Abstract
Background
Improper correction of hyponatremia can cause severe complications, including osmotic demyelination syndrome (ODS). The Adrogué–Madias equation (AM), the Barsoum–Levine (BL) equation, the Electrolyte Free Water Clearance (EFWC) equation and the Nguyen–Kurtz (NK) equation are four derived equations based on the empirically derived Edelman equation for predicting sodium at a later time (Na2) from a known starting sodium (Na1), fluid/electrolyte composition and input and output volumes.
Methods
Our retrospective study included 43 data points from 31 mostly hyponatremic patients. We calculated Na2 based on five sets of rules that were progressively more precisely calculated. Sets A–D included all 31 patients and 43 data points and set E was based on 15 patients and 27 data points.
Results
The root mean square error was calculated and found to be between 4.79 and 6.37 mmol/L (mEq/L) for all sets. Bland–Altman analysis showed high variability and discrepancies between the predicted and actual Na2.
Conclusions
Like similar studies in hypernatremic patients, the data suggest that hyponatremic modeling equations are not reliably accurate in predicting Na2 from Na1 and available clinical data regarding sodium, potassium and fluid balance over longer time frames (12–30 h). Our study was retrospective and was done in an inpatient setting and thus was subject to limitations and laboratory measurement variability, but showed that all four equations are not able to reliably predict Na2 from Na1 and inputs across a 12–30 h period.
Keywords: clearance, hyponatremia, intensive care, nutrition, vasopressin
Introduction
The use of mathematical models to predict changes in sodium (Na) dates back to the Edelman equation, which was described nearly 50 years ago [1]. Using isotopic measurements, Edelman et al. defined the relationship between the serum sodium concentration and the body's content of sodium, potassium and total body water [1]. Subsequent derivations of the Edelman equation have resulted in the Adrogué–Madias (AM), Barsoum–Levine (BL), Electrolyte Free Water Clearance (EFWC) and Nguyen–Kurtz (NK) equations. The Nguyen–Kurtz equation is the most developed derivation and includes the original slope of the Edelman equation (1.03) and the original x-intercept of −23.8, which have physiological significance. This would theoretically be expected to lead to better predictions of changes in serum sodium [1–8].
The importance of being able to predict the sodium level at a later time (Na2) and thus the change in sodium levels clinically is to avoid rapid correction. Correction of serum sodium of >8–12 mmol/day (mEq/day) can result in osmotic demyelination syndrome (ODS) [9–12].
Methods
We designed a retrospective study to compare the predicted Na2 with the observed Na2 using four equations. Our resulting cohort was composed of 31 patients: 29 hyponatremic patients and 2 hypernatremic patients. We reviewed 156 charts and included 31 patients, from which 43 data points were calculated. Eight patients had multiple serial non-overlapping data points calculated (between two and four), but only one data point was calculated on 23 of the 31 patients (Table 1).
Table 1.
The Adrogué–Madias, Barsoum–Levine, EFWC and Nguyen–Kurtz equations
| Adrogué–Madias equation | Na2 = [(Na1 × TBW) + (Vi × ([Na]i + [K]i)]/(TBW + Vi) |
| Barsoum–Levine equation | Na2 = [(TBW × Na1) + ((Vi × ([Na]i + [K]i)) − (Vo × ([Na]o + [K]o))]/(TBW + ΔV) |
| Electrolyte Free Water Clearance equation | Na2 = (Na1 × TBW)/[(TBW − (Vurine*EFWC)], where EFWC = 1 − (([Na]urine + [K]urine)/Na1)) |
| Nguyen–Kurtz equation | Na2 = [[[(Na1 + 23.8) × TBW] + [1.03 × ([Na]i + [K]i) − ([Na]o + [K]o)]]/[(TBW + ΔV)]] − 23.8 |
| Nguyen–Kurtz equation hyperglycemia correction | Add [0.016 × (s[Glucose] − 120)] |
| Total body water | 0.6 × Body Weight |
ΔV, net change in volume; EFWC, Electrolyte Free Water Clearance; [K]i, concentration of potassium in input fluid; [K]o, concentration of potassium in output fluid; [K]urine, urinary potassium; Na1, original sodium; Na2, predicted sodium; [Na]i, concentration of sodium input fluid; [Na]o, concentration of sodium in output fluid; [Na]urine, urinary sodium concentration; s[Glucose], serum glucose concentration; TBW, total body water; Vi, volume of input fluids; Vo, volume of output fluids; Vurine, volume of urine. All volume units, unless otherwise noted, are as follows: volume as liters, concentration units as millimoles/liter or equivalently as milliequivalents/liter, body weight and total body water weights in kilograms.
To be included in the data set, the patient was required to be dysnatremic at the time of presentation (Na <135 or >145 mmol/L), have been admitted into the step-down unit or the intensive care unit, have available serial chemistry panels and urinary electrolytes including urine sodium and potassium, have available accurate weights and have strict ins and outs recorded. In addition, the electrolyte content and volume of all infused and ingested fluid had to be available, with preference given to patients with little to no intake by mouth (see Table 2). The requirements to be included in the idealized set E were more rigorous, as outlined in Table 2. The serum electrolytes were generally checked every 6–8 h and urine electrolytes had to be checked at least every 12–24 h. Since this was a retrospective study, standardization of serum and urine electrolyte monitoring was not possible. The initial serum sodium on presentation, etiologies of dysnatremia and intravenous fluids given are presented in Table 3. The term intravenous fluids refers to 3%, 0.9%, 0.45% or D5 0.225% normal saline. It is important to note that none of the 31 patients in the study required 3% normal saline.
Table 2.
Inclusion and exclusion criteria for a retrospective, observational study of the hyponatremia correction calculation
| Sets A–D: Number of eligible patients 31, number of data points 43, ≥18 years of age, Na <135 or >145 mmol/L, serial chemistry values available between Na1 and Na2, urinary sodium and potassium available, weights available, step-down unit or intensive care unit admission, no blood products. Set E: Number of eligible patients 15, number of data points 27, as well as all the aforementioned criteria and also the ability to calculate all sodium/potassium input and output and to keep track of all fluid infused and eliminated from the patient. |
Table 3.
Etiologies of hyponatremia, sodium values, intake data and output data
| DP # | Pt # | Na1 | Set E? | Etiology of dysnatremia | Wt | uNa | uK | IV fluid | FH2O | Liquid food intake | ONS/TF | K intake | Na intake | UOP | Gluc | Na2 | T |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 115 | No | Hypervolemia | 54 | 10 | 11 | 0 | 0.336 L | 0 | 0 | 0 | 0 | 1.54 | 7.94 (143) | 121 | 16 |
| 2 | 2 | 129 | No | SIADH | 72.9 | 52 | 16 | 3.75 L NS, 0.5 L 1/2NS | 0 | 0 | 0 | 0 | 0 | 2.3 | 6.5 (117) | 127 | 12 |
| 3 | 3 | 119 | No | SIADH | 56.8 | 36 | 24 | 0.2 L (NS with 40 mmol/L kcl) | 0.38 L | 0.39 L tea, 0.24 L coffee | 0 | 13.79 | 0 | 0.45 | 6.22 (112) | 123 | 24 |
| 4 | 4 | 117 | Yes | Hypovolemia | 70 | 10 | 11 | 0.325 L NS | 0.1 L | 0.36 L milk | 0 | 17.96 | 0 | 2.3 | 8.78 (158) | 125 | 16 |
| 5 | 5 | 125 | No | Hypervolemia | 81.4 | 10 | 55 | 0.5 L NS | 0 | 0 | 0 | 0 | 0 | 0.1 | 8.06 (145) | 123 | 23 |
| 6 | 6 | 128 | Yes | Hypovolemia | 63.6 | 10 | 27 | 0 | 0.47 L | 0 | 0 | 40 | 0 | 0.75 | 11.33 (204) | 131 | 14 |
| 7 | 7 | 148 | No | Free water deficit | 40.2 | 110 | 12 | 0.45 L NS, 1.34 L 1/2NS | 0.435 L | 0 | 0.48 L Ensure | 20.21 | 0 | 3.82 | 5.89 (106) | 152 | 24 |
| 8 | 8 | 126 | No | SIADH | 43.7 | 60 | 29 | 0 | 0.44 L | 0.24 L milk | 0 | 11.97 | 0 | 1.05 | 9.78 (176) | 126 | 19 |
| 9 | 9 | 125 | No | SIADH | 78.8 | 125 | 73 | 0 | 1.5 L | 0.24 L tea, 0.58 L coffee | 0 | 11.28 | 0 | 0.45 | 8.94 (161) | 127 | 19 |
| 10 | 10 | 131 | No | SIADH | 74.3 | 50 | 13 | 0.1 L NS | 1.36 L | 0.4 L coffee | 0 | 8.48 | 0 | 2.65 | 7.22 (130) | 133 | 24 |
| 11 | 11 | 121 | No | Thiazide induced | 51.6 | 182 | 18 | 2.235 L NS | 0.1 L | 0 | 0 | 0 | 0 | 1.7 | 8.33 (150) | 137 | 30 |
| 12 | 12 | 132 | Yes | Hypervolemia | 144 | 15 | 18 | 0 | 1.05 L | 0 | 0 | 0 | 0 | 2.35 | 8 (144) | 135 | 24 |
| 13 | 13 | 150 | No | Free water deficit | 56.7 | 35 | 14 | 1.3 L NS, 3.466 1/2NS | 1.2 L | 0 | 0.3 L Ensure | 12.64 | 0 | 1.73 | 7.44 (134) | 136 | 24 |
| 14 | 14 | 115 | No | Hypovolemia | 45.6 | 15 | 54 | 0.5 L NS | 0.42 L | 0 | 0 | 0 | 0 | 0.75 | 9.39 (169) | 118 | 16 |
| 15 | 15 | 128 | Yes | SIADH | 82.7 | 109 | 65 | 3.7 L NS | 0.4 L | 0 | 0 | 0 | 0 | 2.71 | 6 (108) | 132 | 24 |
| 16 | 16 | 122 | No | Thiazide induced | 55.5 | 22 | 91 | 1.27 L NS | 0.472 L | 0.15 L coffee | 0 | 2.68 | 0 | 1.07 | 11.56 (208) | 122 | 24 |
| 17 | 17 | 115 | Yes | SIADH | 42.6 | 93 | 21 | 2.375 L NS | 0.7 L | 0.24 L milk | 0 | 81.97 | 34.48 | 2.15 | 7.28 (131) | 120 | 21 |
| 18 | 18 | 117 | No | SIADH | 67.2 | 31 | 21 | 0.335 L NS | 0.27 L | 0.48 L coffee, 0.72 L milk | 0 | 44.49 | 0 | 1.82 | 5.5 (99) | 122 | 22 |
| 19 | 19 | 122 | Yes | Hypovolemia | 58.5 | 13 | 60 | 1 L NS | 0.21 L | 0 | 0 | 0 | 0 | 0.5 | 7.78 (140) | 118 | 18 |
| 20 | 20 | 124 | No | Hypovolemia | 68.9 | 24 | 21 | 1.15 L NS, 1.4 L 1/2NS | 0.78 L | 0.6 L milk, 0.24 L tea | 0 | 150.89 | 0 | 1.45 | 43.78 (788) | 129 | 27 |
| 21 | 21 | 134 | No | SIADH | 211 | 96 | 22 | 0.25 L NS | 2.375 L | 0.18 L tea | 0 | 0.69 | 0 | 1.68 | 5.67 (102) | 133 | 20 |
| 22 | 22 | 116 | No | Hypervolemia | 77.8 | 5 | 41 | 0 | 0.53 L | 0.24 L milk, 0.48 L coffee | 0 | 20.54 | 0 | 1.58 | 6.61 (119) | 120 | 23 |
| 23 | 23 | 128 | Yes | SIADH | 72.6 | 100 | 18 | 0 | 0.63 L | 0.12 L apple juice | 0 | 4.05 | 0 | 2 | 11.44 (206) | 137 | 12 |
| 24 | 23 | 137 | – | Data point #2, patient #23 | 72.6 | 100 | 18 | 0 | 0.7 L | 0 | 0 | 0 | 0 | 2.6 | 11.44 (206) | 130 | 12 |
| 25 | 24 | 119 | Yes | SIADH | 55 | 67 | 9.4 | 1.4 L NS | 0.12 L | 0.24 L lemonade | 0 | 1.24 | 0 | 0.7 | 5.56 (100) | 129 | 12 |
| 26 | 24 | 129 | – | data point #2, patient #24 | 55 | 42 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0.75 | 5.56 (100) | 128 | 12 |
| 27 | 25 | 125 | Yes | Hypovolemia | 61.4 | 10 | 49 | 1.5 L NS | 0.06 L | 0.06 L orange juice | 0 | 2.98 | 17.24 | 0.6 | 6.28 (113) | 126 | 12 |
| 28 | 26 | 112 | Yes | Hypovolemia | 62.9 | 17 | 3.4 | 1.425 L NS | 1.545 L | 0 | 0 | 0 | 0 | 1.65 | 8.33 (150) | 122 | 12 |
| 29 | 26 | 122 | – | Data point #2, patient #26 | 62.9 | 16 | 3.6 | 0 | 0.31 L | 0 | 0 | 0 | 0 | 0.29 | 6.89 (124) | 122 | 12 |
| 30 | 27 | 116 | Yes | Hypovolemia | 44.1 | 19 | 18 | 0.5 L NS | 0 | 0 | 0 | 0 | 0 | 2 | 3.94 (71) | 122 | 14 |
| 31 | 27 | 122 | – | Data point #2, patient #27 | 44.1 | 41 | 8.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0.3 | 5.61 (101) | 125 | 12 |
| 32 | 28 | 112 | Yes | Hypovolemia | 74.2 | 10 | 10 | 0.568 L NS | 0.3 L | 0 | 0 | 0 | 0 | 2.34 | 6.17 (111) | 113 | 12 |
| 33 | 28 | 113 | – | Data point# 2, patient #28 | 74.2 | 10 | 52 | 0.932 L NS | 0.1 L | 0 | 0 | 0 | 0 | 0.42 | 6.17 (111) | 116 | 12 |
| 34 | 28 | 116 | – | Data point# 3, patient #28 | 74.8 | 19 | 52 | 1.305 L NS | 0.55 L | 0 | 0 | 0 | 0 | 0.73 | 5.67 (102) | 124 | 12 |
| 35 | 28 | 124 | – | Data point# 4, patient #28 | 74.8 | 19 | 52 | 1.475 L NS | 0.325 L | 0 | 0 | 0 | 0 | 0.56 | 5.67 (102) | 123 | 15 |
| 36 | 29 | 120 | Yes | SIADH | 51 | 29 | 53 | 0.5 L NS | 0.63 L | 0 | 0.64 L 2calHN | 80 | 0 | 0.85 | 5.11 (92) | 118 | 11 |
| 37 | 29 | 118 | – | Data point #2, patient #29 | 51 | 51 | 25 | 0.02 L NS | 0.184 L | 0 | 0.24 L Osmolyte | 12.37 | 0 | 0.74 | 88 (4.89) | 122 | 19 |
| 38 | 30 | 121 | Yes | SIADH | 43.7 | 30 | 66 | 0.544 L NS | 0.414 L | 0.18 L tea | 0 | 0.7 | 0 | 0.58 | 5.33 (96) | 119 | 12 |
| 39 | 30 | 119 | – | Data point #2, patient #30 | 43.7 | 10 | 45 | 0.457 L NS | 0.357 L | 0 | 0 | 0 | 0 | 0.3 | 9.28 (167) | 120 | 12 |
| 40 | 30 | 120 | – | Data point #3, patient #30 | 42.9 | 10 | 23 | 1 L NS | 0.117 L | 0 | 0 | 0 | 7.8 | 0.56 | 9.28 (167) | 122 | 14 |
| 41 | 31 | 118 | Yes | Thiazide induced | 130 | 123 | 85 | 0.475 L NS | 1.105 L | 0 | 0 | 40 | 0 | 5 | 5.94 (107) | 123 | 12 |
| 42 | 31 | 123 | – | Data point #2, patient #31 | 130 | 10 | 65 | 0.5 L NS | 1.701 L | 0 | 0 | 40 | 0 | 2.27 | 5.94 (107) | 125 | 14 |
| 43 | 31 | 125 | – | Data point #3, patient #31 | 129 | 10 | 6.3 | 0 | 0.61 L | 0 | 0 | 0 | 0 | 1.64 | 5.94 (107) | 126 | 12 |
DP #, data point number; Pt#, patient number; Na1, initial serum sodium (mmol/L); set E?, identifies if data point is in idealized set E; Wt, weight (kg); uNa, urine sodium (mmol/L); uK, urine potassium (mmol/L); IV fluid, IV fluid type and amount (L); FH2O, free water intake (oral or intravenous); ONS/TF, oral nutritional supplements or tube feeds; K intake, oral potassium intake (mmol); Na intake, oral sodium intake (from sodium tabs or sodium phosphate; mmol) UOP, urine output (L); Gluc, serum glucose (mmol/L and mg/dL); Na2, final serum sodium; NS, 0.9% normal saline; 1/2NS, 0.45% normal saline; free water, D5W or oral water; KCl potassium chloride; T, time in hours between initial and final serum sodium; Na, sodium; 1 mmol = 1 mEq [standard units] in univalent ions like sodium or potassium.
The predictions were then calculated for all four equations in five sets (A–E) with increasingly complex rules for determining Na2 from Na1, input sodium, input potassium and volume inputs of hypotonic fluid as well as infused intravenous fluids. Intravenous fluids are typically the most important determinant of the change of serum sodium. Set A included only intravenous fluids in the calculations. Sets B, C and D use progressively more input information, which is detailed in Table 4.
Table 4.
How sets were calculated
| Fluid volume considered in calculations? | Electrolyte content considered in calculations? | |
|---|---|---|
| Set A | ||
| IV fluids (NS, 1/2NS etc.) | Y | Y |
| PO/IV KCl repletion | N | N |
| PO/IV free water | N | N |
| Sodium content of Na tabs, electrolyte repletion | N | N |
| Juice/PO liquids (coffee, tea etc.) | N | N |
| Tube feeds | N | N |
| IV packing for IV medications | N | N |
| Solid food intake | N | N |
| Set B | ||
| IV fluids (NS, 1/2NS etc.) | Y | Y |
| PO/IV KCl repletion | N | Y |
| PO/IV free water | N | N |
| Sodium content of Na tabs, electrolyte repletion | N | Y |
| Juice/PO liquids (coffee, tea etc.) | N | N |
| Tube feeds | N | N |
| IV packing for IV medications | N | N |
| Solid food intake | N | N |
| Set C | ||
| IV fluids (NS, 1/2NS etc.) | Y | Y |
| PO/IV KCl repletion | Y | Y |
| PO/IV free water | Y | Y |
| Sodium content of Na tabs, electrolyte repletion | Y | Y |
| Juice/PO liquids (coffee, tea etc.) | Y treated as free water | N |
| Tube feeds | Y treated as free water | N |
| IV packing for IV medications | Y treated as free water | N |
| Solid food intake | N | N |
| Set D | ||
| IV fluids (NS, 1/2NS etc.) | Y | Y |
| PO/IV KCl repletion | Y | Y |
| PO/IV free water | Y | Y |
| Sodium content of Na tabs, electrolyte repletion | Y | Y |
| Juice/PO liquids (coffee, tea etc.) | Y | Y |
| Tube feeds | Y | Y |
| IV packing for IV medications | Y treated as free water | N |
| Solid food intake | N | N |
| Set E | ||
| IV fluids (NS, 1/2NS etc.) | Y | Y |
| PO/IV KCl repletion | Y | Y |
| PO/IV free water | Y | Y |
| Sodium content of Na tabs, electrolyte repletion | Y | Y |
| Juice/PO liquids (coffee, tea etc.) | Y | Y |
| Tube feeds | Y | Y |
| IV packing for IV medications | Y | Y |
| Solid food intake | Minimal solid food intake | Minimal solid food intake |
IV, intravenous; PO, oral; KCl, potassium chloride; NS, 0.9% normal saline; 1/2NS, 0.45% normal saline; Na, sodium; Y, yes; N, no.
For sets A–D, there were 43 data points calculated from the data of 31 patients, which means that for 8 patients multiple non overlapping 12–24 h periods were analyzed and the equations used to make serial predictions about changes in the patient's serum sodium. This varied from two data points for five patients to three data points for two patients and four data points for one patient. The range of initial serum sodium at presentation was 112–132 mmol/L (mEq/L) (sets A–D) for our hyponatremic patients and 148–150 mmol/L (mEq/L) (sets A–D) for our hypernetremic patients.
We then used 27 data points from 15 patients from the aforementioned cohort of 31 patients to make up a set of ‘idealized’ patients where the sodium and potassium intake as well as the fluid intake and output could be most rigorously accounted for (set E). For uniformity, total body water was assessed at 60% of total body weight. For these 15 patients, 7 had one data point and 8 had multiple serial non overlapping data points calculated for their clinical course. For five patients, two non overlapping data points were calculated, for two patients, three data points were calculated and for one patient, four data points were calculated. These 15 patients also did not have significant solid oral intake (liquid oral electrolyte intake was accounted for) or other unaccounted fluid losses.
The range of initial serum sodium on presentation for set E patients was 112–132 mmol/L (mEq/L), but there were no clearly hypernatremic patients in set E. However, there was one patient who had a serum sodium increase from the initial value of 128 to 137 mmol/L (after the first 12 h of therapy), which was faster than optimal guidelines. This patient was corrected downwards with dextrose 5% in water (D5W) (as a hypernatremic patient would be) and this subsequent 12 h period of correction was included in the analysis in set E.
Intermediate calculations and the predicted Na2 were calculated using Excel 2010 (Microsoft, Redmond, WA, USA) and checked using SAS 9.3 (SAS, Cary, NC, USA). Root mean square errors (RMSEs), R2 values and Bland–Altman plots for Na2 were also calculated using both Excel and SAS. Excel 2010 and Illustrator CS2 (Adobe Systems, San Jose, CA, USA) were used to construct the tables and figures.
RMSE and R2 (proportion of variance accounted for) were chosen as the metrics to evaluate the performance of each equation on each data set. RMSE is defined as the square root of the average squared differences (errors) between the observed and predicted Na2 value. The RMSEs were calculated for sets A–E and for each of the four equations (AM, BL, EFWC and NK). Please see Appendix 1 for details on how the RMSEs were calculated.
The AM equation is an output-independent equation and the EFWC equation is an input-independent equation. The values for sodium concentration, potassium concentration and total input volume used for predicting Na2 varied depending on the rules of the set. The RMSE for the EFWC equation was identical for sets A–D since this equation is input independent, while set E's RMSE for the EFWC equation differed from the EFWC RMSE for sets A–D only because fewer data points were used.
Results
The data sets consist of 29 hyponatremic patients and 2 hypernatremic patients (sets A–D) and 43 data points derived from their cases. Of the hyponatremic patients, 9 had hypovolemic hyponatremia, 4 had hypervolemic hyponatremia, 13 had the syndrome of inappropriate antidiuretic hormone secretion (SIADH) and 3 had thiazide-induced hyponatremia. Set E consisted of 15 patients with hyponatremia, with no hypernatremic patients, and 27 data points derived from their cases. Of the hyponatremic patients, six had hypovolemic hyponatremia, six had SIADH, two had hypervolemic hyponatremia and one had thiazide-induced hyponatremia.
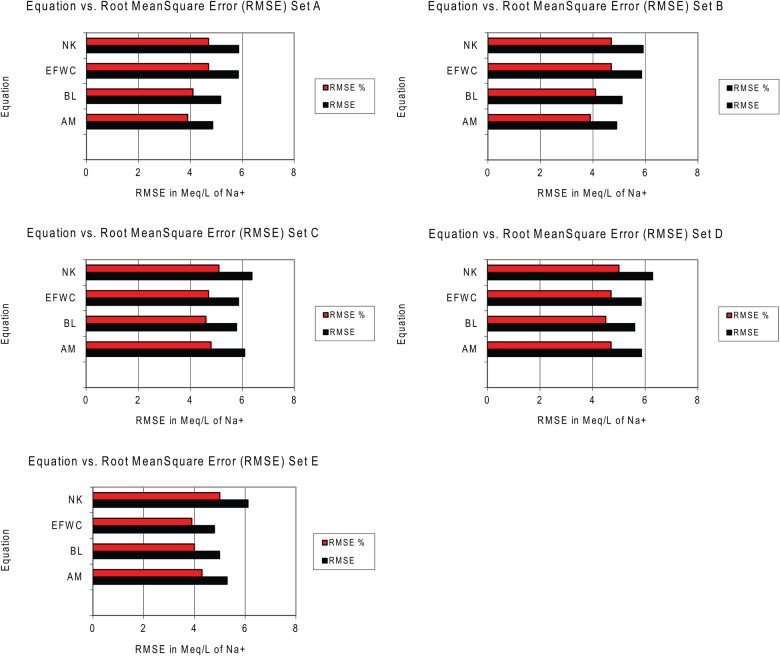
The predicted and calculated serum Na2 were analyzed to assess the accuracy of the four equations. Accuracy was assessed through Bland–Altman analysis, but more importantly the RMSE was used as the parameter of equation accuracy. The RMSEs are presented in Table 5. Across all five sets, the RMSEs were very consistent, between 4.79 and 6.37 mmol/L (mEq/L), and the RMSEs as a percent of the mean Na2 were similar, between 3.9 and 5.1%. The RMSEs are all very similar across all four equations for all data sets. The incorporation of more input information did not seem to systematically lower the RMSE. The RMSE remained similar and was actually slightly lower for the AM equation in some of the simpler sets.
Table 5.
Actual Na2 minus predicted Na2 differences and statistical analysis for sets A–E
| Equation | Set | n | RMSE (RMSE%) | R2 |
|---|---|---|---|---|
| AM | A | 43 | 4.86 (3.9%) | 66.2% |
| BL | A | 43 | 5.17 (4.1%) | 58.9% |
| EFWC | A | 43 | 5.85 (4.7%) | 59.3% |
| NK | A | 43 | 5.86 (4.7%) | 57.4% |
| AM | B | 43 | 4.9 (3.9%) | 64.5% |
| BL | B | 43 | 5.11 (4.1%) | 57.5% |
| EFWC | B | 43 | 5.85 (4.7%) | 59.3% |
| NK | B | 43 | 5.91 (4.7%) | 56.8% |
| AM | C | 43 | 6.09 (4.8%) | 64.5% |
| BL | C | 43 | 5.78 (4.6%) | 54.6% |
| EFWC | C | 43 | 5.85 (4.7%) | 59.3% |
| NK | C | 43 | 6.37 (5.1%) | 56.1% |
| AM | D | 43 | 5.86 (4.7%) | 60.9% |
| BL | D | 43 | 5.6 (4.5%) | 53.9% |
| EFWC | D | 43 | 5.85 (4.7%) | 59.3% |
| NK | D | 43 | 6.28 (5%) | 56.7% |
| AM | E | 27 | 5.29 (4.3%) | 50.8% |
| BL | E | 27 | 4.99 (4%) | 44.3% |
| EFWC | E | 27 | 4.79 (3.9%) | 49.8% |
| NK | E | 27 | 6.11 (5%) | 43.2% |
AM, Adrogué–Madias equation; BL, Barsoum–Levine equation; EFWC, Electrolyte Free Water Clearance equation; NK, Nguyen–Kurtz equation; RMSE, root mean square error. n, number of data points. Units of RMSE in mmol/L, which is equal to mEq/L.
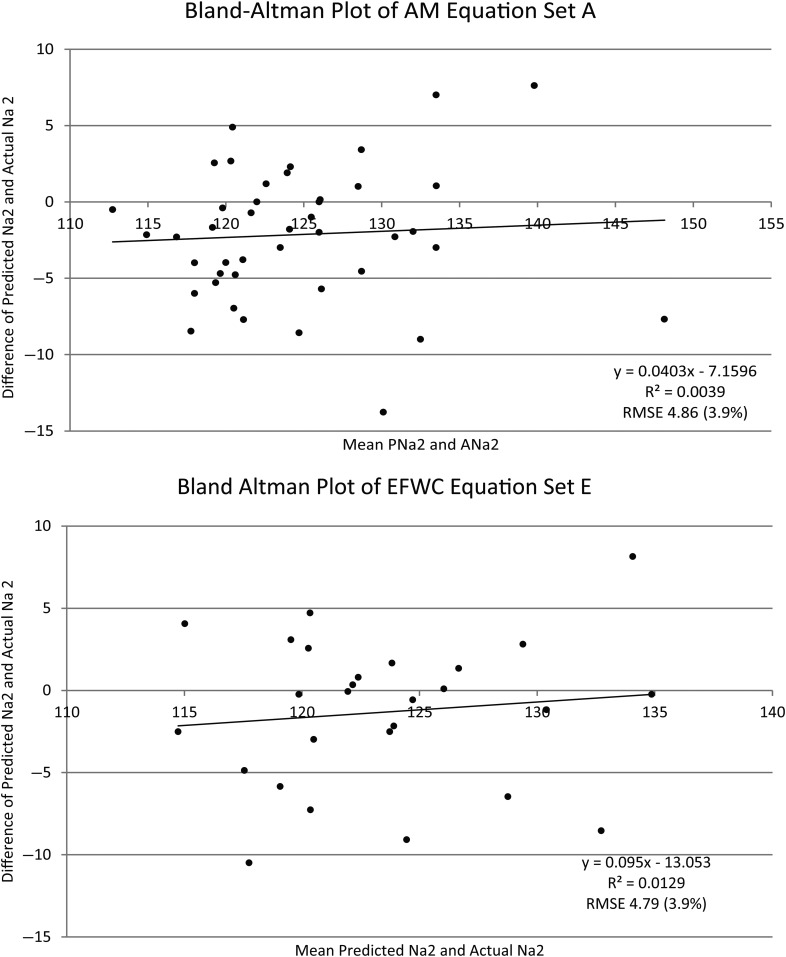
The predicted Na2 versus actual Na2 error ranged from −20.19 to +17.19 mmol/L (mEq/L). The average difference in absolute value between Na2 and Na1 was 4.09 for sets A–D and 3.93 for set E. The range of change in sodium (Na2 − Na1) was −14 to +16 mmol/L (mEq/L). Bland–Altman plots are presented for the two equation data set combinations with the smallest RMSEs (AM equation set A and EFWC equation set E). Even in these best cases, the equation's predictions vary significantly from the observed Na2 (Figure 1).
Fig. 1.
Bland–Altman plots for AM equation set A and EFWC equation set E. AM, Adrogué–Madias equation; EFWC, Electrolyte Free Water Clearance equation; Na2, final serum sodium; RMSE, root mean square error.
Figure 2 shows bar graphs of the RMSEs. While the equations themselves do not change, the inputs and outputs for the same patient and observation can differ across data sets since each data set uses progressively more information. In Figure 2 and Table 5, RMSE values were very similar across all equations and across all data sets. Table 6 shows all the data for the 15 patients and 27 data points for set E, including input sodium, potassium, fluid volume intake and fluid volume output.
Fig. 2.
Bar graphs of the root mean square error (RMSE) for sets A–E. AM, Adrogué–Madias equation; BL, Barsoum–Levine equation; EFWC, Electrolyte Free Water Clearance equation; NK, Nguyen–Kurtz equation; Na2, actual Na2; RMSE, root mean square error, RMSE units in mmol/L or mEq/L (equivalent metric and standard units).
Table 6.
Set E data
| DP # | Pt # | Na1 | Wt | uNa | uK | IV fluid | FH2O | Liquid food intake | ONS/TF | K intake | Na intake | UOP | Gluc | T | Na2 | PNa2 AM | PNa2 BL | PNa2 EFWC | PNa2 NK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 4 | 117 | 70 | 10 | 11.4 | 0.325 L NS | 0.1 L | 0.36 L milk | 0 | 17.96 | 0 | 2.3 | 8.78 (158) | 16 | 125 | 116.65 | 122.08 | 122.41 | 124.87 |
| 6 | 6 | 128 | 63.6 | 10 | 27.2 | 0 | 0.47 L | 0 | 0 | 40 | 0 | 0.75 | 11.33 (204) | 14 | 131 | 127.48 | 129.27 | 129.8 | 131.8 |
| 12 | 12 | 132 | 144 | 15 | 17.9 | 0 | 1.05 L | 0 | 0 | 0 | 0 | 2.35 | 8 (144) | 24 | 135 | 130.4 | 133.11 | 134.75 | 134.38 |
| 15 | 15 | 128 | 82.7 | 109 | 65.3 | 3.7 L NS | 0.4 L | 0 | 0 | 0 | 0 | 2.705 | 6 (108) | 24 | 132 | 128.84 | 126.43 | 125.52 | 123.05 |
| 17 | 17 | 115 | 42.64 | 93 | 20.6 | 2.375 L NS | 0.7 L | 0.24 L milk | 0 | 81.97 | 34.48 | 2.15 | 7.28 (131) | 21 | 120 | 118.71 | 119.16 | 115.11 | 110.56 |
| 19 | 19 | 122 | 58.51 | 13 | 59.6 | 1 L NS | 0.21 L | 0 | 0 | 0 | 0 | 0.5 | 7.78 (140) | 18 | 118 | 122.18 | 122.87 | 122.71 | 121.06 |
| 23 | 23 | 128 | 72.57 | 100 | 18.1 | 0 | 0.63 L | 0.12 L apple juice | 0 | 4.05 | 0 | 2 | 11.44 (206) | 12 | 137 | 125.93 | 126.3 | 128.46 | 131.21 |
| 24 | 23 | 137 | 72.57 | 100 | 18.1 | 0 | 0.7 L | 0 | 0 | 0 | 0 | 2.6 | 11.44 (206) | 12 | 130 | 134.83 | 135.88 | 138.14 | 142.88 |
| 25 | 24 | 119 | 55 | 67 | 9.4 | 1.4 L NS | 0.12 L | 0.24 L lemonade | 0 | 1.24 | 0 | 0.7 | 5.56 (100) | 12 | 129 | 119.23 | 120.1 | 119.91 | 115.73 |
| 26 | 24 | 129 | 55 | 42 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0.75 | 5.56 (100) | 12 | 128 | 129 | 130.81 | 130.8 | 130.65 |
| 27 | 25 | 125 | 61.4 | 10 | 49.2 | 1.5 L NS | 0.06 L | 0.06 L orange juice | 0 | 2.98 | 17.24 | 0.6 | 6.28 (113) | 12 | 126 | 126.27 | 127.33 | 126.08 | 123.53 |
| 28 | 26 | 112 | 62.9 | 17 | 3.4 | 1.425 L NS | 1.545 L | 0 | 0 | 0 | 0 | 1.65 | 8.33 (150) | 12 | 122 | 109.22 | 112.97 | 116.15 | 109.32 |
| 29 | 26 | 122 | 62.9 | 16 | 3.6 | 0 | 0.31 L | 0 | 0 | 0 | 0 | 0.29 | 6.89 (124) | 12 | 122 | 121.01 | 121.79 | 122.79 | 121.47 |
| 30 | 27 | 116 | 44.1 | 19 | 17.6 | 0.5 L NS | 0 | 0 | 0 | 0 | 0 | 2 | 3.94 (71) | 14 | 122 | 116.7 | 123.12 | 122.33 | 128.51 |
| 31 | 27 | 122 | 44.1 | 41 | 8.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0.3 | 5.61 (101) | 12 | 125 | 122 | 122.83 | 122.82 | 121.46 |
| 32 | 28 | 112 | 74.2 | 10 | 10 | 0.568 L NS | 0.3 L | 0 | 0 | 0 | 0 | 2.338 | 6.17 (111) | 12 | 113 | 111.79 | 116.77 | 117.05 | 118.44 |
| 33 | 28 | 113 | 74.2 | 10 | 52.4 | 0.932 L NS | 0.1 L | 0 | 0 | 0 | 0 | 0.42 | 6.17 (111) | 12 | 116 | 113.59 | 114.07 | 113.48 | 112.79 |
| 34 | 28 | 116 | 74.8 | 19 | 52.4 | 1.305 L NS | 0.55 L | 0 | 0 | 0 | 0 | 0.73 | 5.67 (102) | 12 | 124 | 115.7 | 116.4 | 116.73 | 113.17 |
| 35 | 28 | 124 | 74.8 | 19 | 52.4 | 1.475 L NS | 0.325 L | 0 | 0 | 0 | 0 | 0.56 | 5.67 (102) | 15 | 123 | 124.08 | 124.72 | 124.66 | 121.01 |
| 36 | 29 | 120 | 51 | 29 | 52.5 | 0.5 L NS | 0.63 L | 0 | 0.64 L 2calHN | 80 | 0 | 0.85 | 5.11 (92) | 11 | 118 | 119.55 | 120.57 | 121.08 | 116.42 |
| 37 | 29 | 118 | 51 | 51 | 25.2 | 0.02 L NS | 0.184 L | 0 | 0.24 L Osmolyte | 12.37 | 0 | 0.735 | 88 (4.89) | 19 | 122 | 117.21 | 118.2 | 119.01 | 118.47 |
| 38 | 30 | 121 | 43.7 | 30 | 65.9 | 0.544 L NS | 0.414 L | 0.18 L tea | 0 | 0.7 | 0 | 0.575 | 5.33 (96) | 12 | 119 | 119.06 | 119.56 | 121.55 | 116.85 |
| 39 | 30 | 119 | 43.7 | 10 | 44.5 | 0.457 L NS | 0.357 L | 0 | 0 | 0 | 0 | 0.3025 | 9.28 (167) | 12 | 120 | 118.02 | 118.74 | 119.75 | 118.31 |
| 40 | 30 | 120 | 42.9 | 10 | 23 | 1 L NS | 0.117 L | 0 | 0 | 0 | 7.8 | 0.56 | 9.28 (167) | 14 | 122 | 121.03 | 122.91 | 121.92 | 122.12 |
| 41 | 31 | 118 | 130 | 123 | 84.9 | 0.475 L NS | 1.105 L | 0 | 0 | 40 | 0 | 5 | 5.94 (107) | 12 | 123 | 117.08 | 111 | 112.51 | 122.5 |
| 42 | 31 | 123 | 130 | 10 | 64.5 | 0.5 L NS | 1.701 L | 0 | 0 | 40 | 0 | 2.265 | 5.94 (107) | 14 | 125 | 123.04 | 122.44 | 124.42 | 122.66 |
| 43 | 31 | 125 | 129.4 | 10 | 6.3 | 0 | 0.61 L | 0 | 0 | 0 | 0 | 1.635 | 5.94 (107) | 12 | 126 | 125.01 | 126.32 | 127.33 | 126.57 |
DP #, data point number; Pt #, patient number; Na1, initial serum sodium (mmol/L); Wt, weight (kg); uNa, urine sodium (mmol/L); uK, urine potassium (mmol/L); IV fluid, IV fluid type and amount in (L); FH2O, free water intake (oral or intravenous); ONS/TF, oral nutritional supplements or tube feeds; K intake, oral potassium intake (mmol); Na intake, oral sodium intake (from Na tabs or Na phosphate; mmol); UOP, urine output (L); Gluc, serum glucose (mmol/L and mg/dL); T, time in hours from Na1 to Na2; Na2, final serum sodium; NS, 0.9% normal saline; 1/2NS, 0.45% normal saline; KCl, potassium chloride, 17.24 mEq Na in 1 g sodium chloride tab; Na, sodium, K, potassium; 1 mmol = 1 mEq[standard units] in univalent ions like sodium or potassium; PNa2, predicted Na2; AM, Adrogue–Madias equation; BL, Barsoum–Levine equation; EFWC, Electrolyte Free Water Clearance equation; NK, Nguyen–Kurtz equation.
Discussion
The statistical analysis that we have submitted was focused on the RMSE since this is the clinically relevant measure of the prediction's error. In order to be able to predict if the change in sodium will exceed the recommended limit of 8–12 mmol/L/day (mEq/L/day), the Na2 must be known to within 2–3 mmol/L/day (mEq/L/day) or 1–1.5 mmol/L/12 h (mEq/L/12 h). Since the RMSE or average error of the four studied equations is ∼5–6 mmol/L (mEq/L), this implies that they may not be sufficiently accurate to predict the change in sodium in an inpatient population across 12–24 h time intervals, although one data point had an interval of 30 h. That is, our results show that the RMSEs are too large over the examined time frame to reliably predict the change in serum sodium as precisely as required. For reference, the R2 values would need to be >91% for an RMSE of 2–3 mmol/L/day (mEq/L/day), while the observed R2 values in Table 5 are only ∼43–66%.
Since we cannot expect to predict Na2 from Na1 clinically using these equations over a 12–24 h period, this argues that the equations are not accurate enough to be depended on for prognostication of changes in serum sodium over these time frames. Previous studies have shown that these equations have Pearson coefficient correlations that are highly significant [2], although this may only mean that there is some correlation that is significantly different from zero. It is important that previous studies have shown that these equations are not accurate [2, 13] and that large variations between predicted and observed sodium have long been noted [13]. The RMSE is a better and more clinically relevant parameter for assessing the clinical utility of the equations than correlation coefficients and confirms these observed trends.
The RMSEs observed in the range of 4.79–6.37 mmol/L (mEq/L) of sodium also show that these equations cannot be used to predict a change in sodium in our cohort, where the average observed change in sodium was ∼4 mmol/L (mEq/L) over 12–24 h. Furthermore, since the recommended rate of correction of sodium in hyponatremia is limited to <8–12 mmol/L/day (mEq/L/day), these equations are still too inaccurate to be used alone to calculate an exact infusion rate for intravenous fluids without depending on further close observation of urinary and serum electrolyte parameters. It is also notable that the RMSE did not change as additional factors, such as dietary factors, free fluid intake, etc., were included in the analysis across sets B/C and D/E. Thus, a more rigorous evaluation of input data did not increase the observed correlation or reduce the RMSE of these equations.
This study does not attempt to address the theoretical validity of the four equations used for sodium modeling. Rather, we tested their utility in predicting the change in sodium over a longer time frame (12–24 h) and measured the RMSE as a parameter to evaluate how useful these equations are in clinical practice. Theoretically, these models are based on sound physiology and on the work of Edelman et al. [1] and many other renowned nephrologists. The main problems stem from (i) the difficulty in obtaining input values with the accuracy and precision possible in research laboratory settings; (ii) using constant inputs like urine electrolytes that change over time, although how rapidly they change is unclear and may be different depending on the etiology of hyponatremia and other pathophysiological and physiological factors; and (iii) the possible need for further modifications to one or more of these equations to predict Na2 from Na1 over longer time periods or the provision of a time frame where these linear equations can provide an accurate approximation of changes in serum sodium. Of crucial importance is how long urine electrolytes can be assumed to be constant or nearly so.
Many patients with hyponatremia have a dynamic volume status and changing urine electrolytes as the kidneys adjust to treatment (i.e. antidiuretic hormone suppression after volume resuscitation or diuretic withdrawal). Thus, this may limit the usefulness of these equations, which presume static volume status and urinary electrolytes and therefore ignore changes in these parameters over time. Limitations of patient charting and lab error, which are intrinsic to any inpatient setting, must also be taken into account.
Another matter to consider is error related to weight measurement and difficulties in precisely ascertaining the percentage of total body water. For uniformity, total body water was assessed as 60% of total body weight, though in actuality total body water can vary among patients due to physiologic differences of age, gender, volume status, lean body mass, nutritional status and the effects of underlying pathophysiological processes.
Certainly these equations may be accurate or provide a better approximation of the change in sodium in a laboratory setting or if used to predict the change in sodium across a shorter time interval (which would make the assumption of static conditions more accurate). This remains to be seen in other studies, but for now, sodium modeling based on these four equations must be supplemented by frequent laboratory analysis and an attentive nephrologist who will pay close attention to these dynamic conditions.
This is especially important given the greater precision required in correcting hyponatremia that is advocated in recent literature. Previously, a correction of ≤12 mmol/24 h (mEq/24 h) was allotted; now, however, new recommendations are to keep serum sodium correction at ≤10 mmol/L/day (mEq/L/day). There are even more conservative recommendations of maintaining serum sodium correction at <6–8 mmol/24 h (mEq/24 h), since different groups may be at lower or higher risk of complications such as ODS [14–18]. Arginine vasopressin is also being used in patients who have begun to inadvertently overcorrect, or in high-risk cases, as prophylaxis to prevent water diuresis and untoward complications [14, 19]. The risk of overcorrection with the new vasopressin 2 (V2) receptor antagonists is still notable, and ODS has been observed in conjunction with the accompanying water diuresis. Thus, even these new therapeutic options are not exempt from the traditional risks of hyponatremia correction [20, 21].
This points to the importance of preventing inadvertent overcorrection and shows the need for vigilant monitoring of serum electrolytes as the accepted standard of care. The need for better equations, or limits on the use of known equations, to predict Na2 from Na1 are still needed in a clinical environment where precise knowledge of the magnitude of sodium correction can be lifesaving. Alternative approaches of frequent sodium monitoring and the use of D5W and desmopressin to prevent, attenuate or reverse overcorrection are helpful for now [14, 19, 22]. New research regarding the use of minocycline and inositol to prevent the clinical development of ODS after overcorrection is ongoing, but these agents have only been used in animal subjects thus far [23, 24].
Limitations
The data presented are from a retrospective, mostly cross-sectional study and are also impaired by the limitations of charting, though we tried to minimize this by using only extremely clearly charted cases. Laboratory error in the measurement of serum and urine sodium and potassium is also a factor in this study. Moreover, the time interval between the collection of Na1 and Na2 varied from 12 to 24 h (one measurement at 11 h and one measurement at 30 h) and could vary from observation to observation. Further, monitoring of serum sodium and urinary parameters varied and was not able to be standardized given the retrospective nature of the study.
Future directions
A prospective study of eligible hyponatremic or hypernatremic patients may help evaluate these equations better in the inpatient setting and in obtaining more concurrent longitudinal and cross-sectional data. Also possible is an investigation of how extensively and rapidly urinary electrolytes change in patients with hyponatremia caused by different etiologies. This is an important point since it is an implicit assumption of using these linear equations that the urinary electrolytes are not changing, particularly over longer time periods like 12–24 h. If the urinary electrolytes are changing, the rapidity of their change may help predict how long a laboratory test for urinary sodium and potassium is valid for. Another approach would be to study how different etiologies of hyponatremia respond to repletion, and the validity of these equations in each particular patho physiological cause of hyponatremia. A study that includes patients who have been repleted with 3% normal saline as part of the cohort or one that exclusively uses patients who have been repleted with 3% normal saline may also be helpful.
It may prove to be very challenging to verify these equations in the inpatient setting for the reasons stated above, and more controlled settings may be required. Controlled experimental avenues of validating these theoretically derived equations through animal studies, as was recently successfully done with the Edelman equation [25], may ultimately provide more decisive data regarding their accuracy.
Author contributions
R.M.H. helped guide study design, was primarily responsible for the original research, wrote the paper and is the corresponding author. W.-T.Y. contributed to the original research and analyzed the data. E.A.L. contributed to the original research and analyzed the data. J.N.R. contributed to the original research and analyzed the data. J.W. is the principal investigator of the group, created the concept and helped guide the study design.
Conflict of interest statement
None declared.
Acknowledgements
We thank Jeffery Gornbein, DrPH (UCLA SBS) for his assistance with statistical analysis. We acknowledge and thank the staff members of the Olive View Medical Center (OVMC-UCLA) pharmacy, especially Jeffery L. McBath, PharmD, for their assistance in obtaining the concentrations of electrolytes for all intravenous medications. We also would like to thank the members of the Morrison Dietary Department at OVMC-UCLA, especially Cori C. Tempchin, R.D. and Jessica Zakarian-Faber, R.D., for their help in calculating the concentration of electrolytes in liquid food items and tube feeding formulas.
Appendix 1
A) Root Mean Square Error (RMSE): a measurement of average variation of predicted from observed values:
where i is the number of an individual data points,
n is the number of the last observed data point or the total number of observed data points,
yi is the observed value for observation i,
is the predicted value for observation i and
Σ is the sum of the above formula from i = 1 to i = n.
B) Adapting the formula for our calculations :
where Na2 is the actual final sodium for a given data point and PNa2 is the predicted final sodium for a given data point.
References
- 1.Kurtz I, Nguyen MK. Evolving concepts in the quantitative analysis of the determinants of the plasma water sodium concentration and the pathophysiology and treatment of the dysnatremias. Kidney Int 2005; 68: 1982–1993 [DOI] [PubMed] [Google Scholar]
- 2.Lindner G, Schwarz C, Kneidinger N et al. Can we really predict the change in serum sodium levels? An analysis of currently proposed formulae in hypernatraemic patients. Nephrol Dial Transplant 2008; 23: 3501–3508 [DOI] [PubMed] [Google Scholar]
- 3.Nguyen MK. Quantitative approaches to the analysis and treatment of the dysnatremias. Semin Nephrol 2009; 29: 216–226 [DOI] [PubMed] [Google Scholar]
- 4.Nguyen MK, Kurtz I. Determinants of plasma water sodium concentration as reflected in the Edelman equation: role of osmotic and Gibbs-Donnan equilibrium. Am J Physiol Renal Physiol 2004; 286: F828–F837 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen MK, Kurtz I. New insights into the pathophysiology of the dysnatremias: a quantitative analysis. Am J Physiol Renal Physiol 2004; 287: F172–F180 [DOI] [PubMed] [Google Scholar]
- 6.Nguyen MK, Kurtz I. Role of potassium in hypokalemia-induced hyponatremia: lessons learned from the Edelman equation. Clin Exp Nephrol 2004; 8: 98–102 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen MK, Kurtz I. Derivation of a new formula for calculating urinary electrolyte-free water clearance based on the Edelman equation. Am J Physiol Renal Physiol 2005; 288: F1–F7 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen MK, Kurtz I. Quantitative interrelationship between Gibbs-Donnan equilibrium, osmolality of body fluid compartments, and plasma water sodium concentration. J Appl Physiol 2006; 100: 1293–1300 [DOI] [PubMed] [Google Scholar]
- 9.de Souza A, Desai PK. More often striatal myelinolysis than pontine? A consecutive series of patients with osmotic demyelination syndrome. Neurol Res 2012; 34: 262–271 [DOI] [PubMed] [Google Scholar]
- 10.King JD, Rosner MH. Osmotic demyelination syndrome. Am J Med Sci 2012; 34: 262–271 [DOI] [PubMed] [Google Scholar]
- 11.Martin RJ. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry 2004; 75(Suppl 3): iii22–iii28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatewaki Y, Kato K, Tanabe Y et al. MRI findings of corticosubcortical lesions in osmotic myelinolysis: report of two cases. Br J Radiol 2012; 85: e87–e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adrogue HJ, Madias NE. Aiding fluid prescription for the dysnatremias. Intensive Care Med 1997; 23: 309–316 [DOI] [PubMed] [Google Scholar]
- 14.Sood L, Sterns RH, Hix JK et al. Hypertonic saline and desmopressin: a simple strategy for safe correction of severe hyponatremia. Am J Kidney Dis 2013; 61: 571–578 [DOI] [PubMed] [Google Scholar]
- 15.Soupart A, Penninckx R, Stenuit A et al. Azotemia (48 h) decreases the risk of brain damage in rats after correction of chronic hyponatremia. Brain Res 2000; 852: 167–172 [DOI] [PubMed] [Google Scholar]
- 16.Sterns RH, Hix JK, Silver S. Treatment of hyponatremia. Curr Opin Nephrol Hypertens 2010; 19: 493–498 [DOI] [PubMed] [Google Scholar]
- 17.Sterns RH, Hix JK, Silver SM. Management of hyponatremia in the ICU. Chest 2013; 144: 672–679 [DOI] [PubMed] [Google Scholar]
- 18.Sterns RH, Silver S, Kleinschmidt-DeMasters BK et al. Current perspectives in the management of hyponatremia: prevention of CPM. Expert Rev Neurother 2007; 7: 1791–1797 [DOI] [PubMed] [Google Scholar]
- 19.Perianayagam A, Sterns RH, Silver SM et al. DDAVP is effective in preventing and reversing inadvertent overcorrection of hyponatremia. Clin J Am Soc Nephrol 2008; 3: 331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterns R, Hix J. Hyponatremia: vasopressin antagonists in hyponatremia: more data needed. Nat Rev Nephrol 2011; 7: 132–133 [DOI] [PubMed] [Google Scholar]
- 21.Sughrue ME, McDermott M, Blevins LS. Extreme correction of hyponatremia in a patient treated with intravenous conivaptan. J Clin Neurosci 2010; 17: 1331–1334 [DOI] [PubMed] [Google Scholar]
- 22.Soupart A, Penninckx R, Stenuit A et al. Reinduction of hyponatremia improves survival in rats with myelinolysis-related neurologic symptoms. J Neuropathol Exp Neurol 1996; 55: 594–601 [DOI] [PubMed] [Google Scholar]
- 23.Silver SM, Schroeder BM, Sterns RH et al. Myoinositol administration improves survival and reduces myelinolysis after rapid correction of chronic hyponatremia in rats. J Neuropathol Exp Neurol 2006; 65: 37–44 [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Sugimura Y, Iwama S et al. Minocycline prevents osmotic demyelination syndrome by inhibiting the activation of microglia. J Am Soc Nephrol 2010; 21: 2090–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overgaard-Steensen C, Larsson A, Bluhme H et al. Edelman's equation is valid in acute hyponatremia in a porcine model: plasma sodium concentration is determined by external balances of water and cations. Am J Physiol Regul Integr Comp Physiol 2010; 298: R120–R129 [DOI] [PubMed] [Google Scholar]