagerweij T, et al. WEE1 kinase inhibition enhances the radiation response of diffuse intrinsic pontine gliomas. Mol Cancer Ther. 2013;12:141–50. doi: 10.1158/1535-7163.MCT-12-0735. [DOI] [PubMed] [Google Scholar]
- 73.De Witt Hamer PC, Mir SE, Noske D, et al. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res. 2011;17:4200–7. doi: 10.1158/1078-0432.CCR-10-2537. [DOI] [PubMed] [Google Scholar]
- 74.Xu B, Kim ST, Lim DS, et al. Two molecularly distinct G(2)/M checkpoints are induced by ionizing irradiation. Molecular and Cellular Biology. 2002;22:1049–1059. doi: 10.1128/MCB.22.4.1049-1059.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu B, O'Donnell H, Kim ST, et al. Phosphorylation of serine 1387 in brca1 is specifically required for the Atm-mediated S-phase checkpoint after ionizing irradiation. Cancer Research. 2002;62:4588–4591. [PubMed] [Google Scholar]
- 76.Dillon MT, Good JS, Harrington KJ. Selective Targeting of the G2/M Cell Cycle Checkpoint to Improve the Therapeutic Index of Radiotherapy. Clinical Oncology. 2014;26:257–265. doi: 10.1016/j.clon.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 77.Gray LH, Conger AD, Ebert M, et al. The Concentration of Oxygen Dissolved in Tissues at the Time of Irradiation as a Factor in Radiotherapy. British Journal of Radiology. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 78.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–37. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carlson DJ, Keall PJ, Loo BW, et al. Hypofractionation Results in Reduced Tumor Cell Kill Compared to Conventional Fractionation for Tumors with Regions of Hypoxia. International Journal of Radiation Oncology Biology Physics. 2011;79:1188–1195. doi: 10.1016/j.ijrobp.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hall EJ, Brenner DJ. The Radiobiology of Radiosurgery - Rationale for Different Treatment Regimes for Avms and Malignancies. International Journal of Radiation Oncology Biology Physics. 1993;25:381–385. doi: 10.1016/0360-3016(93)90367-5. [DOI] [PubMed] [Google Scholar]
- 81.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25:4066–74. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]
- 82.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 83.Karasawa K, Sunamura M, Okamoto A, et al. Efficacy of novel hypoxic cell sensitiser doranidazole in the treatment of locally advanced pancreatic cancer: long-term results of a placebo-controlled randomised study. Radiother Oncol. 2008;87:326–30. doi: 10.1016/j.radonc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 84.Huang Q, Li F, Liu X, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–6. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feng X, Tian L, Zhang ZX, et al. Caspase 3 in dying tumor cells mediates post-irradiation angiogenesis. Oncotarget. 2015;6:32353–32367. doi: 10.18632/oncotarget.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim KW, Hwang M, Moretti L, et al. Autophagy upregulation by inhibitors of caspase-3 and mTOR enhances radiotherapy in a mouse model of lung cancer. Autophagy. 2008;4:659–668. doi: 10.4161/auto.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moretti L, Kim KW, Jung DK, et al. Radiosensitization of solid tumors by Z-VAD, a pan-caspase inhibitor. Mol Cancer Ther. 2009;8:1270–9. doi: 10.1158/1535-7163.MCT-08-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwachofer JH, Hoogenhout J, Kal HB, et al. Radiosensitivity of different human tumor lines grown as xenografts determined from growth delay and survival data. In Vivo. 1990;4:253–7. [PubMed] [Google Scholar]
- 89.Gerweck LE, Zaidi ST, Zietman A. Multivariate determinants of radiocurability. I: Prediction of single fraction tumor control doses. Int J Radiat Oncol Biol Phys. 1994;29:57–66. doi: 10.1016/0360-3016(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 90.Navin N, Kendall J, Troge J, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–4. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Driessens G, Beck B, Caauwe A, et al. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–30. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen J, Li Y, Yu TS, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schepers AG, Snippert HJ, Stange DE, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–5. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 94.Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R's of radiobiology revisited. Stem Cells. 2010;28:639–48. doi: 10.1002/stem.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8:545–54. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 96.Kaelin WG., Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–98. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 97.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 98.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 99.Loser DA, Shibata A, Shibata AK, et al. Sensitization to radiation and alkylating agents by inhibitors of poly(ADP-ribose) polymerase is enhanced in cells deficient in DNA double-strand break repair. Mol Cancer Ther. 2010;9:1775–87. doi: 10.1158/1535-7163.MCT-09-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]