Abstract
Rituximab is frequently used in systemic lupus erythematosus; however, side effects such as infusion-related reactions limit its use. In this case report, we describe, for the first time, treatment with ofatumumab in four patients with lupus nephritis. The treatment was well tolerated in three of the patients, and a reduction of proteinuria was seen in all cases. This emphasizes the importance of alternative B-cell-depleting therapies in patients with an initial good response to rituximab, but who develop side effects.
Keywords: infusion reactions, lupus nephritis, ofatumumab, rituximab, systemic lupus erythematosus
Background
Rituximab (RTX) treatment is increasingly used in severe systemic lupus erythematosus (SLE), although randomized controlled trials have failed to prove efficacy [1, 2]. The main side effects of RTX are infections, which occur with a frequency of 9–19% [1–3], but infusion-related reactions, reported in ∼10% of patients [1–8], may limit further use in patients with an initial good response to RTX. Alternative B-cell-depleting therapies for patients in whom repeated treatment cycles are contraindicated are thus needed.
We report four patients from the Karolinska SLE cohort with refractory lupus nephritis (LN), who initially experienced beneficial effects of RTX. At retreatment, all patients developed infusion reactions, and due to suspicion that reactions were directed to the mouse component in RTX, ofatumumab, a fully humanized monoclonal anti-CD20 antibody, was given as an alternative B-cell-depleting treatment. All four patients have given their consent to participate in this case report. The study was approved by the Karolinska Institute ethics committee.
Case reports
Case 1
A female patient born in 1989 had a diagnosis of SLE since the age of 8 years. Previous treatment consisted of repeated courses of RTX at a different hospital due to severe lupus. At the age of 23 years, she was given RTX due to LN class V, which did not respond to mycophenolate mofetil (MMF). A minor infusion reaction with chills and fever occurred during the second RTX infusion, but the entire dose could be given.
Because of persistent albuminuria, she was retreated with RTX 9 months later. At the second infusion, she developed chills and fever, and the infusion was stopped. Further therapy with RTX was not given. She continued with antimalarials, prednisolone and MMF in varying doses.
Since the patient still demonstrated signs of active LN [urinary albumin creatinine ratio (U-ACR) 153 mg/mmol], treatment with ofatumumab was initiated 7 months after the second RTX course. It was administered intravenously at a dose of 300 mg on Day 0 and 700 mg on Day 15. No adverse events occurred. MMF was maintained, but as the disease remained active (U-ACR 76–99 mg/mmol), she was retreated with ofatumumab after 14 months (700 mg given twice 3 weeks apart). Since the last infusion, she has continued with antimalarials and MMF. Today, 18 months after the last infusion, U-ACR is 19 mg/mmol.
Case 2
A female patient born in 1975 had a diagnosis of SLE since the age of 29 years. At disease onset, she was given induction therapy with MMF for an LN class IV-G (A). The treatment was later switched to cyclophosphamide (CYC) since nephritis activity was persistent. Repeated biopsy after the induction treatment revealed an LN class IV-S (A). RTX was added with favourable effect, and disease remission was maintained for several years.
Due to disease relapse at the age of 36 years with LN class III (A/C), arthritis and fever, RTX treatment was restarted. The second dose was not given as a delayed infusion reaction (fever, shivering and erythema) occurred 1 week after the infusion. As the disease was active and required further therapy, RTX was switched to ofatumumab, given as a starting dose of 100 mg followed by 600 mg 1 day later and 700 mg 2 weeks later. The U-ACR was 362 mg/mmol. In spite of an initial response to treatment, she relapsed after 3 months (U-ACR 282 mg/mmol), and ofatumumab was repeated. Five months later, U-ACR had decreased to 108 mg/mmol. However, she continued to suffer from a severe non-renal flare, and another dose of 700 mg ofatumumab was given. In the following day, she developed widespread urticaria, and ofatumumab was not repeated.
Case 3
A female born in 1970 had a diagnosis of SLE since the age of 25 years. She was treated with CYC and RTX due to nephritis class IV/V at the age of 31 years. After the third of four planned infusions, she developed chills, fever, hypertension and tachycardia, and the treatment was stopped. Despite the adverse event, she had a good and longstanding treatment effect over the next 7 years.
At the age of 38 years, she presented with arthritis and nephrotic syndrome. Renal biopsy confirmed an LN class V. She was given high doses of corticosteroids in combination with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Because of persisting disease activity with hypoalbuminaemia and skin flare, MMF and antimalarials were added.
Three years later, she relapsed with U-ACR 710 mg/mmol, and RTX was restarted. Despite premedication, she developed chills, fever and back pain requiring observation overnight in the inpatient ward.
Considering the previous beneficial response to B-cell-depleting therapy, ofatumumab was then given in a dose of 700 mg on Days 0 and 14. U-ACR was 645 mg/mmol. She has since then received ofatumumab as maintenance treatment every 6 months in a total of seven cycles. Today, 3 years after initiation of ofatumumab treatment, the U-ACR is 32 mg/mmol.
Case 4
A female born in 1980 had a diagnosis of SLE since the age of 27 years. At the age of 28 years, treatment with RTX in monotherapy was given as a result of LN class V and central nervous system manifestations, which did not respond to conventional therapy. A year later, she was retreated with RTX, now in combination with CYC. During the second infusion, she developed shortness of breath, cough and erythaema, and RTX was discontinued. In the following 3 years, the disease remained in remission.
At the age of 34 years, she relapsed with a U-ACR of 24 mg/mmol. Renal biopsy showed LN class IV-S (A). Given the previous good response to RTX, ofatumumab treatment was initiated with 200 mg at Day 0 without adverse effects and further doses of 700 mg the following day and after 2 weeks. Five months later, cyclosporine A was added due to non-renal lupus activity. Today, 1 year later, her U-ACR is 3 mg/mmol.
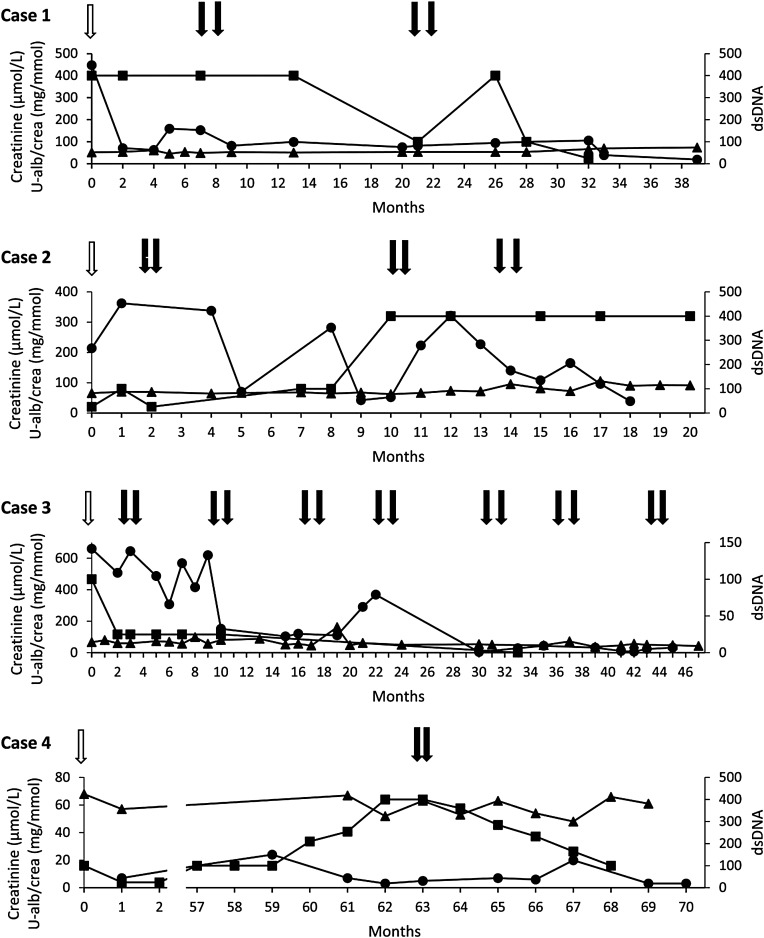
A summary of all cases with previous organ manifestations, treatment and serological profiles is shown in Table 1. Further information on the infusion reactions are given in Table 2. Figure 1 illustrates the time course for treatment and follow-up of all cases with anti-double-stranded sDNA, serum creatinine and U-ACR.
Table 1.
Treatment, disease manifestations and serological profile before and at onset of ofatumumab treatment
| Case | Treatment before onset of ofatumumab | Ongoing treatment at onset of ofatumumab | Disease manifestations before ofatumumab | Disease manifestations at ofatumumab | Autoantibody profile |
|---|---|---|---|---|---|
| 1 | CS, AMA, MMF, RTX, CYC, AZA, MTX | Pred 10 mg/day AMA 200 mg/day MMF 2 g/day |
Arthritis, cytopaenias, LN (class V), malar rash, oral ulcers, photosensitivity, Raynaud's, serositis | Nephritis (no biopsy performed) | ANA, anti-dsDNA, anti-SSA, anti-RNP, anti-Sm, anti-Scl-70, anti-centromere |
| 2 | CS, AMA, MMF, RTX, CYC, AZA | Pred 30 mg/day | Arthritis, fever, hair loss, LN class IV G-(A), class V S-(A) lymphadenopathy, serositis | Arthritis, fever, LN class III (A-C) | ANA, anti-dsDNA, anti-RNP, anti-Sm |
| 3 | CS, AMA, MMF, RTX, CYC, AZA, CyA | Pred 5 mg/day CyA 200 mg/day |
Arthritis, cytopaenias, hair loss, malar rash, nephritis class IV/V, oral ulcers, photosensitivity, Raynaud's | Skin flare, arthritis, LN class V | ANA, anti-ds DNA, anti-RNP, anti-SSA |
| 4 | CS, AMA, MMF, RTX, CYC, BEL, CyA | Pred 10 mg/day AMA 400 mg/day |
Arthritis, cytopaenias, CNS, hair loss, LN class V, meningitis, oral ulcers, photosensitivity | Musculoskeletal, CNS, LN class IV S-(A) | ANA, anti-ds DNA, anti-SSA, anti-RNP, anti-Sm |
CS, corticosteroids; Pred, prednisolone; AMA, antimalarial; MMF, mycophenolate mofetil; RTX, rituximab; CYC, cyclophosphamide; AZA, azathioprin; MTX, methotrexate; CyA, cyclosporine A; Pred, prednisolone; BEL, belimumab; ANA, antinuclear antibody; anti-dsDNA, anti-double-stranded DNA; anti-SSA, anti-Sjögren's-syndrome-related antigen A; anti-RNP, ribonucleoprotein; anti-Sm, anti-Smith; LN, lupus nephritis; CNS, central nervous system.
Table 2.
Cumulative rituximab usage, prophylactic therapy, side effects and consequences of rituximab
| Case | Treatment cycle of rituximab at reaction | Prophylaxis given before rituximab | Side effects of rituximab | Time of reaction | Consequence |
|---|---|---|---|---|---|
| 1 | 6 | Pred 50 mg Paracetamol 500 mg Cetirizine 10 mg |
Fever, shivering | During infusion | Termination of infusion |
| 2 | 2 | Pred 50 mg Paracetamol 500 mg Cetirizine 10 mg |
Fever, shivering, erythema, thrombocytopaenia | After 1 week | Withdrawal |
| 3 | 2 | Pred 50 mg Paracetamol 500 mg Cetirizine 10 mg |
Fever, shivering, back pain | During infusion | Hospitalization over night |
| 4 | 2 | Pred 50 mg Paracetamol 500 mg Cetirizine 10 mg |
Erythema, shortness of breath, coughing, flushing, tachycardia | During infusion | Termination of infusion |
Pred, prednisolone.
Fig. 1.
Follow-up of four patients with lupus nephritis, treated with ofatumumab. The last rituximab dose before switch is indicated by white arrow. Ofatumumab doses are indicated by black arrows. Serum creatinine (triangles, left y-axis), urine albumine/creatinine (U-alb/crea, circles, left y-axis) and anti-double stranded DNA (anti-dsDNA, squares, right y-axis) are shown. Time is indicated on x-axis in months.
Discussion
Infusion reactions following RTX infusions may limit repeated treatment episodes in patients with severe SLE that have insufficient response to conventional therapy. Alternative B-cell-depleting agents could thus increase the treatment choices. Switching to fully humanized monoclonal antibodies directed against CD20 could decrease the risk of reactivity directed against the chimeric component of RTX.
Today, two fully humanized anti-CD20 antibodies are available, ofatumumab and ocrelizumab, but neither is approved for use in SLE. Ocrelizumab in combination with CYC or MMF has been studied in the treatment of proliferative LN. The combination with CYC demonstrated a trend towards a higher renal response rate compared with CYC alone, whereas no difference was found for the combination with MMF compared with MMF monotherapy [9]. The study was terminated early due to more serious infections in the ocrelizumab-treated patients. Data on the use of ofatumumab in SLE are limited [10], and data on treatment effects in LN are lacking.
We report for the first time the use of ofatumumab for the treatment of LN in a series of four adults. All our patients had a history of LN and developed adverse events to RTX, thus limiting further use despite an initial good clinical effect. The reactions observed were mainly type I reactions, but one serum sickness-like reaction was also observed [11].
The optimal dose of ofatumumab in LN has not been determined. In the current report, ofatumumab was mainly given in a dose of 700 mg given twice 2 weeks apart in accordance with a dosing regimen used in a recent study on rheumatoid arthritis [12]. In our case series, the treatment was well tolerated in general, but one patient, who previously demonstrated a delayed reaction to RTX, developed generalized urticaria. We observed a reduction, but not total normalization, of proteinuria in all our cases. The findings are in line with a recent report by Basu [13] on ofatumumab treatment in four children with rituximab-resistant nephrotic syndrome. However, in that report, the dose regimen was substantially higher than the doses used in our report.
In the randomized controlled trials of RTX, infusion reactions were seen in 9.5–16.4% of SLE patients [1, 2]; however, similar frequencies were observed in the placebo groups. Infusion reactions following RTX have been described in several observational studies and registry reports at a frequency of ∼10% [3–8]; however, both lower [14] and higher frequencies have been reported [15]. In some patients, the reactions have been associated with the occurrence of human anti-chimeric antibodies (HACAs), which have been described in patients with serum sickness [1, 16]. Occurrence of HACA was examined in the patient with a serum sickness reaction to RTX, however with negative findings (Case 2, data not shown).
In conclusion, ofatumumab reduced albuminuria in all four cases. We therefore suggest that ofatumumab be considered as an alternative treatment for LN in patients who, after initial good response, have developed reactions to RTX. Randomized controlled trials are needed to prove the efficacy of ofatumumab and to determine the optimal treatment regimen in LN.
Conflict of interest statement
None declared.
References
- 1.Merrill JT, Neuwelt CM, Wallace DJ et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010; 62: 222–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rovin BH, Furie R, Latinis K et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012; 64: 1215–1226 [DOI] [PubMed] [Google Scholar]
- 3.Terrier B, Amoura Z, Ravaud P et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum 2010; 62: 2458–2466 [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Nebro A, de la Fuente JL, Carreno L et al. Multicenter longitudinal study of B-lymphocyte depletion in refractory systemic lupus erythematosus: the LESIMAB study. Lupus 2012; 21: 1063–1076 [DOI] [PubMed] [Google Scholar]
- 5.Gottenberg JE, Guillevin L, Lambotte O et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann Rheum Dis 2005; 64: 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Looney RJ, Anolik JH, Campbell D et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum 2004; 50: 2580–2589 [DOI] [PubMed] [Google Scholar]
- 7.Lu TY, Ng KP, Cambridge G et al. A retrospective seven-year analysis of the use of B cell depletion therapy in systemic lupus erythematosus at University College London Hospital: the first fifty patients. Arthritis Rheum 2009; 61: 482–487 [DOI] [PubMed] [Google Scholar]
- 8.Witt M, Grunke M, Proft F et al. Clinical outcomes and safety of rituximab treatment for patients with systemic lupus erythematosus (SLE)—results from a nationwide cohort in Germany (GRAID). Lupus 2013; 22: 1142–1149 [DOI] [PubMed] [Google Scholar]
- 9.Mysler EF, Spindler AJ, Guzman R et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum 2013; 65: 2368–2379 [DOI] [PubMed] [Google Scholar]
- 10.Thornton CC, Ambrose N, Ioannou Y. Ofatumumab: a novel treatment for severe systemic lupus erythematosus. Rheumatology (Oxford) 2015; 54: 559–560 [DOI] [PubMed] [Google Scholar]
- 11.Gell PGH, Coombs RRA. The classification of allergic reactions underlying disease. In: Gell PGH, Coombs RRA (eds). Clinical Aspects of Immunology. Oxford: Blackwell Scientific, 1963, 317–337 [Google Scholar]
- 12.Taylor PC, Quattrocchi E, Mallett S et al. Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Ann Rheum Dis 2011; 70: 2119–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu B. Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med 2014; 370: 1268–1270 [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Lagares C, Croca S, Sangle S et al. Efficacy of rituximab in 164 patients with biopsy-proven lupus nephritis: pooled data from European cohorts. Autoimmun Rev 2012; 11: 357–364 [DOI] [PubMed] [Google Scholar]
- 15.Catapano F, Chaudhry AN, Jones RB et al. Long-term efficacy and safety of rituximab in refractory and relapsing systemic lupus erythematosus. Nephrol Dial Transplant 2010; 25: 3586–3592 [DOI] [PubMed] [Google Scholar]
- 16.Goto S, Goto H, Tanoshima R et al. Serum sickness with an elevated level of human anti-chimeric antibody following treatment with rituximab in a child with chronic immune thrombocytopenic purpura. Int J Hematol 2009; 89: 305–309 [DOI] [PubMed] [Google Scholar]