Atrial fibrillation (AF) affects more than 33 million people worldwide.1 The prevalence in high-income countries is 1–4%, but rises to more than 13% in individuals over 80 years of age.2 Although embolic stroke is the most feared complication, over the past few decades AF has been associated with increased risk of myocardial infarction,3 heart failure,4 dementia,5 chronic kidney disease,6,7 venous thromboembolism,8 and mortality.9 Conversely, biologically plausible bidirectional relations have been reported, such that myocardial infarction,10 heart failure,10 chronic kidney disease,6,7 and venous thromboembolism11 are associated with increased risk of incident AF.
An association between AF and malignancy has been reported but incompletely defined.12 The earliest publications of cancer predisposing to AF came in the 1940s–50s with reports of neoplastic cardiac infiltration or mechanical pressure on the heart,13 and with oncologic thoracic surgery.14,15 Subsequently, multiple studies have reported an increased risk of AF after cancer therapy with surgery (particularly thoracic) and chemotherapy.12 However, the prevalence of AF appears to be higher in patients with cancer at the time of diagnosis even before undergoing therapy.16,17 Patients with cancer are also at increased risk of developing AF, particularly in the first 90 days after diagnosis suggesting an overlap in pathophysiological processes.17,18
There have been periodic reports of AF preceding the diagnosis of cancer. A case-control study among veterans published in 1994 appears to be the first report that antecedent AF was more common (odds ratio, 1.34; 95% CI, 1.16–1.55) among individuals with cancer (colon).19 In 2014, investigators published a registry study of all Danish patients, and observed that those with AF had a 2.5% (95% CI, 2.4%–2.5%) absolute risk of a cancer diagnosis in the first 3 months after diagnosis of AF, which represented a 5 fold increased risk. They observed that the standardized incidence ratio of cancer was elevated at 1.11 even 24 months after AF diagnosis.20 The Danish investigators noted that the cancers were more likely to be metastatic (57%) at the time of diagnosis, which might suggest that the AF was unlikely to have caused the cancer. The authors suggested that AF may act as a marker for occult cancer.20
In this issue of JAMA Cardiology, Conen et al. investigated if the relationship between AF and cancer in the Women’s Health Study (WHS) is bidirectional.21 In the large WHS cohort, 1467 women developed AF; the authors reported that the incidence of cancer was significantly higher in women with AF compared to those without AF. The risk of cancer was 3 fold greater within 3 months of AF diagnosis but still elevated beyond 1 year (hazard ratio 1.42). Further, they investigated the risk of incident AF after diagnoses of cancer and only found a 20% increased risk in the first 3 months, but not beyond.
The study by Conen et al. has several strengths, including the large sample size, low amount of missing data, routine longitudinal surveillance and adjudication for cancer and AF, ability to account for multiple potential confounders, adjustment for cancer screening tests, and the authors’ multiple sensitivity analyses. The longitudinal nature of the study also is an advantage, facilitating the ability to examine the relative timing of the AF and cancer diagnoses. However, as noted by the authors both conditions frequently have long latency periods, wherein they may remain clinically unrecognized, which may preclude precise assessments of temporality.
The study by Conen et al. raises the question as to whether AF is a risk factor for cancer. The term risk factor often implies a causal relation between the exposure and the outcome. We concur with the investigators’ conclusion that the modest effect size of AF for cancer after 3 months suggests that AF most likely serves as a risk marker for future diagnosis of cancer. The mechanisms underlying the interrelations are probably multifactorial and include shared risk factors, increased detection due to bleeding with anticoagulation (suggested by the prominence of colon cancer), or other systemic processes (Figure 1). Although cancer screening was adjusted for, it is possible that patients with AF are more likely to have increased surveillance with other investigations including imaging studies (e.g. CT or MRI) than patients without new-onset AF. In addition, AF is often undiagnosed or asymptomatic22 and patients who are more likely to be diagnosed with AF may have significant differences than those who go clinically undetected. For example, patients who are more symptomatic with AF may be more symptomatic for cancer, or may be more likely to seek care for other ailments.
Figure 1. Possible components underlying the association between atrial fibrillation and cancer.
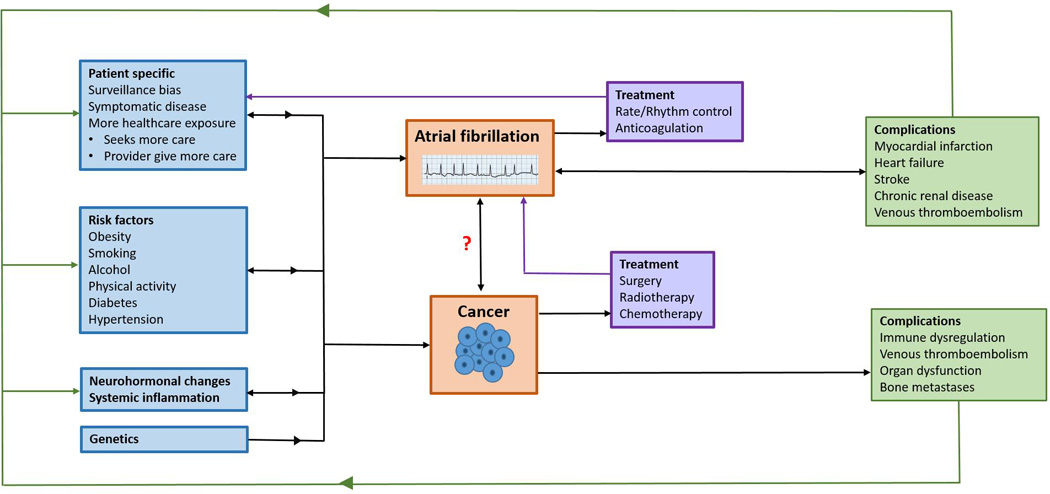
The factors contributing to the development, diagnosis, treatment, and complications of atrial fibrillation (AF) and cancer have complex interrelations, many of which are bidirectional. The development of AF and cancer are promoted by genetics, risk factors, systemic inflammation, and neurohormonal changes. Patient specific factors affect the likelihood of both conditions’ diagnosis. AF and cancer may in turn modify patient specific factors, the original risk factors, neurohormonal systems, and systemic inflammation. AF treatment is associated with increased healthcare exposure such as INR monitoring, follow-up outpatient visits, and increased risk of bleeding, which may hasten cancer diagnosis. Cancer treatments may also promote AF. Both AF and cancer can lead to multiple complications, which can further modify healthcare exposure, the original risk factors, systemic inflammation, and neurohormonal systems. The mechanisms underlying the bidirectional relations AF and cancer are incompletely understood and require further research.
The distinction of AF being a risk marker for cancer, bears a contrast with the relationship between AF and myocardial infarction, heart failure, chronic kidney disease, and venous thromboembolism, which are more likely to be truly causally bidirectional. The underlying mechanisms explaining the association between AF and cancer may be even more complicated with possible interlinking bidirectional relationship of a wide variety of factors (Figure 1).
The provocative work raises both clinical and research questions. Clinically, should a diagnosis of AF prompt a search for occult cancer? Several factors argue against routine screening, including the low absolute risk of cancer (in WHS 1.4 vs. 0.8 per 100 person years of follow-up in individuals with and without AF), and the potential cost and burden of cancer screening. Similar to the literature regarding screening in unprovoked VTE,23 based on available data cancer screening beyond standard routine health care is currently not merited with a new diagnosis of AF.
Clearly many research questions regarding the complex interrelations between AF and cancer remain, and with an aging population represent important areas for future research. Further investigation is required to identify if the presence of AF and cancer should modify management strategies given the increased risk of bleeding and thromboembolism observed with both conditions.12 In addition, understanding the intermediate steps that link AF and cancer in the bidirectional associations reported by Conen et al. may provide valuable mechanistic and therapeutic insights for both conditions.
Acknowledgments
None
Funding Sources
Dr. Ko is supported by the National Heart, Lung, and Blood Institute award 5T32HL007224-38 and the National Institutes of Health Clinical and Translational Science Award program award UL1-TR000157. Dr. Benjamin is supported through NIH/NHLBI HHSN268201500001I; N01-HC25195, 2R01HL092577, 1R01 HL102214, 1R01HL128914, and 1RC1HL101056.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11(11):639–654. doi: 10.1038/nrcardio.2014.118. [DOI] [PubMed] [Google Scholar]
- 3.Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA internal medicine. 2014;174(1):107–114. doi: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnabel RB, Rienstra M, Sullivan LM, et al. Risk assessment for incident heart failure in individuals with atrial fibrillation. Eur J Heart Fail. 2013;15(8):843–849. doi: 10.1093/eurjhf/hft041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158(5 Pt 1):338–346. doi: 10.7326/0003-4819-158-5-201303050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. American heart journal. 2009;158(4):629–636. doi: 10.1016/j.ahj.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Bansal N, Fan D, Hsu C-y, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. 2013;127(5):569–574. doi: 10.1161/CIRCULATIONAHA.112.123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enga KF, Rye-Holmboe I, Hald EM, et al. Atrial fibrillation and future risk of venous thromboembolism:the Tromso study. J Thromb Haemost. 2015;13(1):10–16. doi: 10.1111/jth.12762. [DOI] [PubMed] [Google Scholar]
- 9.Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2(2):e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hald EM, Enga KF, Lochen ML, et al. Venous thromboembolism increases the risk of atrial fibrillation: the Tromso study. J Am Heart Assoc. 2014;3(1):e000483. doi: 10.1161/JAHA.113.000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. Journal of the American College of Cardiology. 2014;63(10):945–953. doi: 10.1016/j.jacc.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Rawls WB, Ancona VC. An unusual case of paroxysmal auricular fibrillation due to mediastinal malignant lymphoma. American heart journal. 1951;41(2):311–315. doi: 10.1016/0002-8703(51)90109-3. [DOI] [PubMed] [Google Scholar]
- 14.Bailey CC, Betts RH. Cardiac arrhythmias following pneumonectomy. New England Journal of Medicine. 1943;229(9):356–359. [Google Scholar]
- 15.Cohen MG, Pastor BH. Delayed cardiac arrhythmias following non-cardiac thoracic surgery. CHEST Journal. 1957;32(4):435–440. doi: 10.1378/chest.32.4.435. [DOI] [PubMed] [Google Scholar]
- 16.O'Neal WT, Lakoski SG, Qureshi W, et al. Relation between cancer and atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke Study) The American journal of cardiology. 2015;115(8):1090–1094. doi: 10.1016/j.amjcard.2015.01.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzzetti S, Costantino G, Sada S, Fundarò C. Colorectal cancer and atrial fibrillation: a case-control study. The American journal of medicine. 2002;112(7):587–588. doi: 10.1016/s0002-9343(02)01029-x. [DOI] [PubMed] [Google Scholar]
- 18.Erichsen R, Christiansen CF, Mehnert F, Weiss NS, Baron JA, Sørensen HT. Colorectal cancer and risk of atrial fibrillation and flutter: a population-based case–control study. Internal and emergency medicine. 2012;7(5):431–438. doi: 10.1007/s11739-011-0701-9. [DOI] [PubMed] [Google Scholar]
- 19.Muller AD, Sonnenberg A, Wasserman IH. Diseases preceding colon cancer. A case-control study among veterans. Dig Dis Sci. 1994;39(11):2480–2484. doi: 10.1007/BF02087670. [DOI] [PubMed] [Google Scholar]
- 20.Ostenfeld EB, Erichsen R, Pedersen L, Farkas DK, Weiss NS, Sørensen HT. Atrial fibrillation as a marker of occult cancer. PloS one. 2014;9(8):e102861. doi: 10.1371/journal.pone.0102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conen D, Wong J, Sandhu R, et al. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiology. 2016 doi: 10.1001/jamacardio.2016.0280. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost. 2013;110(2):213–222. doi: 10.1160/TH13-02-0165. [DOI] [PubMed] [Google Scholar]
- 23.Carrier M, Lazo-Langner A, Shivakumar S, et al. Screening for Occult Cancer in Unprovoked Venous Thromboembolism. N Engl J Med. 2015;373(8):697–704. doi: 10.1056/NEJMoa1506623. [DOI] [PubMed] [Google Scholar]


