Abstract
Quinoa (Chenopodium quinoa Willd., Amaranthaceae) is a grain-like, stress-tolerant food crop that has provided subsistence, nutrition, and medicine for Andean indigenous cultures for thousands of years. Quinoa contains a high content of health-beneficial phytochemicals, including amino acids, fiber, polyunsaturated fatty acids, vitamins, minerals, saponins, phytosterols, phytoecdysteroids, phenolics, betalains, and glycine betaine. Over the past 2 decades, numerous food and nutraceutical products and processes have been developed from quinoa. Furthermore, 4 clinical studies have demonstrated that quinoa supplementation exerts significant, positive effects on metabolic, cardiovascular, and gastrointestinal health in humans. However, vast challenges and opportunities remain within the scientific, agricultural, and development sectors to optimize quinoa's role in the promotion of global human health and nutrition.
Keywords: biological activity, cereal products, dietary supplements, nutritional quality, quinoa
Introduction
Human health and food security have become increasingly important with the advents of climate change, accelerated human population growth, rise of metabolic disease, and increasing median age of the population. In upcoming years, ecosystems are expected to experience increased year-to-year climatic variation and frequency of extreme events (Perez and others 2010), putting pressure on reliable food production. Meanwhile, food demand is projected to increase between 70% and 100% by 2050 as the human population increases from 6 billion to over 9 billion (Tilman and others 2002; Godfray and others 2010). Today, about 1 in 8 individuals already suffers from chronic undernourishment (FAO, IFAD, WFP 2014), while diabetes, obesity, and other metabolic disorders have reached global epidemic proportions (Nguyen and Lau 2012; Zimmet and others 2014). Furthermore, the median age of the world's population is estimated to increase from 26.6 in 2000 to 31.1 in 2050 (Lutz and others 2008), likely resulting in a mounting prevalence of age-related disorders such as frailty, cardiovascular diseases, and osteoporosis (Lunenfeld and Stratton 2013).
As a strategy to combat metabolic diseases and age-related disorders through affordable, integrative strategies, food can play a stronger role in disease treatment and prevention. Food products that confer specific beneficial health effects, termed “functional foods” in 1984, have made their presence all over the world. The effects of functional foods range from improvement of general well-being to reduction of disease risk to treatment of illness. Functional foods can include conventional foods with specific health-beneficial properties, or foods containing enhanced levels of a particular functional nutrient as a result of natural enrichment, fortification, or processing (Bigliardi and Galati 2013).
Humans currently derive 30% to 70% of their daily energy from cereal-based foods, indicating that innovation of grain or grain-like functional foods plays a “gate-keeping” role in the conversion of agricultural crops to consumables (Poutanen and others 2014). Quinoa (Chenopodium quinoa Willd.) (Figure 1) is a grain-like food crop that has provided nutrition and sustenance to Andean indigenous cultures for thousands of years and now plays an increasing role in human diets worldwide. Quinoa has been promoted as an alternative agricultural crop due to its stress-tolerant characteristics and marketed as a “superfood” for its nutritious qualities. A plethora of research has recently emerged on quinoa's chemical constituents and therapeutic properties, depicting the crop as an important resource for functional food development.
Figure 1.
Photograph of Chenopodium quinoa plants with varying fruit colors. Courtesy of David Wu, Jiaqi Agri, China.
However, additional scientific inquiry and modern innovation are necessary to further understand and promote the role that quinoa can play in human health. Quinoa production must also meet demand through sustainable agricultural strategies in order to improve global access to its health benefits. The objectives of this study were to conduct a comprehensive and up-to-date review of quinoa's ethnobotanical, nutritional, phytochemical, and pharmacological aspects, while outlining recent advancements associated with its use in foods, botanical supplements, cosmetics, and pharmaceuticals.
Ethnobotanical Overview of Quinoa
Botanical distinction from cereal grains
Though quinoa is a dicot crop, it is often mistaken for a cereal grain like rice, corn, and wheat (monocots of the Poaceae family), and has therefore acquired the term “pseudocereal.” However, as a member of the Amaranthaceae family (previously Chenopodiaceae) (APG 1998; Kadereit and others 2003), quinoa is systematically and morphologically distinct from cereal grains. This distinction is especially notable by quinoa's unique fruit and seed anatomy. Quinoa fruits are achenes, comprised of a single seed enclosed by an outer pericarp (FAO 2011). The quinoa seed contains a central perisperm where carbohydrate reserves are localized, surrounded by the circular oil-rich and protein-rich embryo, endosperm, and seed coat (Prego and others 1998). The pericarp of the quinoa fruit is rich in bitter saponins and must be removed via mechanical abrasion or washing before consumption of the seeds (Prego and others 1998; Vega-Galvez and others 2010). This process, termed desaponification (saponin removal), has also been referred to as de-husking (Miranda and others 2012a), pearling (Gomez-Caravaca and others 2014), or milling (Kumpun and others 2011).
From a nutritional perspective, quinoa is included in the “whole grains” category (McKeown and others 2013). However, unlike traditional cereal grains, which are commonly processed to strip away the nutrient-rich germ and bran, quinoa desaponification leaves the nutrient-rich embryo and endosperm intact. The embryo, which constitutes up to 60% of the seed weight, confers a balanced nutritional profile of protein, lipid, and carbohydrate (Valencia-Chamorro 2003).
Traditional use
Quinoa has been traditionally used by several indigenous peoples of South America, including the Quechua, Aymara, Tiahuancota, Chibcha, and Mapuche (Vega-Galvez and others 2010; Bhargava and Srivastava 2013). The seeds have been consumed similarly to rice, prepared in soup, puffed to make breakfast cereal, or ground to flour to produce toasted and baked goods (cookies, breads, biscuits, noodles, flakes, tortillas, pancakes) (Popenoe and others 1989; Bhargava and others 2006). Quinoa leaves have also been eaten similarly to spinach (Oelke and others 1992), and the germinated quinoa seedlings (quinoa sprouts) have been incorporated in salads (Schlick and Bubenheim 1996). Furthermore, quinoa seeds can be fermented to make beer, or a traditional ceremonial alcoholic beverage from South America called “chicha” (Healy 2001; FAO 2011). The whole plant has also been used as a rich nutritional source to feed livestock, including cattle, pigs, and poultry (Bhargava and others 2006).
Records indicate a wide variety of medicinal uses of quinoa, from the treatment of wounds and fractures to the promotion of digestive health (Mujica 1994; Bhargava and others 2006; FAO 2011). Quinoa has widely been considered an invigorating, wellness-promoting, and endurance-enhancing food (Popenoe and others 1989; Lafont 1998; Gorelick-Feldman and others 2008; Kokoska and Janovska 2009; FAO 2011). Pungent ashes prepared from quinoa stems, called “llipta,” have been mixed with coca leaves (Erythroxylum coca Lam.) and chewed by Andean farmers to sustain their energy (Martindale 1894). Mixtures of quinoa and fat, called “war balls,” were used to sustain the Incan armies as they marched over the Andes Mountains (Small 2013).
Global production
Quinoa is thought to have been domesticated in the Lake Titicaca region near the Peruvian–Bolivian border and to have been spread through the Andes in parallel with the spread of Incan civilization (Cusack 1984; Dillehay and others 2007). Today, quinoa is cultivated from sea level up to 4500 m in altitude in Argentina, Bolivia, Chile, Colombia, Ecuador, and Peru (Cusack 1984; Fuentes and others 2009; Vega-Galvez and others 2010). An estimated 5000 different accessions of quinoa are currently held in seed banks around the world (Christensen and others 2007). Due to quinoa's water use efficiency, halophytic properties, and wide genetic diversity, the crop can survive in an extreme range of temperatures (−4 to 38 °C), frost, low rainfall (as little as 50 mm/y), nutrient-poor soils with pH ranging from 6.0 to 8.5, and high salinity (40 mS/cm) (Hellin and Higman 2003; Valencia-Chamorro 2003; Bhargava and others 2006; Hariadi and others 2010; Vega-Galvez and others 2010; FAO 2011). In fact, the largest global production region of quinoa is the Salar de Uyuni (salt flats of Oruro and Potosí provinces in Bolivia), where quinoa is the only crop capable of withstanding the extreme environmental conditions (Cusack 1984; FAO 2011; Cusicanqui and others 2013).
Following a long period of marginalized quinoa production and consumption, the demand for quinoa has recently escalated worldwide. However, total production, which is largely limited to small areas in South America, has not been able to meet demand. Therefore, market prices have skyrocketed and access to quinoa's nutritional benefits has become increasingly limited among low-income communities (Jacobsen 2011).
In order to promote quinoa's capacity to improve the livelihoods of diverse communities around the world, improved access to and awareness of quinoa's health value are critical. One key strategy to achieve improved access is the expansion of quinoa cultivation among other continents, especially regions in Africa and Asia where food production is threatened by global climate change and desertification. A second strategy is the dissemination of information regarding quinoa's health value, uses, biodiversity, and sustainable cultivation methods. Recognizing these needs, the United Nations initiated a $2.9 million collaborative program encompassing the public, private, and academic sectors in 2013 entitled “the International Year of Quinoa (IYQ2013)” (FAO 2012, 2014). In upcoming years, generation of further scientific knowledge of quinoa's chemical composition and therapeutic effects, paired by innovation in product development, will facilitate continued steps toward the sustainability of quinoa production and utilization.
Phytochemistry and Nutritional Value of Quinoa
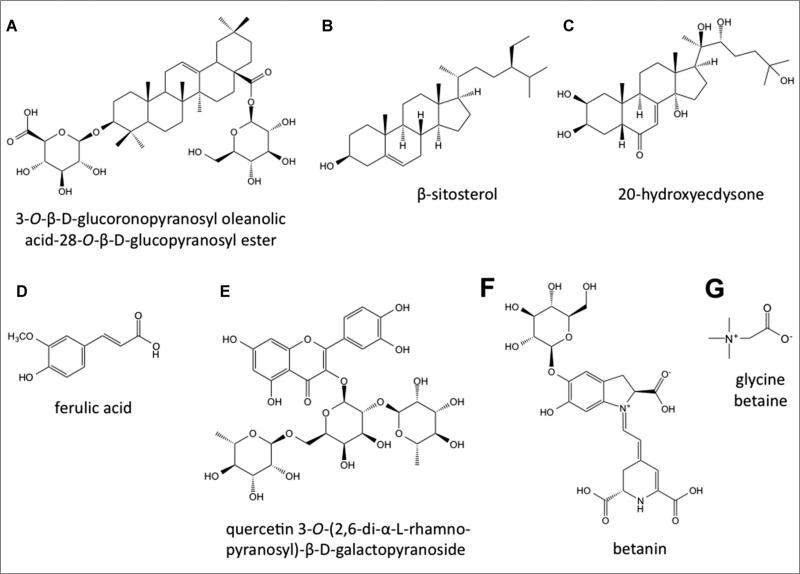
Quinoa has a unique amino acid, carbohydrate, lipid, and micronutrient profile, with nutrient levels often surpassing those in cereal products. Though much of the focus on quinoa's health benefits has centered upon its macro- and micronutrient profiles, quinoa's secondary metabolites may also contribute to the maintenance of human health and wellness. The major groups of secondary metabolites reported in quinoa are triterpenoids (saponins, phytosterols, and phytoecdysteroids), phenolics, beta-lains, and glycine betaine. Representative chemical structures for the major classes of quinoa-derived compounds are depicted in Figure 2.
Figure 2.
Representative chemical structures of the major pharmacologically active secondary metabolites present in quinoa seeds. A: triterpene saponin, B: phytosterol, C: phytoecdysteroid, D: phenolic acid, E: flavonol glycoside, F: betalain, G: glycine betaine.
Protein
The protein quantity and quality of quinoa are generally superior to those of cereal grains, while offering gluten-free property and high digestibility. Quinoa has a higher total protein content (12.9% to 16.5%) than barley (10.8% to 11.0%), oat (11.6%), rice (7.5% to 9.1%), and maize (10.2% to 13.4%), and a total protein content equal to that of wheat (14.3% to 15.4%) (Wright and others 2002; Repo-Carrasco and others 2003; Comai and others 2007; Abugoch James 2009; Jancurová and others 2009; Peiretti and others 2013). The storage proteins of quinoa consist mostly of globulin and albumin, with little to no presence of prolamins, the major storage proteins in many cereal crops. Prolamins, such as gliadin from wheat, secalin from rye, and hordein from barley (collectively referred to as “glutens”), induce autoimmune responses in celiac patients (Zevallos and others 2012; Biesiekierski and others 2013). A recent in vitro study of 15 cultivars of quinoa demonstrated that only 2 cultivars showed any detectable levels of celiac-toxic prolamin epitopes (Zevallos and others 2012), while immune responses were not replicated by whole food consumption in vivo. These results suggest that quinoa is a safe gluten-free substitutes for cereal grains (Zevallos and others 2014).
Among quinoa total protein, 37% is constituted by chenopodin, a globulin 11S-type protein (Repo-Carrasco and others 2003) that has become a reference source of leucine, isoleucine, and phenylalanine and tyrosine by the FAO (Abugoch James 2009). According to FAO/WHO recommendations, quinoa protein can supply over 180% of the daily recommended intake of essential amino acids for adult nutrition (Wright and others 2002; Abugoch James 2009; Vega-Galvez and others 2010), with adequate proportions of all 10 essential amino acids (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, tyrosine, and valine) (Vega-Galvez and others 2010; Miranda and others 2012b). Quinoa's essential amino acid profile is reported to be equivalent to that of casein and dried whole milk by FAO (2011). Furthermore, lysine, one of the limiting amino acids in cereal grains, is found at levels twice as high as those in wheat or maize (Valcárcel-Yamani and Caetano da Silva Lannes 2012).
According to animal feeding experiments, quinoa protein also has high digestibility. Among raw quinoa proteins, 91.6% is absorbable. Heat treatment (cooking) improves protein digestibility to 95.3% (Ruales and others 2002). Quinoa's high bioavailability is partially due to its relatively low content of trypsin inhibitors (1.36 to 5.04 TIU/mg) (Vega-Galvez and others 2010), which reduce protein enzymatic digestion and absorption (Valencia-Chamorro 2003).
Carbohydrate and fiber
Quinoa starch comprises 58.1% to 64.2% of dry seed weight, but has a low glycemic index (Vega-Galvez and others 2010). The starch is constituted mainly by D-xylose (120 mg/100 g) and maltose (101 mg/100 g) with low glucose (19 mg/100 g) and fructose (19.6 mg/100 g) content (Bhargava and others 2006). The starch is highly branched and consists of small granules (particle size less than 2 μm in diameter), which are smaller than the particle sizes of common cereal grains (Vega-Galvez and others 2010).
According to Lamothe and others (2015), quinoa contains 10% total dietary fiber. Fiber is the carbohydrate fraction which is resistant to enzymatic digestion and absorption in the small intestine, and which usually undergoes full or partial fermentation in the large intestine. Dietary fiber is considered essential for optimal digestive health, and also imparts various functional benefits (Brownawell and others 2012). Dietary fiber can promote satiety, reduce cholesterol and lipid absorption, modulate postprandial insulin response, promote endogenous cholesterol conversion to bile acids, improve intestinal microbiota, and reduce risk and severity of gastrointestinal infection and inflammation (Brownawell and others 2012; De Carvalho and others 2014). Furthermore, epidemiological studies have shown an inverse relationship between dietary fiber consumption and the development of cardiovascular disease, obesity, and type 2 diabetes (Brownawell and others 2012).
Though total fiber content in quinoa is comparable to other cereals, the monosaccharide subunit composition of quinoa fiber more closely resembles that of fruits, vegetables, and legumes. Insoluble quinoa fiber, which is comprised mainly of galacturonic acid, arabinose, galactose, xylose, and glucose subunits, represents 78% of total fiber content in quinoa. Meanwhile, soluble quinoa fiber, comprised mainly of glucose, galacturonic acid, and arabinose subunits, constitutes 22% of total fiber. The soluble fiber content, which is higher than that of wheat or maize (~15% each), may play a role in quinoa's health-promoting potential since the fermentability of soluble fiber by colonic microbiota has been recognized for its functional properties (Lamothe and others 2015). Soluble, well-fermentable fibers, such as inulin, fructooligosaccharides, and galactooligosaccharides, can lead to microbial production of short-chain fatty acids (acetic, butyric, and propionic) that lower luminal pH and increase the colonization of favorable bacteria, thereby serving as “prebiotics” (Biesiekierski and others 2013).
While conferring beneficial effects as prebiotics, some readily-fermentable short-chain carbohydrates, referred to as “fodmaps,” can induce symptoms of irritable bowel syndrome. Fodmaps include fructans present in wheat and rye, and foods with fructose content that exceeds glucose content, such as apples. Quinoa, contrarily, does not contain fructans and has a low fructose content. Therefore, quinoa is an integral part of the “low fodmap diet” that has been shown to wield marked beneficial impacts on irritable bowel symptoms in a recent human clinical study (Biesiekierski and others 2013). Further studies are needed to determine the specific types and concentrations of quinoa fibers, paired with their effects on colonic microbiota, in order to gain a better understanding of quinoa fibers’ potential to serve as a prebiotic and prevent disease.
Lipid
The oil content in quinoa ranges from 2% to 10% (average 5% to 7%), which is higher than that of maize (3% to 4%) (Vega-Galvez and others 2010). A recent study found that quinoa seed oil contains 89.4% unsaturated fatty acids and 54.2% to 58.3% polyunsaturated fatty acids (PUFAs). PUFAs are mostly 18:2n-6 and 18:3n-3, with an omega-6/omega-3 ratio of 6/1, which is generally more favorable than that of other plant oils (Tang and others 2015a).
The primary essential fatty acids in quinoa include linoleic acid and linoleneic acid, which are metabolized to arachidonic acid and eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA), respectively. These fatty acids are well protected from oxidation by vitamin E and other antioxidant components of quinoa seeds. Essential fatty acids play important roles in brain development, insulin sensitivity, cardiovascular health, prostaglandin metabolism, immunity, inflammation, and membrane function (Kim and others 2006; McCusker and Grant-Kels 2010; Vega-Galvez and others 2010). Essential fatty acids may exert beneficial physiological effects in humans by altering cell membrane phospholipid fatty acid composition, disrupting lipid rafts, inhibiting pro-inflammatory transcription factor activation, and binding to the G protein coupled receptor GPR120 (Calder 2012).
Vitamins
Quinoa seeds are a rich source of vitamins, which are required in the human diet to act as enzymatic cofactors in metabolism, regulate cell growth and development, protect against oxidative damage, improve vision, and play beneficial roles in various other physiological processes (Fitzpatrick and others 2012). Documented vitamins in quinoa include vitamin A precursor β-carotene (0.39 mg/100 g), thiamin/vitamin B1 (0.4 mg/100 g), riboflavin/vitamin B2 (0.39 mg/100 g), niacin/vitamin B3 (1.06 mg/100 g), panthothenic acid/vitamin B5 (0.61 mg/100 g), pyridoxine/vitamin B6 (0.20 mg/100 g), folic acid/vitamin B9 (23.5 to 78.1 mg/100 g), ascorbic acid/vitamin C (4.0 to 16.4 mg/100 g), and tocopherols/vitamin E (3.7 to 6.0 mg/100 g) (Ruales and Nair 1993; Bhargava and others 2006; Vega-Galvez and others 2010; Tang and others 2015a). Quinoa also contains a variety of carotenoids, predominantly luteins and zeaxanthins, with total concentrations ranging from 1.2 to 1.8 mg/100 g (Tang and others 2015a). Concentrations of many of these vitamins and provitamins in quinoa are higher than typical cereal grains (Bhargava and others 2006; Vega-Galvez and others 2010; Tang and others 2015a).
Minerals
Quinoa has a higher total mineral (ash) content (3.4%) than rice (0.5%), wheat (1.8%), and other cereals (Bhargava and others 2006). The micronutrients calcium (275 to 1487 mg/kg), copper (2 to 51 mg/kg), iron (14 to 168 mg/kg), magnesium (260 to 5020 mg/kg), phosphorus (1400 to 5300 mg/kg), potassium (75 to 12000 mg/kg), and zinc (28 to 48 mg/kg) are present in sufficient quantities in quinoa to maintain a balanced human diet (Repo-Carrasco and others 2003; Bhargava and others 2006; Vega-Galvez and others 2010).
Phytic acid (also known as phytate or IP6) has become a concern among various cereal grain and vegetable crops because it has a strong binding affinity to minerals like iron, thereby reducing their bioavailability (Valencia and others 1999). However, quinoa contains lower levels of phytic acid (1.18 g/100 g seed) than most cereal crops (Valencia-Chamorro 2003), and it has almost twice the soluble iron concentration (Valencia and others 1999). Processing of quinoa by cooking, soaking, and fermentation further reduces phytic acid content and improves iron solubility. Furthermore, germination of quinoa seedlings activates endogenous phytase, an enzyme which hydrolyzes phytic acid–mineral complexes (Valencia and others 1999).
Saponins
Quinoa's outer seed coat (pericarp) is rich in bitter saponins, natural detergent molecules found throughout the plant kingdom (Figure 2A). Saponins are comprised of a steroidal or triterpenoid aglycone (mainly oleanolic acid, hederagenin, phytolaccagenic acid, and serjanic acid), with one or more sugar moieties (Yendo and others 2010). Sugars can be linked to the aglycone at the C-3 and C-28 positions. The major sugar moities include glucose, galactose, arabinose, glucuronic acid, and xylose. Saponin chemistry of quinoa seeds is highly diverse. Madl and others (2006) identified 19 previously reported and 68 novel saponin compounds in quinoa using nano-HPLC electrospray ionization multistage tandem mass spectrometry (nLC-ESI-MS/MS) from a complex triterpene saponin crude extract of quinoa seed coats.
Saponins possess several common properties, including high foaming capacity in aqueous solutions, hemolytic activity when in direct contact with blood cells, and the formation of complexes with cholesterol and other steroidal components of cell membranes (Stuardo and San Martin 2008). Therefore, they are useful for crop protection from microbial infection and insect/bird herbivory, which facilitates organic production of this crop. The synthesis of secondary metabolites, including saponins, is a response to environmental factors and part of an adaptative strategy for abiotic stress tolerance (Ramakrishna and Ravishankar 2011). Triterpenoid saponins are synthesized via the isoprenoid pathway, leading to the formation of triterpenoid skeletons such as oleanane, ursane, lupeol, or dammarane. Subsequent structural modifications, including oxidation, substitution, and glycosylation, are mediated by various enzymes (Cammareri and others 2008).
Since saponins interfere with quinoa's palatability and digestibility, they must be removed before consumption. Some varieties of quinoa have been bred to contain lower saponin content, termed “sweet” quinoa (<0.11% free saponins), though these varieties are often less pest-resistant and experience increased bird herbivory. Despite their unpalatable characteristics, saponins also have a wide range of biological activities relevant to human health, including antifungal, antiviral, anticancer, hypocholesterolemic, hypoglycemic, antithrombotic, diuretic, and anti-inflammatory activities (Madl and others 2006; Vega-Galvez and others 2010). Recently, Yao and others (2014) showed that saponin-rich quinoa seed extracts decreased the production of the inflammatory mediator nitric oxide and inhibited the release of the inflammatory cytokines TNFα and IL-6 in lipopolysaccharide-stimulated RAW 264.7 macrophages.
Phytosterols
Though the phytosterol content of quinoa seeds has not received much attention, quinoa seeds contain phytosterol levels up to 118 mg/100 g quinoa seed, whereby the major constituents were β-sitosterol (Figure 2B), campesterol, brassicasterol, and stigmasterol (Villacrés and others 2013). Ryan and others (2007) found that quinoa seeds contain β-sitosterol (63.7 mg/100 g), campesterol (15.6 mg/100 g), and stigmasterol (3.2 mg/100 g), and these levels were higher than those found in barley, rye, millet, and maize.
Phytosterols are lipophilic compounds structurally similar to cholesterol. Epidemiological evidence, intervention studies, and meta-analyses have consistently concluded significant hypocholesterolemic effects of phytosterols in humans (Marangoni and Poli 2010). Phytosterols lower serum cholesterol levels by competing for cholesterol intestinal absorption, and possibly also by decreasing atherogenic lipoprotein production in the liver and intestines (Ho and Pal 2005). Furthermore, phytosterols have demonstrated anti-inflammatory, antioxidative, and anticarcinogenic effects (Ryan and others 2007).
Phytoecdysteroids
Among edible agricultural crops, quinoa seeds contain the highest levels of phytoecdysteroids (Table 1), polyhydroxlyated steroids that are structurally related to insect molting hormones involved in plant defense (Dinan 2009) and documented to exert a myriad of biological activities in mammals relevant to human health. Quinoa has been shown to contain a range of 138 to 570 μg/g total phytoecdysteroids (Graf and others 2015b). At least 13 different phytoecdysteroids have been isolated from quinoa seeds, of which 20-hydroxyecdysone (20HE) (Figure 2C) was the most abundant (62% to 90% of total phytoecdysteroids). The second most abundant set of phytoecdysteroids in quinoa include makisterone A, 24-epi-makisterone A, and 24(28)-dehydromakisterone A (Zhu and others 2001; Kumpun and others 2011; Graf and others 2014, 2015b).
Table 1.
Phytoecdysteroid content among edible agricultural crop plants.
| Common name | Scientific name | Plant part | Phytoecdysteroid content | Reference |
|---|---|---|---|---|
| Quinoa | Chenopodium quinoa Willd. | Seed | 138 to 570 μg/g total phytoecdysteroids; 109 to 497 μg/g 20HE | Graf and others 2015a, 2015b; Kumpun and others 2011 |
| Spinach | Spinacia oleracea L. | Dry leaves | 40 μg/g 20HE | Gorelick-Feldman and others 2008 |
| Fresh whole plant | 100 to 200 μg/g total phytoecdysteorids | Bakrim and others 2008 | ||
| Fresh leaves | ≤40 μg/g total phytoecdysteroids | Dinan 1995 | ||
| Fresh roots and stem | ≤100 μg/g total phytoecdysteroids | Dinan 1995 | ||
| Beets | Beta vulgaris L. | nd | Báthori and others 1984 | |
| Bitter yam | Dioscorea dumetorum Kunth (Pax) | Dried rhizome | 22 μg/g (20)-5β,11α,20-trihydroxyecdysone; 266 μg/g ajugasterone C; 32 μg/g herkesterone | Sautour and others 2008 |
nd, not determined, but the presence was confirmed.
Extracts from several phytoecdysteroid-containing plants, especially traditional medicinal Chinese and Siberian herbs (Cheng and others 2008; Kokoska and Janovska 2009; Lafont 1998), have been used as adaptogens, muscle builders, and stress reducers (Báthori 2002). Previous reviews on the bioactivities of phytoecdysteroids highlight their growth-promoting, antidiabetic, immunomodulatory, hepatoprotective, neuroprotective, hypocholesterolemic, wound healing, antidepressive, and antioxidant activities (Lafont and Dinan 2003; Dinan and Lafont 2006; Dinan 2009). Evidence also suggests that phytoecdysteroids may be safe for oral and topical application in humans without inducing the unwanted effects associated with sex hormone supplementation. 20HE has been shown to have very low toxicity (LD50 of 6.4 g/kg after intraperitoneal injection; LD50 of 9 g/kg after oral application) (Dinan 2009) and is rapidly cleared from the blood following 14-dehydroxylation metabolism (Dinan and Lafont 2006). Furthermore, 20HE does not demonstrate in vitro androgen binding (Gorelick-Feldman and others 2008) or in vivo estrogenic effects (such as increased uterine weight) in ovariectomized rats (Seidlova-Wuttke and others 2010a).
The most recent studies on the biological effects of 20HE have focused on its potential role in the treatment or prevention of metabolic syndrome and postmenopausal disorders. Kizelsztein and others (2009) demonstrated that 20HE (10 mg/kg for 13 weeks) reduced adiposity 41%, increased insulin sensitivity, and lowered blood glucose levels in high-fat diet-induced obese, hyperglycemic C57Bl/6J mice. Following that study, Foucault and others (2011, 2014) demonstrated that 20HE (6 mg/kg for 3 weeks) and a 20HE-enriched quinoa extract both induced similar anti-obesity and antidiabetic effects. In a third study among high-fat diet mice, 20HE treatment (25 or 50 mg/kg for 12 weeks) lowered body weight, improved insulin sensitivity, and reduced muscle lipid accumulation (Wang and others 2011). Meanwhile, 20HE administration (18 to 121 mg/d/animal for 12 weeks) prevented fat gain and bone density loss, reduced hot flashes, and improved dermal thickness in ovariectomized rats, the standard rodent model for postmenopausal studies (Seidlova-Wuttke and others 2010a, 2010b; Ehrhardt and others 2011; Seidlova-Wuttke and Wuttke 2012). Future studies are needed to test the effect of 20HE on metabolic markers associated with diabetes, obesity, cardiovascular health, and postmenopausal symptoms in humans.
Due to their steroidal structures, 20HE and other phytoecdysteroids have been investigated for their potential anabolic and performance-enhancing abilities, with mixed results. Studies show that 20HE treatment induces protein synthesis in vitro (Gorelick-Feldman and others 2008) and enhances physical performance in rodents, as assessed by forced swim tests and grip strength measurement (Gorelick-Feldman and others 2008; Dinan 2009). However, no significant improvement in performance was found in a double-blind, placebo-controlled human clinical trial among resistance-trained males treated with 20HE (30 mg/d for 8 weeks) (Wilborn and others 2006). This study may have been limited by its small treatment group size (9 individuals in each group) and/or the duration of treatment. Furthermore, the effect of 20HE on performance may be greater among untrained individuals as opposed to trained athletes.
20HE's primary mechanism of action has not yet been determined. 20HE's neuroprotective effects have been attributed to its modulation of GABAA receptors, and its hypocholesterolemic activity has been explained by its ability to increase the conversion of cholesterol to bile acids (Dinan and Lafont 2006). Studies in high-fat-diet mice have linked 20HE's anti-obesity and antidiabetic activity to reduced dietary lipid absorption, increased glucose oxidation, increased energy expenditure (Foucault and others 2014), and enhanced mitochondrial oxidative phosphorylation (Wang and others 2011). In addition, 20HE has been shown to attenuate oxidative stress in 3 different mammalian cell lines (Hu and others 2010, 2012; Graf and others 2015a). Meanwhile, 20HE may affect mammalian gene expression, resulting in activation of the anabolic P13K/Akt pathway (Dinan and Lafont 2006; Gorelick-Feldman and others 2010), and downregulation of the hepatic gluconeogenesis pathway (Kizelsztein and others 2009). 20HE also modulates cell differentiation, as shown in mesenchymal stem cells and keratinocytes (Detmar and others 1994; Gao and others 2008). 20HE's transcriptional effects are likely mediated by nuclear hormone receptor interaction, although evidence of direct binding has not yet been reported.
Phenolics
Phenolics are a large, diverse class of compounds consisting of hydroxyl group(s) attached to at least one aromatic hydrocarbon ring, a highly stable chemical structure that gives these molecules their well-known antioxidant activity (Harborne and Williams 2000). Phenolics also possess a range of biological activities due to their effects on cell-signaling and metabolism, including anti-inflammatory, anticancer, antidiabetic, anti-obesity, and cardioprotective effects (Harborne and Williams 2000; Da-Silva and others 2007; Kelly 2011; Jeong and others 2012).
Phenolics exist as simple single-ringed structures called phenolic acids (Figure 2D), or multiringed structures termed polyphenols (Figure 2E). Phenolic acids can exist in free form or as bound phenolics, associated with pectins in the cell wall (Renard and others 1999; Gomez-Caravaca and others 2011). Several phenolic acids, including derivatives of hydroxycinnamic acid and hydrobenzoic acid, have been identified in quinoa seeds and leaves (Supp. Table 1). Individual phenolic acids have been reported in quinoa seeds at concentrations as high as 251.5 μg/g dry weight (Gorinstein and others 2008).
Polyphenols are divided into several subgroups, among which the flavonoids (including flavonol glycosides and isoflavones) have been most extensively investigated (Tsao 2010). Flavonol glycosides constitute the most abundant phenolics in quinoa seeds and leaves (Gomez-Caravaca and others 2011). More than a dozen different flavonol glycosides have been identified in this plant, constituted mainly by quercetin and kaempferol derivatives, with concentrations of individual compounds occurring at levels as high as 839 μg/g dry weight (Dini and others 2004; Hirose and others 2010).
Isoflavones were identified for the first time in quinoa by Lutz and others (2013), who reported the presence of genistein (0.05 to 0.41 mg/100 g) and daidzein (0.70 to 2.05 mg/100 g) among 10 different seed varieties. However, compared to soybean isoflavone concentrations (genistein, 83.8 mg/100 g; daidzein, 58.3 mg/100 g), the concentration of isoflavones in quinoa is very low (Lutz and others 2013).
Total flavonoid, phenolic, and antioxidant contents have been assessed by spectrometry among several quinoa varieties, indicating that differences in phytochemical content may be due to genotypic or environmental factors (Supp. Table 2). However, spectrophotometric analysis methods are often influenced by interfering compounds, such as vitamins, amino acids, and sugars, which make reliable quantification difficult (George and others 2005). There is need for further studies on specific phenolics in quinoa using HPLC or MS (Gomez-Caravaca and others 2012).
Betalains
There are not many reports regarding the content of betalains (Figure 2F) in quinoa. These molecules give quinoa seeds and vegetative parts their varied yellow, red, and black colors (Figure 1) (Bhargava and others 2006). Betalain pigments, including red-violet betacyanins and yellow-orange betaxanthins, are nitrogen-containing aromatic indole derivatives synthesized from tyrosine (Tang and others 2015b). They are structurally and biosynthetically distinct from phenolics and are restricted to the order Caryophyllales, to which Amaranthaceae crops belong (Moreno and others 2008).
The most abundant betalains in quinoa seeds are betanin and isobetanin, which possess a range of health-promoting properties similar to those of phenolic compounds, including antioxidant and anti-inflammatory effects (Tang and others 2015b). However, studies have shown that some betalains possess antioxidant activity higher than that of polyphenols (Neagu and Barbu 2014).
Betalains are stable between pH 3 and 7, and can therefore be used as a natural dye. There is a growing consumer and industrial demand for natural, safe, stable alternatives to synthetic color ingredients in foods (Neagu and Barbu 2014). Today, betalains are approved for use as colorants in dairy products, sauces, soups, cosmetics, and pharmaceuticals by the U.S. FDA and European Union (E-162) (Esatbeyoglu and others 2015). Quinoa is a candidate crop for the extraction of natural color ingredients, particularly from the nonconsumed vegetative parts. However, additional research is needed to determine the concentration, extractability, and stability of quinoa-derived betalains in order to develop value-added products for use in food coloring.
Glycine betaine
Glycine betaine (Figure 2G), also known as betaine or N,N,N-trimethylglycine, is an N-trimethylated amino acid. Betaine and its precursor, choline, are important for homocysteine regulation and have been implicated in the treatment and prevention of diabetes, obesity, and cardiovascular disease (Olthof and Verhoef 2005). Cereal crops are the largest contributing source of betaine in the Western diet. However, quinoa contains a higher amount of betaine (3930 to 6000 μg/g) compared to other cereal and cereal-like crops (174 to 706 μg/g among wheat products, 50 to 150 μg/g for millet and teff, less than 20 μg/g in buckwheat, 646 μg/g in amaranth) (Ross and others 2014).
Clinical Evidence of Health Benefits of Quinoa-Derived Products
The high nutritional value, medicinal properties, and gluten-free quality of quinoa may benefit several at-risk consumer populations, including children, the elderly, high-performance athletes, lactose-intolerant consumers, osteoporosis-prone women, and people with anemia, diabetes, dyslipidemia, obesity, or celiac disease (Bhargava and others 2006; Vega-Galvez and others 2010). Though the number of animal and human clinical trials on quinoa's therapeutic potential is limited, several studies indicate various benefits associated with quinoa consumption (Table 2).
Table 2.
Clinical trials on the effect of quinoa products in human health.
| Therapeutic application | Study participants and location | Treatment | Endpoints (measured before and after intervention) and outcomes | Conclusions | Reference |
|---|---|---|---|---|---|
| Child growth and development | Boys aged 50–65 months from low-income families in Ecuador | Infant food formulated from quinoa (100 g × 2/d for 15 days) compared with no treatment | ↑ Plasma levels of IGF-1, a marker of malnutrition, known to increase body weight gain | Quinoa-based infant food may play a role in reducing childhood malnutrition | Ruales and others 2002 |
| Celiac disease | 19 celiac patients | Cooked quinoa (50 g/d for 6 weeks) | All gastrointestinal parameters (villus height:crypt depth), surfacen-enterocyte cell height, number of intra-epithelial lymphocytes per 100 enterocytes) improved following quinoa diet; serum lipid levels remained normal with small decrease in total cholesterol, LDL, HDL, and triglycerides | Quinoa is safe for consumption by celiac patients | Zevallos and others 2014 |
| Risk of cardiovascular disease | 22 students aged 18 to 45 years | Quinoa cereal bar daily for 30 days | ↓ Triglycerides ↓ Cholesterol ↓ LDL | Quinoa intake may reduce risk of developing cardiovascular disease | Farinazzi-Machado and others 2012 |
| Postmenopausal symptoms | 35 postmenopausal women with excess weight (menopasual for ≥2 years, waist circumference > 80 cm, serum estradiol 10–20 pg/mL, follicle-stimulating hormone ≥35 mIU/mL, not undergoing hormone therapyor isoflavone supplements in the past 6 months, not taking lipid lowering drugs in the last 2 weeks) | Quinoa flakes (QF) compared with corn flakes (CF), 25 g/d for 4 weeks | QF consumption increased protein and fiber intake but not total caloric intake ↓ Triglycerides, ↓ TBARS ↓ Cholesterol, ↓ LDL, ↑ GSH | Quinoa intake beneficially modulates metabolic parameters | De Carvalho and others 2014 |
IFG-1, insulin-like growth factor 1; TBARS, thiobarbituric acid reactive substances; GSH, glutathione; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
In a childhood nutrition study among boys aged 50 to 65 months from low-income families in Ecuador, the consumption of infant food formulated with quinoa (100 g twice per day for 15 days) significantly increased plasma IGF-1 levels, whereas IGF-1 levels were unchanged in the control group. IGF-1 is a hepatic peptide that promotes growth, increasing body weight and bone length. IGF-1 has been suggested as a marker of malnutrition or a measure of response to nutritional therapy. The positive effects observed for the quinoa-treated group were attributed to the complete essential amino acid profile of the quinoa-formulated baby food, as well as its high digestibility (95.3%), which was higher than that of 5 commercially available infant foods derived from milk and soy. This study indicates that infant food derived from quinoa provides sufficient protein and other essential nutrients crucial for reducing child malnutrition (Ruales and others 2002).
The use of quinoa seeds as a safe, gluten-free alternative to cereal grains was evaluated in a human clinical trial among celiac patients. Nineteen celiac patients consumed 50 g quinoa daily for 6 weeks. Gastrointestinal parameters (villus height:crypt depth, surface-enterocyte cell height, and number of intra-epithelial lymphocytes per 100 enterocytes) and serum lipid levels were evaluated before and after intervention. The study found that gastrointestinal parameters improved following the quinoa diet, while serum lipid levels remained within a normal range, with small decreases observed in total cholesterol, LDL, HDL, and triglycerides (Zevallos and others 2014).
The hypolipidemic potential of quinoa products has been further demonstrated in human trials. Daily consumption of a quinoa cereal bar for 30 days significantly lowered triglyceride, total cholesterol, and LDL levels among 22 students aged 18 to 45 years. Meanwhile, blood glucose levels, body weight, and blood pressure each decreased, though nonsignificantly (Farinazzi-Machado and others 2012).
In a prospective, double-blind human clinical trial among post-menopausal women with excess weight, quinoa flake consumption (25 g/d for 4 weeks) also modulated metabolic parameters posttreatment compared to baseline. In this study, serum triglycerides (112.3 ± 35 to 107.9 ± 33.1 mg/dL) and TBAR values (3.06 ± 0.6 to 2.89 ± 0.5 μmol/L; TBARs are substances produced in response to lipid peroxidation-induced oxidative stress) were both significantly reduced. Furthermore, quinoa intervention nonsignificantly reduced total cholesterol (191 ± 35 to 181 ± 28 mg/dL) and LDL-cholesterol (129±35 to 121±26 mg/dL), while glutathione (GSH, a marker of antioxidant defense) was increased (1.78 ± 0.4 to 1.91 ± 0.4 mmol/L). In a parallel intervention group that consumed corn flakes, similar decreases in triglycerides and TBAR values were observed, but total cholesterol, LDL, and GSH levels were not affected, indicating a possible unique benefit from quinoa consumption (De Carvalho and others 2014).
Though the antidiabetic effects of quinoa have not been studied in humans, co-administration of quinoa seeds (310 g/kg feed for 5 weeks) significantly reduced plasma glucose levels and oxidative stress in Wistar rats fed a high-fructose diet (31% fructose) compared to the control group (Pasko and others 2010a, b). Secondly, phytochemically enriched products derived from quinoa seeds (described in detail below) significantly lowered levels of fasting blood glucose, adiposity, and cholesterol in vivo (Foucault and others 2011, 2014; Graf and others 2014).
The present evidence for quinoa's health benefits warrants future studies on this crop's role in the treatment and prevention of complex human diseases. Randomized, controlled trials are the gold standard for determining the effect of diet on health outcomes, though optimal study design and length of the study are crucial factors in determining trial outcomes. Epidemiological studies and observational cohort studies, which have not yet been conducted on quinoa consumption, can also be useful to generate hypothesis-driven research questions.
Within clinical intervention studies, it is important to utilize quinoa products with well-characterized and standardized phytochemical contents so that observed effects can be linked to specific constituents. Previous clinical studies collectively suggest that the major cholesterol-lowering and antioxidant components of quinoa were proteins, fiber, vitamins (tocopherols and carotenoids), minerals (iron, zinc, magnesium), saponins, phytosterols, phytoecdysteroids, and phenolics (Ruales and others 2002; Farinazzi-Machado and others 2012; De Carvalho and others 2014; Zevallos and others 2014). However, other quinoa phytochemicals, such as polyunsaturated fatty acids and betalains, may also play an important role. Furthermore, individual quinoa constituents may positively interact with each other, leading to enhanced bioactivity through a phenomenon referred to as synergy or potentiation (Schmidt and others 2008). Many studies on whole grains have shown that their health benefits are likely the result of the “whole-grain package” rather than single constituents (McKeown and others 2013). A single serving of quinoa (approximately 40 g) will deliver a large portion of the Recommended Daily Allowance (RDA) of key nutrients and health-beneficial compounds to the consumer (Supp. Table 3). Given the complex nature of quinoa's phytochemical profile, it is important to consider the “whole-grain package” hypothesis when designing or analyzing the results of future clinical studies.
Patient compliance is another factor to consider when drawing conclusions from human clinical trials. Quinoa products used in feeding studies should be appealing and convenient for consumption, such as packaged snacks and energy bars. Biomarkers of quinoa consumption should also be used as tools to measure compliance. De Carvalho and others (2014) utilized urinary enterolignan concentration as a biomarker of quinoa intake. Enterolignans (enterodiol and enterolactone) are the compounds that result from the metabolism of plant lignans by gut microbiota. However, enterolignan concentration may not be a robust measure of quinoa intake since many plant-based foods (various whole grains, vegetables, tea, and coffee) are also rich in lignans and several variables affect the efficiency of gut microbiota conversion of lignans to enterolignans (intestinal integrity, stress, genetics, antibiotic intake) (van Dam and Hu 2008; De Carvalho and others 2014).
A recent study found that alkylresorcinols, phenolic lipids previously thought to be present only in the bran of cereal grains, were also present in quinoa (Ross and Savolainen 2014). Alkylresorcinols have been suggested to be a better measure of whole grain intake in humans (van Dam and Hu 2008). Furthermore, in this study on quinoa, the alkylresorcinol homologs C18:0, C22:0, and C24:0 were identified for the first time in nature. Therefore, specific alkylresorcinol homologs may serve as unique biomarkers of quinoa intake for future clinical intervention and epidemiological studies.
Technological Innovations in Quinoa Processing and Applications
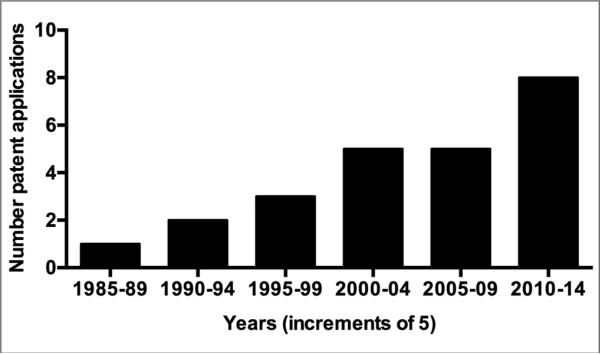
Technological innovations that speed cooking time, generate ready-to-eat packaged products, or deliver phytochemicals in concentrated form can facilitate the intake of health-beneficial phytochemicals in today's fast-paced society. With the growing interest in quinoa's nutritional and medicinal value, methods for producing, concentrating, and/or utilizing several value-added products from quinoa have been developed over the past 25 years. This phenomenon has been demonstrated by the steady rise in publications generated over this time period, especially patents and patent applications (Table 3, Figure 3). Within the functional food, botanical supplement, cosmetic, and pharmaceutical sectors, quinoa-based technologies have been developed to target the following therapeutic areas of human health: (1) celiac disease, (2) sports performance and fitness enhancement, (3) weight loss and/or metabolic parameters associated with diabetes, obesity, hypertension, hyper-lipidemia, and postmenopause, (4) skin and hair care, and (5) drug absorption.
Table 3.
Patents and patent applications related to quinoa.
| Quinoa-derived product | Production method and/or use | Year(s) filed | Reference(s) |
|---|---|---|---|
| Treated seeds | Superheated steam treatment to expand the seeds and reduce cooking time | 1992 | Thomas 1995 |
| Mechanical abrasion, washing, or a combination to debitter seeds for use as flavoring or texturizing agents in food products | 2010a | Scanlin and Burnett 2010 | |
| Flour | Substitute for food additives | 2010a | Weinreich and Konecny 2011 |
| Vegetative portion | Dried and heat treated for incorporation into animal foodstuffs | 1996 | Haaber 1999 |
| Beverages | Boiling of quinoa seeds with other ingredients, including buckwheat, for production of a milk/tea/wine with a balanced and easily absorbable nutritional profile to promote human health; organic quinoa buckwheat tea production using certified organic quinoa | 2013a; 2013 | 余磊 2014; 谢振文 2014 |
| Seeds are soaked, malted, kilned, mashed, cooled, and fermented with yeast to produce a gluten-free (and Kosher, optionally) fermented alcoholic beverage | 2011a | Kamelgard 2013 | |
| Mixing of quinoa extract, tiger nut (Cyperus esculentus), and α-amylases for hydrolysis of starches to thermostable maltodextrins within a beverage formulation that can act as a substitute for animal or plant-derived milk | 2001a | Felipe and others 2003 | |
| Seeds milled to specific particle size, mixed with liquid in specific ratio, and heated within specific temperature range for automated commercial-scale beverage production | 2006a | Edwards 2007 | |
| Protein concentrate | Extraction and precipitation via alkali or enzyme treatment for use in foods, cosmetics, animal food, or sports performance and recovery | 2012a; 2011a; 2012a; 2004 | Enrione and others 2013; Kruger 2012; Pouvreau and others 2014; Scanlin and Stone 2009 |
| Incorporation in formulation for hair treatment | 2011a,b | Kruger 2012 | |
| Lipid | Extraction and molecular distillation to obtain a refined oil for dermatological use | 2007a | Msika 2012 |
| Carbohydrate | Extraction of maltodextrin via alkali or enzyme treatment of quinoa flour to produce a gel-form to deliver quinoa-derived peptides for sports performance and recovery | 2012a,b | Enrione and others 2013 |
| Use of quinoa starches of specific shape and particle size to act as cream substitute that mimics the mouth feel of fat/cream in foods | 1988 | Singer and others 1990 | |
| Saponins | Extraction and precipitation of saponins | 1999; 2007a | Gamboa 2008; Muir and others 2002 |
| Protection of crop plants, especially potatoes, tomatoes, and rice, from fungal, bacterial, and mollusk pathogens | 2007a; 2003; 2004a; 2001; 2008a,b | Bengtsson and others 2008; Dutcheshen 2004, 2005; Dutcheshen and Danyluk 2002; Gamboa 2008 | |
| Growth promotion of crop plants | 2007a,b | Bengtsson and others 2008 | |
| Enhancement of mucosal absorption of pharmaceutical drugs/vaccines | 1995 | Estrada and others 1997 | |
| Phytoecdysteroids | Reduction of fat associated with metabolic syndrome | 2008 | Veillet and Lafont 2012 |
| Cytoplasmic male sterile plants derived from Apelawa quinoa variety | Breeding of new high-yielding quinoa varieties | 1992 | Ward and Johnson 1994 |
indicates application status (not granted).
Patent was listed earlier in the table.
Figure 3.
Incremental increase in quinoa-related patent applications from 1985 to 2014.
Food processing, packaging, and formulation
Superheated steam treatment has been optimized to remove quinoa saponins while also expanding the seeds so as to reduce cooking time for the consumer (Thomas 1995). Tempe production from quinoa seeds has been developed via solid-state fermentation with Rhizopus oligosporus Saito, thereby producing a tempe product which contains all essential amino acids and lower levels of the estrogenic isoflavone compounds than those found in traditional soy-based tempe (Penaloza and others 1992).
The small size and thermostability of quinoa starch granules make them useful in frozen food packaging, emulsion-type products (thickeners), and malted beverages (Bhargava and others 2006). Recently, an antimicrobial biofilm comprised of gold nanoparticles incorporated in quinoa starch has been suggested for use in food packaging to prevent food-born pathogens Escherichia coli and Staphylococcus aureus (Pagno and others 2015). Furthermore, quinoa maltodextrins, derived from the hydrolysis of quinoa starches, possess a gel-like consistency for the delivery of performance-enhancing nutrients or use as a fat/cream substitute (Singer and others 1990; Enrione and others 2013).
Methods to improve the quality of baked goods via substitution of cereal grains for quinoa flour have been investigated. Up to 10% quinoa flour was used in wheat-based bread to improve nutritional quality without negatively affecting loaf volume. Gluten-free spaghetti made from quinoa flour instead of wheat flour had reasonable physical properties and acceptable taste. Gluten-free bread made from quinoa flour as opposed to potato starch had improved bread volume, softer crumb structure, and higher contents of protein, fiber, vitamins, minerals, polyphenols, and antioxidants (Valcárcel-Yamani and Caetano da Silva Lannes 2012). Loaf volume and texture were also improved by the substitution of quinoa flour for rice or corn flour in gluten-free bread production (Elgeti and others 2014). Considering the low nutritional value of gluten-free products currently available in the market, further work on the behavior of quinoa proteins and carbohydrates in bread- and pasta-making is warranted.
Concentration/purification of chemical constituents
Various methods to concentrate or purify quinoa proteins, oils, saponins, and phytoecdysteroids have been developed for food and pharmaceutical applications. One of the most highly studied quinoa extraction methods has been the production of quinoa protein concentrate via alkali or enzyme hydrolysis and precipitation. Hydrolyzed quinoa protein showed higher protein solubility and may be useful for the treatment of protein deficiency, enhancement of sports performance, promotion of exercise recovery, and skin/hair care (Scanlin and Stone 2009; Kruger 2012; Enrione and others 2013; Pouvreau and others 2014). Quinoa protein isolate also demonstrated a hypocholesterolemic effect in vivo by inhibiting bile acid re-absorption in the small intestine and regulating cholesterol synthesis and catabolism (Takao and others 2005).
Extraction methods and uses of quinoa-derived lipids and triterpenoids have also been studied. For example, refined quinoa seed oils have been proposed for dermatological applications (Msika 2012). Quinoa-derived saponins may enhance the mucosal absorption of pharmaceutical drugs and vaccines due to their interactions with cell membranes (Estrada and others 1997). Lastly, quinoa extracts enriched in phytoecdysteroids (1.0% to 2.0% 20HE w/w) lowered adiposity and blood glucose levels associated with metabolic syndrome in vivo (Foucault and others 2011; Veillet and Lafont 2012; Graf and others 2014).
Rapid determination and breeding of improved crop qualities
Quinoa breeding programs in the Americas, Asia, and Europe have successfully improved quinoa crop qualities to facilitate seed production and mechanical harvesting, while improving protein content and minimizing saponin levels (Cusack 1984; Jancurová and others 2009; Bhargava and Srivastava 2013; Bendevis and others 2014). Quinoa is a challenging crop to breed via mass selection or hybridization because the small, tightly clustered flowers are difficult to manually emasculate and are mainly self-pollinating. Nonetheless, outcrossing occurs at rates of 0.5% to 9.9%, and both mass selection and hybridization have been practiced (Bhargava and Srivastava 2013). An alternative method for quinoa hybrid production is the use of cytoplasmic and genetic male sterile lines as maternal parents (Ward and Johnson 1994; Bhargava and Srivastava 2013).
These breeding approaches, combined with efficient methods of biochemical analysis, may facilitate the development of quinoa varieties with enhanced nutritional and medicinal properties. Researchers have begun to investigate variation in carbohydrate, lipid, vitamin, mineral, total polyphenol, and antioxidant contents among quinoa varieties using traditional biochemical analysis technologies (Bhargava and others 2006; Christensen and others 2007; Shen and others 2009; Gonzalez and others 2012; Miranda and others 2012a, 2012b; Yousef and others 2013). However, rapid, simple, low-cost techniques that require no sample preparation and generate no waste are preferable to traditional methods of sample analysis.
Near-infrared spectroscopy (NIRS) is a rapid technology that may be applicable for quinoa quality analysis. Previously used to monitor quality of cereal grains and beans, NIRS has been optimized to accurately determine protein, carbohydrate, lipid, ash, and moisture contents in quinoa seeds, as well as the concentrations of 12 specific amino acids (Escuredo and others 2014; Ferreira and others 2015). NIRS can also be developed to assess concentrations of fiber, fatty acids, vitamins, and minerals (Ferreira and others 2015).
Though NIRS may not be applicable for the determination of secondary metabolite concentration in quinoa seeds, a method to rapidly leach the total amount of phytoecdysteroids and flavonol glycosides available in quinoa seeds, without sample destruction, has recently been developed by Graf and others (2014, 2015b) for application in phytochemical analysis of quinoa varieties. Further work is needed to determine the variation in phytosterols, phenolic acids, betalains, and glycine betaine among quinoa sources, as well as the genetic and environmental factors that influence their concentrations.
Conclusion
Quinoa is a culturally important, stress-tolerant crop with high nutritional value and unique phytochemical composition. Quinoa-derived products and their individual chemical constituents have demonstrated biological activities with various applications to benefit human health. Quinoa therefore has the potential to provide nutrition and medicine to millions of malnourished or health-impaired people worldwide. Further research, including additional human clinical trials, is required to understand quinoa's functional benefits, mechanisms of action, and phytochemical interactions. Lastly, integrative strategies to improve access to, awareness of, and deliverability of quinoa's health benefits among the scientific, agricultural, and industrial sectors are critical.
Supplementary Material
Acknowledgments
BLG was supported by the U.S. Fulbright Student IIE Fellowship, the Robert and Lillian White-Stevens Fellowship from the School of Environmental and Biological Sciences of Rutgers University, and the New Jersey Agricultural Experiment Station. PRS was funded trough a doctoral scholarship from SENESCYT-Ecuador (Beca 2011). LER and JDH were funded by Proyecto Biomasa 2012–2014, Gobierno Regional de Tarapacá, Fondo de Innovación para la Competitividad (FIC), and FONDECYT No 11140915. MEB was funded by a professor/researcher grant from the Universidad de Las Américas-Quito. IR was funded by a Botanical Research Center Pilot Program Sub award 5P50AT002776-08 S12-50318 and P50AT002776-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS). The authors thank Drs. Mary Ann Lila, Lena Struwe, and Joan Bennett for editing the manuscript drafts.
Abbreviations
- AFLPs
amplified fragments length polymorphisms
- CE
catechin equivalents
- CF
corn flakes
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FAO
Food and Agriculture Organization of the United Nations
- FRAP AA
ferric-reducing ability of plasma antioxidant activity
- GAE
gallic acid equivalents
- GSH
glutathione
- HDL
high-density lipoprotein
- 20HE
20-hydroxyecdysone
- HPLC
high-performance liquid chromatography
- HPLC-DAD-ESI-TOF-MS
HPLC-photodiode array coupled with electrospray ionization time-of-flight mass spectrometry
- IGF-1
insulin-like growth factor-1
- IL-6
interleukin-6
- IYQ2013
International Year of Quinoa 2013
- LDL
low-density lipoprotein
- MS
mass spectrometry
- NIRS
near-infrared spectroscopy
- nLC-ES-MS/MS
nano-HPLC electrospray ionization multistage tandem mass spectrometry
- PUFAs
polyunsaturated fatty acids
- RDA
recommended daily allowance
- QF
quinoa flakes
- SD
standard deviation
- TAE
tannic acid equivalents
- TBARs
thiobarbituric acid-reactive substances
- TE
trolox equivalents
- TNFα
tumor necrosis factor alpha
- WHO
World Health Organization of the United Nations
Footnotes
Practical Application: This work can be used to identify specific research studies necessary to further understand the role that quinoa and its individual chemical constituents play in human health. The information presented also highlights open avenues for technological innovation and quinoa product improvement within the industrial sector.
Author Contributions
BLG and IR conceived of and outlined the manuscript. BLG wrote the manuscript under the supervision of IR with contributions on biochemical aspects from LER (protein and other), MEB (phystosterol), PRS (phenolics), and JHD (saponins). All authors contributed to editing of the final manuscript.
Conflict of Interest
IR owns equity in Nutrasorb, L.L.C., which is involved in quinoa R&D.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Contributor Information
Brittany L. Graf, Dept. of Plant Biology and Pathology, Rutgers Univ., 59 Dudley Rd., New Brunswick, NJ 08901, U.S.A.
Patricio Rojas-Silva, Dept. of Plant Biology and Pathology, Rutgers Univ., 59 Dudley Rd., New Brunswick, NJ 08901, U.S.A..
Leonel E. Rojo, Facultad de Ciencias de la Salud, Univ. Arturo Prat, Casilla 121, Iquique, Chile.
Jose Delatorre-Herrera, Facultad de Recursos Naturales Renovables, Univ. Arturo Prat, Casilla 121, Iquique, Chile..
Manuel E. Baldeón, Centro de Investigación Traslacional, Univ. de Las Américas, Quito, Ecuador.
Ilya Raskin, Dept. of Plant Biology and Pathology, Rutgers Univ., 59 Dudley Rd., New Brunswick, NJ 08901, U.S.A..
References
- Abugoch James LE. Quinoa (Chenopodium quinoa Willd.): composition, chemistry, nutritional and functional properties. Adv Food Nutr Res. 2009;58:1–31. doi: 10.1016/S1043-4526(09)58001-1. [DOI] [PubMed] [Google Scholar]
- APG. An ordinary classification for the families of flowering plants. Ann Mo Bot Gard. 1998;85(4):531–53. [Google Scholar]
- Bakrim A, Maria A, Sayah F, Lafont R, Takvorian N. Ecdysteroids in spinach (Spinacia oleracea L.): biosynthesis, transport and regulation of levels. Plant Physiol Biochem. 2008;46(10):844–54. doi: 10.1016/j.plaphy.2008.06.002. 10.1016/j.plaphy.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Báthori M. Phytoecdysteroids effects on mammalians, isolation and analysis. Mini Rev Med Chem. 2002;2:285–93. doi: 10.2174/1389557023406269. [DOI] [PubMed] [Google Scholar]
- Báthori M, Tóth I, Szendrei K, Rattai M, Minker E, Blazsó G. Determination and isolation of ecdysteroids in native goosefoot species. Herba Hungarica. 1984;23:131–45. [Google Scholar]
- Bendevis MA, Sun Y, Rosenqvist E, Shabla S, Liu F, Jacobsen S-E. Photoperiodic effects on short-pulse 14C assimilation and overall carbon and nitrogen allocation patterns in contrasting quinoa cultivars. Environ Exp Bot. 2014;104:9–15. [Google Scholar]
- Bengtsson MV, Hockenhull JR, Elgaard T, Nielsen BKK, Damso M, inventors A natural product having a fungus inhibiting effect on specific fungal pathogens and a growth promoting effect for improving plant production. 2008 Patent Application EP1867230 A3.
- Bhargava A, Shukla S, Ohri D. Chenopodium quinoa – An Indian perspective. Ind Crops Prod. 2006;23:73–87. [Google Scholar]
- Bhargava A, Srivastava S. Quinoa: Botany, production and uses. CABI; Boston, MA: 2013. [Google Scholar]
- Biesiekierski JR, Muir JG, Gibson PR. Is gluten a cause of gastrointestinal symptoms in people without celiac disease? Curr Allergy Asthma Rep. 2013;13:631–38. doi: 10.1007/s11882-013-0386-4. [DOI] [PubMed] [Google Scholar]
- Bigliardi B, Galati F. Innovation trends in the food industry: the case of functional foods. Trends in Food Sci Technol. 2013;31(2):118–29. [Google Scholar]
- Brend Y, Galili L, Badani H, Hovav R, Galili S. Total phenolic content and antioxidant activity of red and yellow quinoa (Chenopodium quinoa Willd.) seeds as affected by baking and cooking conditions. Food Nutr Sci. 2012;3:1150–5. [Google Scholar]
- Brownawell AM, Caers W, Gibson GR, Kendall CWC, Lewis KD, Ringel Y, Slavin JL. Prebiotics and the health benefits of fiber: current regulatory status, future research, and goals. J Nutr. 2012;142:962–974. doi: 10.3945/jn.112.158147. [DOI] [PubMed] [Google Scholar]
- Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition of pharmacology? Br J Clin Pharmacol. 2012;75(3):645–62. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammareri M, Consiglio MF, Pecchia P, Corea G, Lanzotti V, Ibeas JI, Tava A, Conicella C. Molecular characterization of β-amyrin synthase from Aster sedifolius L. and triterpenoid saponin analysis. Plant Sci. 2008;175(3):255–61. [Google Scholar]
- Cheng DM, Yousef GG, Grace MH, Rogers RB, Gorelick-Feldman J, Raskin I, Lila MA. In vitro production of metabolism-enhancing phytoecdysteroids from Ajuga turkestanica. Plant Cell Tissue Organ Cult. 2008;93(1):73–83. DOI: 10.1007/s11240-008-9345-5. [Google Scholar]
- Christensen SA, Pratt DB, Pratt C, Nelson PT, Stevens MR, Jellen EN, Coleman CE, Fairbanks DJ, Bonifacio A, Maughan PJ. Assessment of genetic diversity in the USDA and CIP-FAO international nursery collections of quinoa (Chenopodium quinoa Willd.) using microsatellite markers. Plant Genetic Res. 2007;5(2):82–95. [Google Scholar]
- Comai S, Bertazzo A, Bailoni L, Zancato M, Costa CVL, Allegri G. The content of proteic and nonproteic (free and protein-bound) tryptophan in quinoa and cereal flours. Food Chem. 2007;100:1350–5. [Google Scholar]
- Cusack DF. Quinua: grain of the Incas. Ecologist. 1984;14(1):21–31. [Google Scholar]
- Cusicanqui J, Dillen K, Garcia M, Geerts S, Raes D, Mathijs E. Economic assessment at farm level of the implementation of deficit irrigation for quinoa production in the Southern Bolivian Altiplano. Spanish J Agric Res. 2013;11(4):894–907. [Google Scholar]
- Da-Silva WS, Harney JW, Kim BW, Li JM, Bianco SDC, Crescenzi A, Christoffolete MA, Huang SA, Bianco AC. The small polyphenolic molecule kaempferol increases cellular energy expenditure and thyroid hormone activation. Diabetes. 2007;56:767–76. doi: 10.2337/db06-1488. [DOI] [PubMed] [Google Scholar]
- De Carvalho FG, Ovidio PP, Padovan GJ, Jordao Junior AA, Marchini JS, Navarro AM. Metabolic parameters of postmenopausal women after quinoa or corn flakes intake – a prospective and double-blind study. Int J Food Sci Nutr. 2014;65(3):380–5. doi: 10.3109/09637486.2013.866637. [DOI] [PubMed] [Google Scholar]
- De Simone F, Dini A, Pizza C, Saturnino P, Schettino O. Two flavonol glycosides from Chenopodium quinoa. Phytochemistry. 1990;29(11):3690–2. doi: 10.1016/0031-9422(90)85310-c. [DOI] [PubMed] [Google Scholar]
- Dȩebski B, Gralak MA, Bertrandt J, Klos A. Minerals and polyphenols content of quinoa (Chenopodium quinoa Willd.) plant. Probl Hig Epidemiol. 2013;94(2):300–4. [Google Scholar]
- Detmar M, Dumas M, Bonte F, Meybeck A, Orfanos CE. Effects of ecdysterone on the differentiation of normal human keratinocytes in-vitro. Eur J Dermatol. 1994;4(7):558–62. [Google Scholar]
- Dillehay TD, Rossen J, Andres TC, Williams DE. Preceramic adoption of peanut, squash, and cotton in northern Peru. Science. 2007;316:1890–3. doi: 10.1126/science.1141395. [DOI] [PubMed] [Google Scholar]
- Dinan L. Distribution and levels of phytoecdysteroids within individual plants of species of the Chenopodiaceae. Eur J Entomol. 1995;92:295–300. [Google Scholar]
- Dinan L. The Karlson Lecture. Phytoecdysteroids: what use are they? Arch Insect Biochem Physiol. 2009;72(3):126–41. doi: 10.1002/arch.20334. DOI: 10.1002/arch.20334. [DOI] [PubMed] [Google Scholar]
- Dinan L, Lafont R. Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals. J Endocrinol. 2006;191:1–8. doi: 10.1677/joe.1.06900. [DOI] [PubMed] [Google Scholar]
- Dini I, Tenore GC, Dini A. Phenolic constituents of Kancolla seeds. Food Chem. 2004;84:163–8. [Google Scholar]
- Dini I, Tenore GC, Dini A. Antioxidant compound contents and antioxidant activity before and after cooking in sweet and bitter Chenopodium quinoa seeds. Food Sci Technol. 2010;43:447–51. [Google Scholar]
- Dutcheshen J, inventor Applying saponin/terpenes derived from quinoa or quillaja plants to tomato plants. 2004 U.S. Patent 6743752 B2.
- Dutcheshen J, inventor Method of protecting plants from bacterial and fungal diseases. 2005 U.S. Patent Application 20050261129 A1.
- Dutcheshen J, Danyluk TA, inventors Method and composition for protecting plants from disease. 2002 U.S. Patent 6482770 B2.
- Edwards M, inventor Quinoa-containing beverages and methods of manufacture. 2007 U.S. Patent Application 20070264416 A1.
- Ehrhardt C, Wessels JT, Wuttke W, Seidlova-Wuttke D. The effects of 20-hydroxyecdysone and 17ß-estradiol on the skin of ovariectomized rats. Menopause. 2011;18(3):323–7. doi: 10.1097/gme.0b013e3181f322e3. [DOI] [PubMed] [Google Scholar]
- Elgeti D, Nordlohne SD, Foste M, Besl M, Linden MH, Heinz V, Jekle M, Becker T. Volume and texture improvement of gluten-free bread using quinoa white flour. J Cereal Sci. 2014;59:41–7. [Google Scholar]
- Enrione CJI, Diaz CP, Osorio LF, inventors Method for the formulation of a gel-format foodstuff for use as a nutritional foodstuff enriched with peptides and maltodextrins obtained from quinoa flour. 2013 Patent Application CA2860041 A1.
- Esatbeyoglu T, Wagner AE, Schini-Kerth VB, Rimbach G. Betanin-A food colorant with biological activity. Mol Nutr Food Res. 2015;59(1):36–47. doi: 10.1002/mnfr.201400484. DOI: 10.1002/mnfr.201400484. [DOI] [PubMed] [Google Scholar]
- Escuredo O, González Martín MI, Moncada GW, Fischer S, Hernández Hierro JM. Amino acid profile of the quinoa (Chenopodium quinoa Willd.) using near infrared spectroscopy and chemometric techniques. J Cereal Sci. 2014;60:67–74. [Google Scholar]
- Estrada A, Redmond MJ, Laarveld B, inventors Quinoa saponin compositions and methods of use. 1997 U.S. Patent 5688772 A.
- FAO. [2014 August 8];Quinoa: an ancient crop to contribute to world food security. 2011 Available from: http://www.fao.org/docrep/017/aq287e/aq287e.pdf.
- FAO. [2014 August 8];Master plan for the international year of quinoa: a future sown thousands of years ago. 2012 Available from: http://www.fao.org/alc/file/media/aiq/pubs/master_plan.pdf.
- FAO. [2014 December 15];Assessment of the International Year of Quinoa 2013. 2014 Available from: http://www.fao.org/docrep/meeting/030/mk172E.pdf.
- FAO, IFAD, WFP [2014 December 11];The state of food security in the world 2014: strengthening the enabling environment to improve food security and nutrition. 2014 http://www.fao.org/B37BC637-A0D5-4792-9D59-76BED47AA439/FinalDownload/DownloadId-D5639E627FFE7206EFBE682D322CD9A2/B37BC637-A0D5-4792-9D59-76BED47AA439/3/a-i4030e.pdf.
- Farinazzi-Machado FMV, Barbalho SM, Oshiiwa M, Goulart R, Pessan Junior O. Use of cereal bars with quinoa (Chenopodium quinoa W.) to reduce risk factors related to cardiovascular diseases. Cienc Technol Aliment Campinas. 2012;32(3):239–44. [Google Scholar]
- Felipe CX, Guamis LB, Quevedo TJM, Trujillo MAJ, inventors Liquid product of vegetable origin as milk substitute. 2003 Patent Application EP1338206 A1.
- Ferreira DS, Pallone JAL, Poppi RJ. Direct analysis of the main chemical constituents in Chenopodium quinoa grain using Fourier transform near-infrared spectroscopy. Food Contr. 2015;48:91–5. [Google Scholar]
- Fitzpatrick TB, Basset GJC, Borel P, Carrari F, DellaPenna D, Fraser PD, Hellmann H, Osorio S, Rothan C, Valpuesta V, Caris-Veyrat C, Fernie AR. Vitamin deficiencies in humans: can plant science help? Plant Cell. 2012;24:395–414. doi: 10.1105/tpc.111.093120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucault AS, Even P, Lafont R, Dioh W, Veillet S, Tome D, Huneau JF, Herman WH, Quignard-Boulange A. Quinoa extract enriched in 20-hydroxyecdysone affects energy homeostasis and intestinal fat absorption in mice fed a high-fat diet. Physiol Behav. 2014;128:226–31. doi: 10.1016/j.physbeh.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Foucault AS, Mathe V, Lafont R, Even P, Dioh W, Veillet S, Tome D, Huneau JF, Hermier D, Quignard-Boulange A. Quinoa extract enriched in 20-hydroxyecdysone protects mice from diet-induced obesity and modulates adipokines expression. Obesity. 2011;20:270–7. doi: 10.1038/oby.2011.257. DOI: 10.1038/oby.2011.257. [DOI] [PubMed] [Google Scholar]
- Fuentes F, Martinez EA, Hinrichsen PV, Jellen EN, Maughan PJ. Assessment of genetic diversity patterns in Chilean quinoa (Chenopodium quinoa Willd.) germplasm using multiplex fluorescent microsatellite markers. Conserv Genet. 2009;10:369–77. [Google Scholar]
- Gamboa SMR, inventor Modified saponin molluscicide. 2008 U.S. Patent 20070196517A1.
- Gao L, Cai G, Shi X. ß-Ecdysterone induces osteogenic differentiation in mouse mesenchymal stem cells and relieves osteoporosis. Biol Pharm Bull. 2008;31(12):2245–9. doi: 10.1248/bpb.31.2245. [DOI] [PubMed] [Google Scholar]
- Gawlik-Dziki U, Swieca M, Sulkowski M, Dziki D, Baraniak B, Czyz J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts—in vitro study. Food Chem Toxicol. 2013;57:154–60. doi: 10.1016/j.fct.2013.03.023. [DOI] [PubMed] [Google Scholar]
- George S, Brat P, Alter P, Amiot MJ. Rapid determination of polyphenols and vitamin C in plant-derived products. J Agric Food Chem. 2005;53:1370–3. doi: 10.1021/jf048396b. [DOI] [PubMed] [Google Scholar]
- Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C. Food security: the challenge of feeding 9 billion people. Science. 2010;327:812–8. doi: 10.1126/science.1185383. DOI: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- Gomez-Caravaca AM, Iafelice G, Lavini A, Pulvento C, Caboni MF, Marconi E. Phenolic compounds and saponins in quinoa samples (Chenopodium quinoa Willd.) grown under different saline and nonsaline irrigation regimens. J Agric Food Chem. 2012;60:4620–7. doi: 10.1021/jf3002125. [DOI] [PubMed] [Google Scholar]
- Gomez-Caravaca AM, Iafelice G, Verardo V, Marconi E, Caboni MF. Influence of pearling process on phenolic and saponin content in quinoa (Chenopodium quinoa Willd). Food Chem. 2014;157:174–8. doi: 10.1016/j.foodchem.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Gomez-Caravaca AM, Segura-Carretero A, Fernandez-Gutierrez A, Caboni MF. Simultaneous determination of phenolic compounds and saponins in quinoa (Chenopodium quinoa Willd) by a liquid chromatography-diode array detection-electrospray ionization-time-of-flight mass spectrometry methodology. J Agric Food Chem. 2011;59:10815–25. doi: 10.1021/jf202224j. [DOI] [PubMed] [Google Scholar]
- Gonzalez JA, Konishi Y, Bruno M, Valoy M, Prado FE. Interrelationships among seed yield, total protein and amino acid composition of ten quinoa (Chenopodium quinoa) cultivars from two different agroecological regions. J Sci Food Agric. 2012;92:1222–9. doi: 10.1002/jsfa.4686. [DOI] [PubMed] [Google Scholar]
- Gorelick-Feldman J, Cohick W, Raskin I. Ecdysteroids elicit a rapid Ca2+ flux leading to Akt activation and increased protein synthesis in skeletal muscle cells. Steroids. 2010;75:632–7. doi: 10.1016/j.steroids.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick-Feldman J, MacLean D, Ilic N, Poulev A, Lila MA, Cheng D, Raskin I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J Agric Food Chem. 2008;56(10):3532–7. doi: 10.1021/jf073059z. [DOI] [PubMed] [Google Scholar]
- Gorinstein S, Lojek A, Ciz M, Pawelzik E, Delgado-Licon E, Medina OJ, Moreno M, Salas IA, Goshev I. Comparison of composition and antioxidant capacity of some cereals and pseudocereals. Int J Food Sci Technol. 2008;43:629–37. [Google Scholar]
- Graf BL, Cheng DM, Esposito D, Shertel T, Poulev A, Plundrich N, Itenberg D, Dayan N, Lila MA, Raskin I. Compounds leached from quinoa seeds inhibit matrix metalloproteinase activity and intracellular reactive oxygen species. Int J Cosmetic Sci. 2015a;37(2):212–21. doi: 10.1111/ics.12185. 10.1111/ics.12185. [DOI] [PubMed] [Google Scholar]
- Graf BL, Poulev A, Kuhn P, Grace M, Lila MA, Raskin I. Quinoa seeds leach phytochemicals and other compounds with anti-diabetic properties. Food Chem. 2014;163:178–85. doi: 10.1016/j.foodchem.2014.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf BL, Rojo LE, Delatorre-Herrera J, Poulev A, Calfio C, Raskin I. Phytoecdysteroids and flavonoid glycosides among Chilean and commercial sources of Chenopodium quinoa: variation and correlation to physicochemical characteristics. J Sci Food Agric. 2015b doi: 10.1002/jsfa.7134. DOI: 10.1002/jsfa.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaber J, inventor Preserved feeding/food stuff, a method of preparing it and the use thereof. 1999 Patent EP0768040 B1.
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Hariadi Y, Marandon K, Tian Y, Jacobsen S-E, Shabala S. Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J Exp Biol. 2010;62(1):185–93. doi: 10.1093/jxb/erq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy K. The quinoa trail: from South American salt flats to Western health food stores. Llamas, weavings, and organic chocolate: Multicultural grassroots development in the Andes and Amazon of Bolivia. University of Notre Dame Press; Notre Dame, IN: 2001. [Google Scholar]
- Hellin J, Higman S. Quinoa and food security. Feeding the market: South American farmers, trade and globalization. Kumarian Press; Bloomfield, CT: 2003. pp. 89–114. [Google Scholar]
- Hirose Y, Fujita T, Ishii T, Ueno N. Antioxidantive properties and flavonoid composition of Chenopodium quinoa seeds cultivated in Japan. Food Chem. 2010;119:1300–6. [Google Scholar]
- Ho SS, Pal S. Margarine phytosterols decrease the secretion of atherogenic lipoproteins from HepG2 liver and Caco2 intestinal cells. Atherosclerosis. 2005;182(1):29–36. doi: 10.1016/j.atherosclerosis.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Hu J, Luo CX, Chu WH, Shan YA, Qian Z, Zhu G, Yu YB, Feng H. 20-Hydroxyecdysone protects against oxidative stress-induced neuronal injury by scavening free radicals and modulating NF-κB and JNK pathways. PLOS ONE. 2012;7(12):e50764. doi: 10.1371/journal.pone.0050764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhao TZ, Chu WH, Luo CX, Tang WH, Yi L, Feng H. Protective effects of 20-hydroxyecdysone on CoCl2-induced cell injury in PC12 cells. J Cellular Biochem. 2010;111:1512–21. doi: 10.1002/jcb.22877. [DOI] [PubMed] [Google Scholar]
- Jacobsen SE. The situation for quinoa and its production in Southern Bolivia: from economic success to environmental disaster. J Agron Crop Sci. 2011;197:390–9. [Google Scholar]
- Jacobsen SE. What is wrong with the sustainability of quinoa production in Southern Bolivia—a reply to Winkel et al. J Agron Crop Sci. 2012;198:320–3. 2012. [Google Scholar]
- Jancurová M, Minarovicová L, Dandar A. Quinoa—a review. Czech J Food Sci. 2009;27:71–9. [Google Scholar]
- Jeong SM, Kang MJ, Choi HN, Kim JH, Kim JI. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr Res Pract. 2012;6(3):201–7. doi: 10.4162/nrp.2012.6.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadereit G, Borsch T, Weising K, Freitag H. Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C4 photosynthesis. Int J Plant Sci. 2003;164(6):959–86. [Google Scholar]
- Kamelgard JI, inventor Quinoa-based beverages and method of creating quinoa-based beverages. 2013 Patent Application EP2670833 A1.
- Kelly GS. Quercetin: monograph. Altern Med Rev. 2011;16(2):172–94. [PubMed] [Google Scholar]
- Kim HH, Cho S, Lee S, Kim KH, Cho KH, Eun HC, Chung JH. Photoprotective and anti-skin-aging effects of eicosapentaenoic acid in human skin in vivo. J Lipid Res. 2006;47:921–30. doi: 10.1194/jlr.M500420-JLR200. [DOI] [PubMed] [Google Scholar]
- Kizelsztein P, Govorko D, Komarnytsky S, Evans A, Wang Z, Cefalu WT, Raskin I. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am J Physiol Endocrinol Metab. 2009;296(3):E433–9. doi: 10.1152/ajpendo.90772.2008. DOI: 10.1152/ajpendo.90772.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoska L, Janovska D. Chemistry and pharmacology of Rhaponticum carthamoides: a review. Phytochemistry. 2009;70:842–55. doi: 10.1016/j.phytochem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Kruger M, inventor Composition, useful for treating hair, comprises quinoa protein a quaternary ammonium compound comprising e.g. esterquats and quaternary imidazoline compound, and a nourishing fatty component comprising silicone and/or oil body. 2012 Patent Application DE102011085217 A1.
- Kumpun S, Maria A, Crouzet S, Evrard-Todeschi N, Girault J-P, Lafont R. Ecdysteroids from Chenopodium quinoa Willd., an ancient Andean crop of high nutritional value. Food Chem. 2011;125(4):1226–34. DOI: 10.1016/j.foodchem.2010.10.039. [Google Scholar]
- Lafont R. Phytoecdysteroids in world flora: diversity, distribution, biosynthesis and evolution. Russ J Plant Physiol. 1998;45(3):276–95. [Google Scholar]
- Lafont R, Dinan L. Practical uses for ecdysteroids in mammals including humans: an update. J Insect Sci. 2003;3(7):1–30. doi: 10.1093/jis/3.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamothe LM, Srichuwong S, Reuhs BL, Hamaker BR. Quinoa (Chenopodium quinoa W.) and amaranth (Amaranthus caudatus L.) provide dietary fibres high in pectic substances and xyloglucans. Food Chem. 2015;167:490–6. doi: 10.1016/j.foodchem.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Lunenfeld B, Stratton P. The clinical consequences of an ageing world and preventative strategies. Best Pract Res Clin Obstet Gynaecol. 2013;27(5):643–59. doi: 10.1016/j.bpobgyn.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M, Martinez A, Martinez EA. Daidzein and genistein contents in seeds of quinoa (Chenopodium quinoa Willd.) from local ecotypes grown in arid Chile. Ind Crops Prod. 2013;49:117–21. [Google Scholar]
- Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451:716–9. doi: 10.1038/nature06516. [DOI] [PubMed] [Google Scholar]
- Madl T, Sterk H, Mittelbach M, Rechberger GN. Tandem mass spectrometric analysis of a complex triterpene saponin mixture of Chenopodium quinoa. J Am Society Mass Spec. 2006;17(6):795–806. doi: 10.1016/j.jasms.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Marangoni F, Poli A. Phytosterols and cardiovascular health. Pharmacol Res. 2010;61:193–9. doi: 10.1016/j.phrs.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Martindale W. 3rd edn. H. K. Lewis; London: 1894. Coca and cocaine: Their history, medical and economic uses, and medicinal preparations. [Google Scholar]
- McCusker MM, Grant-Kels JM. Healing fats of the skin: the structural and immunologic role of the ω-6 and ω-3 fatty acids. Clin Dermatol. 2010;28:440–51. doi: 10.1016/j.clindermatol.2010.03.020. [DOI] [PubMed] [Google Scholar]
- McKeown NM, Jacques PF, Seal CJ, de Vries J, Jonnalagadda SS, Clemens R, Webb D, Murphy LA, van Klinken JW, Topping D, Murray R, Degeneffe D, Marquart LF. Whole grains and health: from theory to practice – highlights of the Grains for Health Foundation's Whole Grains Summit 2012. J Nutr. 2013;143(5):744S–58S. doi: 10.3945/jn.112.172536. [DOI] [PubMed] [Google Scholar]
- Miranda M, Vega-Galvez A, Martinez E, Lopez J, Rodriguez MJ, Henriquez K, Fuentes F. Genetic diversity and comparison of physiochemical and nutritional characteristics of six quinoa (Chenopodium quinoa willd.) genotypes cultivated in Chile. Cienc Technol Aliment Campinas. 2012a;32(4):835–43. [Google Scholar]
- Miranda M, Vega-Galvez A, Quispe-Fuentes I, Rodriguez MJ, Maureira H, Martinez EA. Nutritional aspects of six quinoa (Chenopodium quinoa Willd.) ecotypes from three geographical areas of Chile. Chilean J Agric Res. 2012b;72(2):175–81. [Google Scholar]
- Moreno DA, Garcia-Viguera C, Gil JI, Gil-Izquierdo A. Betalains in the era of global agri-food science, technology and nutritional health. Phytochemistry Rev. 2008;7:261–80. [Google Scholar]
- Msika P, inventor Composition containing a quinoa grain extract for dermatological use. 2012 U.S. Patent Appliction 20100136144 A1.
- Muir AD, Paton D, Ballantyne K, Aubin AA, inventors Process for recovery and purification of saponins and sapogenins from quinoa (Chenopodium quinoa). 2002 U.S. Patent 6355249 B2.
- Mujica A. Andean grains and legumes. In: Hernando Bermujo JE, Leon J, editors. Neglected crops: 1492 from a different perspective. Vol. 26. FAO; Rome, Italy: 1994. [2012 October 16]. pp. 131–48. http://www.hort.purdue.edu/newcrop/1492/grains.html. [Google Scholar]
- Neagu C, Barbu V. Principal component analysis of the factors involved in the extraction of beetroot betalains. J Agroalimentary Process Technol. 2014;20(4):311–8. [Google Scholar]
- Nguyen T, Lau DCW. The obesity epidemic and its impact on hypertension. Can J Cardiol. 2012;28(3):326–33. doi: 10.1016/j.cjca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Nsimba RY, Kikuzaki H, Konishi Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008;106:760–6. [Google Scholar]
- Oelke EA, Putnam DH, Teynor TM, Oplinger ES. Quinoa. Alternative Field Crops Manual. University of Wisconsin-Extension; 1992. [August 8, 2014]. https://www.hort.purdue.edu/newcrop/afcm/quinoa.html. [Google Scholar]
- Olthof MR, Verhoef P. Effects of betaine intake on plasma homocysteine concentrations and consequences for health. Curr Drug Metab. 2005;8:15–22. doi: 10.2174/1389200052997366. [DOI] [PubMed] [Google Scholar]
- Pagno CH, Costa TMH, de Menezes EW, Benvenutti EV, Hertz PF, Matte CR, Tosati JV, Monteiro AR, Rios AO, Flores SH. Development of active biofilms of quinoa (Chenopodium quinoa W.) starch containing gold nanoparticles and evaluation of antimicrobial activity. Food Chem. 2015;173:755–62. doi: 10.1016/j.foodchem.2014.10.068. [DOI] [PubMed] [Google Scholar]
- Pasko P, Bartón H, Zagrodzki P, Gorinstein S, Folta M, Zachwieja Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009;115:994–8. [Google Scholar]
- Pasko P, Barton H, Zagrodzki P, Izewska A, Krosniak M, Gawlik M, Gawlik M, Gorinstein S. Effect of diet supplemented with quinoa seeds on oxidative status and selected tissues of high fructose-fed rats. Plant Foods Hum Nutr. 2010a;65:146–51. doi: 10.1007/s11130-010-0164-6. [DOI] [PubMed] [Google Scholar]
- Pasko P, Zagrodzki P, Barton H, Chlopicka J, Gorinstein S. Effect of quinoa seeds (Chenopodium quinoa) in diet on some biochemical parameters and essential elements in blood of high fructose-fed rats. Plant Foods Hum Nutr. 2010b;65:333–8. doi: 10.1007/s11130-010-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiretti PG, Gai F, Tassone S. Fatty acid profile and nutritive value of quinoa (Chenopodium quinoa Willd.) seeds and plants at different growth stages. Anim Feed Sci Technol. 2013;183(1–2):56–61. [Google Scholar]
- Penaloza W, Davey CL, Hedger JN, Kell DB. Physiological studies on the solid-state quinoa tempe fermentation, using on-line measurements of fungal biomass production. J of Sci Food Ag. 1992;59(2):227–35. [Google Scholar]
- Perez C, Nicklin C, Dangles O, Vanek S, Sherwood S, Halloy S, Garrett K, Forbes G. Climate change in the high Andes: implications and adaptation strategies for small-scale farmers. Int J Environ Cult Econ Soc Sustain. 2010;6:71–88. [Google Scholar]
- Popenoe H, King SR, Leon J, Kalinowski LS. Lost crops of the Incas. In: Vietmeyer ND, editor. Little known plants of the Andes with promise for worldwide cultivation. National Academy Press; Washington, DC: 1989. [Google Scholar]
- Poutanen K, Sozer N, Della Valle G. How can technology help to deliver more of grain in cereal foods for a healthy diet? J Cereal Sci. 2014;59(3):327–36. [Google Scholar]
- Pouvreau LA, Kanning MW, Van de Velde F, inventors Processing quinoa for improved protein-to-carbohydrate formulations. 2014 U.S. Patent Application 20140161950 A1.
- Prego I, Maldonado S, Otegui M. Seed structure and localization of reserves in quinoa. Ann Bot. 1998;82:481–8. [Google Scholar]
- Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6(11):1720–31. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard CM, Wende G, Booth EJ. Cell wall phenolics and polysaccharides in different tissues of quinoa (Chenopodium quinoa Willd.). J Sci Food Agric. 1999;79:2029–34. [Google Scholar]
- Repo-Carrasco R, Espinoza E, E. JS. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kaniwa ~ (Chenopodium pallidicaule). Food Rev Int. 2003;19:179–89. [Google Scholar]
- Ross A, Savolainen O. A new biomarker for quinoa intake. FASEB. 2014;28(1)(Suppl):1044.25. [Google Scholar]
- Ross AB, Zangger A, Guiraud SP. Cereal foods are the major source of betaine in the Western diet – analysis of betaine and free choline in cereal foods and updated assessments of betaine intake. Food Chem. 2014;145:859–65. doi: 10.1016/j.foodchem.2013.08.122. [DOI] [PubMed] [Google Scholar]
- Ruales J, De Grijalva Y, Lopez-Jaramillo P, Nair BM. The nutritional quality of infant food from quinoa and its effect on the plasma level of insulin-like growth factor-1 (IGF-1) in undernourished children. Int J Food Sci Nutr. 2002;53:143–54. doi: 10.1080/09637480220132157. [DOI] [PubMed] [Google Scholar]
- Ruales J, Nair BM. Content of fat, vitamins and minerals in quinoa (Chenopodium quinoa, Willd) seeds. Food Chem. 1993;48(2):131–6. [Google Scholar]
- Ryan E, Galvin K, O'Connor TP, Maguire AR, O'Brien NM. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Food Hum Nutr. 2007;62:85–91. doi: 10.1007/s11130-007-0046-8. [DOI] [PubMed] [Google Scholar]
- Sautour M, Canon F, Miyamoto T, Dongmo A, Lacaille-Dubois M-A. A new ecdysteroid and other constituents from two Dioscorea species. Biochem Syst Ecol. 2008;36:559–63. [Google Scholar]
- Scanlin L, Stone M, inventors Purified plant protein is separated from solvent extraction; as food ingredients, infant formula ingredients, cosmetic ingredients, pet food ingredients, and animal feed supplements. 2009 U.S. Patent 7563473 B2.
- Scanlin LA, Burnett C, inventors Quinoa grain processing and products. 2010 U.S. Patent Application 20100196569 A1.
- Schlick G, Bubenheim DL. Quinoa: candidate crop for NASA's controlled ecological life support systems. In: Janick J, editor. Progress in new crops. ASHS Press; Arlington, VA: 1996. pp. 630–40. [Google Scholar]
- Schmidt B, Ribnicky D, Poulev A, Logendra S, Cefalu WT, Raskin I. A natural history of botanical therapeutics. Metabolism. 2008;57:S3–S9. doi: 10.1016/j.metabol.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidlova-Wuttke D, Christel D, Kapur P, Nguyen BT, Jarry H, Wuttke W. Beta-ecdysone has bone protective but no estrogenic effects in ovariectomized rats. Phytomedicine. 2010a;17(11):884–9. doi: 10.1016/j.phymed.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Seidlova-Wuttke D, Ehrhardt C, Wuttke W. Metabolic effects of 20-OH-ecdysone in ovariectomized rats. J Steroid Biochem Mol Biol. 2010b;119(3–5):121–6. doi: 10.1016/j.jsbmb.2010.01.006. DOI: 10.1016/j.jsbmb.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Seidlova-Wuttke D, Wuttke W. In a placebo-controlled study ß-Ecdysone (ECD) prevented the development of the metabolic syndrome. Planta Med. 2012;78(11):CL37. (Abstract) [Google Scholar]
- Shen Y, Jin L, Xiao P, Lu Y, Bao J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J Cereal Sci. 2009;49:106–11. [Google Scholar]
- Singer NS, Tang P, Chang H, Dunn JM, inventors Carbohydrate cream substitute. 1990 U.S. Patent 4911946 A.
- Small E. Quinoa—is the United Nations’ featured crop of 2013 bad for biodiversity? Biodiversity. 2013;14(3):169–79. [Google Scholar]
- Stuardo M, San Martin R. Antifungal properties of quinoa (Chenopodium quinoa Willd) alkali treated saponins against Botrytis cinerea. Ind Crops Prod. 2008;27:296–302. [Google Scholar]
- Takao T, Watanabe N, Yuhara K, Itoh S, Suda S, Tsuruoka Y, Nakatsugawa K, Konishi Y. Hypocholesterolemic effect of protein isolated from quinoa (Chenopodium quinoa Willd.) seeds. Food Sci Technol Res. 2005;11(2):161–7. [Google Scholar]
- Tang Y, Li X, Chen PX, Zhang B, Hernandez M, Zhang H, Marcone MF, Liu R, Tsao R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015a;174:502–8. doi: 10.1016/j.foodchem.2014.11.040. [DOI] [PubMed] [Google Scholar]
- Tang Y, Li X, Zhang B, Chen PX, Liu R, Tsao R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015b;166:380–8. doi: 10.1016/j.foodchem.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Thomas R, inventor Process for the treatment of quinoa seeds, and product obtained. 1995 U.S. Patent 5264231 A.
- Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–7. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–46. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcárcel-Yamani B, Caetano da Silva Lannes S. Applications of quinoa (Chenopodium quinoa Willd.) and amaranth (Amaranthus spp.) and their influence in the nutritional value of cereal based foods. Food Public Health. 2012;2(6):265–75. [Google Scholar]
- Valencia US, Sandberg AS, Ruales J. Processing of quinoa (Chenopodium quinoa, Willd): effects on in vitro iron availability and phytate hydrolysis. Int J Food Sci Nutr. 1999;50:203–11. doi: 10.1080/096374899101247. [DOI] [PubMed] [Google Scholar]
- Valencia-Chamorro SA. Quinoa. In: Caballero B, editor. Encyclopdia of food science and nutrition. Academic Press; Amsterdam: 2003. pp. 4895–902. [Google Scholar]
- van Dam RM, Hu FB. Are alkylresorcinols accurate biomarkers for whole grain intake? Am J Clin Nutr. 2008;87:797–8. doi: 10.1093/ajcn/87.4.797. [DOI] [PubMed] [Google Scholar]
- Vega-Galvez A, Miranda M, Vergara J, Uribe E, Puente L, Martinez EA. Nutrition facts and functional potential of quinoa (Chenopodium quinoa Willd.). an ancient Andean grain: a review. J Sci Food Agric. 2010;90:2541–7. doi: 10.1002/jsfa.4158. [DOI] [PubMed] [Google Scholar]
- Veillet S, Lafont R, inventors Use of phytoecdysones in the preparation of a composition for acting on the metabolic syndrome. 2012 U.S. Patent 8236359 B2.
- Villacrés E, Pástor G, Quelal MB, Zambrano I, Morales SH. Effect of processing on the content of fatty acids, tocopherols and sterols in the oils of quinoa (Chenopodium quinoa Willd), lupine (Lupinus mutablis Sweet), amaranth (Amaranthus caudatus L.) and sangorache (Amaranthus quitensis L.). Global Adv Res J Food Sci Technol. 2013;2(4):44–53. [Google Scholar]
- Wang ZQ, Yu Y, Zhang XH, Ribnicky D, Cefalu WT. Ecdysterone enhances muscle insulin signaling by modulating acylcarnitine profile and mitochondrial oxidative phosphorylation complexes in mice fed a high-fat diet. Diabetes. 2011:1–10. doi: 10.2337/db10-1605. DOI: 10.2337/db10-1605. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ward SM, Johnson DL, inventors Cytoplasmic male sterile quinoa. 1994 U.S. Patent 5304718 A.
- Weinreich B, Konecny S, inventors Flour made of amaranth and/or quinoa as a substitute for food additives in food production. 2011 Patent Application WO2010112216 A3.
- Wilborn CD, Taylor LW, Campbell BI, Kerksick C, Rasmussen CJ, Greenwood M, Kreider RB. Effects of methoxyisoflavone, ecdysterone, and sulfo-polysaccharide supplementation on training adaptations in resistance-trained males. J Int Soc Sports Nutr. 2006;3(2):19–27. doi: 10.1186/1550-2783-3-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel T, Bertero HD, Bommel P, Bourliaud J, Chevariia Lazo M, Cortes G, Gasselin P, Geerts S, Joffre R, Martinez Avisa B, Rambal S, Riviere G, Tichit M, Tourrand JF, Vassas Toral A, Vacher JJ, Vieira Pak M. The sustainability of quinoa production in Southern Bolivia: from misrepresentations to questionable solutions. Comments on Jacobsen (2011, J Agron Crop Sci 197: 390–399). J Agron Crop Sci. 2012;198:314–9. [Google Scholar]
- Wright KH, Pike OA, Fairbanks DJ, Huber SC. Composition of Atriplex hortensis, sweet and bitter Chenopodium quinoa seeds. Food Chem Toxicol. 2002;67:1383–5. [Google Scholar]
- Yao Y, Yang X, Shi Z, Ren G. Anti-inflammatory activity of saponins from quinoa (Chenopodium quinoa Willd.) seeds in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. J Food Sci. 2014;79(5):H1018–23. doi: 10.1111/1750-3841.12425. [DOI] [PubMed] [Google Scholar]
- Yendo ACA, De Costa F, Gosmann G, Fett-Neto AG. Production of plant bioactive triterpenoid saponins: elicitation strategies and target genes to improve yields. Mol Biotechnol. 2010;46:94–104. doi: 10.1007/s12033-010-9257-6. [DOI] [PubMed] [Google Scholar]
- Yousef GG, Brown AF, Funakoshi Y, Mbeunkui F, Grace MH, Ballington JR, Loraine A, Lila MA. Efficient quantification of the health-relevant anthocyanin and phenolic acid profiles of commercial cultivars and breeding selections of blueberries (Vaccinium spp.). J Agric Food Chem. 2013;61:4806–15. doi: 10.1021/jf400823s. [DOI] [PubMed] [Google Scholar]
- Zevallos VF, Ellis HJ, Suligoj T, Herencia LI, Ciclitira PJ. Variable activation of immune response by quinoa (Chenopodium quinoa Willd.) prolamins in celiac disease. Am J Clin Nutr. 2012;96:337–44. doi: 10.3945/ajcn.111.030684. [DOI] [PubMed] [Google Scholar]
- Zevallos VF, Herencia LI, Chang F, Donnelly S, Ellis HJ, Ciclitira PJ. Gastrointestinal effects of eating quinoa (Chenopodium quinoa Willd.) in celiac patients. Am J Gastroenterol. 2014;109:270–8. doi: 10.1038/ajg.2013.431. [DOI] [PubMed] [Google Scholar]
- Zhu N, Kikuzaki H, Vastano BC, Nakatani N, Karwe MV, Rosen RT, Ho CT. Ecdysteroids of quinoa seeds (Chenopodium quinoa Willd.). J Agric Food Chem. 2001;49(5):2576–8. doi: 10.1021/jf0014462. [DOI] [PubMed] [Google Scholar]
- Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endrocrinol. 2014;2:56–64. doi: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
- 余磊, inventor Bitter buckwheat and quinoa milk tea wine and preparation method thereof. 2014 Patent Application CN103540467 A.
- 谢振文, inventor Quinoa organic buckwheat tea and production methods. 2014 Patent CN103315115 B.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.