Abstract
Anemia is not uncommon in the post-renal transplant period and has been reported in up to 40% of renal transplant recipients. It is commonly due to drugs and infections. While post-transplantation anemia is usually due to graft dysfunction and drugs such as mycophenolate and cotrimoxazole, tacrolimus is an uncommon cause. Tacrolimus is usually not believed to be significantly myelosuppressive, but it can cause anemia due to thrombotic microangiopathy. A literature review shows a very small number of reported cases of pure red cell aplasia (PRCA) where tacrolimus seemed to be a causative agent. We report a case series of three renal transplant recipients who were on tacrolimus and presented with chronic transfusion requiring anemia due to PRCA.
Keywords: anemia, hemoglobin, pure red cell aplasia, tacrolimus, transplantation
Background
Renal transplantation is possibly the best form of renal replacement therapy that helps in reversal or amelioration of many effects of end-stage renal disease (ESRD), an important one of these being anemia and requirement of erythropoietin (EPO). Post-transplantation anemia (PTA) may still occur due to different etiologies but is commonly due to the drugs and infections [1]. While anemia due to drugs such as mycophenolate, cotrimoxazole and valgancyclovir, which are widely used in post-renal transplantation, is well known, tacrolimus is an uncommon cause. Tacrolimus can cause microangiopathic hemolytic anemia besides its other known adverse effects. Pure red cell aplasia (PRCA) is a less common cause of anemia, and the etiologies range from congenital diseases to acquired causes such as viral infections, drugs, EPO therapy, malignancies, connective tissue disorders and idiopathic [2]. Tacrolimus is believed to be an immunosuppressive agent without a significant potential for myelosuppression. A literature review shows a very small number of reported cases of PRCA where tacrolimus was suspected to be a causative agent [3, 4]. We report a case series of three renal transplant recipients who were on tacrolimus and presented with chronic transfusion requiring anemia due to PRCA, and recovered completely after tacrolimus withdrawal.
Case study
Here we describe three post-renal transplant patients who presented with severe anemia requiring multiple blood transfusions and not responding to routine management.
Case 1
A 40-year-old woman with ESRD, native kidney disease (NKD) being adult polycystic kidney disease, had been on twice weekly maintenance hemodialysis (MHD) and EPO therapy for 10 months. She underwent live unrelated renal transplantation in August 2013 and was on triple drug immunosuppression with tacrolimus, mycophenolate mofetil (MMF) and prednisolone, along with valgancyclovir prophylaxis for 3 months and cotrimoxazole prophylaxis for 6 months. In the early post-transplantation period, she had antibody-mediated and borderline cellular rejection, which responded to pulse steroid and plasmapheresis with her creatinine reaching 1.3 mg/dL. She was discharged with hemoglobin (Hb) of 12.1 g%. Nine months later, she presented with symptoms suggestive of anemia ongoing for the previous 3 months and she had received 22 units of packed red cell transfusions during this period. The Hb was 4.0 g%, while total leucocyte counts and platelet counts were normal. The serum creatinine at this presentation was 0.9 mg/dL. The peripheral blood smear showed normocytic and normochromic anemia with a reticulocyte count of 0.1%. The patient was advised after developing anemia to stop MMF for a brief period of time, but had no improvement.
Case 2
A 30-year-old man with ESRD, for whom NKD was unknown, had been on twice weekly MHD and EPO therapy for 2 years and had undergone uncomplicated live related renal transplantation in August 2014. He was put on the same post-transplantation medication protocol as above. His anemic symptoms started 2 months after transplantation with a drop of Hb from 9.1 to 5.5 g%. Further investigations revealed nomocytic normochromic peripheral smear with a reticulocyte count of 0.15%. The serum creatinine was 1.0 mg/dL. Suspecting a drug-induced cause, valgancyclovir and cotrimoxazole were stopped, but he continued to be transfusion-dependent.
Case 3
A 35-year-old woman who had undergone live unrelated renal transplantation in February 2011 in another center, for whom NKD was unknown, had been on twice weekly MHD and EPO therapy for 1 year before transplantation. She was referred to our center with unexplained transfusion-dependent severe anemia requiring 20 blood transfusions in the previous 4 months. She was on tacrolimus 1.5 mg BD, MMF 1 g BD and prednisolone 7.5 mg OD. She had a similar investigation profile with a Hb level of 6.3 g%, nomocytic normochromic peripheral smear and a reticulocyte count of 0.2%. The graft function was good with a serum creatinine of 1.2 mg/dL.
Each of these three cases was evaluated for anemia. The clinical examination was unremarkable except for pallor. None of them had received EPO in the post-transplantation period, and serum EPO levels were not done. All had good graft function. A detailed review of all prior medication was done. Except for the drugs mentioned above, there had been no exposure to any other drug reported to be associated with PRCA. There was no evidence of blood loss or hemolysis (including the Coombs test). The serum iron studies, vitamin b12 and folic acid levels were normal. Tests for parvovirus B19, Epstein–Barr virus and cytomegalovirus by a polymerase chain reaction were negative. To rule out autoimmune cause antinuclear antibodies, complement levels were done, which did not reveal any abnormal results. Liver function tests and serum lactate dehydrogenase were normal. Bone marrow examination revealed severe hypoplasia of erythroid series with normal maturation of other cell lines without any abnormal cells. The findings were suggestive of PRCA (Figures 1–3). Imaging of chest and abdomen to look for thymoma and findings suggestive of lymphoproliferative disorders were normal.
Fig. 2.
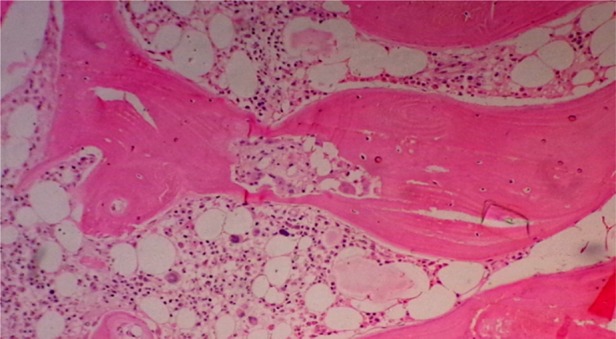
Bone marrow biopsy findings of Case 2, showing a decrease in erythroid series and normal myeloid series (H&E in 100×).
Fig. 1.
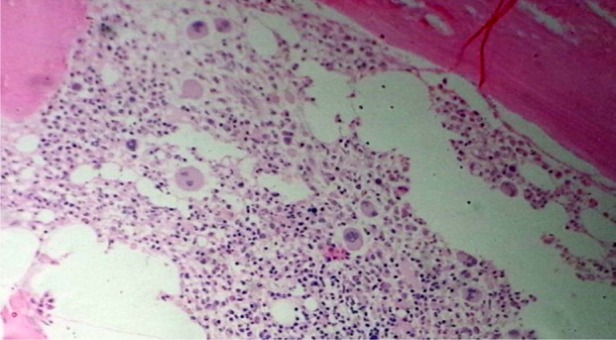
Bone marrow biopsy findings of Case 1, showing severe reduction in red cell precursors (H&E in 100×).
Fig 3.
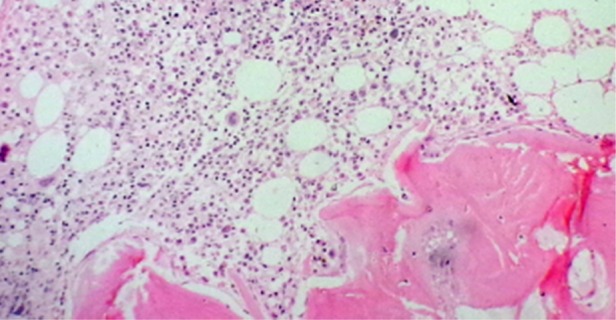
Bone marrow biopsy findings of Case 3, showing reduction in erythroid precursors (H&E in 100×).
Despite withdrawal of the likely drugs that could cause anemia, patients continued to have severe transfusion-requiring anemia. We suspected tacrolimus as a possible etiology after the literature review, which revealed a very few cases of tacrolimus-associated PRCA [3–6]. Tacrolimus was replaced with cyclosporine in all three cases. Each of them improved gradually over 2 months and completely by 3–4 months, and none required blood transfusions after stopping tacrolimus (Table 1 and Figure 4). MMF was reinstituted without any further drop in Hb.
Table 1.
Clinical profiles of patients with anemia on tacrolimus
| Clinical features | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Time period between transplantation and presentation | 9 months | 2 months | 40 months |
| Mean Hb at presentation | 4.0 g% | 5.5 g% | 6.3 g% |
| Reticulocyte count at presentation | 0.1% | 0.15% | 0.2% |
| Creatinine at presentation | 0.9 mg/dL | 1.0 mg/dL | 1.2 mg/dL |
| Duration of anemia | 3 months | 2 months | 4 months |
| Number of blood transfusions | 22 | 6 | 20 |
| Time taken for recovery | 4 months | 2 months | 3 months |
Fig. 4.
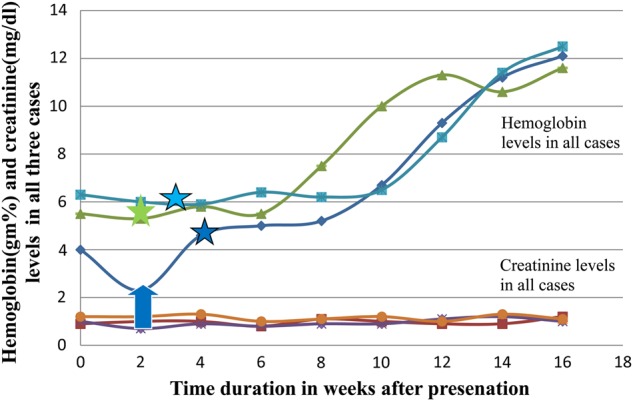
The line diagram showing the Hb and creatinine levels over the course of treatment. The blue arrow indicates time of blood transfusion in the first case. The star marks with respective colors indicate the time at which tacrolimus was changed to cyclosporine.
Discussion
PTA can be due to multiple causes and PRCA is an uncommon cause. PRCA is characterized by anemia with the almost complete absence of red cell precursors in the bone marrow but essentially normal granulopoiesis and megakaryopoiesis [3]. The patients were diagnosed as PRCA on the basis of chronic-persistent anemia of apparently unknown etiology, blood transfusion dependency, sparing of other cell lines except for erythrocytes, low reticulocyte counts and bone marrow biopsy findings consistent with PRCA. Acquired PRCA can be a solitary diagnosis or in association with immunologic diseases, chronic lymphocytic leukemia, thymoma or as idiosyncratic drug reaction. Evaluation for the possible causes of PRCA should include a previous history of drug use, exposure to toxins or infections, liver and kidney functions, immunological examination including auto-antibodies, a bone marrow examination including morphology, chromosome and rearrangement of T-cell receptor analysis, peripheral blood flow cytometry, virological examination including parvovirus B19 DNA and computed tomography and/or magnetic resonance imaging examinations to rule out the presence of thymoma and neoplasms [1]. It would be preferable to wait for at least a month before instituting specific treatment, with the rationale that 10–12% of idiopathic PRCA patients run a short- and self-limited course [2]. Cyclosporine has become established as one of the leading drugs for treatment of PRCA. Also effective are anti-thymocyte globulin, azathioprine, cyclophosphamide and daclizumab. Idiopathic PRCA also occasionally undergoes spontaneous remission, but temporal relation of withdrawal of tacrolimus with improvement of anemia suggests an etiological role of tacrolimus. EPO is one of the important causes of PRCA in renal patients, but none of our patients was on EPO supplementation. All patients improved after tacrolimus was replaced by cyclosporine. Cyclosporine is also a treatment option for idiopathic PRCA. Therefore, to say that the clinical improvement was because of therapeutic effect of cyclosporine rather than withdrawal of tacrolimus is very difficult. Safety concerns and ethical issues prevented us from restarting tacrolimus to conclusively prove that the tacrolimus was a culprit in causing PRCA.
Conclusion
The evaluation of anemia in transplant recipients can be a challenging task, considering the multiple myelosuppressive drugs and risk of opportunistic infections to which these patients are exposed. Severe anemia requiring multiple blood transfusions in post-transplant period begets the risk for rejection as well. Therefore, timely evaluation and management of anemia in these patients are very important. Tacrolimus needs to be kept in the suspect box in the management of PRCA in these patients, but further evidence is needed to prove whether tacrolimus is a real culprit in causing PRCA in post-transplant patients.
Conflict of interest statement
The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Vanrenterghem Y, Ponticelli C, Morales JM et al. . Prevalence and management of anemia in renal transplant recipients: a European survey. Am J Transplant 2003; 3: 835. [DOI] [PubMed] [Google Scholar]
- 2.Sawada K, Fujishima N, Hirokawa M. Acquired pure red cell aplasia: updated review of treatment. Br J Haematol 2008; 142: 505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winkler M, Schulze F, Jost U et al. . Anaemia associated with FK-506 immunosuppression. Lancet 1993; 341: 1035–1036 [PubMed] [Google Scholar]
- 4.Suzuki S, Osaka Y, Nakai Y et al. . Pure red cell aplasia induced by FK-506. Transplantation 1996; 61: 831–832 [DOI] [PubMed] [Google Scholar]
- 5.Gregoor PS, Weimar W. Tacrolimus and pure red-cell aplasia. Am J Transplant 2005; 5: 195–196 [DOI] [PubMed] [Google Scholar]
- 6.Misra S, Moore TB, Ament ME et al. . Red cell aplasia in children on tacrolimus after liver transplantation. Transplantation 1998; 65: 575–577 [DOI] [PubMed] [Google Scholar]


