Abstract
The intestinal epithelium of insects is exposed to xenobiotics and entomopathogens during the feeding developmental stages. In these conditions, an effective enterocyte turnover mechanism is highly desirable to maintain integrity of the gut epithelial wall. As in other insects, the gut of lepidopteran larvae have stem cells that are capable of proliferation, which occurs during molting and pathogenic episodes. While much is known on the regulation of gut stem cell division during molting, there is a current knowledge gap on the molecular regulation of gut healing processes after entomopathogen exposure. Relevant information on this subject is emerging from studies of the response to exposure to insecticidal proteins from the bacterium Bacillus thuringiensis (Bt) as model intoxicants. In this work we discuss currently available data on the molecular cues involved in gut stem cell proliferation, insect gut healing, and the implications of enhanced healing as a potential mechanism of resistance against Bt toxins.
Introduction to the larval intestine of Lepidoptera
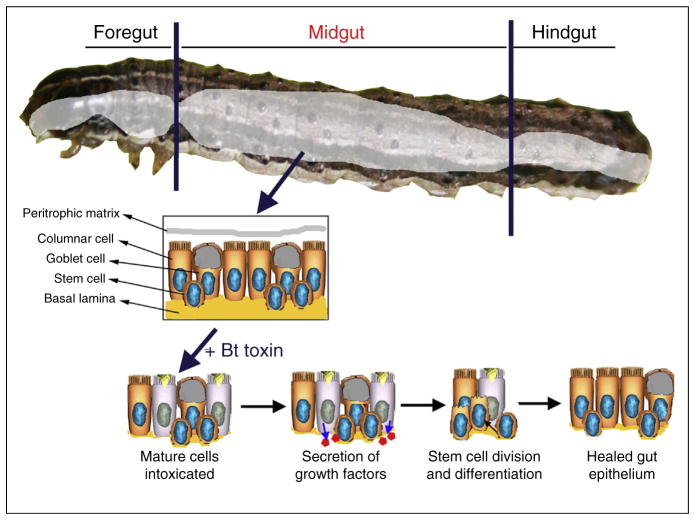
The insect intestinal epithelium has two overarching functions; provide a barrier between ingested items (including microorganisms) and the main body cavity (hemocoel), and nutrient uptake [1]. The monolayer epithelium of Lepidoptera larvae includes four major cell types: intestinal stem cells (ISCs), goblet cells (GCs), columnar cells or enterocytes (ECs), and enteroendocrine cells (EEs) (Figure 1). Basal to the epithelial cell layer is an extracellular matrix (ECM) of circular and longitudinal muscle fibers interwoven with trachea that provide oxygen used during peristaltic muscle contractions that move the food bolus along the digestive tube [2].
Figure 1.
Diagram of the main cell types in the midgut of lepidopteran larvae and the steps in the process of epithelial healing in response to intoxication with toxins from Bacillus thuringiensis (Bt). Less abundant enteroendocrine cells are also present in the midgut are not represented in the figure.
Each cell type in the gut epithelium has a defined role and contributes to unique microenvironments in the tissue. For instance, the unique physicochemical conditions in the gut lumen of Lepidoptera larvae are mostly maintained by the action of vacuolar ATPase pumps and secretions from GCs [3], while the absorptive role of ECs is evidenced by their elongated apical microvilli. Endoreplication of ECs results in polyploidy, further contributing to increased cell size and digestive capabilities [4]. Homeostasis and epithelial renewal are ISC-mediated, since these stem cells are the only gut cell type capable of division and thus represent the only source of new cells during tissue repair and growth. The ability of ISC to proliferate is remarkable, as the gut surface area increases approximately 200-fold during larval development [5]. The role of EEs in insects is secretory in nature and regulates the immune response [6], metabolic/endocrine functions associated with growth [7], lipid metabolism [8], and paracrine/endocrine peptide secretion [9].
Intestinal regenerative mechanisms in Lepidoptera
Midgut growth at each larval instar is initiated by increasing rates of stem cell proliferation [10] and subsequent differentiation to increase the total cell number [5]. This process has been best characterized in Drosophila adult gut epithelium as a relevant genetic model. In Drosophila epithelium, asymmetric ISC divisions assure maintenance of a constant number of ISC cells. Alternatively, gut ISCs may also undergo symmetrical division which may be followed by differentiation to provide a net increase in the number of midgut cells in response to abundant nutrients [11]. However, once gut stem cells differentiate they are incapable of reverting to stem cells [12], in contrast to dedifferentiation processes documented in alternative insect tissues [13].
In Lepidoptera, much progress has been made using primary midgut cell cultures from larvae. These cultures are optimal models to study gut regeneration, as they preserve the proliferative and differentiation features observed during molting [14•] and during the regenerative response to gut injury [15]. Similar to observations in Drosophila, isolated Lepidoptera ISCs undergo asymmetric cell division during epithelial growth and repair (Figure 1), and ISC symmetric differentiation has also been observed with some midgut differentiation factors (MDFs), as detailed below [16]. This dual fate of stem cells is also detected in cultured midgut stem cells from Heliothis virescens larvae; differentiation progressed in the presence of fetal bovine serum, while proliferation was observed in the presence of Albumax II [17]. Other mitogens for cultured stem cell systems were identified from conditioned media and hemolymph (reviewed in [18]). The first MDF identified from conditioned media was a 30 amino acid peptide with high identity to the C-terminus of fetuin [19], a protein that promotes cell attachment and growth in mammals [20]. Undigested fetuin did not have an effect on H. virescens midgut cell cultures and only after tryptic digestion one of the resulting peptides was identified as midgut growth factor MDF2 [19]. Additional peptides inducing midgut stem cell differentiation (MDF3 and MDF4) were isolated from chymotryptic digestion of Lymantria dispar hemolymph [21]. However, 100% differentiation of Lepidoptera stem cell cultures has never been observed with these MDFs, suggesting the existence of additional differentiation factors, including ecdysone [22], α-arylphorin [23] and insulin-related bombyxin [24]. In the case of α-arylphorin there is also evidence for mitogenic activity on gut cells in vivo [25], where 4th instar Manduca sexta larvae displayed weight gain after feeding on arylphorin.
Apart from ISCs, regeneration of midgut epithelia in Lepidoptera is also regulated by tracheal stem cells (TSCs) within the ECM and basal lamina. These cells are cued to undergo cell division during the larval molt to increase the amount of trachea supporting the muscle layer as the size of the epithelium increases with ISCs division and differentiation [26].
Similar to the gut growth process observed during molting, ISCs proliferate and differentiate to restore gut epithelial integrity after diverse biotic and abiotic injuries. However, at least in some cases gut healing may involve additional processes distinct from ISC proliferation. For example, gut healing in Bombyx mori larvae after physical perforation involved recruitment of hemocytes and production of a melanized scab, and stem cell proliferation detected as DNA duplication [27]. In contrast, the response to infection with the bacterium Bacillus thuringiensis (Bt) involved a regenerative mechanism [28,29], which in vitro it has been shown to depend on asymmetrical ISC division [15]. Interestingly, an increase in the number of midgut cells producing MDF1 peptide was detected after treatment with Bt toxins [15], suggesting a potential role for this peptide in response to intoxication.
Ingestion of plant xenobiotics can also have a drastic effect on these healing defensive responses to concurrent entomopathogen ingestion. For instance, the sloughing of virus-infected midgut cells occurred at a higher rate in insects that fed on cotton compared to artificial diet. Cellular sloughing contributed to the prevention of spread of infection and resulted in decreased susceptibility to nucleopolyhedrovirus [30]. Further support for this mechanism was provided by the inhibition of midgut cell sloughing with stilbene-derived brighteners, which restored susceptibility to nucleopolyhedrovirus in Trichoplusia ni and H. virescens [31].
Control of the midgut regenerative response
The adult Drosophila intestine has been the premier model for the genetic characterization of regeneration and homeostasis. Readers are directed to an in-depth recent review of relevant Drosophila literature [32•]; as only main concepts are described in this section. In contrast to the four gut cell types of lepidopteran larvae, a fifth cell type named transient amplifying (TA) cells, appears only during pathogenic episodes in Drosophila. The gene expression profiles and intracellular regulators of ISCs have been described in-depth to ascertain the molecular characteristics defining the intestinal stem cell condition [33]. In Drosophila ISCs the proliferation or differentiation fate depend on an interaction between expression of nuclear binding transcription factors [33]. Another relevant factor in determining ISC fate in Drosophila is the directionality of ISC secretion and uptake. Thus, the apical and basal localization of receptors and ligand secretion dictates the differentiation cues underlying the Notch signaling pathway [34]. High levels of Notch ligand in differentiating cells promotes EC differentiation corresponding to high levels of its Delta receptor. Alternatively, low levels of Notch result in differentiation to EE [35]. The cytosolic release of Ca2+ from intracellular storage in response to extracellular cues has been described as necessary during ISC responding to growth factors and cytokines [36]. These molecular cues are produced by ISCs, the ECM and basal visceral muscle, damaged mature epithelial cells, and not fully differentiated EBs to activate proliferation and differentiation. Pathways involved in homeostasis and repair of gut epithelial damage include the janus kinase signal transducer activator of transcription (JAK/STAT) pathway and its interaction with the epidermal growth factor receptor (EGFR) [37,38]. The role of surrounding differentiated cells on modulating gut homeostasis by ISCs has also been established. For instance, enteroendocrine cells are the only secretory cells in the Drosophila intestine and regulate insulin peptide production in the basal lamina muscle, directly affecting ISC proliferation [39].
In contrast to the detailed models of gut homeostasis emerging in Drosophila, little is known about the specific molecular signals involved in controlling the gut healing response in other insect orders. In this review, we concentrate on response to Bt toxins as an area of research in which relevant progress has been made to describe the gut healing response in mostly non-model insects of agricultural importance. While specific events modulating the gut healing response to Bt toxins are unknown, relevant information is emerging from recent transcriptome, proteomic and functional genomic analyses of exposure to Bt toxins in diverse insects. In general, insects usually reduce their digestive activity, concomitant with an increase in immune related function.
In mosquito (Aedes aegypti) larvae, exposure to mosquitocidal Bt toxins has been shown to induce upregulation of components of the mitogen-activated protein kinase (MAPK) cascade, while cell proliferation was among the down-regulated functions [40]. More specifically, the MAPK p38 pathway was reported to be activated in response to Cry toxins from Bt in both Diptera and Lepidoptera [41•]. In fact, genetic knockdown of p38 resulted in increased insect susceptibility to Bt toxins. Studies evaluating the response of Caenorhabditis elegans to nematocidal Cry toxins, identified downstream responses to activation of the p38 MAPK pathway, including the upregulation of stress response genes and ion transporters [42,43]. One of these stress responses is the activation of the unfolded protein response (UPR) pathway, which has been reported to be activated in response to Cry11Aa in A. aegypti [44].
Studies in Coleoptera have also identified putative immune-related genes and proteins with increased levels after exposure to Cry proteins. However, their role in the gut regenerative response has not been experimentally tested. In the genetic model Tribolium castaneum, proteomic and transcriptomic studies have identified apolipophorin III as being upregulated in response to Cry3 proteins [45,46]. Selective up-regulation of apolipophorin III only occurs after exposure to Cry proteins active against T. castaneum [47], supporting the important role of this protein in the gut response to intoxication. While the specific role of this protein in the gut defense response is not known, reports in the lepidopteran Helicoverpa armigera suggest sequestration of circulating Cry toxins by lipids associated to lipophorins, preventing damage to enterocytes [48]. Transcriptome profiling identified up-regulation of genes involved in signaling, detoxification and cell structure as up-regulated in Tenebrio molitor larvae after intoxication with Cry3Aa toxin [49•]. Genes with reduced expression were involved in diverse metabolic pathways, suggesting shutdown of digestion and a concomitant up-regulation of energy production through respiration in response to intoxication. A similar response was observed in larvae of western corn rootworm, Diabrotica virgifera virgifera, during exposure to Cry3Bb [50]. Interestingly, alteration in gene expression in T. molitor almost ceased after 24 h, supporting tight control of the midgut response to intoxication [49•].
Lack of reliable genomic resources has hindered the ability to identify genes responding to Cry intoxication in Lepidoptera, yet some gene families are emerging as critical components for a successful response to Cry intoxication. For instance, the response to pathogens (REPAT) genes identified in Spodoptera exigua have been commonly reported to display differential expression in response to Cry [51•] or vegetative insecticidal proteins (Vip) [52•] from Bt. While the specific role for REPAT genes in gut healing is unknown, their activity as transcription factors, high sequence diversity, and specific expression profiles in response to selected pathogens, suggest that they are involved in multiple defensive processes [53]. Another gene family that has been associated with midgut response to Bt intoxication are hexamerins, more specifically arylphorins. These genes are believed to be produced in fat body and involved in transport/storage functions [54]. However, there is evidence for arylphorin being secreted basally by midgut cells [55] into the area occupied by stem cell nidi. Specifically, α-arylphorin is of particular interest because it has direct mitogenic properties on ISCs [23] and can induce gut hyperplasia by feeding [25]. Furthermore, the role of arylphorin in midgut healing is supported by levels increasing after ingestion of bacteria [56], although this increase may be related to a role as inducible effector protein in insect immunity [57]. However, arylphorin transcripts were highly downregulated in larval midguts of L. dispar after ingestion of a Bt pesticide [58], after intoxication of S. exigua larvae with Vip toxin [52•] or in Ostrinia nubilalis larvae exposed to Cry1Fa protoxin [59]. While these reports may contradict a role for arylphorin in midgut healing, it is important to consider that the mitogenic effect of arylphorin is highly dependent on concentration. Thus, low concentrations induce mitogenic activity [23], while increasing arylphorin levels result in lack of proliferative effects [22,25]. Consequently, it is plausible that discrepancies in detecting association between increased arylphorin and gut regeneration in response to Bt intoxication may represent the down-regulation (not an increase) of arylphorin necessary to exert its mitogenic function. Interestingly, this hypothesis would help explain the dual function described for arylphorin; an immune function (tissue healing) at low concentrations and a storage function at higher concentrations. Further research is needed to test this hypothesis.
As discussed in the previous section, the MDF1 peptide was also suggested as mitogen involved in the midgut healing response to Bt toxins in vitro [15]. While this peptide is identical of the C-terminus of bovine fetuin, BLASTp searches do not detect significant matches of MDF1 to any insect protein (data not shown). Consequently, it is plausible that this peptide was generated by hydrolysis of media components. However, increased detection of MDF1-positive cells suggests this peptide or a similar protein is produced by midgut cells after exposure to Bt toxins. Unfortunately, no research has been performed on the functional participation of MDF1 in midgut regeneration after exposure to Bt toxins.
Intestinal regeneration and resistance to Bt
The efficacy of Bt pesticides and transgenic Bt crops depends on insecticidal proteins such as the Cry or Vip toxins. Consequently, alterations in any of the steps in the mode of action of these toxins could potentially result in resistance [60]. The multi-step mode of action of Cry proteins has been recently reviewed [61] and includes: First, an activation step in the midgut fluids of the host, second, binding conducive to toxin insertion on the enterocyte membrane and pore formation, third, osmotic enterocyte death, and fourth, collapse of the intestinal barrier that allows resident gut bacteria to invade the hemocoel resulting in septicemia and ultimately insect death. In the vast majority of cases resistance to Cry toxins involves alterations in midgut toxin receptors, which results in cross-resistance to Cry toxins sharing recognition of the altered receptor site [60]. However, cases of resistance to Cry toxins involving alterations in toxin processing by midgut fluids [62] or an enhanced gut healing response [63] have also been reported in Lepidoptera. Non-receptor related resistance mechanisms represent a serious threat to Bt pesticides and Bt crops, since they affect steps common to all Cry toxins and would result in cross-resistance to a wide range of Bt insecticidal proteins. Moreover, a mechanism preventing resident gut bacteria from invading the hemocoel and causing septicemia could also affect efficacy of alternative entomopathogens infecting per os.
There are only two reports in the literature of resistance to Cry toxins involving an enhanced midgut healing response, both cases involved resistance to Cry1Ac in the tobacco budworm, H. virescens [63,64]. In both cases, cross-resistance to multiple Cry toxins with different binding sites was observed [65]. These results further supported the hypothesis of resistance by alterations in a common step in the mode of action of the toxins such as enhanced healing or toxin processing by midgut fluids [66]. This enhanced midgut regenerative response in resistant larvae is suggestive of increased production of mitogens or new midgut growth factors, or differences in the sensitivity of stem cells to mitogens. While a number of mitogens with activity on midgut stem cells have been reported (reviewed in [18]), their relative production in larvae from the susceptible and resistant H. virescens strains has not been determined.
Based on the information described in the previous section on genes hypothesized to participate in the mid-gut response to Bt toxins, candidate genes involved in an enhanced regenerative response in Cry-resistant insects include REPAT and arylphorin. In fact, constitutive increased expression of both REPAT and arylphorin genes was detected in a strain of S. exigua resistant to Xentari [67], a Bt pesticide containing Cry toxins with different binding receptors in the larval midgut. As explained above, this cross-resistance phenotype would suggest that resistance involved alterations in common steps in the mode of action of the toxins, such as processing or effective midgut epithelium disruption. However, staining of midgut cells for DNA synthesis as proxy for proliferation determined a decreased number of proliferative cells in resistant versus susceptible larvae [67]. Moreover, no increase in ISC proliferation was detected after exposure of susceptible larvae to the Bt pesticide, suggesting that S. exigua larvae may not respond to exposure to Cry toxins by activating the gut healing response. In agreement with this observation, feeding of Spodoptera frugiperda larvae on Bt cotton producing the Cry1Ac toxin resulted in epithelial damage, which was not associated with an increase in ISCs when comparing to feeding on non-Bt cotton isoline [68]. Because diverse time points were not examined in those reports, an alternative explanation for these observations of no ISC increase is that the ISC differentiation was predominant over proliferation during the time of observation, as suggested from observations of Alabama argillacea exposed to Bt cotton [69] or exposure to Bt pesticides in susceptible and resistant strains of Plutella xylostella [70].
While not experimentally tested to date, the observation that selected REPAT and arylphorin genes appear to display similar expression profiles during exposure to Bt toxins [52•,67], and that REPAT genes have been proposed as transcriptional regulators [71] may be suggestive of REPAT genes participating in arylphorin expression. Interestingly, silencing expression of the ATP binding cassette (ABCC) transporter gene expression in S. exigua larvae resulted in up-regulation of the same REPAT and arylphorin genes that respond to Bt intoxication and were found constitutively up-regulated in the Xentari-resistant strain [72]. Although speculative [73], these observations support a model in which genetic pathways in the insect are activated during exposure to the Cry toxin generating a direct down-regulation of toxin receptors and up-regulation of putative gut healing factors (such as arylphorin) to reduce epithelial damage and avoid gut disruption. In agreement with this hypothesis, resistance to Cry1Ac in P. xylostella was genetically linked to down-regulation of Cry1Ac receptor genes ABCC and alkaline phosphatase, which was trans-regulated by a gene in the MAPK kinase signaling pathway [73•]. The potential trans-regulation of arylphorin expression by the MAPK kinase pathway in response to Bt intoxication needs to be further explored.
Conclusions and future perspectives
Damage to the insect digestive system by entomopathogens and their toxins activates a defensive response that seems to be conserved among distinct insect groups. One of the most relevant processes of this defensive response is the regeneration of the epithelium by replacing diseased with newly differentiated midgut cells. This mechanism depends on midgut stem cell proliferation and differentiation and seems capable of allowing insects to survive exposure to entomopathogens. An enhanced gut regenerative response was proposed as resistance mechanism to diverse Cry toxins in H. virescens, although evidence of similar resistance mechanism in other insects is lacking. It is expected that this enhanced regenerative response is controlled by increased production of mitogenic factors.
The genes REPAT and arylphorin have been reported to differentially change expression in response to exposure to Bt and other entomopathogens. Arylphorin is a candidate protein to regulate regeneration after intoxication given that it is expressed by midgut cells, it has mitogenic effect on gut stem cells, and displays altered expression during infective processes. Discrepancies in the literature in regards to changes to arylphorin expression during gut healing may be explained by differential functions of arylphorin which are concentration dependent; lower concentrations appear critical for a mitogenic effect, thus requiring a tight regulation during response to intoxication. There are no data available on gut stem cells detection of arylphorin and the specific molecular pathways activated by arylphorin in gut stem cells, yet there is preliminary evidence of potential regulation of arylphorin expression by REPAT genes. A model for a coordinated response after Bt exposure regulated by MAPK pathways that includes downregulation of Bt toxin receptors coupled to upregulation of genes involved in gut healing is emerging from recent publications. Characterization of the gut regenerative process will help shed more light on a defensive mechanism that can result in resistance to diverse Bt toxins and other entomopathogens targeting the insect gut epithelium, and potentially identify targets for novel insecticidal technologies.
Acknowledgments
The authors would like to acknowledge funding through the National Institute of Health training grant number 1K12 GM 00708 IRACDA Program in support of A. Castagnola during the time of the writing of this manuscript. This manuscript represents a contribution from the Multistate Research Project S1052: The working Group on Improving Microbial Control of Arthropod Pests by JLJ-F.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Huang J-H, Jing X, Douglas AE. The multi-tasking gut epithelium of insects. Insect Biochem Mol Biol. 2015;67:15–20. doi: 10.1016/j.ibmb.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane NJ, Dallai R, Ashhurst DE. Structural macromolecules of the cell membranes and the extracellular matrices of the insect midgut. Biol Insect Midgut. 1996 [Google Scholar]
- 3.Wieczorek H, Gruber G, Harvey WR, Huss M, Merzendorfer H, Zeiske W. Structure and regulation of insect plasma membrane H+V-ATPase. J Exp Biol. 2000;203:127–135. doi: 10.1242/jeb.203.1.127. [DOI] [PubMed] [Google Scholar]
- 4.Lee HO, Davidson JM, Duronio RJ. Endoreplication: polyploidy with purpose. Genes Dev. 2009;23:2461–2477. doi: 10.1101/gad.1829209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin KM, Hakim RS. Growth and differentiation of the larval midgut epithelium during molting in the moth, Manduca sexta. Tissue Cell. 1991;23:411–422. doi: 10.1016/0040-8166(91)90058-2. [DOI] [PubMed] [Google Scholar]
- 6.Wong ACN, Vanhove AS, Watnick PI. The interplay between intestinal bacteria and host metabolism in health and disease: lessons from Drosophila melanogaster. Dis Models Mech. 2016;9:271–281. doi: 10.1242/dmm.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scopelliti A, Cordero JB, Diao F, Strathdee K, White BH, Sansom OJ, Vidal M. Local control of intestinal stem cell homeostasis by enteroendocrine cells in the adult Drosophila midgut. Curr Biol. 2014;24:1199–1211. doi: 10.1016/j.cub.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song W, Veenstra JA, Perrimon N. Control of lipid metabolism by tachykinin in Drosophila. Cell Rep. 2014;9:40–47. doi: 10.1016/j.celrep.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiher W, Shirras C, Kahnt J, Baumeister S, Isaac RE, Wegener C. Peptidomics and peptide hormone processing in the Drosophila midgut. J Proteome Res. 2011;10:1881–1892. doi: 10.1021/pr101116g. [DOI] [PubMed] [Google Scholar]
- 10.Hakim RS, Baldwin KM, Loeb M. The role of stem cells in midgut growth and regeneration. In Vitro Cell Dev Biol Animal. 2001;37:338–342. doi: 10.1007/BF02577567. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Li Z. No intestinal stem cell regeneration after complete progenitor ablation in Drosophila adult midgut. J Genet Genom. 2015;42:83–86. doi: 10.1016/j.jgg.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Raff M. Adult stem cell plasticity: fact or artifact? Ann Rev Cell Dev Biol. 2003;19:1–22. doi: 10.1146/annurev.cellbio.19.111301.143037. [DOI] [PubMed] [Google Scholar]
- 14•.Sadruddin SY, Hakim RS, Loeb M. Proliferation and differentiation of midgut epithelial cells from Manduca sexta. In Vitro Invertebr Reprod Dev. 1994;26:197–204. Described the preparation and maintenance of primary cell cultures derived from insect midgut. Since these cultured cells display phenotypes observed in vivo, their development opened new avenues of research using these cultured cells as a physiological and developmental insect gut model. [Google Scholar]
- 15.Loeb MJ, Martin PAW, Hakim RS, Goto S, Takeda M. Regeneration of cultured midgut cells after exposure to sublethal doses of toxin from two strains of Bacillus thuringiensis. J Insect Physiol. 2001;47:599–606. doi: 10.1016/s0022-1910(00)00150-5. [DOI] [PubMed] [Google Scholar]
- 16.Loeb MJ. Factors affecting proliferation and differentiation of Lepidopteran midgut stem cells. Arch Insect Biochem Physiol. 2010;74:1–16. doi: 10.1002/arch.20349. [DOI] [PubMed] [Google Scholar]
- 17.Castagnola A, Eda S, Jurat-Fuentes JL. Monitoring stem cell proliferation and differentiation in primary midgut cell cultures from Heliothis virescens larvae using flow cytometry. Differentiation. 2011;81:192–198. doi: 10.1016/j.diff.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Hakim RS, Baldwin K, Smagghe G. Regulation of midgut growth, development, and metamorphosis. Ann Rev Entomol. 2010:593–608. doi: 10.1146/annurev-ento-112408-085450. [DOI] [PubMed]
- 19.Loeb MJ, Jaffe H, Gelman DB, Hakim RS. Two polypeptide factors that promote differentiation of insect midgut stem cells in vitro. Arch Insect Biochem Physiol. 1999;40:129–140. doi: 10.1002/(SICI)1520-6327(1999)40:3<129::AID-ARCH2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Nie ZT. Fetuin: its enigmatic property of growth promotion. Am J Physiol. 1992;263:C551–C562. doi: 10.1152/ajpcell.1992.263.3.C551. [DOI] [PubMed] [Google Scholar]
- 21.Loeb MJ, Jaffe H. Peptides that elicit midgut stem cell differentiation isolated from chymotryptic digests of hemolymph from Lymantria dispar pupae. Arch Insect Biochem Physiol. 2002;50:85–96. doi: 10.1002/arch.10033. [DOI] [PubMed] [Google Scholar]
- 22.Smagghe G, Vanhassel W, Moeremans C, De Wilde D, Goto S, Loeb MJ, Blackburn MB, Hakim RS. Stimulation of midgut stem cell proliferation and differentiation by insect hormones and peptides. In. In: Vaudry H, Roubos E, Schoofs L, Fiik G, Larhammar D, editors. Trends in Comparative Endocrinology and Neurobiology. 2005. pp. 472–475. [DOI] [PubMed] [Google Scholar]
- 23.Blackburn MB, Loeb MJ, Clark E, Jaffe H. Stimulation of midgut stem cell proliferation by Manduca sexta alpha-arylphorin. Arch Insect Biochem Physiol. 2004;55:26–32. doi: 10.1002/arch.10119. [DOI] [PubMed] [Google Scholar]
- 24.Goto S, Loeb MJ, Takeda M. Bombyxin stimulates proliferation of cultured stem cells derived from Heliothis virescens and Mamestra brassicae larvae. In Vitro Cell Dev Biol – Animal. 2005;41:38–42. doi: 10.1290/0312092.1. [DOI] [PubMed] [Google Scholar]
- 25.Hakim RS, Blackburn MB, Corti P, Gelman DB, Goodman C, Elsen K, Loeb MJ, Lynn D, Soin T, Smagghe G. Growth and mitogenic effects of arylphorin in vivo and in vitro. Arch Insect Biochem Physiol. 2007;64:63–73. doi: 10.1002/arch.20155. [DOI] [PubMed] [Google Scholar]
- 26.Nardi JB, Bee CM, Miller LA, Mathur D, Ohlstein B. Cell renewal in adjoining intestinal and tracheal epithelia of Manduca. J Insect Physiol. 2011;57:487–493. doi: 10.1016/j.jinsphys.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Huang W, Zhang J, Yang B, Beerntsen BT, Song H, Ling E. DNA duplication is essential for the repair of gastrointestinal perforation in the insect midgut. Sci Rep. 2016:6. doi: 10.1038/srep19142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang AS, Yen DF, Peng WK. Defense reaction of midgut epithelial cells in the rice oth larva (Corcyra cephalonica) infected with Bacillus thuringiensis. J Invertebr Pathol. 1986;47:333–339. [Google Scholar]
- 29.Spies AG, Spence KD. Effect of sublethal Bacillus thuringiensis crystal endotoxin treatment on the larval midgut of a moth, Manduca sexta. Tissue Cell. 1985;17:379–394. doi: 10.1016/0040-8166(85)90056-4. [DOI] [PubMed] [Google Scholar]
- 30.Hoover K, Washburn JO, Volkman LE. Midgut-based resistance of Heliothis virescens to baculovirus infection mediated by phytochemicals in cotton. J Insect Physiol. 2000;46:999–1007. doi: 10.1016/s0022-1910(99)00211-5. [DOI] [PubMed] [Google Scholar]
- 31.Washburn JO, Kirkpatrick BA, Haas-Stapleton E, Volkman LE. Evidence that the stilbene-derived optical brightener M2R enhances Autographa californica M nucleopolyhedrovirus infection of Trichoplusia ni and Heliothis virescens by preventing sloughing of infected midgut epithelial cells. Biol Control. 1998;11:58–69. [Google Scholar]
- 32•.Royet J. Epithelial homeostasis and the underlying molecular mechanisms in the gut of the insect model Drosophila melanogaster. Cell Mol Life Sci. 2011;68:3651–3660. doi: 10.1007/s00018-011-0828-x. Complete review of processes involved in maintaining gut homeostasis in the genetic model Drosophila. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naszai M, Carroll LR, Cordero JB. Intestinal stem cell proliferation and epithelial homeostasis in the adult Drosophila midgut. Insect Biochem Mol Biol. 2015;67:9–14. doi: 10.1016/j.ibmb.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Koch U, Lehal R, Radtke F. Stem cells living with a Notch. Development. 2013;140:689–704. doi: 10.1242/dev.080614. [DOI] [PubMed] [Google Scholar]
- 35.Guo Z, Ohlstein B. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science. 2015;350:aab0988–1–aab0988–8. doi: 10.1126/science.aab0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng H, Gerencser AA, Jasper H. Signal integration by Ca2+ regulates intestinal stem-cell activity. Nature. 2015;528:212. doi: 10.1038/nature16170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amcheslavsky A, Song W, Li Q, Nie Y, Bragatto I, Ferrandon D, Perrimon N, Ip YT. Enteroendocrine cells support intestinal stem-cell-mediated homeostasis in Drosophila. Cell Rep. 2014;9:32–39. doi: 10.1016/j.celrep.2014.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canton PE, Cancino-Rodezno A, Gill SS, Soberon M, Bravo A. Transcriptional cellular responses in midgut tissue of Aedes aegypti larvae following intoxication with Cry11Aa toxin from Bacillus thuringiensis. BMC Genom. 2015;16:1042. doi: 10.1186/s12864-015-2240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Cancino-Rodezno A, Alexander C, Villasenor R, Pacheco S, Porta H, Pauchet Y, Soberon M, Gill SS, Bravo A. The mitogen-activated protein kinase p38 is involved in insect defense against Cry toxins from Bacillus thuringiensis. Insect Biochem Mol Biol. 2010;40:58–63. doi: 10.1016/j.ibmb.2009.12.010. First report of MAPK pathways as involved in insect defense to Cry toxins. Silencing of p38 in mosquito larvae resulted in hypersensitive phenotypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huffman DL, Abrami L, Sasik R, Corbeil J, van der Goot FG, Aroian RV. Mitogen-activated protein kinase pathways defends against bacterial pore-forming toxins. Proc Natl Acad Sci U S A. 2004;101:10995–11000. doi: 10.1073/pnas.0404073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bischof LJ, Kao C-Y, Los FCO, Gonzalez MR, Shen Z, Briggs SP, van der Goot FG, Aroian RV. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. Plos Pathogens. 2008;4:e1000176. doi: 10.1371/journal.ppat.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bedoya-Perez LP, Cancino-Rodezno A, Flores-Escobar B, Soberon M, Bravo A. Role of UPR pathway in defense response of Aedes aegypti against Cry11Aa toxin from Bacillus thuringiensis. Int J Mol Sci. 2013;14:8467–8478. doi: 10.3390/ijms14048467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Contreras E, Rausell C, Real MD. Tribolium castaneum Apolipophorin-III acts as an immune response protein against Bacillus thuringiensis Cry3Ba toxic activity. J Invertebr Pathol. 2013;113:209–213. doi: 10.1016/j.jip.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Contreras E, Rausell C, Real MD. Proteome response of Tribolium castaneum larvae to Bacillus thuringiensis toxin producing strains. PLOS ONE. 2013:8. doi: 10.1371/journal.pone.0055330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Contreras E, Benito-Jardon M, Jose Lopez-Galiano M, Dolores Real M, Rausell C. Tribolium castaneum immune defense genes are differentially expressed in response to Bacillus thuringiensis toxins sharing common receptor molecules and exhibiting disparate toxicity. Dev Comp Immunol. 2015;50:139–145. doi: 10.1016/j.dci.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Ma G, Roberts H, Sarjan M, Featherstone N, Lahnstein J, Akhurst R, Schmidt O. Is the mature endotoxin Cry1Ac from Bacillus thuringiensis inactivated by a coagulation reaction in the gut lumen of resistant, Helicoverpa armigera larvae? Insect Biochem Mol Biol. 2005;35:729–739. doi: 10.1016/j.ibmb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 49•.Oppert B, Dowd SE, Bouffard P, Li L, Conesa A, Lorenzen MD, Toutges M, Marshall J, Huestis DL, Fabrick J, et al. Transcriptome profiling of the intoxication response of Tenebrio molitor larvae to Bacillus thuringiensis Cry3Aa protoxin. PLOS ONE. 2012:7. doi: 10.1371/journal.pone.0034624. First transcriptome profiling of response to Bt toxins in Coleoptera. Presents data on differential gene expression at diverse time points during the intoxication process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sayed A, Wiechman B, Struewing I, Smith M, French W, Nielsen C, Bagley M. Isolation of transcripts from Diabrotica virgifera virgifera LeConte responsive to the Bacillus thuringiensis toxin Cry3Bb1. Insect Mol Biol. 2010;19:381–389. doi: 10.1111/j.1365-2583.2010.00998.x. [DOI] [PubMed] [Google Scholar]
- 51•.Herrero S, Ansems M, Van Oers MM, Vlak JM, Bakker PL, de Maagd RA. REPAT, a new family of proteins induced by bacterial toxins and baculovirus infection in Spodoptera exigua. Insect Biochem Mol Biol. 2007;37:1109–1118. doi: 10.1016/j.ibmb.2007.06.007. Discovery of the REPAT family of genes as involved in response to gut damage by entomopathogens. Data are emerging that suggest a critical role for these genes in the gut defense response. [DOI] [PubMed] [Google Scholar]
- 52•.Bel Y, Jakubowska AK, Costa J, Herrero S, Escriche B. Comprehensive analysis of gene expression profiles of the beet armyworm Spodoptera exigua larvae challenged with Bacillus thuringiensis Vip3Aa Toxin. PLOS ONE. 2013:8. doi: 10.1371/journal.pone.0081927. Presents the first comprehensive analysis identifying relevant gene expression changes in response to gut intoxication with Vip proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Navarro-Cerrillo G, Ferre J, de Maagd RA, Herrero S. Functional interactions between members of the REPAT family of insect pathogen-induced proteins. Insect Mol Biol. 2012;21:335–342. doi: 10.1111/j.1365-2583.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 54.Burmester T. Expression and evolution of hexamerins from the tobacco hornworm, Manduca sexta, and other Lepidoptera. Insect Biochem Mol Biol. 2015;62:226–234. doi: 10.1016/j.ibmb.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Palli SR, Locke M. The synthesis of hemolymph proteins by the larval midgut of an insect Calpodes ethlius (Lepidoptera, Hesperiidae) Insect Biochem. 1987;17:561–572. [Google Scholar]
- 56.Freitak D, Wheat CW, Heckel DG, Vogel H. Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biol. 2007;5:56. doi: 10.1186/1741-7007-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beresford PJ, BasinskiGray JM, Chiu JKC, Chadwick JS, Aston WP. Characterization of hemolytic and cytotoxic gallysins: a relationship with arylphorins. Dev Compar Immunol. 1997;21:253–266. doi: 10.1016/s0145-305x(97)00011-6. [DOI] [PubMed] [Google Scholar]
- 58.Sparks ME, Blackburn MB, Kuhar D, Gundersen-Rindal DE. Transcriptome of the Lymantria dispar (gypsy moth) larval midgut in response to infection by Bacillus thuringiensis. PLOS ONE. 2013;8:1–9. doi: 10.1371/journal.pone.0061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vellichirammal NN, Wang H, Eyun S-I, Moriyama EN, Coates BS, Miller NJ, Siegfried BD. Transcriptional analysis of susceptible and resistant European corn borer strains and their response to Cry1F protoxin. BMC Genomics. 2015:16. doi: 10.1186/s12864-015-1751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferre J, Van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Ann Rev Entomol. 2002;47:501–533. doi: 10.1146/annurev.ento.47.091201.145234. [DOI] [PubMed] [Google Scholar]
- 61.Adang MJ, Crickmore N, Jurat-Fuentes JL. Diversity of Bacillus thuringiensis crystal toxins and mechanism of action. In: Dhadialla TS, Gill SS, editors. Insect Midgut and Insecticidal Proteins. 2014. pp. 39–87. [Google Scholar]
- 62.Oppert B, Kramer KJ, Johnson D, Upton SJ, McGaughey WH. Luminal proteinases from Plodia interpunctella and the hydrolysis of Bacillus thuringiensis CryIA(c) protoxin. Insect Biochem Mol Biol. 1996;26:571–583. doi: 10.1016/s0965-1748(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 63.Forcada C, Alcacer E, Garcera MD, Tato A, Martinez R. Resistance to Bacillus thuringiensis Cry1Ac toxin in three strains of Heliothis virescens: proteolytic and SEM study of the larval midgut. Arch Insect Biochem Physiol. 1999;42:51–63. doi: 10.1002/(SICI)1520-6327(199909)42:1<51::AID-ARCH6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 64.Martinez-Ramirez AC, Gould F, Ferre J. Histopathological effects and growth reduction in a susceptible and a resistant strain of Heliothis virescens (Lepidoptera: Noctuidae) caused by sublethal doses of pure Cry1A crystal proteins from Bacillus thuringiensis. Biocontrol Sci Technol. 1999;9:239–246. [Google Scholar]
- 65.Jurat-Fuentes JL, Gould FL, Adang MJ. Dual resistance to Bacillus thuringiensis Cry1Ac and Cry2Aa toxins in Heliothis virescens suggests multiple mechanisms of resistance. Appl Environ Microbiol. 2003;69:5898–5906. doi: 10.1128/AEM.69.10.5898-5906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karumbaiah L, Oppert B, Jurat-Fuentes JL, Adang MJ. Analysis of midgut proteinases from Bacillus thuringiensis-susceptible and -resistant Heliothis virescens (Lepidoptera: Noctuidae) Compar Biochem Physiol B – Biochem Mol Biol. 2007;146:139–146. doi: 10.1016/j.cbpb.2006.10.104. [DOI] [PubMed] [Google Scholar]
- 67.Hernández-Martínez P, Navarro-Cerrillo G, Caccia S, de Maagd RA, Moar WJ, Ferre J, Escriche B, Herrero S. Constitutive activation of the midgut response to Bacillus thuringiensis in Bt-resistant Spodoptera exigua. PLoS ONE. 2010:e12795. doi: 10.1371/journal.pone.0012795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cunha FM, Wanderley-Teixeira V, Torres JB, Teixeira AAC, Alves TJS, Brayner FA. Impact of Bt cotton on the immune system and histology of the midgut of the fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) Animal Biol. 2013;63:185–197. [Google Scholar]
- 69.Sousa MEC, Santos FAB, Wanderley-Teixeira V, Teixeira AAC, de Siqueira HAA, Alves LC, Torres JB. Histopathology and ultrastructure of midgut of Alabama argillacea (Hubner) (Lepidoptera Noctuidae) fed Bt-cotton. J Insect Physiol. 2010;56:1913–1919. doi: 10.1016/j.jinsphys.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 70.da Solidade Ribeiro LM, Wanderley-Teixeira V, Abreu de Siqueira HA, Batista de Oliveira AC, Jeanine Martins Lemos AJ, Coelho Teixeira AA. Midgut histopathology of resistant and susceptible Plutella xylostella exposed to commercial formulations of Bacillus thuringiensis. Bull Insectol. 2013;66:161–171. [Google Scholar]
- 71.Hernández-Rodríguez CS, Ferré J, Herrero S. Genomic structure and promoter analysis of pathogen-induced repat genes from Spodoptera exigua. Insect Mol Biol. 2009;18:77–85. doi: 10.1111/j.1365-2583.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- 72.Park Y, Gonzalez-Martinez RM, Navarro-Cerrillo G, Chakroun M, Kim Y, Ziarsolo P, Blanca J, Canizares J, Ferre J, Herrero S. ABCC transporters mediate insect resistance to multiple Bt toxins revealed by bulk segregant analysis. BMC Biol. 2014:12. doi: 10.1186/1741-7007-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73•.Guo Z, Kang S, Chen D, Wu Q, Wang S, Xie W, Zhu X, Baxter SW, Zhou X, Jurat-Fuentes JL, et al. MAPK signaling pathway alters expression of midgut ALP and ABCC genes and causes resistance to Bacillus thuringiensis Cry1Ac toxin in diamondback moth. PLoS Genetics. 2015;11 doi: 10.1371/journal.pgen.1005124. First demonstration of a transregulatory linkage between a MAPK pathway and Bt toxin receptor genes. This was a relevant finding considering that MAPK pathways had been proposed to have a key role in midgut response to Cry intoxication and alteration of the MAPK gene expression resulted in resistance to Bt toxins. [DOI] [PMC free article] [PubMed] [Google Scholar]