Abstract
A biomaterial with both antithrombin and antiplatelet properties is the ideal surface for use in extracorporeal circulation (ECC) as it targets both fibrin generation and platelet adhesion. A hemocompatible surface coating avoids the need for systemic anticoagulation by providing a local anticoagulant effect at the polymer-blood interface. Previous work has demonstrated the potential use of argatroban, a direct thrombin inhibitor, as a nonthrombogenic material for extracorporeal devices. The work reported here focuses on the characterization of argatroban linked to a polyurethane-silicone polymer, CarboSil®. Chemical immobilization, the amount of argatroban, incubation times, and saturation point were evaluated to achieve maximal antithrombin activity at the polymer surface. Cross-linked polymer coatings reacted with 10 and 30 µmole of argatroban were prepared and tested. These coatings resulted in argatroban activity levels of 0.131 µM and 0.446 µM, respectively. After refining the cross-linking process, argatroban activity increased by approximately 3.6 fold. Maintenance of activity and leaching from the polymer surface were also evaluated. Using the refined process for linking argatroban to polymer, the resulting polymer was applied as a surface coating to the inner lumen of poly(vinyl chloride) ECC circuit tubing and its antithrombin effect evaluated using a 4 h rabbit ECC model. Following 4 h of blood exposure, the argatroban circuit demonstrated significantly less thrombus formation compared to the control CarboSil® coating with a 4.1 cm2 thrombus average area for the control coating compared to 1.2 cm2 for the argatroban coating (n=4). There was no significant change in thrombin time from baseline in plasma from animals in which the argatroban coated circuit was used, with a thrombin time of 16.2 s at t=0 and 14.5 s after 4 h. These results demonstrate the potential efficacy of immobilized argatroban as a hemocompatible biomaterial for extracorporeal life support devices.
Keywords: argatroban, direct thrombin inhibitor, extracorporeal circulation, polymer, surface coating, thrombogenicity
Introduction
The major limitation of current extracorporeal circulatory life support (ECLS) devices remains the risk for thrombus formation. The clinical application of extracorporeal devices currently employs systemic anticoagulation to prevent clot formation within the circuit. Systemic anticoagulation, however, places patients at high risk for bleeding, which often can be life-threatening. Although extensive work has been done to develop extracorporeal circuits with nonthrombogenic surface properties, clinical success has been equivocal and the use of such devices has been limited to specific clinical scenarios1–8. The ideal biomaterial for extracorporeal circulatory devices is one that is similar to vascular endothelium. A surface with both a thrombin inhibitor and nitric oxide releasing material is particularly promising as these agents target fibrin generation and platelet activation, respectively, which are both implicit in thrombus formation. A biomaterial with both antithrombin and antiplatelet properties has a superior safety profile over systemic anticoagulation as such a material provides localized anticoagulant activity thereby eliminating the risk of bleeding that occurs with systemic anticoagulation.
The most common systemic anticoagulant used for extracorporeal circulatory life support (ECLS) is unfractionated heparin9–11. Heparin is a complex sulfated glycosaminoglycan with a molecular weight of 3–30 kD12. It produces its anticoagulant effect indirectly by enhancing the activity of antithrombin. Heparin binds to antithrombin via a specific pentasaccharide sequence, and its binding induces a conformational change that potentiates antithrombin’s activity with thrombin by 1000-fold13. Heparin is derived from porcine intestinal mucosa and is a structurally heterogeneous molecule with highly variable pharmacokinetics. The high-affinity pentasaccharide sequence required for the heparin-antithrombin complex is present in only one-third of heparin molecules14, 15. Additional heparin-thrombin binding, however, can occur electrostatically due to heparin’s strong anionic properties. This negative charge also results in nonspecific binding to other molecules including plasma proteins, platelets, and leukocytes16. Close laboratory monitoring is therefore required to ensure that blood heparin levels are within therapeutic range.
In contrast to heparin, argatroban is a highly selective, direct, thrombin inhibitor that reversibly binds to both free and clot-associated thrombin17. Other antithrombotic properties include inhibition of protein C, inhibition of several procoagulant factors (V, VIII, XIII), and inhibition of platelet aggregation17, 18. Argatroban is currently FDA approved for use as an alternative systemic anticoagulant for patients who have a contraindication to heparin due to heparin-induced thrombocytopenia19. Other clinical applications to date include procedural anticoagulation for percutaneous cardiac interventions as well as anticoagulation for extracorporeal membrane oxygenation and renal replacement therapies20–22.
Although both heparin and argatroban function as thrombin inhibitors, argatroban provides several significant benefits over heparin. First, argatroban is a direct thrombin inhibitor that does not require a co-factor (i.e., antithrombin) to impart activity. It also is able to inhibit both circulating and clot-bound thrombin. Second, as a homogeneous synthetic molecule, argatroban offers more predicable pharmacokinetics. Lastly, argatroban does not interact with heparin-induced antibodies and therefore cannot cause the rare but potentially fatal allergic syndrome, heparin-induced thrombocytopenia.
Previous work by Major et al.8 demonstrated the potential for a combined direct thrombin inhibitor and nitric oxide releasing polymer as a nonthrombogenic surface coating for extracorporeal devices. This previous work demonstrated both antithrombin and antiplatelet effects in an ECLS circuit after 4 h blood exposure. However, the level of immobilized argatroban was not optimized for maximum antithrombin activity. In this study, the synthesis of an argatroban-containing polymer for use as a surface coating was refined for in vitro antithrombin activity and further assessed for anticoagulation effects in the 4 h rabbit model of extracorporeal circulation (ECC). The immobilization of argatroban with a cross-linking reagent, incubation times, and saturation point of argatroban were each evaluated in order to increase the yield of argatroban activity at the polymer surface. Surface activity and leaching from the polymer were also measured as a function of time. In vivo activity of tubing coated with the argatroban polymer was evaluated in a 4 h rabbit arterio-venous ECC model.
Materials and Methods
Materials for synthesis of the argatroban polymer solution were obtained from the following: CarboSil® polymer from DSM Biomedical (Berkeley, CA); anhydrous hexane from Fisher Scientific (Fair Lawn, NJ); anhydrous tetrahydrofuran (THF), anhydrous hexamethylene diisocyanate (HMDI), argatroban monohydrate ((2F,4R)-1-[(2S)-5-(diaminomethylideneamino)-2-[[(3R)-3-methyl-1,2,3,4-tetrahydroquinolin-8-yl]sulfonylamino]pentanoyl]-4-methyl-piperidine-2-carboxylic acid) all from Sigma-Aldrich (St. Louis, MO). Hanks Balanced Salt Solution (HBSS) with calcium and magnesium was purchased from Gibco® (Grand Island, NY). Materials for the chromogenic antithrombin assay were obtained from the following: chromogenic substrate (N-p-tosyl-Gly-Pro-Arg-p-nitroanilide acetate) from Sigma-Aldrich (St. Louis, MO); BC thrombin from Siemens (Newark, DE). ECC circuits were constructed from the following: Tygon® poly(vinyl chloride) (PVC) tubing from Fisher Healthcare (Houston, TX); polyurethane angiocatheters, 16-gauge and 14-gauge, from Kendall Monoject Tyco Healthcare (Mansfield, MA).
Synthesis of argatroban coating
Two grams of CarboSil® polymer were dissolved in 25 ml of THF in a flat-bottom cylindrical beaker. For synthesis protocols involving a chemical linker, 2 ml of HMDI (approximately 23.8 mmol) was slowly added and dissolved in the solution by magnetic stirring at room temperature. The duration of incubation varied between experiments (either 1 or 2 h) as described in the text. Excess unbound HMDI was removed by precipitating the polymer and washing with hexane using a filter paper-covered Buchner funnel. The polymer was then allowed to completely air-dry for approximately 15 min. Once dry, the polymer was re-dissolved in 25 ml of THF. Argatroban, purchased in powder form, was dissolved in DMSO to make a stock solution with a concentration of 100 mM. Using the stock solution, the desired amount of argatroban (between 1–30 µmole) was slowly added to the solution containing the polymer with the HMDI cross-linker. Under continuous stirring, the argatroban was incubated at room temperature for the allotted period of time (either overnight or 15 min). Excess argatroban which was not cross-linked to the polymer was removed by precipitating the polymer and washing with deionized water using a filter-covered Buchner funnel. The polymer product was then dried at room temperature overnight. Following this, the product was re-dissolved in 25 ml of THF, yielding the final product for use as a polymer coating.
For the portion of the study aimed at evaluating the need for a chemical cross-linker, a separate solution of polymer containing unlinked argatroban was prepared to compare the antithrombin activity of linked argatroban to free argatroban suspended in polymer. The solution was prepared by dissolving the same amount of CarboSil® in 25 ml of THF. The prescribed amount of argatroban was added directly into the polymer without addition of HMDI and allowed to incubate overnight.
Incorporation of HMDI into the CarboSil® polymer was evaluated by obtaining a 10 µl sample prior to addition of argatroban and analyzing the sample with Fourier transform infrared spectrometry (Nicolet 6700, Thermo Scientific, Madison, WI). The same process was performed to evaluate covalent linkage of argatroban to the polymer. See Figure 1 for representative IR spectra.
Figure 1.
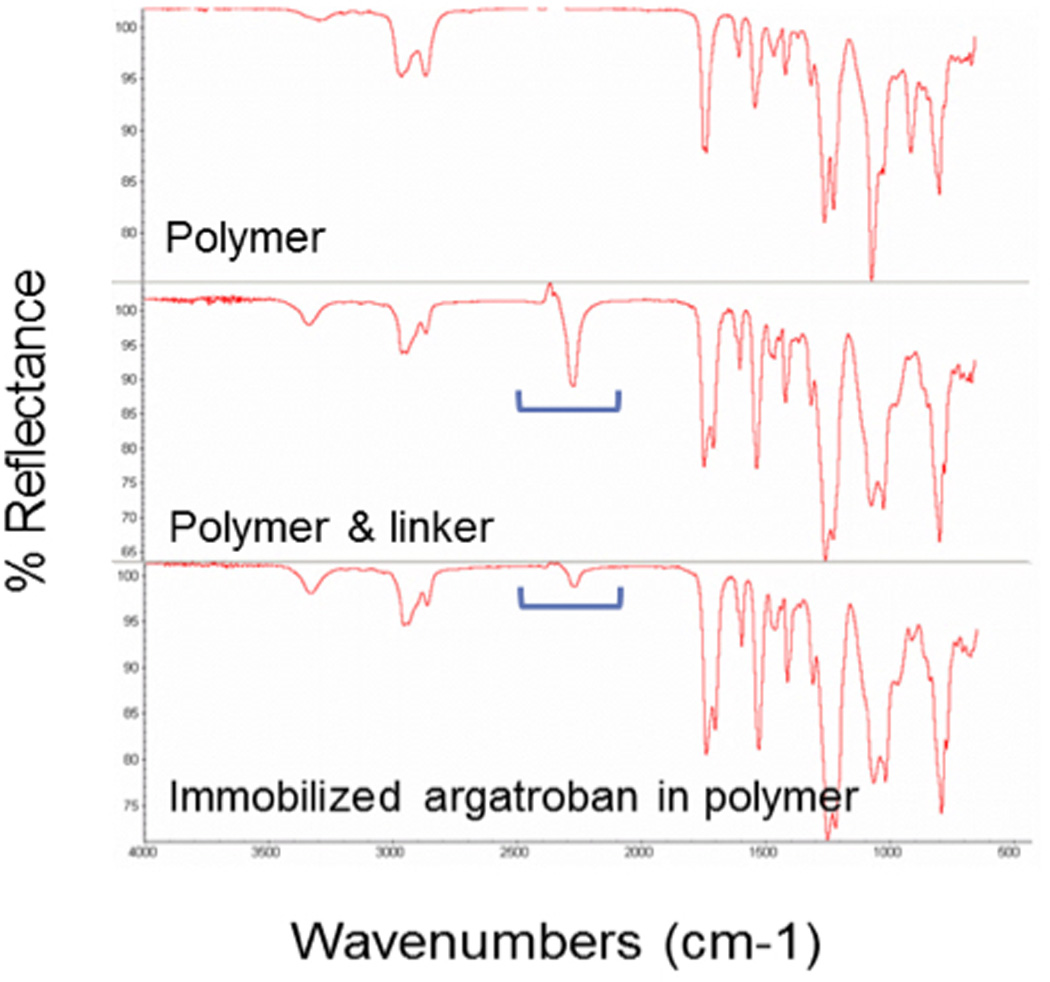
Fourier transformed infrared spectroscopy (FTIR). Top figure illustrates the baseline FTIR spectrum for the polymer, CarboSil®. The peak at 2250 cm−1 occurs in the presence of a NCO group (isocyanate), indicating the incorporation of HMDI into polymer. Reduction of the peak at 2250 cm−1 occurs after incubation of argatroban with HMDI, signifying binding of HMDI’s isocyanate group to argatroban.
Assay of argatroban using an in vitro antithrombin assay
The amount of functional argatroban present in polymer was measured using an in vitro assay. The argatroban polymer solution was applied as a thin film to the bottom of wells within a 96-well microplate using 100 µl of polymer solution. THF was slowly evaporated overnight at room temperature by partially covering the plate with a lid. The amount of argatroban present on the surface of the film was measured by assaying thrombin inhibition using the chromogenic thrombin substrate, N-p-tosyl-Gly-Pro-Arg-p-nitroanilide acetate. Each well was filled with HBSS and left to sit at room temperature for 1 h to allow the tethered argatroban to realign itself in such a way that the active binding site faced the polymer/solution interface. A standard amount of thrombin (0.03 IU/ml) was added to each well and the plate was incubated for 30 min at 37°C. The chromogenic substrate (N-p-tosyl-Gly-Pro-Arg-p-nitroanilide acetate) was then added to each well and incubated for a total of 60 min. Any thrombin that was not inhibited by argatroban hydrolyzed the chromogenic substrate, releasing a chromogen that was measurable photometrically. Change in absorption at 405 nm was quantitated using a Biotek Cytation 3 microplate reader (Winooski, VT). To determine the amount of argatroban present at the surface of the polymer film, results were compared to a standard curve assaying the inhibition of thrombin by free argatroban (0–3 µM).
To evaluate for leaching of argatroban from the film, the wells of a 96-well microplate were filled with 200 µl of HBSS and incubated at 37°C for various times. After the allotted period of time, the buffer solution was removed and a 10 µl sample was analyzed using the thrombin inhibition assay described above.
Preparation of argatroban-coated ECC circuits
The ECC circuit was prepared by connecting two polyurethane angiocatheters, 16-gauge and 14-gauge, to either end of Tygon® poly(vinyl chloride) tubing. The angiocatheters were attached to the tubing by luer-lock PVC connectors and securely adhered via solvent sealing with THF. The Tygon® tubing portion of the ECC circuit consisted of two ¼ inch inner diameter tubing, 16 cm in length, connected by a single ⅜ inch inner diameter tubing, 8 cm in length. This was intentional to create a “thrombogenicity chamber” containing areas within the circuit with turbulent blood flow, in order to promote thrombus formation and better assess the antithrombotic properties of the argatroban containing surface coating.
The inner lumen surfaces of the Tygon® and angiocatheters were coated with the argatroban polymer by instilling the solution into the lumens and then discarding excess solution. The THF solvent was evaporated from the tubing’s inner surface overnight at room temperature. The same process was used to coat the control ECC circuits with CarboSil®. The tubing was then stored at 4°C until ready for use.
Rabbit ECC model
Approval for animal research was provided by the University Committee on the Use and Care of Animals in accordance with University and federal regulations. New Zealand white rabbits (Covance, Battle Creek, MI) were used for this study. Initial anesthesia was administered with intramuscular xylazine at a dose of 5 mg/kg (AnaSed Lloyd Laboratories, Shenandoah, IA) and ketamine at 30 mg/kg (Hospira, Lake Forest, IL). Tracheotomy was performed and anesthesia maintained for the remaining duration of the experiment with the inhaled gas anesthetic, isoflurane, delivered via tracheal tube between 2–3%. Mechanical ventilation was provided with an ADS 2000 Ventilator (Engler Engineering, Hialeah, FL).
Peripheral intravenous access was obtained with a 22-gauge angiocatheter (BD, Franklin Lakes, NJ) placed into a left ear vein. Maintenance IV fluids were run at 10 ml/kg/h. Heart rate and blood pressure were monitored via an arterial line (16-gauge Jelco® angiocatheter, Johnson & Johnson, Cincinnati, OH) placed in the left carotid artery. Core body temperature was measured with a rectal probe. Body temperature was maintained using a water-blanket and an infrared heating lamp.
Arterio-venous ECC was established via the left carotid artery to a branch of the right jugular vein. The 16-gauge angiocatheter was cannulated into the carotid artery and the 14-gauge angiocatheter into the jugular vein using cut-down technique. Flow through the ECC circuit was monitored using an ultrasonic flow probe and flow meter (Transonic HT207, Ithaca, NY). The arterio-venous circuit was run up to 4 h or until the circuit occluded.
After 4 h or at termination of the experiments due to occlusion, the circuit was clamped and removed from the animals. Heparin was administered intravenously (400 U/kg) to prevent postmortem thrombosis. The animals were then euthanized with intravenous pentobarbital (130 mg/kg) (Vortech Pharmaceuticals, Dearborn, MI).
Following completion of the experiment, the circuit was evaluated for gross clot formation by emptying the tubing. It was then rinsed with 60 ml of normal saline in order to assess residual thrombus present on the surface of the inner lumen. A photograph of the tubing was taken and surface area of the thrombus was evaluated by calculating area of clot on the inner surface of the thrombogenicity chamber of the circuit (i.e., the 8 cm length Tygon® tubing with ⅜ inch inner diameter). Clot area was calculated using 2-dimensional image analysis from Image J software (National Institutes of Health, Bethesda, MD), measured as cm2.
Blood sampling
Blood samples were obtained from the arterial line. Samples were obtained at baseline (prior to vessel cannulation for ECC) and hourly following initiation of ECC blood flow. Samples obtained were used to determine arterial blood gas values, activated clotting time, white blood cell count, platelet count, platelet aggregation, fibrinogen levels, and thrombin clotting time. Blood gases were analyzed by an ABL 725 blood-gas analyzer and OSM3 Hemoximeter (Radiometer Copenhagen, Copenhagen, DK) with pH, PaCO2, PaO2, and hemoglobin recorded. Activated clotting time was measured by a Hemochron Blood Coagulation System Model 801 (International Technidyne, Edison, NJ). White blood cell and platelet counts were analyzed by a Coulter Counter Z1 (Coulter Electronics, Hialeah, FL). Platelet aggregation was measured by a Chrono-Log Optimal Aggregometer Model 490 (Chrono-Log, Haverton, PA). Plasma fibrinogen concentrations and thrombin clotting times were measured using a Dade Behring BCS Coagulation Analyzer (Siemens, Deerfield, IL).
Statistical analysis
Data is expressed as mean ± SEM (standard error of the mean). Statistical analysis was performed using SAS JMP (SAS Institute, Cary, NC). Comparison between the in vitro control and argatroban groups was analyzed by the Student’s T-test analysis. Comparison of the parameters from the in vivo CarboSil® controls and the 10 µmole Argatroban/CarboSil® coated ECCs were evaluated using the one-way analysis of variance (ANOVA) with multiple comparisons using Student’s T-test. Statistical significance was determined at the p<0.05 level.
Results
Cross-linking minimizes leaching of argatroban from polymer
As the first step, the need for chemical immobilization to minimize leaching of argatroban from the polymer was evaluated. Argatroban is a dipeptide of arginine and 4-methyl-2-piperidine carboxylic acid with a molecular weight of 509 g/mole23. It possesses two NH2 groups. One group is bound to a methyltetrahydroquinoline sulfonyl group. The second amine was utilized in this study to link argatroban to CarboSil®. Previous work has shown that argatroban maintains its antithrombin properties when immobilized at this free amine site24. In addition, X-ray crystallography studies have demonstrated that occupation of the free NH2 group does not affect argatroban’s antithrombin action as binding of thrombin occurs at the 4-methyl-2-piperidine carboxylic acid and methyltetrahydroquinoline sulfonyl sites25. Therefore, argatroban was immobilized to CarboSil® via chemical modification with the cross-linker, hexamethylene diisocyanate (HMDI). Figure 2a shows the chemical structure of argatroban and figure 2b illustrates how argatroban is linked covalently to CarboSil® via the binding of argatroban’s free amine group to isocyanate of HMDI. Leaching of argatroban from polymer containing immobilized argatroban was compared to leaching from free argatroban suspended within polymer. Polymer solutions were prepared with argatroban either immobilized to polymer or suspended in polymer and were then plated as thin films within wells of a 96-well microplate. After drying to evaporate the solvent, each well was filled with HBSS buffer and incubated at 37°C overnight. The buffer solution was removed the following day. The amount of argatroban present on the surface of the wells and the amount of leached argatroban in the buffer solution were measured. More argatroban activity was found at the surface of the wells coated with free argatroban polymer compared to immobilized argatroban polymer (Figure 3a). This was expected given the additional washing process used to remove excess unbound argatroban during the reaction to link argatroban. Leaching did occur from both polymers but 34 times more leached from the argatroban freely suspended in polymer compared to the immobilized argatroban polymer (Figure 3b). Given these results, covalent linkage with HMDI was selected as the standard procedure for synthesis of the argatroban polymer solution.
Figure 2.
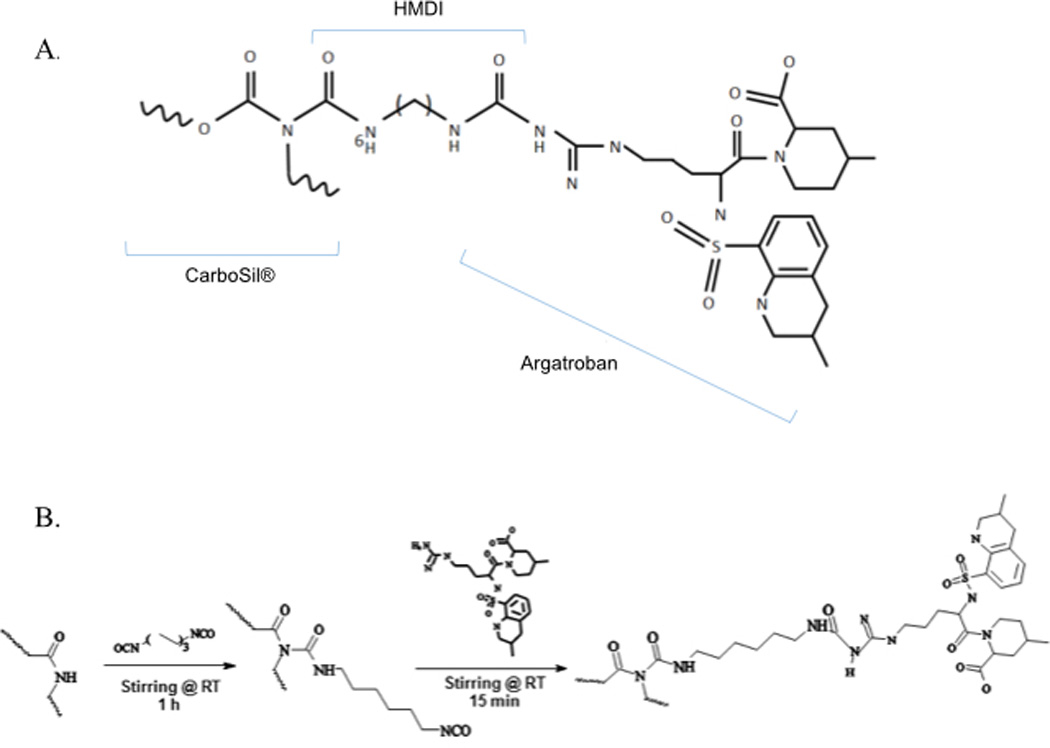
(A) Structure of argatroban linked to CarboSil®. (B) Immobilization of argatroban to CarboSil® is possible due to the presence of a free amine (NH2 group) on argatroban and is shown in the synthesis scheme.
Figure 3.
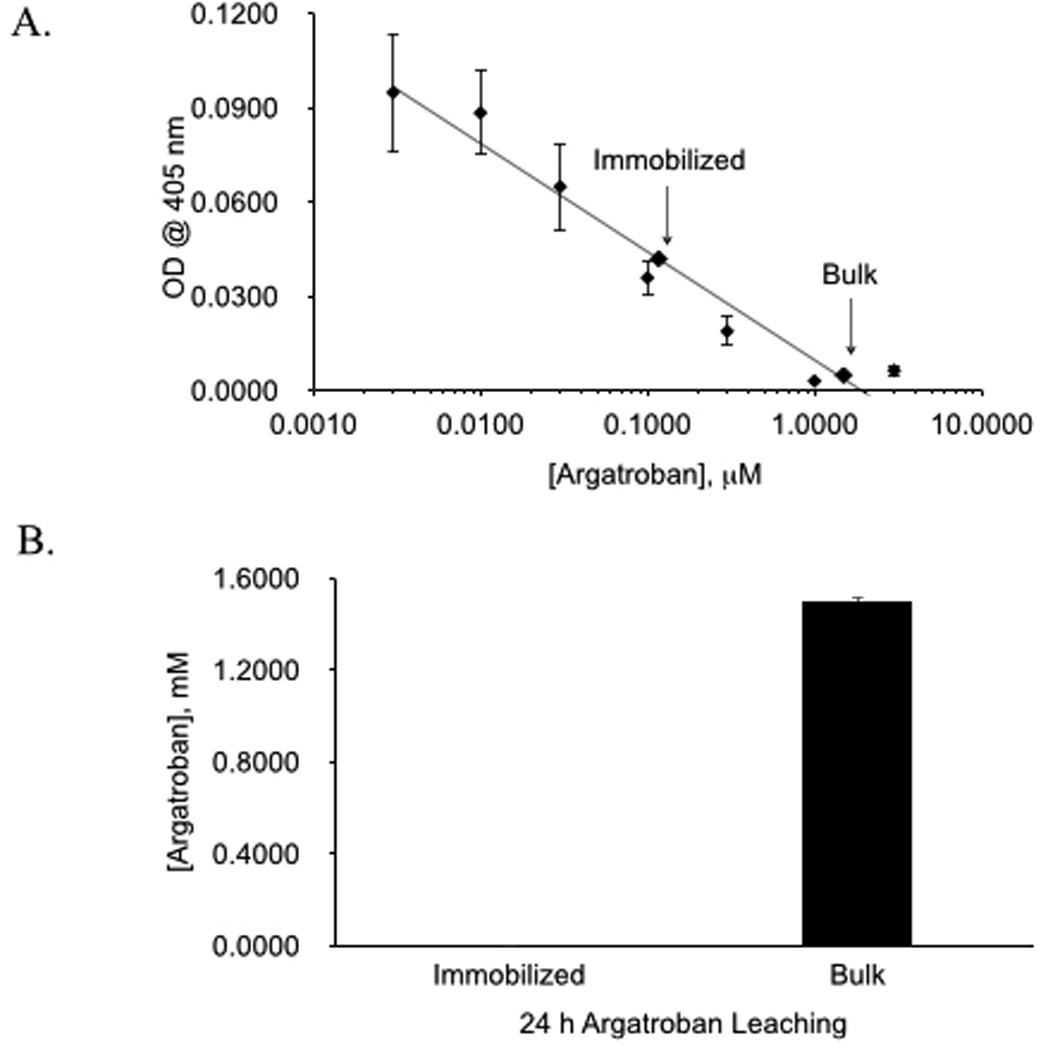
Comparison of surface activity (A) and leaching (B) of argatroban from polymer with immobilized argatroban versus polymer with bulk (unlinked) argatroban. Amount of argatroban was determined using a functional chromogenic thrombin inhibitory assay. Data values are means ± SEM (n=8 replicates per assay with a total of 3 assays). Significance was determined at p<0.05.
Enhancing immobilized argatroban activity in polymer
To determine if there is a threshold amount of argatroban required to exhibit in vitro activity, various amounts of argatroban during cross-linking reaction were evaluated. The antithrombin assay was performed on CarboSil® alone and CarboSil® linked with HMDI to determine background photometric detection levels. OD values were measured and extrapolated to yield a background reading of 0.0036 and 0.0031 µM, respectively, on the argatroban standard curve. Amounts of 1 µmole, 10 µmole, and 30 µmole were used in the reaction for synthesis of the covalently linked argatroban polymer solution. The addition of 1 µmole to the polymer solution resulted in no in vitro activity with an antithrombin assay result of 0.0032 µM (Figure 3). Activity for 10 and 30 µmole of argatroban resulted in proportionally higher levels of activity with levels of 0.131 µM and 0.446 µM, respectively.
The synthesis process was then evaluated in effort to increase argatroban yield at the polymer surface. Modifications were made to the technique established by Major et al.8. Previous work utilized a 2 h incubation period for HMDI incorporation into CarboSil® and overnight incubation for argatroban attachment to CarboSil®/HMDI. The first incubation step between CarboSil® and HMDI was reduced to 1 h. Results showed a higher amount of argatroban activity at the polymer surface with the shorter incubation time, with 0.325 µM argatroban measured at the polymer surface with the standard incubation time and 0.987 µM with the shortened time (Figure 4a). The second incubation step between CarboSil®/HMDI was decreased to 15 min from the previous 24 h. Results showed an even higher argatroban yield at the polymer surface of 1.642 µM with the shorter incubation time (Figure 4b).
Figure 4.
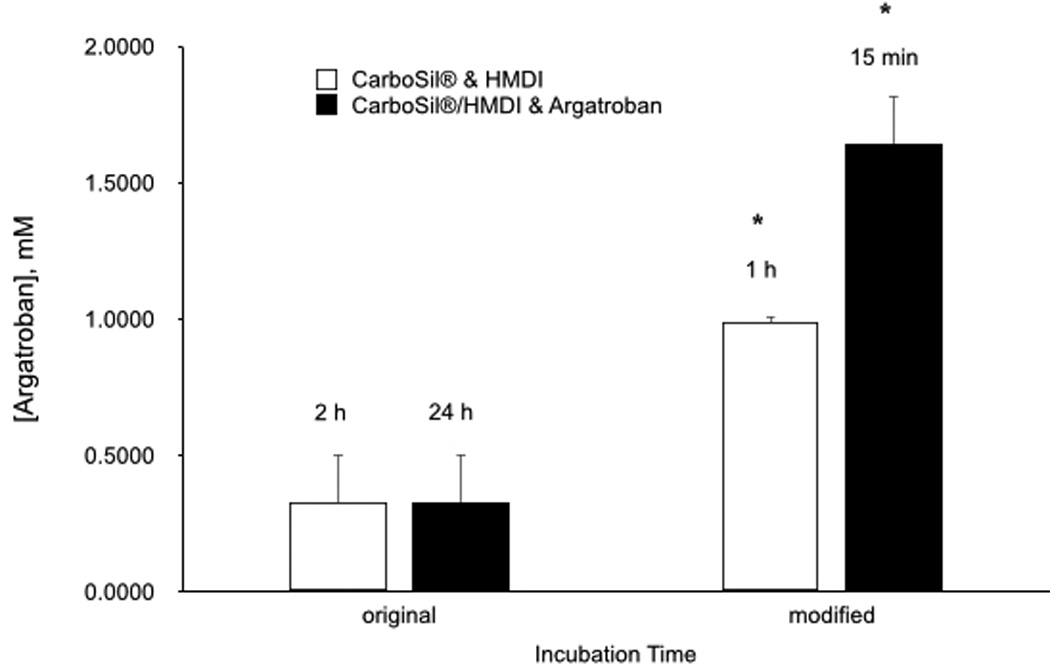
Comparison of in vitro antithrombin activity argatroban activity with modified incubation reaction times. (A) Between polymer and HMDI. (B) Between polymer/HMDI and argatroban. Data values are means ± SEM (n=3). Significance was determined at p<0.05.
In vitro evaluation of surface antithrombin activity was performed to evaluate preservation of argatroban activity over time. Ninety six-well microplates were coated with polymer in which either 1 µmole, 10 µmole, or 30 µmole argatroban was used during linkage reaction. Wells were filled with buffer and incubated at 37°C for allotted lengths of time (1 d, 3 d and 7 d). At the end of the incubation period, the buffer solution was removed from the wells. The plate was evaluated for surface argatroban activity as was the buffer solution. Again, there was a nonsignificant amount of argatroban present for the polymer reacted with 1 µmole of argatroban compared to the no argatroban control. For the polymers reacted with 10 µmole and 30 µmole argatroban, activity progressively decreased at the plate surface with the passage of time (Figure 5). Evaluation of the buffer solution at the same time points showed progressively increased activity, demonstrating that argatroban leached out of the polymer (Figure 7). Leaching occurred over the full 7 d with the most significant amount occurring during the first 24 h. This suggests that some fraction of the argatroban within the polymer is not covalently linked to the CarboSil®.
Figure 5.
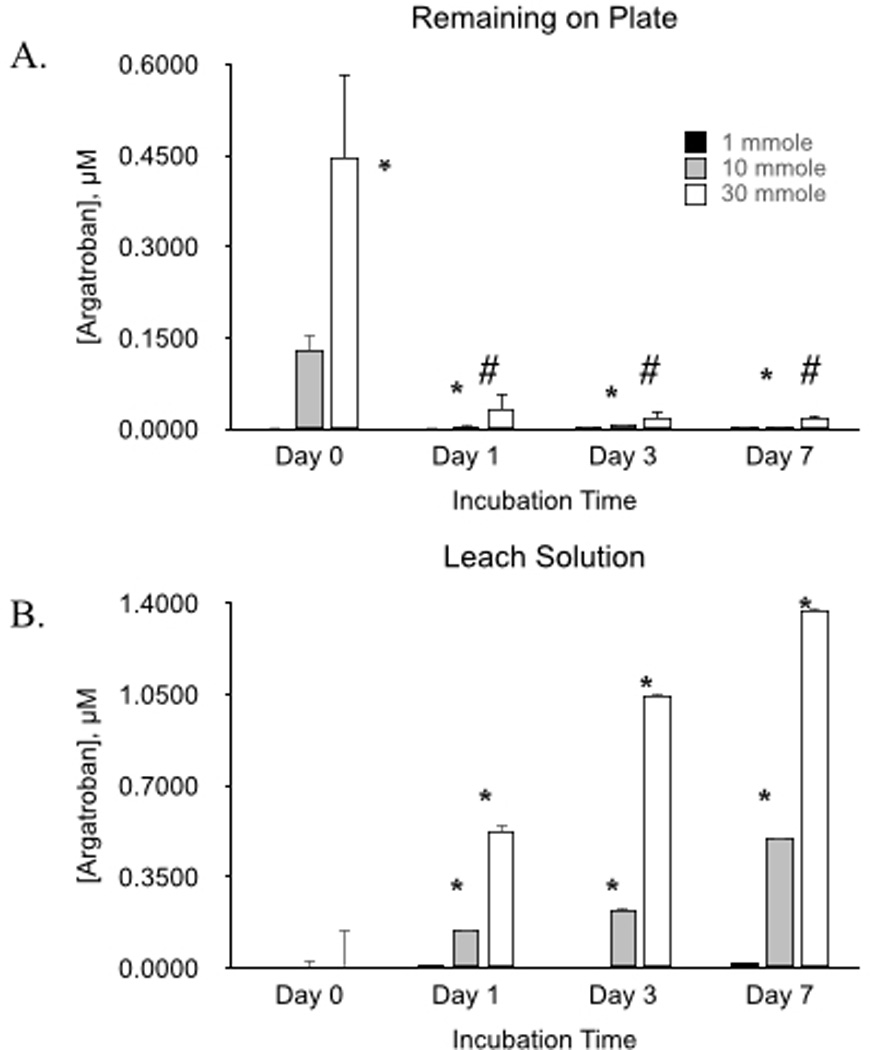
Antithrombin activity of immobilized argatroban (A) and activity of leach solution from coating over time (B). Data values are means ± SEM and n=3. Significance was determined at p<0.05.
Figure 7.
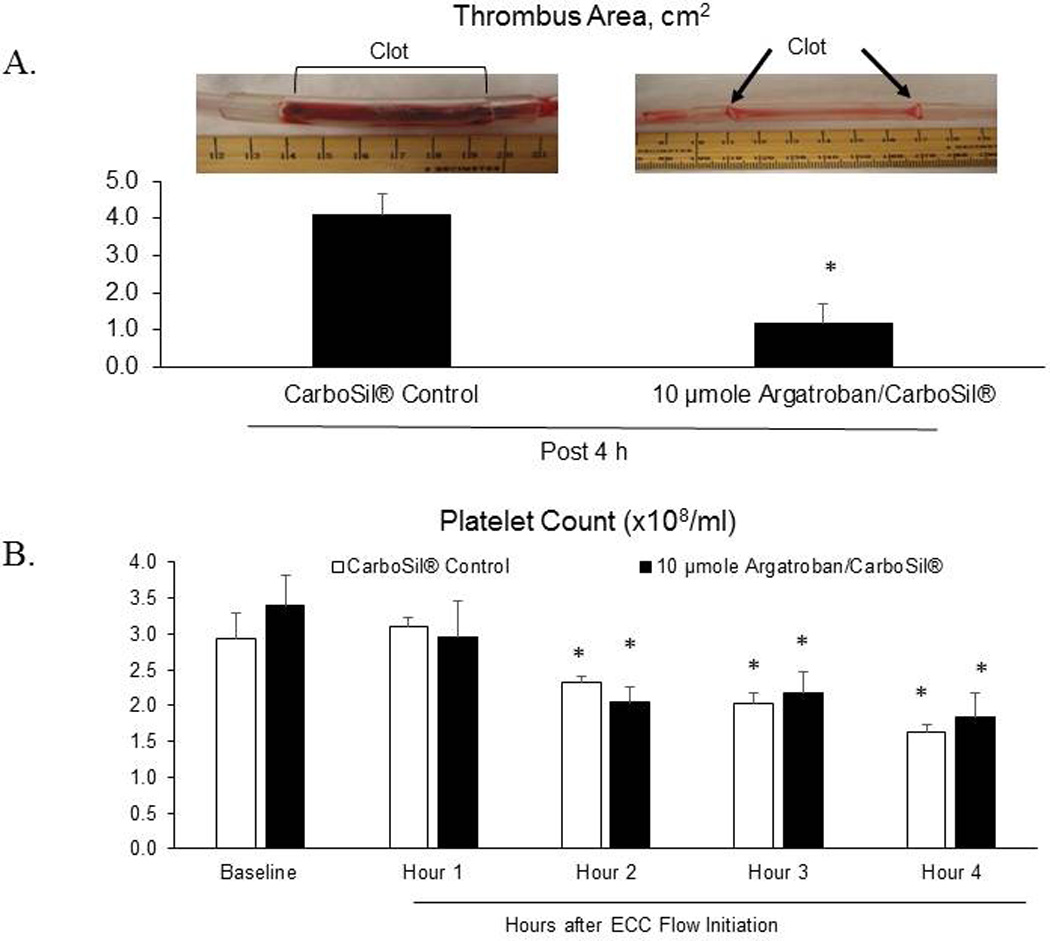
(A) ECC thrombus area and representative images of thrombogenicity chambers showing clot in the CarboSil® control ECC while the 10 µmole Argatroban/ CarboSil® coated chamber had virtually no clot. (B) Platelet count (×108/ml) over the ECC treatment time. Data values are means ± SEM and n=4. Significance was determined at p<0.05.
Saturation of the immobilized argatroban binding sites was evaluated by incubating 96-well coated microplates with 0.03 IU/ml thrombin overnight at 37°C. The plates were evaluated for antithrombin activity the following day. Incubation with thrombin using the same plate was performed for 7 consecutive days. Over the 7 d course of this experiment, argatroban activity did decrease over time, most significantly over the first 24 h period, most likely due to the leaching noted above (Figure 6). Over the subsequent days, there was still activity present albeit at lower levels with a less percentage decrement in activity with each consecutive day (Figure 6b).
Figure 6.
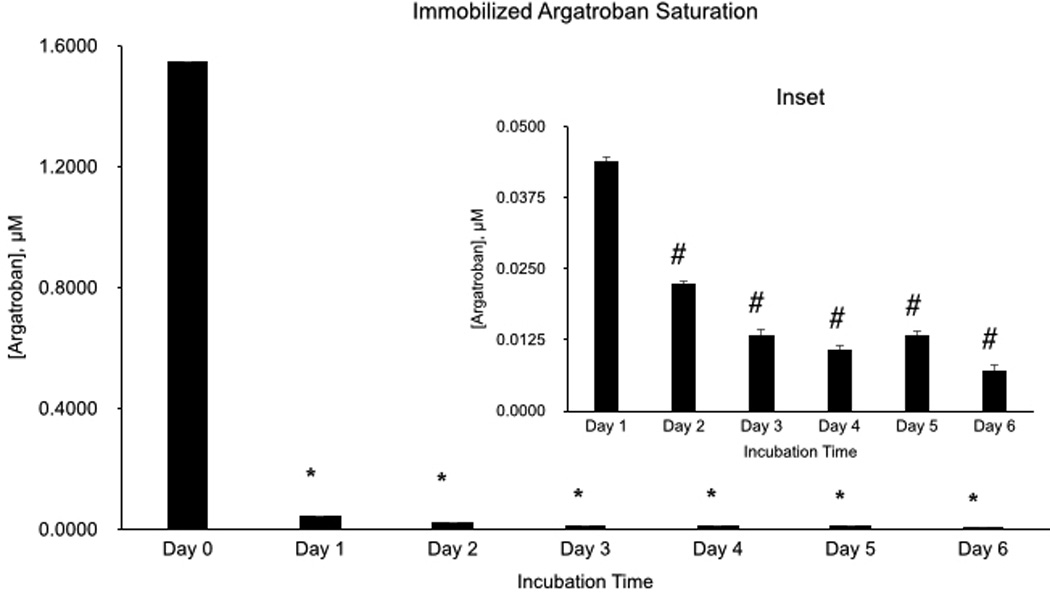
Saturation of argatroban binding sites over time. (Inset) Days 1–7, on a smaller scale. Data values are means ± SEM and n=3. Significance was determined at p<0.05.
Effect of argatroban surface coating on thrombus formation and thrombin clotting time in an in vivo ECC model
Using the refined synthesis process described above and polymer solution cross-linked using 10 µmole argatroban, ECC circuits were coated and tested for up to 4 h in a rabbit model system. The run terminated early if thrombus formation resulted in complete occlusion of blood flow. Previous studies have shown that ECC circuits without antithrombogenic surface coatings clot within 4 h26. Four rabbits were used in both the control group (CarboSil® coating) and the test group (argatroban derivatives CarboSil® coating). Hemodynamic parameters and ECC flow rates were monitored hourly with no significant difference noted between the two groups (Table 1). At the conclusion of the ECC run, surface thrombus area in the tubing was measured. Thrombus formation for the argatroban polymer was significantly reduced compared to the control CarboSil® polymer (Figure 9). Mean area of clot for the control circuits was 4.1 cm2 while mean area of clot for the argatroban circuits was 1.2 cm2 (Figure 7a). Even though the argatroban circuits showed significant reduction in thrombus formation compared to the controls (Figure 7b), argatroban, a direct thrombin inhibitor, is only an anticoagulant; similar to heparin, it has no effect on preventing platelet activation. The platelet count data here confirms this and points to previous work that showed both an anticoagulant and antiplatelet agent, such as nitric oxide, are necessary to completely prevent ECC clotting27.
Table 1.
Hemodynamic parameters and blood counts.
| Treatment | Parameter a | Baseline | Time on ECC (h) b | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
|
CarboSil® control coating |
MAP (mmHg) | 58±4(4) | 39±2(4)* | 38±3(4)* | 36±1(3)* | 37±2(3)* |
| HR (BPM) | 209±13(4) | 198±14(4) | 197±17(4) | 193±14(3) | 196±12(3) | |
| ECC blood flow (ml/min) |
75±1(4) | 73±13(4) | 80±3(3) | 77±10(3) | 66±12(3)* | |
| ACT (s) | 195±8(4) | 199±8(4) | 229±4(4)* | 252±24(3)* | 280±14(3)* | |
| WBC (×103/ml) | 5.2±0.3(4) | 2.8±0.3(4)* | 4.5±0.5(4) | 4.5±0.7(3)* | 3.1±0.4(3)* | |
| Fibrinogen (mg/dl) | 241±11(4) | 225±8(4) | 222±7(4) | 208±10(3) | 199±7(3) | |
|
10 µmole Argatroban coating |
MAP (mmHg) | 58±7(4) | 34±1(4)* | 35±1(4)* | 35±4(4)* | 37±4(4)* |
| HR (BPM) | 214±12(4) | 197±6(4) | 196±6(4) | 204±7(4) | 208±8(4) | |
| ECC blood flow (ml/min) |
77±2(4) | 61±2(4)* | 59±3(4)* | 65±3(4)* | 65±4(3)* | |
| ACT (s) | 182±3(4) | 182±4(4) | 195±5(4) | 223±5(4) | 234±4(4)* | |
| WBC (×103/ml) | 5.4±0.4(4) | 2.9±0.1(4)* | 3.1±0.4(4)* | 3.3±0.5(4)* | 3.9±0.5(4)* | |
| Fibrinogen (mg/dl) | 212±6(4) | 161±7(4)* | 150±2(4)* | 130±7(4)* | 123±7(4)* | |
MAP=mean arterial pressure, HR=heart rate, ACT=activated clotting time, WBC=white blood cell count, PLT=platelet count.
Data values are means ± SEM. (x)= size.
Significance was determined using one-way ANOVA and p<0.05.
As mentioned above, some leaching was observed in vitro despite attempts to optimize the covalent immobilization of argatroban to the polymer. In order to evaluate leaching in vivo, thrombin clotting time of the circulating blood was measured. Thrombin clotting time is the time it takes for fibrin to form in plasma containing an anticoagulant after addition of excess thrombin. The normal reference range is < 20 s28. The mean baselines for the thrombin times in plasma from rabbits with ECC loops containing the argatroban linked polymer or CarboSil® alone were 16.2 s and 14.5 s, respectively (Figure 11). At the end of the 4 h experiment, the mean thrombin times in plasma from rabbits with argatroban linked polymer or CarboSil® alone were 13.7 s or 11.8 s, respectively.
Discussion
Exposure of blood to biomaterials triggers a hypercoagulable response caused by the activation of platelets and dysregulation of the coagulation cascade29, 30. In order to prevent thrombosis within the extracorporeal circuit, antithrombotic therapy is required. The potential for argatroban as a polymer surface coating was first evaluated by Major et al.8. Results from that study showed that argatroban maintains its antithrombin activity when immobilized within a polymer coating, allowing its consideration as a nonthrombogenic coating material for ECLS devices. In that study, Elast-eon™ E2As was used as the polymer backbone and 30 µmole of argatroban was reacted with two types of isocyanate crosslinkers, HMDI and polyethylene glycol-diisocyanate (PEG-DI) to test for maximal argatroban surface activity. Once in vitro surface activity was confirmed, the argatroban polymer was evaluated as a surface coating in a rabbit ECC model with reduced clot formation demonstrated compared to controls.
The studies presented here expand upon this previous work, further testing argatroban linkage to a polymer as an inner surface coating for ECC circuits. For this work, CarboSil®, a polyurethane-silicone copolymer, was used in place of Elast-eon® E2As as Elast-eon® E2A is no longer commercially available. First, the necessity for chemical immobilization of argatroban to prevent excessive leaching was evaluated. As previous comparison of the two isocyanate linkers demonstrated superior activity with HMDI8, HDMI was employed here as the linker of choice. The amount of leaching from polymer containing argatroban incorporated without chemical modification was compared to polymer containing argatroban immobilized to the polymer with HMDI. Significantly more leaching occurred from the unlinked argatroban polymer, 34 times more compared to the immobilized polymer. These results suggest incorporation of argatroban into polymer without chemical cross-linkage is inadequate to prevent mass leaching out of the polymer.
Once requirements for immobilization were established, the threshold amount of argatroban necessary to yield in vitro activity was determined. Polymers cross-linked using 1 µmole, 10 µmole, and 30 µmole of argatroban were evaluated. The addition of 1 µmole to the polymer solution resulted in no activity. Activity from polymer where cross-linking was performed with 10 µmole resulted in an argatroban activity level of 0.131 µM while polymer where cross-linking was performed with 30 µmole resulted in an argatroban activity level of 0.446 µM. Testing of amounts higher than 30 µmole was not performed as previous rabbit studies showed a significant reduction in thrombus formation after 4 h blood exposure from a solution prepared using 30 µmole argatroban8. Results of in vivo testing using 10 µmole are described below.
Next, the argatroban polymer solution synthesis process was analyzed to improve argatroban yield compared to established techniques. Initial synthesis was based on procedures developed by Balakrishnan et al.31 with adjustments made by Major et al.8. This process utilized a 2 h incubation period for HMDI incorporation into CarboSil® and overnight incubation for argatroban incorporation into CarboSil®/HMDI. Because the isocyanate groups of HMDI are highly reactive, there is potential for self-crosslinkage between argatroban molecules as well as the polymer itself. If excessive, the resultant product becomes insoluble in THF. Incubation times were therefore shortened to minimize isocyanate self-crosslinking and increase isocyanate engraftment of argatroban. The first incubation step between CarboSil® and HMDI was reduced to 1 h and the second incubation step between CarboSil®/HMDI and argatroban decreased to 15 min. Antithrombin assay results showed a higher amount of argatroban activity at the polymer surface with the shorter incubations times for both reactions, particularly for the second step (argatroban yield of 1.642 µM) as compared to the work by Major et al.8 which had a yield of 0.03 µM using the previously described procedure. Additional attempts at modifications to the synthesis process may be worthwhile to further enhance immobilized argatroban yield.
In order to assess preservation of activity over time, in vitro activity assays were conducted. For the polymers cross-linked using 10 µmole and 30 µmole argatroban, activity progressively decreased at the plate surface with the passage of time. Evaluation of the buffer solution at the same time points showed progressively increased activity suggesting that the loss of surface activity is due to leached argatroban rather than intrinsic loss of activity from deactivation of the thrombin-binding site. Leaching of argatroban occurred over the full 7 d with the most significant leaching during the first 24 h. For patient safety reasons, leaching from the polymer coating should be avoided as excessive leaching can result in unintended systemic anticoagulation. However, although leaching was noted in vitro, in vivo results described below showed that the degree of leaching was not significant enough to result in levels of systemic anticoagulation. As the goal of nonthrombogenic surface coatings is to locally inhibit any thrombus formation at the blood/biomaterial interface, a small amount of leaching may be tolerable as long as this amount does not reach levels of systemic anticoagulation.
Lastly, the coating’s patency over time was evaluated. Long-term efficacy of antithrombin surface coatings is affected by the availability of thrombin binding sites under dynamic conditions of blood circulation and the constant supply and consumption of clotting factors, including that of platelets and proteases. Although difficult to replicate with an in vitro model, an attempt to evaluate saturation of argatroban binding sites was performed by repeatedly exposing argatroban coatings to thrombin for 7 consecutive days. Over the 7 d course, argatroban activity did decrease over time, most significantly over the first 24 h period, which was most likely due to the leaching noted previously. Over the subsequent days, there was still activity present, albeit at lower levels with a smaller percentage decrement in activity per day. These results suggest that polymer-linked argatroban remains available to bind thrombin via a reversible process after repeated exposure to thrombin.
Using the refined synthesis process described above, the cross-linked argatroban polymer was prepared for in vivo evaluation. An amount of 10 µmole was chosen to compare efficacy of the new preparation vs. the 30 µmole level employed with the E2As polymer in previous work8. The 10 µmole solution prepared with the new synthesis protocol was chosen for direct comparison to previous work rather than the 30 µmole solution as one of the goals of this study was to assess the minimum amount of argatroban necessary to achieve clinical effect. ECC circuits were coated and tested in an arterio-venous ECC rabbit model. Four rabbit circuits were run for both the control group (CarboSil® coating) and the test group (argatroban coating). Hemodynamic parameters and ECC flow rates were monitored hourly with no significant difference noted between the two groups (Table 1). At the conclusion of the ECC run, mean thrombus area for the control circuits was 4.1 cm2 compared to mean thrombus area of 1.2 cm2 for the argatroban circuits, representing a 71% reduction in clot formation for the argatroban containing circuits. Mean thrombus area from previous work using an argatroban coating prepared with 30 µmole was 1.69 cm2 8. This is of particular relevance for cost-effectiveness, as this improvement in synthesis of the argatroban linked polymer using only 10 µmole argatroban during cross-linking resulted in better argatroban incorporation and provided equivalent reduction in thrombus area compared to the previous results reported using 30 µmole.
As mentioned above, for any surface-bound biologically active agents, leaching is of concern as it can result in untoward adverse effects. Leaching was observed in vitro despite attempts to optimize immobilization of argatroban to the polymer by covalent linkage via HMDI. In order to ascertain clinically significant leaching, thrombin clotting time of plasma samples was measured throughout the ECC test period. Thrombin clotting time, measured in s, is the time required for fibrinogen to be converted to fibrin following the addition of excess thrombin. The normal reference range is < 20 s32. Although the thrombin clotting time in plasma from animals that used argatroban coated circuits was slightly longer than that observed in controls, it was still within the normal reference range indicating that the amount of leached argatroban observed at the molecular level during the 4 h time frame is not significant enough to cause an adverse clinical effect. The mechanism of thrombus inhibition demonstrated in this study is likely due to the immobilized argatroban at the blood-biomaterial interface. However, as some leaching of argatroban occurred, the possibility of some antithrombotic effect from small amounts of argatroban leached cannot be ruled out. Further work will include better characterization of the molecular process resulting in the decreased clot formation.
Conclusion
Despite decades of successful extracorporeal device use, the primary obstacle for ECLS applications remains bedside management of anticoagulation. Given the undue and high-risk complication of bleeding associated with systemic anticoagulation, a nonthrombogenic biomaterial surface coating presents itself as a superior alternative. The results presented here further characterize argatroban functionality when linked to a polymer (CarboSil®) demonstrating the feasibility and practicality of synthesis of an argatroban-based polymeric coating. In addition, the evaluation of the argatroban linked polymer coating in an in vivo short-term ECC model demonstrates the potential for success of this approach clinically. Further work is needed to maximize argatroban yield at the polymer/blood interface and minimize leaching in order to preserve activity for longer-term ECLS devices, with the ultimate goal of incorporating these findings to the development of a combined direct-thrombin inhibitor/antiplatelet nitric oxide release surface coating.
Figure 8.
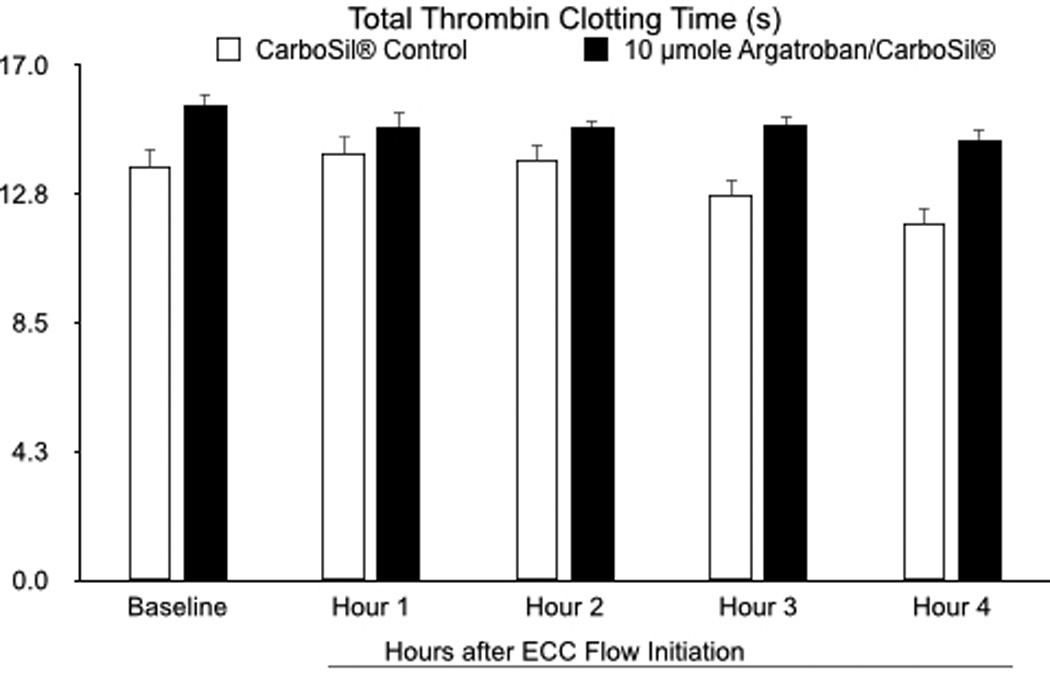
Total thrombin time in s. Data values are means ± SEM and n=3–4. Significance was determined at p<0.05.
Acknowledgments
This work was supported by the National Institutes of Health grant R21 EB016236.
Footnotes
Disclosures
The authors attest that there are no conflicts of interests.
References
- 1.Anton N. Pediatrics (Evanston) 2009;123:e453–e458. doi: 10.1542/peds.2008-1508. [DOI] [PubMed] [Google Scholar]
- 2.Evenepoel P. American journal of kidney diseases. 2007;49:642–649. doi: 10.1053/j.ajkd.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Gorman RC. The Journal of thoracic and cardiovascular surgery. 1996;111:1–11. doi: 10.1016/s0022-5223(96)70395-1. discussion 11–12. [DOI] [PubMed] [Google Scholar]
- 4.Mahmood S, Bilal H, Zaman M, Tang A. Interactive cardiovascular and thoracic surgery. 2012;14:406–414. doi: 10.1093/icvts/ivr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muehrcke DD. The Journal of thoracic and cardiovascular surgery. 1996;112:472–483. doi: 10.1016/S0022-5223(96)70275-1. [DOI] [PubMed] [Google Scholar]
- 6.Ovrum E. Circulation (New York, N.Y.) 1995;92:2579–2584. doi: 10.1161/01.cir.92.9.2579. [DOI] [PubMed] [Google Scholar]
- 7.Shah PS. Cochrane database of systematic reviews. 2014;2:CD005983. doi: 10.1002/14651858.CD005983.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Major TC. Biomaterials. 2014;35:7271–7285. doi: 10.1016/j.biomaterials.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shore-Lesserson L, Manspeizer HE, Bolastig M, Harrington D, Vela-Cantos F, DePerio M. Anesth Analg. 2000;90:813–818. doi: 10.1097/00000539-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14:e77–e84. doi: 10.1097/PCC.0b013e31827127e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchino S. Intensive care medicine. 2007;33:1563–1570. doi: 10.1007/s00134-007-0754-4. [DOI] [PubMed] [Google Scholar]
- 12.Bussey H. Pharmacotherapy. 2004;24:103S–107S. doi: 10.1592/phco.24.12.103s.36109. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg RD. The Journal of biological chemistry. 1973;248:6490–6505. [PubMed] [Google Scholar]
- 14.Wu YI. Blood coagulation & fibrinolysis. 1994;5:83–95. doi: 10.1097/00001721-199402000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Hirsh J. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:1094–1096. doi: 10.1161/hq0701.093686. [DOI] [PubMed] [Google Scholar]
- 16.Hirsh J. The New England journal of medicine. 1991;324:1565–1574. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- 17.McKeage K. Drugs (New York, N.Y.) 2001;61:515–522. doi: 10.2165/00003495-200161040-00005. discussion 523–514. [DOI] [PubMed] [Google Scholar]
- 18.Swan SK. Pharmacotherapy. 2000;20:318–329. doi: 10.1592/phco.20.4.318.34881. [DOI] [PubMed] [Google Scholar]
- 19.Lewis BE. Circulation (New York, N.Y.) 2001;103:1838–1843. doi: 10.1161/01.cir.103.14.1838. [DOI] [PubMed] [Google Scholar]
- 20.Beiderlinden M. Artificial organs. 2007;31:461–465. doi: 10.1111/j.1525-1594.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 21.Rössig L. International journal of cardiology. 2011;148:214–219. doi: 10.1016/j.ijcard.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 22.Link A. Critical care medicine. 2009;37:105–110. doi: 10.1097/CCM.0b013e3181932394. [DOI] [PubMed] [Google Scholar]
- 23.Yeh RW, Jang IK. Am Heart J. 2006;151:1131–1138. doi: 10.1016/j.ahj.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Ueshima S. Blood coagulation & fibrinolysis. 2000;11:631–639. doi: 10.1097/00001721-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Banner DW. The Journal of biological chemistry. 1991;266:20085–20093. [PubMed] [Google Scholar]
- 26.Major TC. Biomaterials. 2010;31:2736–2745. doi: 10.1016/j.biomaterials.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Major TC, Brisbois EJ, Jones AM, Zanetti ME, Annich GM, Bartlett RH, Handa H. Biomaterials. 2014;35:7271–7285. doi: 10.1016/j.biomaterials.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivandic B, Zorn M. Clin Appl Thromb Hemost. 2011;17:549–555. doi: 10.1177/1076029610382651. [DOI] [PubMed] [Google Scholar]
- 29.Gorbet MB, Sefton MV. Biomaterials. 2004;25:5681–5703. doi: 10.1016/j.biomaterials.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Woodman RC, Harker LA. Blood. 1990;76:1680–1697. [PubMed] [Google Scholar]
- 31.Balakrishnan B. Biomaterials. 2005;26:3495–3502. doi: 10.1016/j.biomaterials.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 32.J T, V K, E S. Journal [Google Scholar]


