Abstract
Importance
A substantial proportion of patients with atrial fibrillation (AF) die of non-cardiovascular causes, and recent studies suggest a link between AF and cancer.
Objective
To evaluate the relationships between AF and cancer in a large, long term prospective cohort study.
Design, Setting and participants
A total of 34’691 women aged ≥45 years and free of AF, cardiovascular disease and cancer at baseline were prospectively followed between 1993 and 2014 for incident AF and malignant cancer within the Women’s Health Study, a randomized trial of aspirin and Vitamin E for the prevention of cardiovascular disease and cancer. Cox proportional-hazards models using time-updated covariates were constructed to assess the relationship of new-onset AF with subsequent cancer and to adjust for potential confounders.
Exposure
New-onset AF
Main outcome measure
Incident malignant cancer confirmed by an endpoint committee.
Results
During 19.1 years of follow-up, AF was a significant risk factor for incident cancer in age-adjusted (hazard ratio (HR) 1.58, 95% confidence interval (CI), 1.34, 1.87, p<0.0001) and multivariable adjusted (HR 1.48, 95% CI, 1.25, 1.75, p<0.001) models. The relative risk of cancer was highest in the first 3 months after new-onset AF (HR 3.54, 95% CI 2.05, 6.10, p<0.001) but remained significant beyond 1 year after new-onset AF (adjusted HR 1.42, 95% CI 1.18, 1.71, p<0.001), and a trend toward an increased cancer mortality was observed (adjusted HR 1.32, 95% CI 0.98, 1.79, p=0.07). In contrast, among women with new onset cancer, the relative risk of AF was increased only within the first 3 months (HR 4.67, 95% CI 2.85, 7.64, p<0.001) but not thereafter (HR 1.15, 95% CI 0.95, 1.39, p=0.15).
Conclusions and relevance
In this large initially healthy cohort, women with new-onset AF had an elevated cancer risk beyond one year of AF diagnosis. Shared risk factors and/or common systemic disease processes might underlie this association.
Keywords: Atrial fibrillation, cancer, women, epidemiology, death
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia1,2, and associated with an increased risk of major cardiovascular complications3–5. Recent studies suggest that patients with AF also face a substantial risk of death from non-cardiovascular causes6. In a contemporary AF population treated with oral anticoagulation, over a third of all deaths were due to non-cardiovascular causes, and malignancies accounted for the largest proportion of these deaths6.
In retrospective case-control studies, cancer patients were more likely to have AF documented at the time of their diagnosis7. Recent registry data registry suggested that an AF diagnosis is associated with higher than expected cancer incidence rates, although an internal control group was lacking in this study.8 Prior studies had limited ability to control for shared risk factors, which could underlie the association9–11. In addition, the temporal nature of the relationship between AF and cancer is difficult to discern in retrospective study designs, especially given the potential latency of both diagnoses.
An increased risk of malignant cancer among individuals with AF would be of substantial public health importance, given the high prevalence and associated costs of both disorders. We therefore examined the relationships between AF and cancer in a large, well-characterized prospective cohort of 34691 initially healthy women who were followed for up to 20.4 years.
Methods
Study participants
The study design of the Women’s Health Study, a completed randomized trial examining the effects of low dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer, has been described in detail previously12,13. Between 1993 and 1995, 39’876 female health professionals from the United States who were ≥45 years old and free of cardiovascular disease and cancer at baseline were randomized to 100 mg aspirin every other day, 600 IU vitamin E every other day, both agents or placebo. Randomized treatment ended on March 31st 2004, and all women were subsequently invited to participate in an observational cohort study. Written informed consent was obtained from all participants. The institutional review board of Brigham and Women’s Hospital, Boston approved the study.
For the purpose of this analysis, we excluded women with a history of AF (N=879), malignant cancer (N=52) or a major cardiovascular event (N=56) prior to randomization. Women lost to follow-up during the randomized trial period (n=1’243, 3.1%) or who opted out of the observational follow-up (n=2’955, 7.4%) were also excluded because of the inability to reliably confirm AF and subsequent events in these women, as described below. However, the number of self-reported AF events in the excluded subpopulation was only 129. The final study population consisted of 34’691 women (87.0%).
Ascertainment of new-onset atrial fibrillation
AF confirmation in the Women’s Health Study has been described previously14,15. Women were asked to report diagnoses of incident AF at baseline, at 48 months, and then on each questionnaire thereafter. In 2006, we systematically collected permission to review medical records of women who indicated at least one AF episode on any questionnaire between 1993 and 2006. After 2006, medical records were reviewed biannually if AF was reported on subsequent questionnaires. For deceased participants who had reported AF, family members were contacted to obtain consent and additional details for event confirmation. All medical records were reviewed by an endpoint committee of cardiologists to confirm a self-reported AF diagnosis according to predefined criteria. Electrocardiographic AF documentation or a medical report documenting a diagnosis of AF were criteria used for AF confirmation. The date of AF onset was set as the earliest date in the medical records when AF documentation was believed to have occurred. Only confirmed AF events are included in this study. The AF confirmation rate by medical record review was 86%.
The most severe AF pattern within two years of AF onset was used to classify women as having paroxysmal or non-paroxysmal AF16. Paroxysmal AF was defined as self-terminating AF lasting <7 days that did not require cardioversion16,17.
Ascertainment of other covariates
Information on demographics, risk factors, and study outcomes were obtained from questionnaires distributed every six months during the first year and every 12 months thereafter. A large number of covariates were assessed at study entry and at several points of follow-up. Race/ethnicity was self-reported as white, black, Hispanic American, Asian American or other.
Ascertainment of incident cancer and death
Cancer ascertainment in the Women’s Health Study has been described18. If cancer was reported by questionnaire or death certificate, written consent for medical record review was obtained from the participant, or family members if deceased. Subsequently, medical records were collected from hospitals or treating physicians. An endpoint committee of physicians adjudicated all endpoints according to predefined criteria. Reports of cancer were confirmed on the basis of pathology or cytology reports, or rarely based on strong clinical and radiological or laboratory marker evidence when pathology or cytology review was not conducted. The date of cancer onset was usually set as the date on the histology report confirming the diagnosis. The primary cancer end point for the Women’s Health Study was any invasive cancer, excluding non-melanoma skin cancer. Secondary cancer end points included the incidence of breast, colorectal and lung cancer. The cancer confirmation rate by medical record review was 82%.
Deaths were usually reported by family members or postal authorities or ascertained through the National Death Index. A death was defined to be due to cancer, if it was a consequence of the disease itself or of treatment for the disease, as judged by the endpoint committee.
Statistical analysis
Baseline characteristics were compared using Wilcoxon rank-sum tests for continuous variables and Chi-square tests for categorical variables. Person-years of follow-up were calculated from the date of return of the baseline questionnaire to the occurrence of first endpoint, death, loss to follow-up or October 1st, 2013, whichever came first.
To compare the risk of incident cancer among women with and without new-onset AF, we calculated hazard ratios (HR) and 95% confidence intervals (CI) using multivariable time-varying Cox proportional-hazards models adjusted for age, randomized treatment assignment, education, race/ethnicity and height at study entry, as well as time-dependent measures for body mass index, hypertension, hypercholesterolemia, diabetes mellitus, smoking, number of cigarettes smoked per day, alcohol consumption, physical activity, hormone replacement therapy, presence of a recent breast (mammography) or colon cancer (colonoscopy or sigmoidoscopy) screening test and incident non-fatal cardiovascular events (congestive heart failure, myocardial infarction or stroke). Categorical variables were entered in the regression models as shown in Table 1.
Table 1.
Baseline characteristics stratified by the occurrence of incident atrial fibrillation
| Characteristic (N=34691) | No incident AF (n=33224) | Incident AF (n=1467) | P value* |
|---|---|---|---|
| Age, years | 53 (49–58) | 58 (52–64) | <0.001 |
| Race/ethnicity, % | <0.001 | ||
| White | 31290 (95.0) | 1427 (98.1) | |
| Other | 1647 (5.0) | 28 (1.9) | |
| Body mass index, kg/m2 | 24.9 (22.5–28.3) | 26.1 (23.2–30.3) | <0.001 |
| Height, meters | 1.65 (1.60–1.68) | 1.65 (1.63–1.70) | <0.001 |
| Hypertension, % | <0.001 | ||
| Yes | 8550 (25.7) | 610 (41.6) | |
| No | 24674 (74.3) | 857 (58.4) | |
| Diabetes, % | <0.001 | ||
| Yes | 870 (2.6) | 64 (4.4) | |
| No | 32354 (97.4) | 1403 (95.6) | |
| Hypercholesterolemia, % | <0.001 | ||
| Yes | 9988 (30.1) | 501 (34.2) | |
| No | 23236 (69.9) | 966 (65.9) | |
| Smoking, % | 0.06 | ||
| Current/past | 16020 (48.3) | 744 (50.8) | |
| Never | 17180 (51.8) | 721 (49.2) | |
| Alcohol consumption, % | 0.05 | ||
| Rarely/never | 14682 (44.2) | 667 (45.5) | |
| 1–3 drinks per month | 4404 (13.3) | 179 (12.2) | |
| 1–6 drinks per week | 10723 (32.3) | 444 (30.3) | |
| ≥1 drink per day | 3406 (10.3) | 176 (12.0) | |
| Highest education level, % | 0.003 | ||
| < bachelor’s degree | 18153 (55.6) | 870 (60.1) | |
| Bachelor’s degree | 7779 (23.8) | 305 (21.1) | |
| Master’s degree or doctorate | 6722 (20.6) | 272 (18.8) | |
| Physical activity, kcal/week | 586 (196–1340) | 549 (175–1303) | 0.05 |
| Hormone replacement therapy, % | <0.001 | ||
| Current | 13641 (41.1) | 676 (46.2) | |
| Past | 2845 (8.6) | 156 (10.7) | |
| Never | 16675 (50.3) | 632 (43.2) | |
| Number of screening tests for colon cancer | 2 (0–3) | 2 (1–3) | 0.19 |
| Number of screening tests for breast cancer | 7 (4–8) | 7 (4–8) | 0.85 |
AF=Atrial fibrillation. Data are medians (interquartile range) or counts (percentages). Number of observations across categories may not sum to the given numbers because of missing data.
Continuous and categorical variables were compared using Kruskal-Wallis tests and chi-square tests, respectively.
We performed several additional analyses. First, to determine whether the risk of cancer differs according to AF pattern, we constructed Cox models where our exposure of interest was limited to either new-onset paroxysmal or non-paroxysmal AF16. Second, to evaluate whether the risk of cancer differs according to the time after new-onset AF, we constructed multivariable models for incident cancer using 3 separate AF indicator variables for the time periods 0–3 months, 3–12 months and beyond 12 months of new-onset AF, where the reference category for each variable was patients without AF prior to the cancer event. Third, we assessed the risk of cancer subtypes after new-onset AF. Fourth, to further address the possibility of detection bias among women with an AF diagnosis, we evaluated the relationship between new-onset AF and subsequent cancer mortality. Finally, we assessed the robustness of our findings in two sensitivity analyses, where we removed the adjustment for screening procedures or where we only considered AF events occurring in or after 2006, when the AF validation process started. The same covariates listed above were used in all of these models.
We then assessed the risk of incident AF among women with new-onset cancer using Cox proportional hazards models with the same time-dependent covariates detailed above. We again performed an analysis where we separately assessed the risk of incident AF before or after 3 months of new-onset cancer, and before or after 1 year after new-onset cancer.
To address the issue that aspirin has been variably associated with cancer, we assessed the interactions of new-onset AF or cancer with the randomized treatment assignments, and we did another analysis that was limited to the randomized trial period. As a final sensitivity analysis, we repeated the main models using subdistribution hazards models proposed by Fine and Gray, in order to compare cumulative hazards in the presence of competing risks of AF19. All models were constructed using complete-case analyses without imputation for missing data, and no model excluded more than 902 participants (2.6%) due to missing data. All statistical analyzes were carried out using SAS version 9.4 (SAS Institute Inc, Cary, NC). A two-tailed p value <0.05 was considered to indicate statistical significance.
Results
Baseline characteristics of the study participants stratified by AF status are shown in Table 1. During a median follow-up of 19.1 years (interquartile range 17.6–19.7 years), new-onset AF and malignant cancer was confirmed among 1’467 (4.2%) and 5’130 (14.8%) participants, respectively. Women with new-onset AF were significantly older and taller at baseline, had a higher body mass index, a higher prevalence of hypertension, diabetes and hypercholesteremia, a lower education level and were more often white. Similar differences in risk factors were observed when women with and without incident cancer were compared, with the exception of education level, smoking status and number of screening tests for breast and colon cancer performed (Table 2).
Table 2.
Baseline characteristics stratified by the occurrence of incident cancer
| Characteristic (N=34691) | No incident cancer (n=29561) | Incident cancer (n=5130) | P value* |
|---|---|---|---|
| Age, years | 53 (49–58) | 55 (50–61) | <0.001 |
| Race/ethnicity, % | <0.001 | ||
| White | 27813 (94.9) | 4904 (96.5) | |
| Other | 1495 (5.1) | 180 (3.5) | |
| Body mass index, kg/m2 | 24.9 (22.5–28.3) | 25.1 (22.6–28.9) | 0.002 |
| Height, meters | 1.65 (1.60–1.68) | 1.65 (1.60–1.68) | <0.001 |
| Hypertension, % | <0.001 | ||
| Yes | 7623 (25.8) | 1537 (30.0) | |
| No | 21938 (74.2) | 3593 (70.0) | |
| Diabetes, % | 0.01 | ||
| Yes | 768 (2.6) | 166 (3.2) | |
| No | 28793 (97.4) | 4964 (96.8) | |
| Hypercholesterolemia, % | <0.001 | ||
| Yes | 8793 (29.8) | 1696 (33.1) | |
| No | 20768 (70.3) | 3434 (66.9) | |
| Smoking, % | <0.001 | ||
| Current/past | 14031 (47.5) | 2733 (53.3) | |
| Never | 15505 (52.5) | 2396 (46.7) | |
| Alcohol consumption, % | <0.001 | ||
| Rarely/never | 13173 (44.6) | 2176 (42.4) | |
| 1–3 drinks per month | 3925 (13.3) | 658 (12.8) | |
| 1–6 drinks per week | 9511 (32.2) | 1656 (32.3) | |
| ≥1 drink per day | 2942 (10.0) | 640 (12.5) | |
| Highest education level, % | 0.49 | ||
| < bachelor’s degree | 16163 (55.7) | 2860 (56.5) | |
| Bachelor’s degree | 6909 (23.8) | 1175 (23.2) | |
| Master’s degree or doctorate | 5971 (20.6) | 1023 (20.2) | |
| Physical activity, kcal/week | 592 (197–1342) | 550 (182–1321) | 0.02 |
| Hormone replacement therapy, % | 0.002 | ||
| Current | 12166 (41.2) | 2151 (42.0) | |
| Past | 2500 (8.5) | 501 (9.8) | |
| Never | 14838 (50.3) | 2469 (48.2) | |
| Number of screening tests for colon cancer | 2 (1–3) | 2 (0–3) | <0.001 |
| Number of screening tests for breast cancer | 7 (5–8) | 6 (3–8) | <0.001 |
Data are medians (interquartile range) or counts (percentages). Number of observations across categories may not sum to the given numbers because of missing data.
Continuous and categorical variables were compared using Kruskal-Wallis tests and chi-square tests, respectively.
AF as a Risk Factor for Incident Cancer
Among the 1’467 women with new-onset AF, 147 (10.0%) developed cancer during subsequent follow-up. The incidence of cancer among women with and without new-onset AF was 1.4 and 0.8 events per 100 person-years of follow-up.. As shown in Table 3, AF was significantly associated with incident cancer (multivariable adjusted HR 1.48, 95% CI, 1.25, 1.75, p<0.001). Neither the assignment to aspirin nor to vitamin E modified this association (p for interaction 0.27 and 0.16).
Table 3.
Risk of incident cancer among women with new-onset atrial fibrillation
| Hazard ratio (95% confidence interval) | P value | |
|---|---|---|
| All atrial fibrillation events (N=1467) | ||
| Crude | 1.90 (1.61, 2.24) | <0.001 |
| Age-adjusted | 1.58 (1.34, 1.87) | <0.001 |
| Multivariable adjusted* | 1.48 (1.25, 1.75) | <0.001 |
| Paroxysmal atrial fibrillation (N=947) | ||
| Crude | 1.68 (1.36, 2.07) | <0.001 |
| Age-adjusted | 1.42 (1.15, 1.75) | 0.001 |
| Multivariable adjusted* | 1.37 (1.10, 1.69) | 0.004 |
| Non-paroxysmal atrial fibrillation (N=520) | ||
| Crude | 2.22 (1.71, 2.88) | <0.001 |
| Age-adjusted | 1.75 (1.35, 2.27) | <0.001 |
| Multivariable adjusted* | 1.50 (1.15, 1.96) | 0.003 |
Adjusted for age, randomized treatment assignment, education, race/ethnicity and height at study entry, as well as time-dependent measures for body mass index, hypertension, hypercholesterolemia, diabetes, smoking status, number of cigarettes smoked per day, alcohol consumption, physical activity, hormone replacement therapy use, presence of a recent cancer screening test and incident non-fatal cardiovascular events (congestive heart failure, myocardial infarction or stroke). Due to missing data, multivariable models were based on 33789 participants.
The risk of cancer was significantly elevated in women with either paroxysmal or non-paroxysmal AF (Table 3). With respect to timing of incident cancer events, 14 and 27 cancer diagnoses were made within 3 months and 1 year of AF diagnosis. Although the risk of cancer was highest within the first 3 months of incident AF (incidence 3.8 per 100 person-years; adjusted HR 3.54; 95% CI 2.05, 6.10, p<0.001), the risk remained significant beyond 3 months (incidence 1.4 per 100 person-years; adjusted HR 1.39, 95% CI 1.17, 1.66, p<0.001) and beyond 1 year (incidence 1.3 per 100 person-years; adjusted HR 1.42, 95% CI 1.18, 1.71, p<0.001) after new-onset AF. When we limited our analysis to fatal cancers using total cancer mortality as the outcome (n=1’284), new-onset AF was not significantly associated with cancer mortality in multivariable adjusted models (HR 1.32, 95% CI 0.98, 1.79, p=0.07) over the entire range of follow-up. However, the adjusted association with cancer mortality became of borderline significance in the time period 3 months (HR 1.36, 95% CI 1.00, 1.85; p=0.048) or 1 year (HR 1.38, 95% CI 1.00, 1.90; p=0.048) after new-onset AF.
Similar results were obtained when only AF events in 2006 or thereafter were considered (adjusted HR 1.57, 95% CI 1.13, 2.18), when we removed the adjustment for screening procedures (adjusted HR 1.47, 95% CI 1.24, 1.74) or when the analysis was restricted to the randomized trial period (adjusted HR 1.52, 95% CI 1.15, 2.01).
Results of the models that assessed the risk of cancer subtypes are shown in Table 4. The multivariable adjusted HR associated with new-onset AF was highest and statistically significant for colon cancer, whereas multivariable adjusted associations for lung and breast cancer were not statistically significant.
Table 4.
Risk of incident cancer subtypes among women with new-onset atrial fibrillation
| Hazard ratio (95% confidence interval) | P value | |
|---|---|---|
| Incident breast cancer (N=2106) | ||
| Age-adjusted | 1.35 (1.01, 1.81) | 0.04 |
| Multivariable adjusted* | 1.32 (0.99, 1.77) | 0.06 |
| Incident colon cancer (N=451) | ||
| Age-adjusted | 2.36 (1.51, 3.68) | <0.001 |
| Multivariable adjusted* | 2.11 (1.33, 3.36) | 0.002 |
| Incident lung cancer (N=440) | ||
| Age-adjusted | 1.69 (1.02, 2.81) | 0.04 |
| Multivariable adjusted* | 1.51 (0.90, 2.54) | 0.12 |
Adjusted for age, randomized treatment assignment, education, race/ethnicity and height at study entry, as well as time-dependent measures for body mass index, hypertension, hypercholesterolemia, diabetes, smoking status, number of cigarettes smoked per day, alcohol consumption, physical activity, hormone replacement therapy use, presence of a recent cancer screening test and incident non-fatal cardiovascular events (congestive heart failure, myocardial infarction or stroke). Due to missing data, multivariable models were based on 33789 participants.
Cancer as a Risk Factor for Incident AF
Among the 5’130 women diagnosed with cancer during follow-up, 142 (2.8%) subsequently developed AF. Of these, 16 and 21 incident AF events occurred within 3 months and 1 year of cancer diagnosis. The incidence of AF among women with and without new-onset cancer was 0.38 and 0.24 events per 100 person-years of follow-up. The HR (95% CI) for incident AF after cancer was 1.25 (1.05, 1.48, p=0.01) in age-adjusted and 1.20 (1.01, 1.44, p=0.04) in multivariable adjusted models. However, this was primarily due to an increased AF risk within 3 months after a cancer diagnosis, during which time the multivariable adjusted HR for AF was 4.67 (95% CI 2.85, 7.64, p<0.001). The relative risk of incident AF was not increased either beyond 3 months (HR 1.10, 95% CI 0.91, 1.32, p=0.34) or beyond 1 year (HR 1.15, 95% CI 0.95, 1.39, p=0.15) after new-onset cancer. No significant interaction with randomized treatment assignment was observed (p=0.70 and 0.12).
Competing Risk Models
After a diagnosis of new-onset cancer, the risk of death was 4.2% (n=216) at 3 months and 10.1% (n=516) at 1 year. After a diagnosis of new-onset AF, the risk of death was 0.3% (n=4) and 1.2% (n=18), respectively. In Fine-Gray subdistribution hazards models, the multivariable adjusted HR for subsequent AF associated with a new cancer diagnosis was no longer significant (HR 1.03 (95% CI 0.86, 1.24; p=0.76). In contrast, the HR for the relationship between AF and incident cancer was 1.47 (95% CI 1.23, 1.75; p<0.001) and also the remaining associations for cancer subtypes were not materially different from the standard models (Appendix Tables 1 and 2).
Discussion
In this large prospective study of initially healthy women, participants with new-onset AF had a significantly increased risk of incident cancer during subsequent follow-up, even after extensive multivariable adjustment. The relative increase in risk was higher within 3 months of new-onset AF but more modest elevations in risk persisted over the long term. Of the cancer subtypes examined, AF was most strongly associated with colon cancer. In contrast, among women with new onset cancer, the risk of AF was increased only within the first 3 months but not thereafter.
To our knowledge, our work is the first large prospective cohort study with multivariable adjustment for potential confounders on this important topic. In an AF registry, which lacked a non-AF control group and adjustment for confounders, individuals diagnosed with new onset AF during hospitalization had higher rates of malignant cancers compared to the national average, with the highest rates observed for lung, kidney, and colon cancer8. Similar to our findings, there was a modest absolute increase in cancer, which was highest within the first 3 months after AF diagnosis. However, the registry found relatively lower elevations in the rate of cancer after 3 months. The rapid fall in rate ratio after the initial 3 months suggested that the majority of the cancers were likely to have been present at the time of AF diagnosis, although the ability to estimate dates of diagnosis of AF and cancer with certainty in a hospital-based registry is limited. In contrast, in our prospective cohort where medical records were reviewed and date of onset of both diagnoses was determined by endpoint committees, we found that the relative risk for malignant cancer remained significantly elevated beyond one year of AF diagnosis, suggesting that not all cancers were present at the time of AF diagnosis.
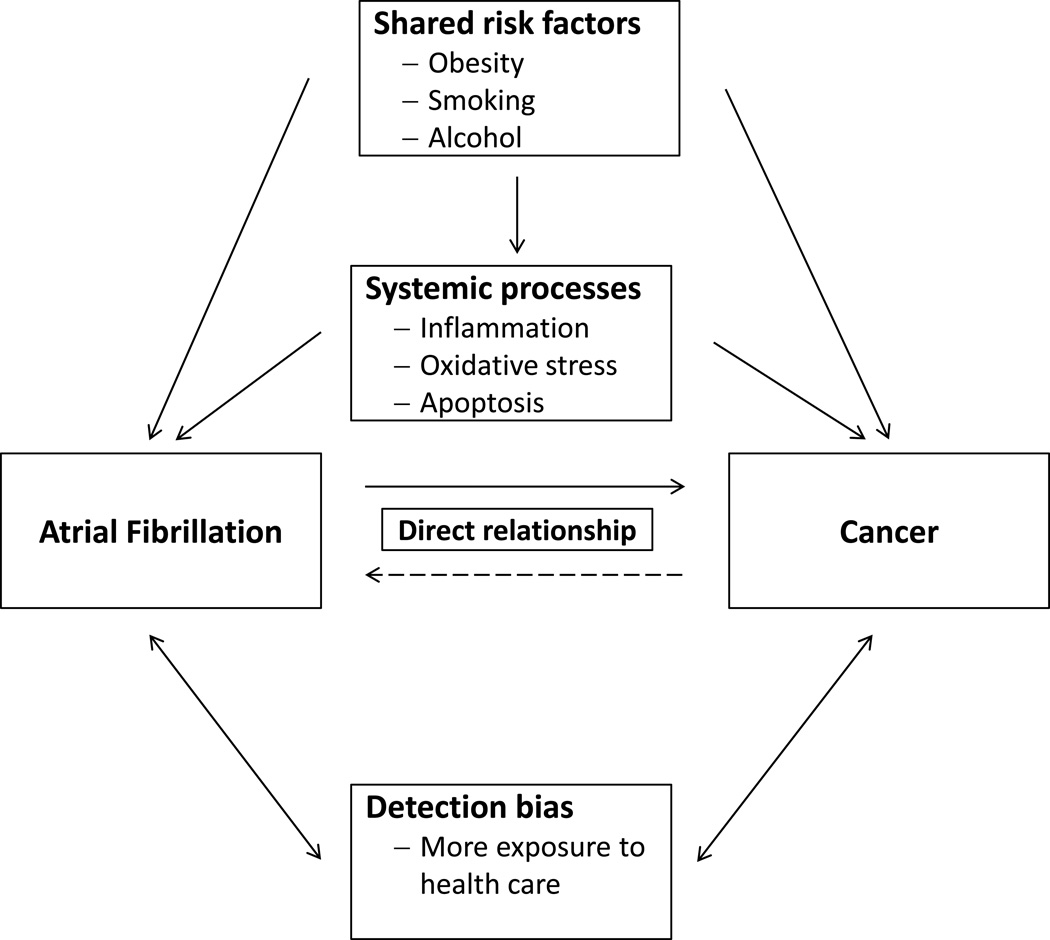
The potential mechanisms underlying the increased long term risk of cancer among individuals with AF are currently unknown. Shared risk factors could be one explanation (Figure)10,11,14,20–23. The similar risk factor profiles among women with new-onset AF and new-onset cancer (Tables 1 and 2) provide support to this concept. These similarities also underscore the importance of properly adjusting for these variables when evaluating this relationship. Although we used comprehensive multivariable adjustment, residual confounding may still persist. In particular body mass index may not ideally represent body fat distribution and metabolic fat activity, which may be more strongly related to certain forms of cancer22.
Figure.
Potential mechanisms for the relationship between atrial fibrillation and cancer
Alternatively, AF may be an early sign of occult cancer or an initial manifestation of a systemic process which increases the risk for both diseases (Figure). Inflammation or oxidative stress could represent combined predisposing processes9,24,25,26,27,28. Both disease processes are associated with pro-thrombotic states29,30. In addition, apoptosis plays a potential role in AF development31, and resultant disruption of the counterregulatory balance between of pro-apoptotic and anti-apoptotic factors could contribute to carcinogenesis by reducing apoptosis in cancer cells32.
Finally, detection bias must be considered as a possible explanation for our findings. Women with new-onset AF and cancer are more exposed to health care and therefore might be more likely to have cancer or AF detected than women without these diseases. The higher short term risk of cancer after new-onset AF could be explained by a diagnostic work-up at the time of AF diagnosis. The longer term elevation in the relative risk of cancer in AF patients is more difficult to explain solely on the basis of detection bias. Cancer screening was not increased in AF patients, and adjustment for screening did not impact the results. We also did not observe an analogous elevation in AF risk among surviving cancer patients, although both diagnoses result in ongoing exposure to the health care system. The long term use of anticoagulants among AF patients could have led to earlier detection of cancers due to bleeding, particularly colon cancer. However, if present, this did not translate into lower cancer mortality. In contrast, cancer mortality tended to be higher beyond 3 months of AF diagnosis. Although cancer may be detected earlier in AF patients, their mortality related to cancer may be higher than in individuals without AF, potentially providing an explanation for the high non-cardiovascular mortality documented in AF patients6.
Our data may have clinical implications. Although the absolute increase in cancer risk among women with new-onset AF was modest in this low risk cohort of initially healthy women, it may be higher in older populations with a higher burden of risk factors. These data further emphasize the importance of risk factor reduction in AF patients to not only reduce recurrent AF episodes33 but to also potentially decrease other adverse outcomes. Additional analyses are needed to evaluate whether incorporating AF into cancer prediction models improve their performance.
Some potential limitations should be taken into account. First, the present study was performed in women who are predominantly white and the generalizability of our findings to other populations is uncertain. Second, despite stringent follow-up methods, some asymptomatic AF cases may have gone undetected. However, if present this should have biased our results toward the null. Nevertheless, it is unclear whether our results also apply to asymptomatic AF. Third, defining the exact date of onset of AF and cancer is challenging, especially in the context of a high short term risk for both disease entities, and likely there is some imprecision in our date of diagnosis. Finally, while AF was assessed on a yearly basis, some other risk factors were less regularly updated, which may have led to some imprecision on the covariate status in time-updated models.
Conclusions
In this large prospective cohort, new-onset AF was a significant risk factor for the subsequent diagnosis of incident cancer. The relative risk was higher early after an AF diagnosis but persisted over the long term. Future studies are needed to assess the mechanisms underlying this association and to determine whether a diagnosis of AF incrementally adds to existing cancer risk prediction algorithms. Regardless, optimal risk factor control in AF patients seems prudent.
Supplementary Material
Acknowledgments
This study was supported by grant HL-093613 from the NHLBI to Dr Albert. The WHS was supported by grants HL-043851, HL-080467, and HL-099355 from the NHLBI and grant CA-047988 from the National Cancer Institute. Dr Conen received research grants from the Swiss National Science Foundation (PP00P3_133681 and PP00P3_159322).
None of the funding sources had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
David Conen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
David Conen conducted and is responsible for the data analysis
Footnotes
Disclosures
None
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–2751. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305(20):2080–2087. doi: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang TS, Gersh BJ, Appleton CP, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40(9):1636–1644. doi: 10.1016/s0735-1097(02)02373-2. [DOI] [PubMed] [Google Scholar]
- 6.Marijon E, Le Heuzey JY, Connolly S, et al. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013;128(20):2192–2201. doi: 10.1161/CIRCULATIONAHA.112.000491. [DOI] [PubMed] [Google Scholar]
- 7.Guzzetti S, Costantino G, Vernocchi A, Sada S, Fundaro C. First diagnosis of colorectal or breast cancer and prevalence of atrial fibrillation. Intern Emerg Med. 2008;3(3):227–231. doi: 10.1007/s11739-008-0124-4. [DOI] [PubMed] [Google Scholar]
- 8.Ostenfeld EB, Erichsen R, Pedersen L, Farkas DK, Weiss NS, Sorensen HT. Atrial fibrillation as a marker of occult cancer. PLoS One. 2014;9(8):e102861. doi: 10.1371/journal.pone.0102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conen D, Ridker PM, Everett BM, et al. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J. 2010;31(14):1730–1736. doi: 10.1093/eurheartj/ehq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123(14):1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tedrow UB, Conen D, Ridker PM, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women's health study) J Am Coll Cardiol. 2010;55(21):2319–2327. doi: 10.1016/j.jacc.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. J Womens Health Gend Based Med. 2000;9(1):19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 14.Conen D, Tedrow UB, Cook NR, Moorthy MV, Buring JE, Albert CM. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA. 2008;300(21):2489–2496. doi: 10.1001/jama.2008.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of Systolic and Diastolic Blood Pressure on the Risk of Incident Atrial Fibrillation in Women. Circulation. 2009;119:2146–2152. doi: 10.1161/CIRCULATIONAHA.108.830042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114(7):e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 17.Sandhu RK, Conen D, Tedrow UB, et al. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc. 2014;3(3):e000916. doi: 10.1161/JAHA.114.000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 20.Everett BM, Cook NR, Conen D, Chasman DI, Ridker PM, Albert CM. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J. 2013;34(29):2243–2251. doi: 10.1093/eurheartj/eht033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299(17):2037–2047. doi: 10.1001/jama.299.17.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62(6):933–947. doi: 10.1136/gutjnl-2013-304701. [DOI] [PubMed] [Google Scholar]
- 23.Nelson DE, Jarman DW, Rehm J, et al. Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am J Public Health. 2013;103(4):641–648. doi: 10.2105/AJPH.2012.301199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108(24):3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 25.Zhang SM, Buring JE, Lee IM, Cook NR, Ridker PM. C-reactive protein levels are not associated with increased risk for colorectal cancer in women. Ann Intern Med. 2005;142(6):425–432. doi: 10.7326/0003-4819-142-6-200503150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Zhang SM, Lin J, Cook NR, et al. C-reactive protein and risk of breast cancer. J Natl Cancer Inst. 2007;99(11):890–894. doi: 10.1093/jnci/djk202. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph V, Andrie RP, Rudolph TK, et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med. 2010;16(4):470–474. doi: 10.1038/nm.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandel NS, Tuveson DA. The promise and perils of antioxidants for cancer patients. N Engl J Med. 2014;371(2):177–178. doi: 10.1056/NEJMcibr1405701. [DOI] [PubMed] [Google Scholar]
- 29.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009;373(9658):155–166. doi: 10.1016/S0140-6736(09)60040-4. [DOI] [PubMed] [Google Scholar]
- 30.Kyriazi V, Theodoulou E. Assessing the risk and prognosis of thrombotic complications in cancer patients. Arch Pathol Lab Med. 2013;137(9):1286–1295. doi: 10.5858/arpa.2012-0490-RA. [DOI] [PubMed] [Google Scholar]
- 31.Cardin S, Li D, Thorin-Trescases N, Leung TK, Thorin E, Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res. 2003;60(2):315–325. doi: 10.1016/j.cardiores.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310(19):2050–2060. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.