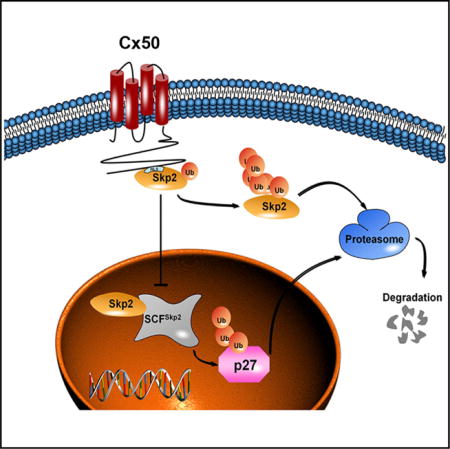
SUMMARY
Connexins and connexin channels play important roles in cell growth/differentiation and tumorigenesis. Here, we identified a relationship between a connexin molecule and a critical cell-cycle regulator. Our data show that connexin (Cx) 50 regulated lens cell-cycle progression and differentiation by modulating expression of cyclin-dependent kinase inhibitor p27/p57 and E3 ubiquitin ligase Skp2. Cx50 directly interacted with and retained Skp2 in the cytosol by masking the nuclear targeting domain of Skp2, and this effect was supported by an increased nuclear localization of Skp2, disruption of Skp2 interaction with importin-7, and decreased levels of p27/p57 in mouse lenses lacking Cx50. As a result, Cx50 increased auto-ubiquitination and subsequent degradation of Skp2. A mutation (V362E) on the C terminus of Cx50 disrupted the interaction between Cx50 and Skp2 and completely abolished such effects. Therefore, this study identifies a role for connexins in regulating cell-cycle modulators and, consequently, cell growth and differentiation.
Graphical abstract
INTRODUCTION
Connexins are membrane proteins that are the structural components of both gap junctions and hemichannels (Goodenough et al., 1996; Goodenough and Paul, 2003). These channels are crucial in maintaining normal cell and tissue functions. Besides forming gap junction channels and hemichannels, increasing studies suggest a gap junction and hemichannel-independent role of connexins in cell growth, differentiation, and tumorigenicity (Jiang and Gu, 2005; Jiang, 2010; Zhou and Jiang, 2014). However, the molecular mechanisms underlying the function of connexins in cell growth control and differentiation remain largely unknown.
The eye lens is a unique organ representing various developmental stages of cells with an enriched gap junction communication network (Lovicu and McAvoy, 2005; Wride, 1996). The mature lens is composed of two major compartmental cell populations: mitotically active epithelial cells at the anterior part and elongated fiber cells differentiated from epithelial cells at the lens bow region, forming the bulk of the lens body (Tholozan and Quinlan, 2007). The epithelial cells in the lens bow region close to the lens equator withdraw themselves from the cell cycle to initiate fiber cell differentiation, which is coordinated by the cell-cycle CDK inhibitors p27 and p57 (Zhang et al., 1998; Rowan et al., 2008). In p27/p57 double knockout (KO) mice, lens differentiation was found to be disrupted; however, enhanced proliferation was observed (Zhang et al., 1998). Ubiquitin-dependent proteolysis via E3 ubiquitin ligase S-phase kinase protein 2 (Skp2) is reported to be primarily responsible for p27 degradation (Pagano et al., 1995; Carrano et al., 1999). The function of Skp2/p27 in cell proliferation has been reported to be influenced by the presence of Cx43 in mouse embryonic fibroblasts (Zhang et al., 2003b).
Three connexins, Cx43, Cx46, and Cx50 are abundantly expressed in the lens. Mice lacking genes encoding either Cx46 or Cx50 develop lens cataracts (Gong et al., 1997; White et al., 1998; Rong et al., 2002); however, only Cx50-deficient mice develop smaller lenses (White et al., 1998; Rong et al., 2002). In Cx50 KO mouse lenses, reduced cell proliferation and delayed denuclearization have also been reported (Sellitto et al., 2004; Rong et al., 2002; Dunia et al., 2006), suggesting that Cx50 plays an important role in cell proliferation. Our earlier studies show that Cx50 is able to promote lens epithelial-fiber differentiation in lens primary cell culture (Gu et al., 2003), a system that closely mimics the differentiation process of lens cells in vivo (Menko et al., 1984; Berthoud et al., 1999). Furthermore, this function is independent of the role of Cx50 in forming gap junctions and hemichannels (Banks et al., 2007, 2009). The intracellular C terminus (CT) of Cx50 is sufficient to promote lens fiber cell differentiation. Moreover, the Val-362 (V362) residue within the CT domain, by maintaining an α-helical structure, functionally participates in lens epithelial-fiber differentiation (Shi et al., 2010).
In this study, we identified a mechanism of Cx50 in regulating lens cell proliferation and differentiation through the direct interaction and cytoplasmic retention of the cell-cycle regulator, Skp2 and consequently, the enhanced stability of cell-cycle inhibitor p27/p57. This mechanistic study establishes a direct functional relationship between connexins and key cell-cycle regulators.
RESULTS
The Stimulatory Role of Cx50 in Lens Cell Differentiation Is Mediated by p27
To determine if lens cell differentiation is coupled to cell-cycle regulation, we examined the expression of a CDK inhibitor, p27 using chick lens primary culture. At the fourth to fifth day of culturing, lens epithelial cells clustered together to form a crown-like structure, named “lentoids,” with characteristics mimicking lens cell differentiation in vivo (Menko et al., 1984; Jiang et al., 1993). The expression level of p27 was elevated at the sixth day of culturing, along with the increased expression level of lens differentiation markers, CP49 and Cx50 (Figure 1A). We have previously showed that Cx50 promotes lens cell differentiation (Gu et al., 2003). Here, we found that the level of p27 was further enhanced in primary lens cells overexpressing exogenous Cx50, indicated by the higher level of total Cx50 protein as compared to vehicle control cells (Figure 1A). The increase of p27 was primarily observed during the early stage of lens cell differentiation and after 8 days of culturing, the level of p27 was comparable to the vehicle control, consistent with the period of exit of cell cycle and initiation of fiber cell differentiation. There was no difference between the mRNA level of p27 in cells expressing Cx50 or vehicle control at day 4 and day 8 of primary lens cell culturing (Figure S1), suggesting that the increased p27 during lens differentiation was regulated at the protein, but not at the mRNA level. Interestingly, p27 was barely detectable in the lens epithelial cells, but was richly expressed in the lentoids, where the differentiated fiber cells predominate (Figure 1B). To determine if p27 is involved in the role of Cx50 in lens cell differentiation, p27 was knocked down using siRNA. The stimulatory effect of Cx50 on the differentiation indicated by increased numbers of lentoids (Figure 1C, middle) and expression of aquaporin 0 (AQP0), a marker for lens cell differentiation (Figure 1C, left) was significantly attenuated. To further validate the role of p27, we took advantage of a Skp2-resistant mutant of p27 (T187A). The comparable expression of p27 and p27(T187A) was observed (Figure 1D, left). Compared to wild-type (WT) p27, expression of p27(T187A) mutant further enhanced lens cell differentiation evidenced by increased total lentoid numbers (Figure 1D, middle) and expression of AQP0 (Figure 1D, right). Co-expression of Cx50 did not further augment the effect possibly due to the saturated level of p27 achieved since overexpression of Cx50 did not further enhance the level of p27 shown by western blots (Figure 1D, left). These results suggest that Cx50 increases the expression of p27, which is correlated with the role of Cx50 in promoting lens cell differentiation and further imply that lens cell differentiation is governed by cell-cycle exit. Moreover, p27 functions as a downstream effector of Cx50 on the lens cell differentiation.
Figure 1. The Stimulatory Effect of Cx50 on Lens Cell Differentiation Is Mediated by the Increased p27 Protein Level.
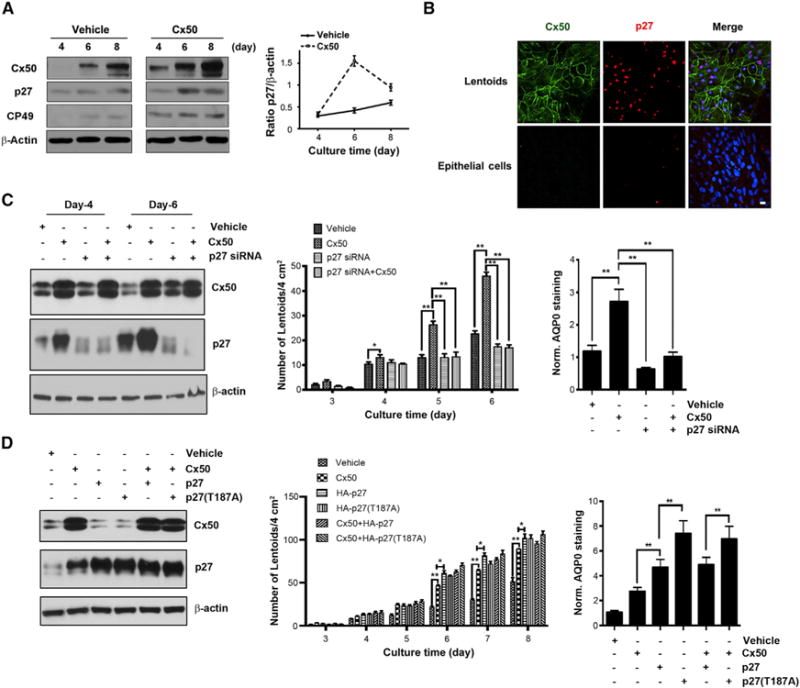
(A) p27 protein expression increases associated with the enhancement of cell differentiation by Cx50 in chick lens primary cells.
(B) p27 localizes in the nuclei of lentoids in lens primary cell culture. Scale bar represents 20 μm.
(C) Knocking down p27 significantly attenuated the stimulatory effect of Cx50 on cell differentiation of primary lens cells. The differentiation level was assessed by quantifying lentoid formation (middle). The AQP0 level was quantified by fluorescence area immunolabeled with anti-AQP0 antibody versus total area and normalized to RCAS(A) vehicle group (right).
(D) Overexpression of p27 and p27(T187A) promotes the lens cell differentiation in chick lens primary cells. Cell lysates at the culturing day 8 were immunoblotted with anti-Cx50, p27, or β-actin antibody (left). The lentoid formation was quantified during 3–8 days of culturing (middle). The AQP0 level was quantified by fluorescence area immunolabeled with anti-AQP0 antibody (right). All data were presented as the means ± SEM (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001, compared to vehicle control group, unless indicated otherwise.
See also Figure S1.
Skp2 Mediates the Effect of Cx50 on Lens Cell Differentiation and Exit of Cell Cycle
The level of p27 is known to be directly regulated by Skp2, a major component of the E3 ligase complex, SCFSkp2, responsible for p27 ubiquitination and subsequent degradation in the nucleus (Carrano et al., 1999; Nakayama et al., 2004). Because upregulation of p27 as shown in Figure 1 in differentiated lens fibers occurs at the protein level (Figure 1A and Figure S1) and Skp2-resistant mutant of p27, p27(T187A) further enhanced lens cell differentiation (Figure 1D), it would be interesting to test if Skp2 is involved in the process of lens differentiation. RCAS(A) retrovirus containing either of the two shRNAs against different regions of Skp2 was used to knock down Skp2 in primary lens cells. The expression of Skp2 mRNA was reduced by ~70%–80% by either of these two shRNAs compared to the vehicle control (Figure S2A). Skp2 knockdown by two shRNAs, as expected, increased p27 protein level, but had no effect on Cx50 and β-actin (Figure S2B). Knocking down Skp2 by either of the two shRNAs in lens primary cells significantly elevated the formation of lentoids (Figure 2A, left), and the expression of AQP0 (Figure 2A, right). The degree of the increase by Skp2 knockdown by either shRNA in lens cell differentiation is comparable, if not greater than that of Cx50 overexpression. Co-expressing Cx50 and either Skp2 shRNA further enhanced the primary lens cell differentiation. We have previously shown that unlike WT Cx50, the Cx50(V362E) mutant has a minimal effect on lens differentiation (Shi et al., 2010). Expression of the Cx50(V362E) mutant, unlike WT Cx50, failed to augment lens differentiation induced by either Skp2 shRNA (Figure 2B). These results suggest that Skp2 is likely involved in the role of Cx50 in promoting lens differentiation.
Figure 2. Skp2, but Not Skp2(ΔF), Attenuates the Stimulatory Effect of Cx50 on Lens Cell Differentiation.
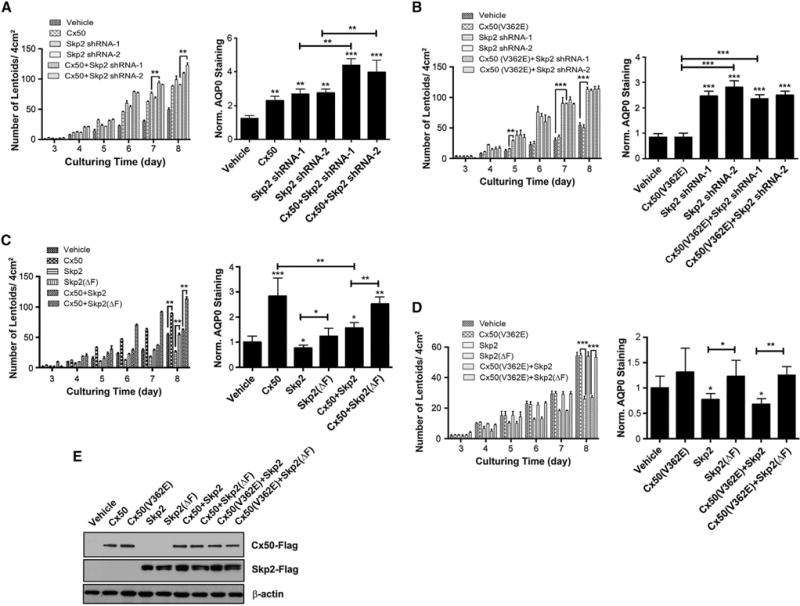
(A) Inhibition of Skp2 stimulates cell differentiation, and expression of Cx50 augments this effect in primary lens cells. Lentoid formation was quantified during 3–8 days of culturing (left) and the level of AQP0 was shown (right).
(B) Expression of mutant Cx50(V362E) fails to augment the stimulatory effect on lens cell differentiation by inhibition of Skp2.
(C) Overexpression of Skp2, but not Skp2(ΔF), inhibits lens cell differentiation, but expression of Cx50 reverses this effect.
(D) Cx50(V362E) fails to stimulate lens cell differentiation suppressed by Skp2, while Skp2(ΔF) failed to suppress lens cell differentiation.
(E) Comparable levels of Cx50, Cx50(V362E), Skp2, and Skp2(ΔF) were expressed alone or co-expressed in lens primary cells. All the data were presented as the means ± SEM (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001, compared to vehicle control group, unless indicated otherwise.
See also Figure S2.
To further confirm the role of Skp2, recombinant RCAS(A) retrovirus containing Skp2 or a E3 ubiquitin ligase-impaired, F-domain deleted mutant, Skp2(ΔF) tagged with FLAG was generated and expressed alone or co-expressed with Cx50 in chick lens primary culture. Overexpression of exogenous Skp2 significantly attenuated the differentiation of lens cells by suppressing lentoid formation (Figure 2C, left) and AQP0 expression (Figure 2C, right). However, this effect was not observed with the overexpression of Skp2(ΔF), suggesting that this effect of Skp2 is likely to be mediated by F-domain of Skp2. In addition, co-expression of Skp2, but not Skp2(ΔF), significantly suppressed the stimulatory effect of Cx50 on lens cell differentiation, showing similar levels of lentoids and AQP0 as compared to the RCAS(A) vehicle control group. This inhibitory effect of Skp2 on Cx50-induced enhancement of lens cell differentiation was not observed when Skp2 was co-expressed with the Cx50(V362E) mutant (Figure 2D). Western blots showed comparable expression levels of Cx50, Skp2, and mutants (Figure 2E). Together, these data support the role of p27/Skp2 in lens cell differentiation, a functional interaction between Skp2 and Cx50 and the involvement of F-domain of Skp2 and Val-362 of Cx50.
We then determined the functional relationship between Cx50 and Skp2 in cell proliferation and cell-cycle control. CEF cells were infected with various recombinant retroviruses containing Cx50, Cx46, Cx43, and/or Skp2 and a bromodeoxyuridine (BrdU) incorporation assay was performed (Figure 3A). Cx50, but not the other two lens connexins, significantly decreased the proliferation rate. In contrast, Skp2 dramatically increased cell proliferation, but this effect was significantly compromised when co-expressed with Cx50 (Figure 3A). The V362E mutation attenuated the ability of Cx50 to suppress cell proliferation and the stimulatory effect of Skp2 on cell proliferation (Figure 3B). Conversely, reduction of Skp2 by Skp2 shRNA decreased the proliferation rates of CEF cells. Interestingly, co-expression of Cx50 and either of the two Skp2 shRNAs did not lead to a further reduction of cell proliferation (Figure 3C). The effects of Cx50 and Skp2 on cell proliferation coincided with their effects on lens cell differentiation, which further suggests that lens cell differentiation and proliferation were coupled. To determine if the effect of Cx50 on cell proliferation is mediated by p27, we knocked down p27 with siRNA. p27 siRNA significantly increased cell proliferation and further blocked the inhibitory effect of Cx50 (Figure 3D).
Figure 3. Skp2-p27 Pathway Mediates Inhibitory Effects of Cx50 on Cell Proliferation and Cell-Cycle Progression.
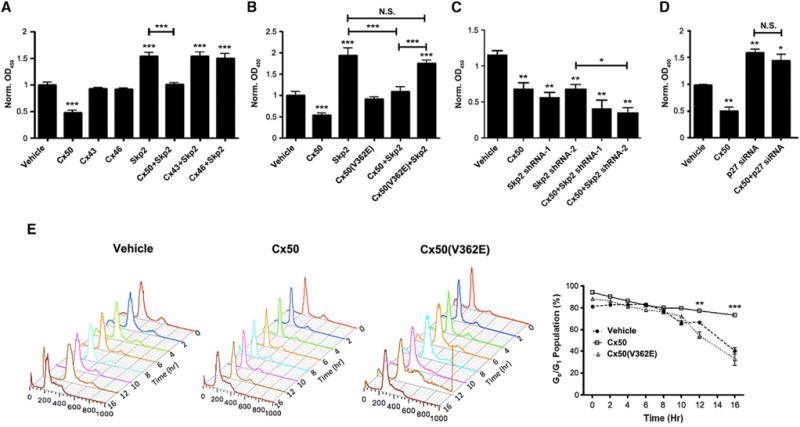
(A–D) The proliferation of CEF cells expressing Cx50 (WT or V362E mutant), Cx43, Cx46, or/and Skp2 (or Skp2 shRNAs) via retroviral infection or p27 siRNA was measured by BrdU labeling assay.
(A) Cx50 inhibits cell proliferation, but not Cx43 or Cx46, and Skp2 suppresses the inhibitory effect by Cx50.
(B) Cx50(V362E) mutant does not inhibit the stimulatory effect by overexpressing Skp2.
(C) Inhibition of Skp2 by either of the two Skp2 shRNAs suppresses the cellular proliferation and augments the inhibitory effect of Cx50.
(D) p27 siRNA increased cell proliferation and attenuated the inhibitory effect of Cx50.
(E) Cx50 delays cell-cycle progression, but Cx50(V362E) mutant does not. CEF cells were labeled with PI and analyzed with FACS.
All data were presented as the means ± SEM (n = 3–5), *p < 0.05; **p < 0.01; ***p < 0.001, compared to vehicle control group, unless marked otherwise. N.S., not significant.
As a prominent marker for cells entering S phase, Skp2 expression level increases whereas p27 decreases (Nakayama et al., 2004). To determine whether Cx50 affects cell-cycle progression and at which stage(s) the effect occurs, we monitored the cell-cycle progression in CEF cells expressing RCAS(A) (vehicle), Cx50, or Cx50(V362E) (Figure 3E). CEF cells were synchronized at G0/G1 phase by serum starvation for 72 hr, and the cell-cycle arrest was released by adding fresh medium containing normal serum. Cell populations in different cycle phases were quantified by flow cytometry analysis (FACS). The results showed that incubation in serum-free media for 72 hr arrested most of cells at the G0/G1 phase (~80%–90% cells in G0/G1). During 0–12 hr after releasing from G0/G1 arrest, there was no significant difference between cells expressing vehicle, Cx50, or Cx50(V362E) mutant. However, 16 hr after G0/G1 arrest, more than 50% of CEF cells expressing vehicle or Cx50(V362E) mutant entered S phase in contrast to only 15% of cells expressing Cx50 (Figure 3E). The results suggest that Cx50 significantly delayed cell-cycle progression after release from the G0/G1 phase, indicating that Cx50 induced a cell-cycle arrest at the G1/S boundary, thereby decreasing cell proliferation.
Cx50-CT Directly Interacts with Skp2-N Terminus
Similar protein sequences were identified between cyclin A-NT and Cx50-CT (Figure S3). Cyclin A has been shown to directly interact with Skp2 (Ji et al., 2006). We hypothesized that Cx50 could interact with Skp2 in a similar manner as cyclin A. The association between Cx50 and Skp2 was shown by a co-immunoprecipitation (coIP) assay. Skp2 and/or Cx50 were expressed in CEF cells (Figure 4A, left). CoIP using an antibody against the Cx50 intracellular loop domain showed that Skp2 was only precipitated in cells co-expressing Skp2 and Cx50, but not in cells expressing Skp2 or Cx50 alone (Figure 4A, right). The coIP result was also confirmed with non-FLAG-tagged Cx50 and Skp2 to exclude the potential issues associated with FLAG tags on both proteins (Figure 5E). Significantly less Skp2 was co-immunoprecipitated in cells expressing Cx50(V362E) mutant (Figure 5E), suggesting that Val-362 plays an important role in the interaction between Cx50 and Skp2. CoIP assay was performed to determine stoichiometry of the interaction by using fixed amount of Cx50-expressing CEF cell lysate with various amount of Skp2-expressing CEF cell lysate. The molar ratio of the Cx50 to Skp2 was estimated to be approximately two to three based on the saturated levels of the binding between these two proteins (Figure S4).
Figure 4. Cx50-CT Directly Interacts with Skp2-NT, whereas the Mutation on V362E Weakens the Interaction.
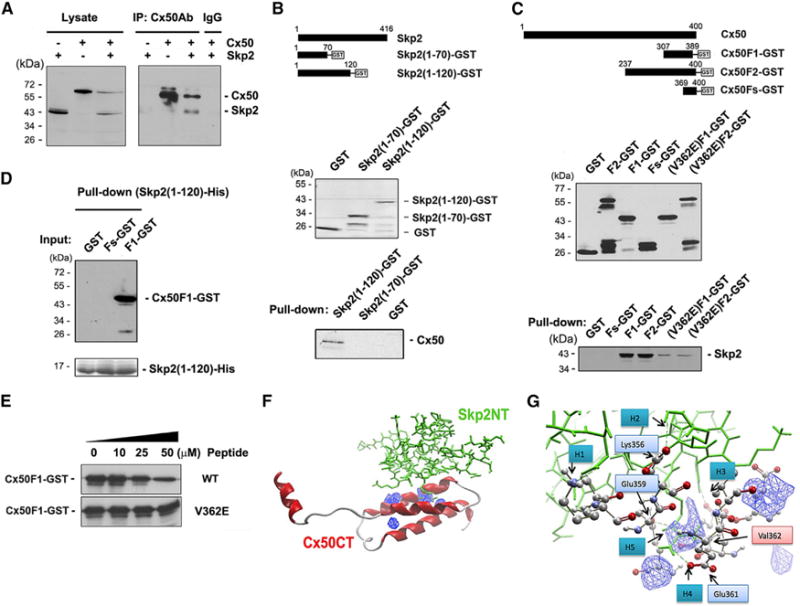
(A) Cx50 associates with Skp2.
(B–D) Cx50-CT directly interacts with Skp2-NT and Cx50(V362E) mutant weakens the interaction. Two GST fusion proteins was generated, Skp2(1-70)-GST and Skp2(1-120)-GST (B, upper). Pull-down assay was conducted with FLAG-tagged Cx50 and Skp2(1-70)-GST or Skp2(1-120)-GST fusion protein (B, lower). Three GST fusion proteins of Cx50-CTs were used for protein pull-down assay (C, upper) with FLAG-tagged Skp2 (C, lower). 9xHis-Skp2-NT was incubated with GST, Fs-GST, or F1-GST-beads (D).
(E) Peptide competition assay by pre-incubating His-Skp2-NT-beads with WT or V362E mutant Cx50-CT, prior to incubation with Cx50-F1-GST.
(F) The hypothetical 3D structure model showing the interaction between Cx50-CT and Skp2-NT. The possible interacting cavities on Cx50-CT were labeled in blue.
(G) Amplified interaction interface between Cx50-CT and Skp2-NT. Amino acids residues on Cx50 involved in this interaction were labeled in light blue boxes and Val-362 in a pink box.
See also Figure S3.
Figure 5. Interaction with Cx50 Retains Skp2 in the Cytosol and Hinders the NLS of Skp2 and Cx50.
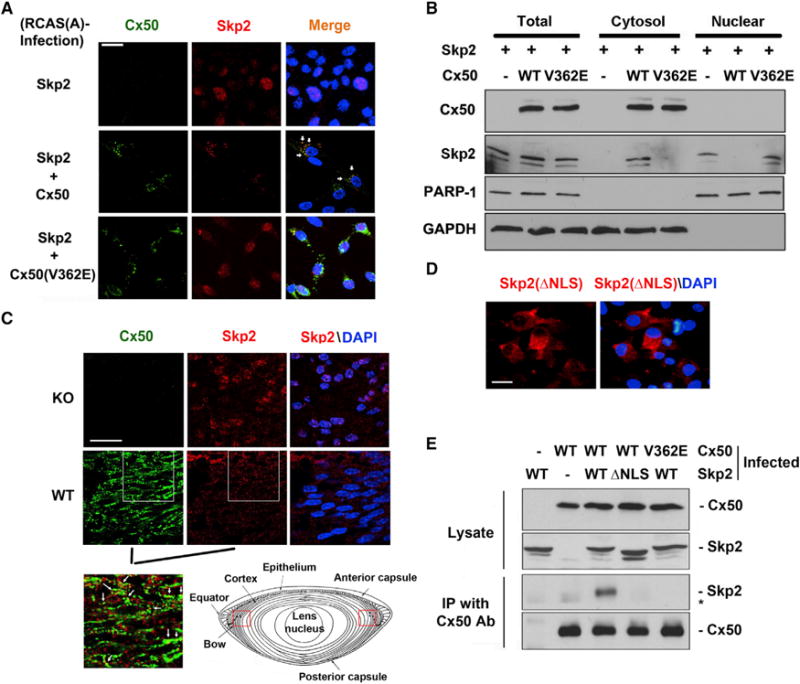
(A) Cx50 and Skp2 are partially co-localized in the cytosol. Co-localization was indicated by white arrow. Scale bar represents 20 μm.
(B) Skp2 is enriched in cytosolic fractions of the cells expressing WT Cx50, but not vehicle or Cx50(V362E) mutant.
(C) Skp2 is predominantly localized in nucleus in the lens bow region of Cx50 KO mice. Scale bar represents 20 μm. The fluorescence images are derived from the bow regions as indicated in red frames (cartoon, lower right). The magnified, overlay image of double labeling with Cx50 and FLAG-Skp2 antibodies of WT lens is shown in lower left (from the images overlaid in white square box, and the co-localization is indicated by white arrows).
(D) Skp2(DNLS) is located in the cytosol of CEF cells. Scale bar represents 20 μm.
(E) Cx50 fails to interact with Skp2(DNLS), and V362E mutation disrupts the interaction between Cx50 and Skp2. *Indicates a non-specific band.
See also Figure 4.
To identify the interaction motifs on Cx50 and Skp2, based on the known interaction between cyclin A-NT and Skp2-NT (Ji et al., 2006), two GST-tagged Skp2 fusion proteins were generated by fusing fragments of Skp2 (amino acids [aa] 1–70 or aa 1–120) with GST (Figure 4B, upper). A pull-down assay with Cx50-CT-GST fusion protein-conjugated beads showed that Cx50 was associated with Skp2(1–120), but not Skp2(1–70) or GST (Figure 4B, lower). This result suggests that the region around aa 70–120 of Skp2 was important for its interaction with Cx50. The reverse pull-down assay was performed using various sizes of Cx50 CT-GST to define the interactive domain on Cx50-CT. GST-tagged Cx50-CT fusion proteins were generated including Cx50-F2 (aa 273–420) containing a full-length CT domain; Cx50-F1 (aa 307–389), a shorter fragment of the CT domain; and Cx50-Fs (aa 369–400), the shortest fragment of CT domain, lacking the PEST domain and Val-362 residue (Figure 4C, upper). Skp2 was pulled down by both Cx50 F1 and F2, but not by Cx50 Fs. A very low level of Skp2 was detected in F1 and F2 containing the V362E mutation (Figure 4C, lower). This result suggests that Skp2 interacted with the CT region (aa 307–369) of Cx50 and Val-362 plays a critical role in this interaction.
To determine the direct interaction between specific domains of Skp2 and Cx50, beads conjugated with His-tagged Skp2-NT (aa 1–120) (Figure 4D, upper) were incubated with Cx50 F1-GST, Fs-GST, or GST. Only the F1 fragment, but not Fs and GST, was able to interact with His-tagged Skp2-NT (aa 1–120). This result demonstrates the direct interaction between specific regions of Skp2-NT and Cx50-CT.
To test if Val-362 is directly involved in this interaction, a binding competition assay was performed (Figure 4E). With increasing concentration, the peptide derived from Cx50-CT (sequence: AEEEEEQEEEQQAPQEEPGVKKAEEEVVSDEVEGPY; aa 336–370) reduced the interaction between Skp2 NT (1–120 aa) and Cx50 F1, while the same peptide with the mutation V362E had no such effect. These data support that Val-362 residue plays a critical role in the interaction between Cx50 and Skp2.
A computer simulation method was used to predict the interaction interface by a molecular docking software MolDock (Thomsen and Christensen, 2006). The hypothetical structure of Cx50-CT contains four hypothetical helical loops (Shi et al., 2010). The fragment, Skp2 (aa 70–120), was predicted to be located in a helical loop (PDB: 1FQV). The docking program predicted that four cavities in Cx50-CT are potential interacting sites with other proteins, primarily locating within or between the third and fourth helical loop regions (labeled in blue, Figure 4F). The model with the highest predictive score suggested that Skp2-NT was very likely to interact with one of these cavities and to form three hydrogen bonds at predicated sites of Glu361, Glu359, and Lys356 on Cx50-CT and a short fragment on Skp2-NT (GVSWDE, aa 115–120). Two additional hydrogen bonds were identified at the other region of helical loops, which were far away from the cavity regions (Figure 4G). Based on the modeling, Val-362 was located in the interaction interface of Cx50 and Skp2. Given the fact that the V362E mutation de-stabilizes the α-helical structures of Cx50-CT, we postulated that the mutation on Val-362 would alter the interaction interface and consequently disrupt the interaction between Cx50 and Skp2. This simulation modeling of the interaction between Cx50 and Skp2 suggests that the Cx50 helical loop motif containing Val-362 is capable of making energetically favorable interactions with the Skp2 molecule, which is consistent with our experimental data.
Cx50 Retains Skp2 in the Cytosol and Prevents Its Nuclear Localization
Skp2, as the substrate recognition subunit of the SCF complex, forms the SCFSkp2 complex in the cytosol and directs it into the nucleus (Lisztwan et al., 1998); therefore, Skp2 is predominantly localized in the nucleus. Consistently, when expressed alone in CEF cells, Skp2 was primarily localized in the nucleus, whereas Cx50 was located in the cytosol (Figure 5A, upper). Interestingly, when co-expressed with Skp2 and Cx50, Skp2 predominantly localized to the cytosol, while nuclear expression of Skp2 was barely detectable (Figure 5A, middle). Cx50 and Skp2 were partially co-localized with each other in the cytosol (indicated by white arrows). However, when co-expressed with Skp2 and the Cx50(V362E) mutant, Skp2 remained in the nucleus while Cx50(V362E) was located in the cytosol. To further confirm the localization of Skp2, we separated the fractions enriched in nuclear (indicated by nuclear marker, PARP-1) and cytosol (cytosolic marker, GAPDH) portions of cells expressing Skp2 alone, both Skp2 and Cx50, or Skp2 and Cx50(V362E) mutant. Skp2 was only detected in the cytosolic fraction of cells expressing both Skp2 and Cx50, but not in the cytosol of control cells or cells expressing Cx50(V362E) (Figure 5B). The absence of Skp2 in nuclear fractions further confirms the redistribution of Skp2 from the nucleus to the cytosol in the presence of Cx50. To confirm the role of Cx50 in preventing nuclear localization of Skp2, we examined the distribution pattern of Skp2 in lenses of WT and Cx50 KO mice. The dual-immunofluorescence of mouse lenses showed that at the lens bow region close to the lens equator (indicated by the frame, Figure 5C, lower right, lens cartoon), the area with the active process of lens cell differentiation, Skp2 was found to locate predominately in the nuclei of lens cells in Cx50 KO mice; however, no apparent nuclear localization was observed in WT control mice (Figure 5C). Moreover, Cx50 and Skp2 are partially co-localized as indicated by white arrows (Figure 5C, enlarged image, lower left). This in vivo result further validated the in vitro observation regarding the role of Cx50 in retaining Skp2 in the cytosol.
Skp2 as a nuclear protein possesses a nuclear localization signal (NLS) sequence (aa 88–100) predicted by PredictNLS (Cokol et al., 2000). This sequence resides within the interactive domain (aa 70–120) of Skp2 with Cx50. It is possible that the interaction with Cx50 would block the accessibility of the NLS domain, thereby preventing the nuclear translocation of Skp2. To test this hypothesis, we generated the recombinant RCAS(A) retrovirus containing Skp2(DNLS) (Skp2 lacking NLS sequence). Unlike WT Skp2, Skp2(DNLS) primarily localized to the cytosol (Figure 5D) and lost the ability of Skp2 to interact with Cx50 (Figure 5E). The results indicate that blocking of the NLS domain because of the interaction with Cx50 is a likely mechanism in preventing the nuclear localization of Skp2.
Cx50 Destabilizes Skp2 and Stabilizes p27 through Protein Ubiquitination
Expression levels of Skp2 are coordinated with progression of the cell cycle starting at very low levels in the early G1 phase, increasing in the late G1 phase and reaching the maximal value in the S and G2/M phases (Michel and Xiong, 1998; Lisztwan et al., 1998). To test if cytosolic retention had any impact on protein stability of Skp2, we determined protein turnover of Skp2 in CEF cells treated with cycloheximide (CHX). CHX, an inhibitor of protein biosynthesis in eukaryotic organisms was used to determine the rate of protein turnover (Ennis and Lubin, 1964; Yin et al., 2008) and induce cell-cycle arrest at G1/S phases (Adolph et al., 1993; Liu et al., 2010). During a 4-hr treatment with CHX, co-expression with Cx50 led to a faster degradation of Skp2 as compared to cells expressing Skp2 alone or co-expressing with the Cx50(V362E) mutant (Figure 6A). Similar results were obtained in mouse cells. In the presence of CHX, expression of mouse Cx50 in mouse embryonic fibroblast (MEF) cells greatly accelerated the degradation of endogenous Skp2 as compared to vehicle controls (Figure S5). We further determined the impact of Cx50 on Skp2 expression level during the cell cycle (Figure 6B). The increase of Skp2 from S to G2/M transition (from 8% S phase cells at 10 hr time point to 30% S phase cells at 14 hr) was significantly suppressed by Cx50 as compared to that of Skp2 in cells without Cx50 expression. To assess if the effect of Cx50 on Skp2 is preserved with reduced progression of cell cycle in the presence of Cx50, we depleted p27 with siRNA. As compared with the scrambled RNA control, deletion of p27 by siRNA had no effect on the reduction of Skp2 by Cx50 (Figure 6C), suggesting that the effect of Cx50 on Skp2 is not influenced by p27 and the reduced rate of cell cycle is due to Cx50. Like most cell-cycle regulators, Skp2 levels are regulated by ubiquitination and subsequent proteasome degradation (Wirbelauer et al., 2000; Bashir et al., 2004; Wei et al., 2004). The level of Skp2 ubiquitination in CEF cells expressing Cx50 or the Cx50(V362E) mutant was examined. Co-expression of Cx50 dramatically increased the level of ubiquitinated Skp2 (Figure 6D), which was consistent with the effects of Cx50 on destabilization of Skp2 and decreased expression of Skp2 during cell-cycle progression. The primary E3 ligase for Skp2 is APCcdh1 complex, which normally functions in the nucleus. To test if Skp2 undergoes another cytosolic ubiquitin-related degradation, specifically auto-ubiquitination by the SCFSkp2 complex (Wirbelauer et al., 2000), we examined that if the SCFSkp2 complex would still remain intact in the presence of Cx50. The coIP experiments demonstrated that indeed, Skp1, another important component of SCFSkp2 complex, was present in the Skp2 and Cx50 complex (Figure 6E), suggesting that Cx50 is unlikely to disturb the integrity of the SCFSkp2 complex. To test the possibility that Cx50 interferes with the association of Skp2 with importin-7, a protein mediating nuclear transport of Skp2, coIP was performed and the data showed the absence of importin-7 in coIP with Cx50, but not with vehicle or Cx50(V362E)-expressing cells (Figure 6E). Cdh1 is a major component for Cdh1-anaphase-promoting complex (APC), which mediates degradation of Skp2 in the nucleus. CoIP showed the lack of association between SCFSkp2 complex and Cdh1 in the presence of Cx50 (Figure 6E). This data further support cytosolic localization of Skp2 with the expression of Cx50. To further exploit the direct role of Cx50 on auto-ubiquitination of Skp2, in vitro ubiquitination assay was performed. The SCFSkp2 complex (containing Skp1/ROC/Cul1 components) was isolated from CEF cells by immunoprecipitation with FLAG antibody as shown in Figure 6E and was incubated with GST fusion proteins containing WT, V362 mutant of Cx50 C-terminal domains or GST protein. Cx50 C-terminal domain (GST-Cx50(CT)) increased the extent of Skp2 ubiquitination and this increase was not observed with GST fusion protein containing Cx50 V362E mutant (Figure 6F). The presence of the components of SCFSkp2 complex, Skp1, Rock1, and Cul1 was also detected with coIP. Moreover, the ubiquitination product was not detected in the reaction lacking ATP, ubiquitin, or E1/E2 enzymes. This result suggests a direct effect of Cx50 on promoting auto-ubiquitination of Skp2, likely through the direct interaction between Cx50 via the involvement of V362 residue and Skp2.
Figure 6. Cx50 De-stabilizes Skp2 via Auto-ubiquitination during Cell-Cycle Progression and Blocks the Association among Skp2, Importin-7, and Cdh1.
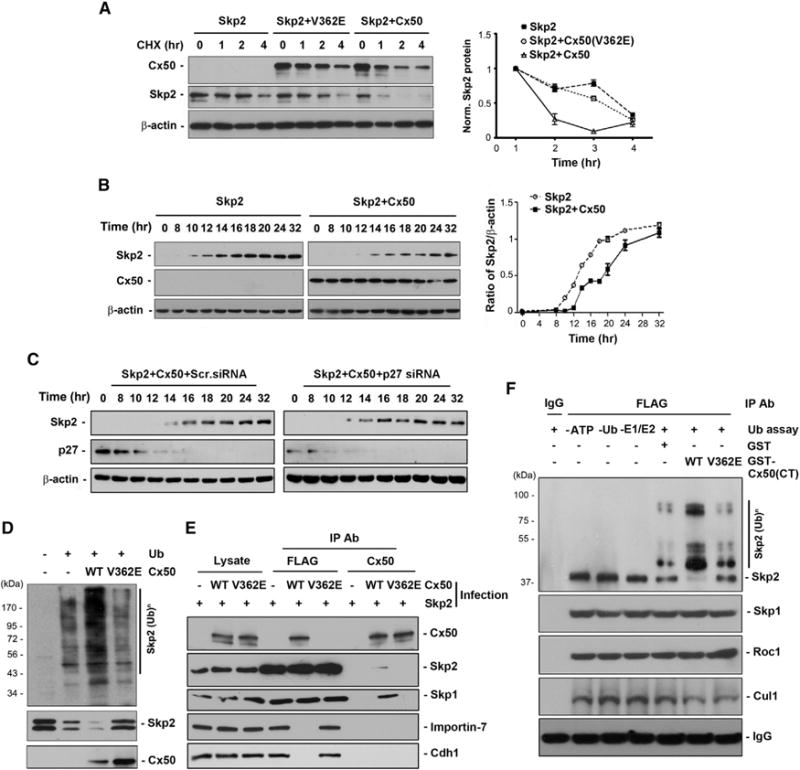
(A) Cx50 de-stabilizes Skp2 at the mitotic phase. The protein level was quantified and normalized to 0 hr.
(B) Cx50 delays the increased expression of Skp2 during cell-cycle progression. The level of protein was quantified and normalized to 0 hr.
(C) The reduction of Skp2 by Cx50 is not affected by p27 siRNA.
(D) Cx50 increases ubiquitination level of Skp2. The protein expression levels were examined with western blotting (lower).
(E) Cx50 has no effect on the association of Skp2 with Skp1, but disrupts Skp1/2 interaction with importin-7 and Cdh1.
(F) Cx50 C terminus increases auto-ubiquitination of Skp2 in vitro. SCFSkp2 complex was used for in vitro ubiquitination assay with isolated GST-fusion containing WT or V362E mutant of Cx50 C terminus or GST. The specificity of the assay was validated containing all assay-required materials except Mg-ATP, ubiquitin (Ub) or E1/E2 enzymes, respectively.
See also Figure S5.
The level of p27 protein during the cell cycle is regulated through the ubiquitin-proteasome pathway (Pagano et al., 1995; Hengst and Reed, 1996). Skp2 as an E3 ligase specifically regulates p27 ubiquitination during cell-cycle progression. The expression level and stability of p27 are inversely correlated to the level of Skp2 during cell-cycle progression; at a very high level in the G0 and early G1 phase, but decreasing in the late G1 phase and reaching the lowest level at the later S and G2/M phases (Besson et al., 2006). We showed that Cx50 delayed cell-cycle transition from the G1 to S phase. It is likely that the delay in cell-cycle progression by Cx50 was caused by the increased level of p27 due to cytosolic retention and destabilization of Skp2 induced by Cx50. We synchronized the cells at G0/G1 phase with serum starvation, and treated cells with CHX for the indicated times (0–6 hr) (Figure 7A). The expression of Cx50 decreased the turnover of p27 as compared to non-Cx50 expressed controls. At the 10 hr time point when cells started to enter S phase (8.6% cells at S phase at 10 hr time point; 30% S phase cells at 12 hr), there was an increase of p27 expression. However, in cells expressing Cx50, the increase of p27 appeared at the 14 hr time point (12.4% cells at S phase; 26.5% S phase cells at 18 hr), indicating a delay of entering the S phase (Figure 7B). The expression of Cx50 enriched percentage of the cells at G1 phase. However, knocking down p27 significantly reduced the cells at G1 phase. This reduction was slightly, but significantly attenuated by the co-expression of Cx50, although the extent of attenuation was lower than that with p27 (Figure 7C, right). This modest attenuation is likely due to incomplete p27 knockdown during initial state of cell-cycle arrest. Consistent with a reduced level of Skp2, the amount of ubiquitinated p27 proteins was much less in cells expressing Cx50 compared to cells without Cx50 or expressing the Cx50(V362E) mutant (Figure 7D), suggesting that Cx50 increases the stabilization of p27 by decreasing its ubiquitination/degradation through the reduction of overall level and cytosolic retention of Skp2. Due to its predominant localization in the cytosol in the presence of Cx50, Skp2 degradation is not mediated by the APC complex as evidenced by the absence of the association between Skp2 and Cdh1 (Figure 6D).
Figure 7. Stabilization of p27 by Cx50 during the Cell Cycle and Reduction of p27/p57 in Cx50-Deficient Lens.
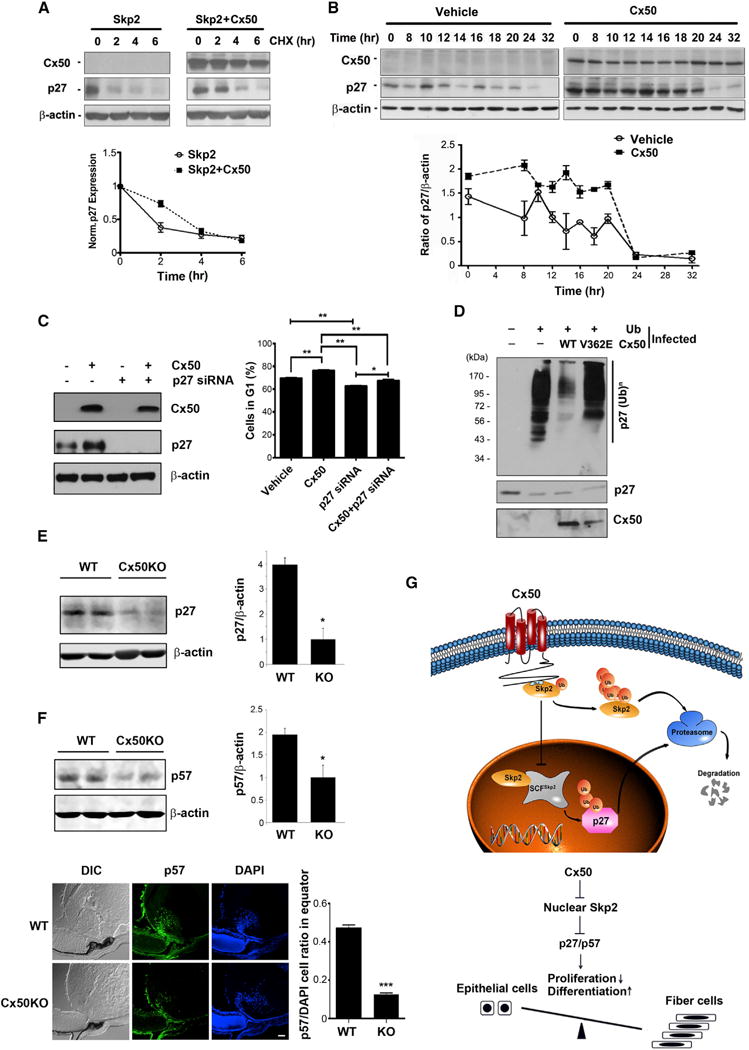
(A) Cx50 stabilizes p27 at G0/G1 phase. The protein levels were normalized to 0 hr.
(B) Cx50 increases the expression level of p27 during cell-cycle progression. The expression level of protein was normalized to 0 hr of RCAS(A) vehicle control.
(C) Knocking down p27 promotes the cell cycle that is arrested by Cx50. The cell population was determined by FACS analysis with PI staining. (D) Cx50 decreases ubiquitination level of p27. The protein levels were examined by western blotting (lower).
(E) Decreased levels of p27 in Cx50-knockout lens. Lens lysates from 3-day-old WT and Cx50 knockout mice were immunoblotted (left) and quantified (right).
(F) Decreased levels of p57 in the bow region of Cx50-knockout lens. Lens lysates of 3-day old WT and Cx50 KO mice were immunoblotted with anti-p57 or β-actin antibody (upper left) and quantified (upper right). The frozen tissue sections of 3-day-old lenses from WT or Cx50 knockout mice were immunofluo-rescence-labeled (lower left) and quantified (lower right). Scale bar represents 50 μm.
(G) Schematic model illustrates the interaction and mechanistic roles of Cx50 and Skp2 in regulating lens cell differentiation. Left: Cx50 interacts with Skp2 in the cytosol, leading to cytoplasmic retention of Skp2, thereby preventing it from translocating into nucleus. Reduced nuclear Skp2 leads to decreased ubiquitination of p27/p57 in nucleus and less degradation of p27/p57. Right: the increased p27 due to the retention of Skp2 in cytosol by Cx50 leads to cell-cycle arrest and promotion of lens cell differentiation.
The lens size of Cx50 knockout is significantly smaller than that of the WT animal (White et al., 1998; Rong et al., 2002). The levels of p27 (Figure 7E) and p57 (Figure 7F) were significantly decreased in the lens of Cx50 knockout mice. Lens cell differentiation occurs in the bow region close to lens equator. Immunofluorescence data showed reduced signals of p57 in the differentiating lens fibers in Cx50 knockout lenses (Figure 7F, lower). Together, these data suggest that Cx50 plays an important role in regulating lens cell differentiation via its direct effect on Skp2 and consequently, the levels of p27/p57.
DISCUSSION
Accumulating data have shown channel-independent roles of connexins in cellular events including cell growth, proliferation and differentiation, tumorigenicity, and wound healing (for review, see Jiang and Gu, 2005; Zhou and Jiang, 2014). However, the molecular mechanism underlying non-conventional functions of connexins remained largely unexplored. Connexin family proteins have a relatively conserved membrane topology, with similar NT and extracellular loop domain sequences, whereas the cytoplasmic CT domain are diverse (Goodenough and Paul, 2009). Increasing evidence indicates that the CT domains of connexin proteins contribute to channel-independent functions. Previous studies show that analogous to full-length Cx43, the CT domain of Cx43 by itself that does not form functional gap junction can inhibit tumor cell growth (Zhang et al., 2003a). We have previously shown that the role of Cx50 in promoting lens cell differentiation is independent of its function in forming gap junctions or hemichannels (Banks et al., 2007, 2009; Gu et al., 2003), and the CT domain and Val-362 residue are primarily responsible for this function (Banks et al., 2007; Shi et al., 2010). Unveiling the sequential similarity between Cx50 and cyclin A inspired us to examine the relationship between Cx50 and the cyclin A-binding partner, Skp2. Here, we demonstrated the direct interaction between Cx50 and Skp2, and identified interactive domains on both proteins. Su et al. showed that Skp2 siRNA inhibits rabbit lens epithelial cell proliferation (Su et al., 2010) . Our study revealed how Skp2 is regulated in the lens epithelial-fiber differentiation. We used two different shRNAs to knock down Skp2. Reduction of Skp2 expression accelerates, whereas overexpression delays differentiation of lens cells, which is inversely correlated with proliferation of lens epithelial cells. Cx50 rescues the lens cell differentiation suppressed by Skp2, but further augments the differentiation induced by Skp2 shRNAs.
Interestingly, the related sequences of Cx50 and cyclin A are similar, but the level of homology is relatively low (Figure S3A). By comparing the sequence, we found that cyclin A-interacting motif on Skp2 (Ji et al., 2006) (Figure S3B, underlined) falls into the 70–120 aa region of chick Skp2. Therefore, consistent with the known interaction between Skp2 and cyclin A, our data supported that this region of Skp2 mediates its interaction with Cx50. Although Cx50 and cyclin A appear to bind to a similar region on Skp2; however, this is unlikely a competitive binding since unlike Cx50, the interaction with cyclin A primarily occurs in the nucleus. We have previously shown that the V362E mutation compromises the ability of Cx50 in promoting lens cell differentiation (Shi et al., 2010). Here, we show that Val-362 is located in the interaction interface of Cx50 and Skp2. The mutation on Val-362 (V362E) uncouples the interaction between Cx50 and Skp2. In contrast to WT Cx50, the expression of this mutant cannot attenuate the inhibitory effect on differentiation by Skp2. This evidence suggests that direct physical interaction with Skp2 is essential for the role of Cx50 in promoting lens cell differentiation.
The NT region (aa 70–120) of Skp2, the identified Cx50-interactive domain, has been shown to be involved in multiple cellular functions. Cyclin A binds to Skp2 at this region (Ji et al., 2006). This region, however, is different from the active domain of Skp2 E3 ligase in the SCFSkp2 complex involved in mediating p27 ubiquitination. Mutations on this cyclin A binding region attenuate the ability of Skp2 to counter cell-cycle arrest through the p53/p21-mediated checkpoint (Ji et al., 2007). Cyclin A is activated during the S to G2/M transition and Skp2-cyclin A interaction is important for protecting cyclin A from binding to p27, since the association with p27 blocks the ability of cyclin A in promoting cell-cycle progression (Ji et al., 2007). Our study suggests that connexin, an atypical cell-cycle regulator, plays a critical role in controlling this process. The interaction with Cx50 in the cytosol consequently reduces the amount of Skp2 in the nucleus and thus its interaction with cyclin A, leading to increased binding of p27 with cyclin A and consequently, the inhibition of cyclin A and suppression of cell-cycle progression. Interestingly, APC/Ccdh1 complex binds to Skp2 through the similar N-terminal domain (aa 46–94 in human Skp2) (Bashir et al., 2004; Wei et al., 2004), which is corresponding to the region on chick Skp2 at aa 77–121. That explains why Cdh1 dissociated with Skp2 in the presence of Cx50, further supporting our observation of translocation of Skp2 with expression of Cx50.
Sequestering Skp2 in the cytosol, thus preventing the transfer to its functional location in nucleus, switches the cells from highly proliferative to a differentiating state. Skp2 is a major component of SCFSkp2 E3 complex catalyzing the ubiquitination of proteins. This complex is located in the cytoplasm until Skp2 is expressed in late G1 phase and inclusion of Skp2 helps the complex SCFSkp2 migrate into the nucleus (Lisztwan et al., 1998). This complex promotes the ubiquitination of selected proteins, including cyclin E/cyclin D, p27, and p57. We found that Cx50 was capable of retaining Skp2 in the cytosol. Moreover, in addition to Skp2, we also detected the presence of Skp1/Roc1/Cul1 in the coIP of Cx50, which supported the notion that intact SCFSkp2 complex is likely to be retained in the cytosol as a result of the interaction between Cx50 and Skp2. Skp2 normally resides in the nuclei; however, cytosolic localization of Skp2 has been reported (Drobnjak et al., 2003; Li et al., 2004; Lim et al., 2002; Radke et al., 2005; Lin et al., 2009). The major question is what causes normally nuclear localized Skp2 to reside in cytoplasm. Recent studies suggest that subcellular localization of Skp2 is regulated by protein phosphorylation (Gao et al., 2009; Lin et al., 2009); however, this observation was not supported by other studies (Bashir et al., 2010; Boutonnet et al., 2010). We showed that Skp2 is absent from the nucleus in the presence of Cx50. Therefore, interaction with Cx50 as demonstrated in this study is likely an important mechanism for cytosolic retention of Skp2.
We show that nuclear targeting of the NLS domain of Skp2 overlaps with the Cx50 interactive domain and deletion of NLS inhibits Skp2 interaction with Cx50. Interaction with Cx50 may hinder the NLS domain of Skp2, thereby preventing its nuclear translocation. Indeed, the interaction with Cx50 prevented the association of Skp2 with importin-7, a critical mediator for nuclear transport of Skp2. Another possibility is that the binding of Skp2 to Cx50 may cause conformational change with the exposure of other possible protein binding sites of Skp2, such as the adjacent nuclear export signal (NES) domain (aa 127–135). Association with other protein(s) may further help retain Skp2 in the cytoplasm; however, continuous association with Cx50 at this stage may not be necessary. This may explain that not all cytosolic Skp2 is co-localized with Cx50 in the cytosol, although the exact mechanism requires further investigation. Moreover, at lens bow region with an active process of epithelial-fiber differentiation, Skp2 is expressed at the cytosol of the WT mouse lens with minimal expression in nucleus, and partially co-localized with Cx50. However, in Cx50 KO lens, Skp2 is predominantly expressed in the nucleus of lens cells. This in vivo observation strengthened the finding that Cx50 retains Skp2 in the cytosol.
Upregulation of Skp2 is a key step required for the passage of cells from the G1 to S phase during the cell cycle (Nakayama et al., 2004). We showed that interaction with Cx50 significantly reduced this upregulation, which subsequently delays cell-cycle progression, and this reduction was mediated by protein ubiquitination. Indeed, more Skp2 protein was modified by ubiquitination in the presence of Cx50. One of the possible explanations is that the protein conformational change of Skp2 because of its interaction with Cx50 may reveal the sites suitable for modification by ubiquitin. Skp2 is targeted for ubiquitination and degradation by APCcdh1 (Bashir et al., 2004; Wei et al., 2004); however, the APCcdh1 complex is primarily localized in the nuclei of cells so it is unlikely that cytosolic Skp2 can be accessed by the APCcdh1 complex. Consistently, we showed that Skp2 failed to interact with Cdh1 only in Cx50, but not in vehicle or Cx50(V362E)-expressing cells. Skp2 undergoes auto-ubiquitination in the SCFSkp2 complex (Wirbelauer et al., 2000) and interaction with Cx50 may promote this reaction. Indeed, our in vitro ubiquitination assay with isolated SCFskp2 complex showed that C terminus of Cx50 directly increased auto-ubiquitination Skp2 and such increase was not observed in V362E mutant that disrupts the interaction between Cx50 and Skp2. Our data also showed that Skp1 was still associated with Skp2 whereas Skp2 formed the complex with Cx50, implying that binding with Cx50 is unlikely to disrupt the formation of SCFSkp2 complex. Previous studies have shown that the ubiquitin-proteasome pathway is active during lens cell proliferation and differentiation (Guo et al., 2004, 2006; Girão et al., 2005; Imai et al., 2010), although the key molecules responsible for this pathway have not been reported. Skp2 could be one of the key molecules involved in protein ubiquitination in the lens. Consistent with the downregulation of Skp2, we observed that protein turnover of p27 was significantly delayed by Cx50, leading to the augmentation of overall p27 level throughout the cell cycle. The protein level of p27 is also tightly regulated by the ubiquitination system, primarily through E3 ligase Skp2 in the nucleus (Nakayama et al., 2004). Kip1 ubiquitination-promoting complex (KPC) is also reported to be responsible for the ubiquitination of cytosolic p27 (Kamura et al., 2004), suggesting an Skp2-independent, cell-cycle phase-specific ubiquitin-proteasome pathway. Downregulation of Skp2 in the nucleus induced by its interaction with Cx50 in cytosol, as expected, decreased enzymatic activity of Skp2 leading to lesser ubiquitination and more stabilization of p27. Therefore, elucidating how the interaction of Cx50 regulates the ubiquitination of Skp2 and the possible involvement of SCFSkp2 complex will be a direction for further investigation.
We observed the predominant expression of p27 in the lentoids, where the differentiated fiber cells accumulated, but not in proliferative epithelial cells. This expression pattern matches the role of p27 in attenuating the progress of the cell cycle. Furthermore, we showed that knocking down p27 by siRNA attenuated the stimulatory effect of Cx50 on lens cell differentiation and inhibitory effort on cell proliferation, suggesting that p27 is a downstream effector of Cx50. The relationship between Skp2 and p27 was supported by the observation that the expression of Skp2-resistant mutant p27(T187A) further augmented lens cell differentiation. Interestingly, expression of this mutant did not further enhance the effect of Cx50 because co-expression with Cx50 mutant did not increase the total amount of p27 as compared to the expression of this mutant alone. Previous studies have reported that p27 is present around lens equator regions and p27/p57 are important regulators in mouse lens development (Kase et al., 2005; Zhang et al., 1998). We showed both p27 and p57 proteins were greatly decreased in Cx50 KO mouse lens and this decrease is well correlated with the reduction of lens size. Correspondingly, the reduction of p57 protein is evident in the Cx50 KO around bow region, a highly differentiating zone of the lens. Together, the present study establishes physical and functional connections between the connexin molecule and core cell-cycle regulators, and delineates a mechanism regarding connexin molecules in orchestrating cell-cycle control, proliferation, and differentiation.
EXPERIMENTAL PROCEDURES
Cell-Cycle Synchronization and Flow Cytometric Analysis
To synchronize the cell at G1/S phase, CEF cells were plated at ~30% confluency, treated with 2 mM thymidine for 18 hr, and followed by incubation with fresh medium containing 10% FBS for 9 hr to release the cells back into cell cycle. Second cell-cycle block was performed by treating with 2 mM thymidine for 17 hr. To synchronize the cells at G2/M phase, CEF cells at ~30% confluency were treated with 2 mM thymidine for 24 hr and followed by the addition of fresh medium and incubation for 3 hr to release cells back into the cell cycle, followed by 100 ng/ml nocodazole for another 12 hr. For synchronizing cells at G0/G1 phase, CEF cells at 30%–40% confluency were incubated with FBS-free medium for 72 hr, and released into cell cycle by incubation with the medium containing 10% FBS.
For FACS analysis, CEF cells were harvested in 0.25% trypsin solution, fixed in cold 75% ethanol, and stored at −20 °C for 16 hr. Fixed cells were subsequently washed once with cold PBS, treated with RNase A (100 mg/ml) in PBS and 50 mg/ml propidium iodide (PI) for 30 min in the dark. DNA content of cells was quantified in a BD Biosciences FACS Calibur (UTHSCSA Flow Cytometry Core) with 10,000 events for G0/G1 cell population using BD Biosciences Cell Quest software, and the data were analyzed by FlowJo software.
Ubiquitination Assay
For in vivo assay, ubiquitin protein was expressed ectopically in cells by using recombinant RCAS(A) retrovirus containing ubiquitin tagged with c-myc. CEF cells were infected with the indicated recombinant retroviruses for 48 hr, treated with 10 μM MG132 for 6 hr, and lysed in IP buffer. The cell extracts were then incubated with monoclonal anti-p27 antibody (1:2,500) or polyclonal anti-FLAG antibody (for Skp2) (1:5,000) and protein G or A beads, respectively, at 4°C for 16 hr. The beads were then washed three times with IP buffer and the proteins were eluted with 50 ml non-reducing laemmli sample buffer before SDS-PAGE and western blotting. The ubiquitinated proteins were depicted by probing blots with c-myc antibody (1:5,000) (Invitrogen).
To assess in vitro ubiquitination in reconstituted system, SCFSkp2 complex was immunoprecipitated with anti-FLAG M2 affinity gel (Sigma) from cell lysate of FLAG-Skp2-expressing CEF cells. The precipitates were then washed with IP buffer twice, PBS three times, and finally ubiquitination assay buffer (5 mM Tris [pH 8], 0.5 mM MgCl2, 0.01% Tween-20 and 0.1 mM β-mercaptoethanol) five times. Then, the precipitates were subjected to auto-ubiquitination assay by using the VIVAbind Ub kit from VIVA Bioscience. Briefly, the precipitates were mixed with E1, E2 mix (UbcH3, UbcH5b, UbcH5C and Ubc13), ubiquitin, Mg-ATP, GST, GST-Cx50-CT, or GST-Cx50-CT(V362E) fusion proteins, 10× ub assay buffer, and then incubated at 37°C for 6 hr.
Statistical Analysis
All the data were analyzed using GraphPad Prism 5.04 software (GraphPad Software). One-way ANOVA and Student-Newman Keul’s test were used for more than two compared groups and paired Student’s t test was used for comparison between two groups. Unless otherwise specified in the figure legends, the data are presented as the mean ± SEM of at least three determinations. Asterisks indicate the degree of significant differences: *p < 0.05, **p < 0.01, ***p < 0.001.
Reagent information and other experimental procedures are included in the Supplemental Experimental Procedures. Experiments related to animals were approved by the Institutional Animal Care and Use Committee of UTHSCSA.
Supplementary Material
Highlights.
Connexin 50 governs cell-cycle existence and differentiation through p27/p57 and Skp2
Direct interaction with connexin 50 retains Skp2 in the cytosol
Connexin 50 promotes auto-ubiquitination and subsequent degradation of Skp2
p27/p57 protein is stabilized through downregulation of Skp2
Acknowledgments
We thank Dr. P. Renee Yew for providing pCS2-myc-ubiquitin plasmid and invaluable suggestions and Dr. Hai Rao at UTHSCSA for his suggestions on in vitro ubiquitination assay. We thank people at J.X.J. laboratory for critical reading of the manuscript. This work was supported by grants from the National Institute of Health (EY012085 to J.X.J.; EY013163 to T.W.W.) and the Welch Foundation (AQ-1507 to J.X.J.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at http://dx.doi.org/10.1016/j.devcel.2015.10.014.
AUTHOR CONTRIBUTIONS
Q.S. designed and conducted experiments and wrote the paper; S.G., X.S.Y., and E.A.B. conducted experiments; T.W.W. provided materials; and J.X.J. designed experiments and wrote the paper.
References
- Adolph S, Brüsselbach S, Müller R. Inhibition of transcription blocks cell cycle progression of NIH3T3 fibroblasts specifically in G1. J Cell Sci. 1993;105:113–122. doi: 10.1242/jcs.105.1.113. [DOI] [PubMed] [Google Scholar]
- Banks EA, Yu XS, Shi Q, Jiang JX. Promotion of lens epithelial-fiber differentiation by the C-terminus of connexin 45.6 a role independent of gap junction communication. J Cell Sci. 2007;120:3602–3612. doi: 10.1242/jcs.000935. [DOI] [PubMed] [Google Scholar]
- Banks EA, Toloue MM, Shi Q, Zhou ZJ, Liu J, Nicholson BJ, Jiang JX. Connexin mutation that causes dominant congenital cataracts inhibits gap junctions, but not hemichannels, in a dominant negative manner. J Cell Sci. 2009;122:378–388. doi: 10.1242/jcs.034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- Bashir T, Pagan JK, Busino L, Pagano M. Phosphorylation of Ser72 is dispensable for Skp2 assembly into an active SCF ubiquitin ligase and its subcellular localization. Cell Cycle. 2010;9:971–974. doi: 10.4161/cc.9.5.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud VM, Bassnett S, Beyer EC. Cultured chicken embryo lens cells resemble differentiating fiber cells in vivo and contain two kinetic pools of connexin56. Exp Eye Res. 1999;68:475–484. doi: 10.1006/exer.1998.0635. [DOI] [PubMed] [Google Scholar]
- Besson A, Gurian-West M, Chen X, Kelly-Spratt KS, Kemp CJ, Roberts JM. A pathway in quiescent cells that controls p27Kip1 stability, subcellular localization, and tumor suppression. Genes Dev. 2006;20:47–64. doi: 10.1101/gad.1384406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutonnet C, Tanguay PL, Julien C, Rodier G, Coulombe P, Meloche S. Phosphorylation of Ser72 does not regulate the ubiquitin ligase activity and subcellular localization of Skp2. Cell Cycle. 2010;9:975–979. doi: 10.4161/cc.9.5.10915. [DOI] [PubMed] [Google Scholar]
- Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- Cokol M, Nair R, Rost B. Finding nuclear localization signals. EMBO Rep. 2000;1:411–415. doi: 10.1093/embo-reports/kvd092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobnjak M, Melamed J, Taneja S, Melzer K, Wieczorek R, Levinson B, Zeleniuch-Jacquotte A, Polsky D, Ferrara J, Perez-Soler R, et al. Altered expression of p27 and Skp2 proteins in prostate cancer of African-American patients. Clin Cancer Res. 2003;9:2613–2619. [PubMed] [Google Scholar]
- Dunia I, Cibert C, Gong X, Xia CH, Recouvreur M, Levy E, Kumar N, Bloemendal H, Benedetti EL. Structural and immunocytochemical alterations in eye lens fiber cells from Cx46 and Cx50 knockout mice. Eur J Cell Biol. 2006;85:729–752. doi: 10.1016/j.ejcb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Ennis HL, Lubin M. Cyclohemimide: Aspects of inhibition of protein synthesis in mammalian cells. Science. 1964;146:1474–1476. doi: 10.1126/science.146.3650.1474. [DOI] [PubMed] [Google Scholar]
- Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol. 2009;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girão H, Pereira P, Taylor A, Shang F. Subcellular redistribution of components of the ubiquitin-proteasome pathway during lens differentiation and maturation. Invest Ophthalmol Vis Sci. 2005;46:1386–1392. doi: 10.1167/iovs.04-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of α3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol. 2009;1:a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- Gu S, Yu XS, Yin X, Jiang JX. Stimulation of lens cell differentiation by gap junction protein connexin 45.6. Invest. Ophthalmol Vis Sci. 2003;44:2103–2111. doi: 10.1167/iovs.02-1045. [DOI] [PubMed] [Google Scholar]
- Guo W, Shang F, Liu Q, Urim L, West-Mays J, Taylor A. Differential regulation of components of the ubiquitin-proteasome pathway during lens cell differentiation. Invest Ophthalmol Vis Sci. 2004;45:1194–1201. doi: 10.1167/iovs.03-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Shang F, Liu Q, Urim L, Zhang M, Taylor A. Ubiquitin-proteasome pathway function is required for lens cell proliferation and differentiation. Invest Ophthalmol Vis Sci. 2006;47:2569–2575. doi: 10.1167/iovs.05-0261. [DOI] [PubMed] [Google Scholar]
- Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- Imai F, Yoshizawa A, Fujimori-Tonou N, Kawakami K, Masai I. The ubiquitin proteasome system is required for cell proliferation of the lens epithelium and for differentiation of lens fiber cells in zebrafish. Development. 2010;137:3257–3268. doi: 10.1242/dev.053124. [DOI] [PubMed] [Google Scholar]
- Ji P, Goldin L, Ren H, Sun D, Guardavaccaro D, Pagano M, Zhu L. Skp2 contains a novel cyclin A binding domain that directly protects cyclin A from inhibition by p27Kip1. J Biol Chem. 2006;281:24058–24069. doi: 10.1074/jbc.M603105200. [DOI] [PubMed] [Google Scholar]
- Ji P, Sun D, Wang H, Bauzon F, Zhu L. Disrupting Skp2-cyclin A interaction with a blocking peptide induces selective cancer cell killing. Mol Cancer Ther. 2007;6:684–691. doi: 10.1158/1535-7163.MCT-06-0538. [DOI] [PubMed] [Google Scholar]
- Jiang JX. Gap junctions or hemichannel-dependent and independent roles of connexins in cataractogenesis and lens development. Curr Mol Med. 2010;10:851–863. doi: 10.2174/156652410793937750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JX, Gu S. Gap junction- and hemichannel-independent actions of connexins. Biochim Biophys Acta. 2005;1711:208–214. doi: 10.1016/j.bbamem.2004.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JX, Paul DL, Goodenough DA. Posttranslational phosphorylation of lens fiber connexin46: a slow occurrence. Invest Ophthalmol Vis Sci. 1993;34:3558–3565. [PubMed] [Google Scholar]
- Kamura T, Hara T, Matsumoto M, Ishida N, Okumura F, Hatakeyama S, Yoshida M, Nakayama K, Nakayama KI. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat Cell Biol. 2004;6:1229–1235. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- Kase S, Yoshida K, Ikeda H, Harada T, Harada C, Imaki J, Ohgami K, Shiratori K, Nakayama KI, Nakayama K, Ohno S. Disappearance of p27(KIP1) and increase in proliferation of the lens cells after extraction of most of the fiber cells of the lens. Curr Eye Res. 2005;30:437–442. doi: 10.1080/02713680590959286. [DOI] [PubMed] [Google Scholar]
- Li P, Li C, Zhao X, Zhang X, Nicosia SV, Bai W. p27(Kip1) stabilization and G(1) arrest by 1,25-dihydroxyvitamin D(3) in ovarian cancer cells mediated through down-regulation of cyclin E/cyclin-dependent kinase 2 and Skp1-Cullin-F-box protein/Skp2 ubiquitin ligase. J Biol Chem. 2004;279:25260–25267. doi: 10.1074/jbc.M311052200. [DOI] [PubMed] [Google Scholar]
- Lim MS, Adamson A, Lin Z, Perez-Ordonez B, Jordan RC, Tripp S, Perkins SL, Elenitoba-Johnson KS. Expression of Skp2, a p27(Kip1) ubiquitin ligase, in malignant lymphoma: correlation with p27(Kip1) and proliferation index. Blood. 2002;100:2950–2956. doi: 10.1182/blood.V100.8.2950. [DOI] [PubMed] [Google Scholar]
- Lin HK, Wang G, Chen Z, Teruya-Feldstein J, Liu Y, Chan CH, Yang WL, Erdjument-Bromage H, Nakayama KI, Nimer S, et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat Cell Biol. 2009;11:420–432. doi: 10.1038/ncb1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisztwan J, Marti A, Sutterlüty H, Gstaiger M, Wirbelauer C, Krek W. Association of human CUL-1 and ubiquitin-conjugating enzyme CDC34 with the F-box protein p45(SKP2): evidence for evolutionary conservation in the subunit composition of the CDC34-SCF pathway. EMBO J. 1998;17:368–383. doi: 10.1093/emboj/17.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yang JM, Zhang SS, Liu XY, Liu DX. Induction of cell cycle arrest at G1 and S phases and cAMP-dependent differentiation in C6 glioma by low concentration of cycloheximide. BMC Cancer. 2010;10:684. doi: 10.1186/1471-2407-10-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Menko AS, Klukas KA, Johnson RG. Chicken embryo lens cultures mimic differentiation in the lens. Dev Biol. 1984;103:129–141. doi: 10.1016/0012-1606(84)90014-9. [DOI] [PubMed] [Google Scholar]
- Michel JJ, Xiong Y. Human CUL-1, but not other cullin family members, selectively interacts with SKP1 to form a complex with SKP2 and cyclin A. Cell Growth Differ. 1998;9:435–449. [PubMed] [Google Scholar]
- Nakayama K, Nagahama H, Minamishima YA, Miyake S, Ishida N, Hatakeyama S, Kitagawa M, Iemura S, Natsume T, Nakayama KI. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell. 2004;6:661–672. doi: 10.1016/s1534-5807(04)00131-5. [DOI] [PubMed] [Google Scholar]
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Radke S, Pirkmaier A, Germain D. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene. 2005;24:3448–3458. doi: 10.1038/sj.onc.1208328. [DOI] [PubMed] [Google Scholar]
- Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, Dunia I, Levy E, Gong X. Disruption of Gja8 (α8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development. 2002;129:167–174. doi: 10.1242/dev.129.1.167. [DOI] [PubMed] [Google Scholar]
- Rowan S, Conley KW, Le TT, Donner AL, Maas RL, Brown NL. Notch signaling regulates growth and differentiation in the mammalian lens. Dev Biol. 2008;321:111–122. doi: 10.1016/j.ydbio.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto C, Li L, White TW. Connexin50 is essential for normal postnatal lens cell proliferation. Invest Ophthalmol Vis Sci. 2004;45:3196–3202. doi: 10.1167/iovs.04-0194. [DOI] [PubMed] [Google Scholar]
- Shi Q, Banks EA, Yu XS, Gu S, Lauer J, Fields GB, Jiang JX. Amino acid residue Val362 plays a critical role in maintaining the structure of C terminus of connexin 50 and in lens epithelial-fiber differentiation. J Biol Chem. 2010;285:18415–18422. doi: 10.1074/jbc.M110.107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Wang F, Yan Q, Teng Y, Cui H. Inhibition of proliferation of rabbit lens epithelial cells by S-phase kinase-interacting protein 2 targeting small interfering RNA. Mol Vis. 2010;16:907–915. [PMC free article] [PubMed] [Google Scholar]
- Tholozan FM, Quinlan RA. Lens cells: more than meets the eye. Int J Biochem Cell Biol. 2007;39:1754–1759. doi: 10.1016/j.biocel.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Thomsen R, Christensen MH. MolDock: a new technique for high-accuracy molecular docking. J Med Chem. 2006;49:3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG., Jr Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol. 1998;143:815–825. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirbelauer C, Sutterlüty H, Blondel M, Gstaiger M, Peter M, Reymond F, Krek W. The F-box protein Skp2 is a ubiquitylation target of a Cul1-based core ubiquitin ligase complex: evidence for a role of Cul1 in the suppression of Skp2 expression in quiescent fibroblasts. EMBO J. 2000;19:5362–5375. doi: 10.1093/emboj/19.20.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wride MA. Cellular and molecular features of lens differentiation: a review of recent advances. Differentiation. 1996;61:77–93. doi: 10.1046/j.1432-0436.1996.6120077.x. [DOI] [PubMed] [Google Scholar]
- Yin X, Liu J, Jiang JX. Lens fiber connexin turnover and caspase-3-mediated cleavage are regulated alternately by phosphorylation. Cell Commun Adhes. 2008;15:1–11. doi: 10.1080/15419060802253663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ. Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev. 1998;12:3162–3167. doi: 10.1101/gad.12.20.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Kaneda M, Morita I. The gap junction-independent tumor-suppressing effect of connexin 43. J Biol Chem. 2003a;278:44852–44856. doi: 10.1074/jbc.M305072200. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Nakayama K, Nakayama K, Morita I. A novel route for connexin 43 to inhibit cell proliferation: negative regulation of S-phase kinase-associated protein (Skp 2) Cancer Res. 2003b;63:1623–1630. [PubMed] [Google Scholar]
- Zhou JZ, Jiang JX. Gap junction and hemichannel-independent actions of connexins on cell and tissue functions–an update. FEBS Lett. 2014;588:1186–1192. doi: 10.1016/j.febslet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


