Abstract
Background
Although the Dietary Approaches to Stop Hypertension (DASH) diet lowers blood pressure (BP) for adults with normal kidney function, evidence is lacking regarding its safety and efficacy in chronic kidney disease (CKD). We aimed to test the effects of the DASH diet on serum electrolytes and BP in adults with moderate CKD.
Methods
In a prospective before–after feeding study, 11 adults with an estimated glomerular filtration rate of 30–59 mL/min/1.73 m2 and medication-treated hypertension were provided a reduced-sodium, run-in diet for 1 week followed by a reduced-sodium, DASH diet for 2 weeks. Changes in serum electrolytes and BP were compared pre–post DASH.
Results
Eleven participants underwent feeding; 1 completed 1 week and 10 completed 2 weeks of DASH. Compared with baseline, DASH modestly increased serum potassium at 1 week (mean ± standard deviation, +0.28 ± 0.4 mg/dL; P = 0.043) but had no significant effect on potassium at 2 weeks (+0.15 ± 0.28 mg/dL; P = 0.13). Serum bicarbonate was reduced (−2.5 ± 3.0 mg/dL; P = 0.03) at 2 weeks. Neither incident hyperkalemia nor new onset metabolic acidosis was observed. Clinic BP and mean 24-h ambulatory BP was unchanged. DASH significantly reduced mean nighttime BP (−5.3 ± 5.8 mmHg; P = 0.018), and enhanced percent declines in both nocturnal systolic BP (−2.1% to −5.1%; P = 0.004) and diastolic BP (−3.7% to −10.0%; P = 0.008).
Conclusions
These pilot data suggest that a reduced-sodium DASH dietary pattern does not cause acute metabolic events in adults with moderate CKD and may improve nocturnal BP. Definitive studies are needed to determine long-term effects of DASH in CKD.
Keywords: blood pressure, DASH diet, kidney disease, metabolic side effects
Introduction
There is a paucity of data on evidence-based dietary therapies that safely lower blood pressure (BP) in adults with chronic kidney disease (CKD). The same diets that lower BP in adults with normal kidney function have potential to adversely affect individuals with CKD. These diets typically emphasize liberal consumption of fruits and vegetables, whole grains, dairy, poultry, fish, legumes and nuts, and thereby have more potassium, phosphorus and protein than is currently recommended for patients with CKD [1, 2]. If consumed in large quantities, these nutrients could cause acute or long-term electrolyte abnormalities, derangements in mineral metabolism and progressive kidney failure. The benefits and risks of evidence-based BP-lowering diets have not been rigorously tested among adults with moderate or severe CKD.
The risks for patients with CKD to develop diet-related complications are influenced by several factors, such as the presence of comorbid conditions, medication use and severity of kidney dysfunction. For example, incidence of hyperkalemia and hyperphosphatemia substantially increases as kidney function worsens and is most prevalent among individuals with end-stage kidney disease [3]. Additionally, factors such as age >65 years, diabetes and use of renin angiotensin aldosterone system (RAAS) inhibitors are associated with earlier onset of metabolic complications [3, 4]. This suggests that perhaps a subset of adults who are younger, non-diabetic, not taking RAAS inhibitors and/or have less severe CKD could safely benefit from diets that improve BP.
The Dietary Approaches to Stop Hypertension (DASH) diet is an evidence-based treatment for hypertension [5, 6]. It is rich in fruits and vegetables, low-fat dairy, whole grains, lean meats, fish, poultry, nuts, seeds and legumes, with reduced sweets and saturated fat. In addition to lowering BP, the DASH diet also improves low-density lipoprotein cholesterol, insulin sensitivity and risk for coronary heart disease, heart failure and stroke [7–9]—all highly important treatment targets for patients with CKD. In the current pilot study, we evaluated the short-term effects of DASH on serum electrolytes, markers of mineral metabolism, and clinic and ambulatory BP in adults with medication-treated hypertension and moderate CKD.
Materials and methods
Study design and participants
We conducted a prospective, before–after pilot feeding study from February to August 2014. Participants were recruited from Duke clinics and the local community. Study entry criteria are shown in Table 1. The study protocol was approved by the Duke Institutional Review Board and all participants provided written informed consent.
Table 1.
Inclusion and exclusion criteria for study participation
| Inclusion criteria |
| ≥18 years old |
| eGFR of 30.0–59.9 mL/min/1.73 m2 |
| SBP ≥130 mmHg or DBP ≥80 mmHg at two out of three screening visits |
| Stable anti-hypertensive medications for at least 2 weeks preceding enrollment |
| Exclusion criteria |
| Evidence of any of the following in the 6 months preceding enrollment: rise in serum creatinine ≥0.5 mg/dL, documented serum potassium >5.1 mEq/L, or a cardiovascular event |
| Baseline serum potassium >4.6 mEq/L |
| Albumin-to-creatinine ratio >1700 mg/g |
| Hemoglobin A1C >10% |
| Inability to stop oral potassium, vitamin and mineral supplements or antacids containing magnesium or calcium |
| Use of aldosterone receptor inhibitors, phosphodiesterase inhibitors, oral corticosteroids, weight loss medications or insulin |
| Pregnant or lactating |
| Ingest >14 servings of alcohol/week |
| Anticipated change in anti-hypertensive medications during study period |
| Inability to follow study diet due to food allergies, intolerances or special diet requirements |
eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Study diets and feeding protocol
The feeding intervention was designed to follow a similar protocol to DASH multicenter trials [5, 6]. Each participant received an isocaloric ‘run-in’ diet for 1 week immediately followed by an isocaloric DASH diet for 2 weeks. The nutrient composition of each diet was estimated using Food Processor Nutrition & Analysis Software (Salem, OR, USA) (Table 2). The run-in diet was fashioned after the average daily intake of most American adults [10]. The reduced sodium content of both diets was equivalent (2400 mg/2100 kcal/day) and intentionally designed to avoid iatrogenically increasing BP in hypertensive adults with diminished capacity to excrete sodium. Menus were pre-set to contain 2100, 2600, 3100, 3600 and 4200 kcal/day. Unit cookies (100 kcal) with a nutrient content comparable to each study diet were provided as needed to achieve intermediate kilocalorie levels. Participants were asked to discontinue all vitamin and mineral supplements and to maintain their usual physical activity.
Table 2.
Composition of study diets based on 2100 kcal/day
| Nutrient | Control diet | DASH diet |
|---|---|---|
| Carbohydrate, %kcal | 51 | 55 |
| Protein, %kcal | 15 | 18 |
| Fat, %kcal | 34 | 27 |
| Fiber, g/day | 12 | 31 |
| Cholesterol, mg/day | 300 | <200 |
| Potassium, mg/day | 1700 | 4700 |
| Magnesium, mg/day | 160 | 496 |
| Calcium, mg/day | 450 | 1240 |
| Sodium, mg/day | 2400 | 2400 |
DASH, Dietary Approaches to Stop Hypertension.
All study food and beverages were prepared in a metabolic kitchen at Duke University's Sarah W. Stedman Nutrition & Metabolism Center. Participants were served dinner menu items, which was the largest meal provided, each weekday at the study center regardless of their scheduled feeding time. All other study food and beverages were packaged ‘to go’ and sent home with participants. Adherence was monitored by weekday attendance, direct observation of on-site meals, and daily food diaries that participants used to document all uneaten ‘take home’ food and also document consumption of non-study food or beverages.
Measurements
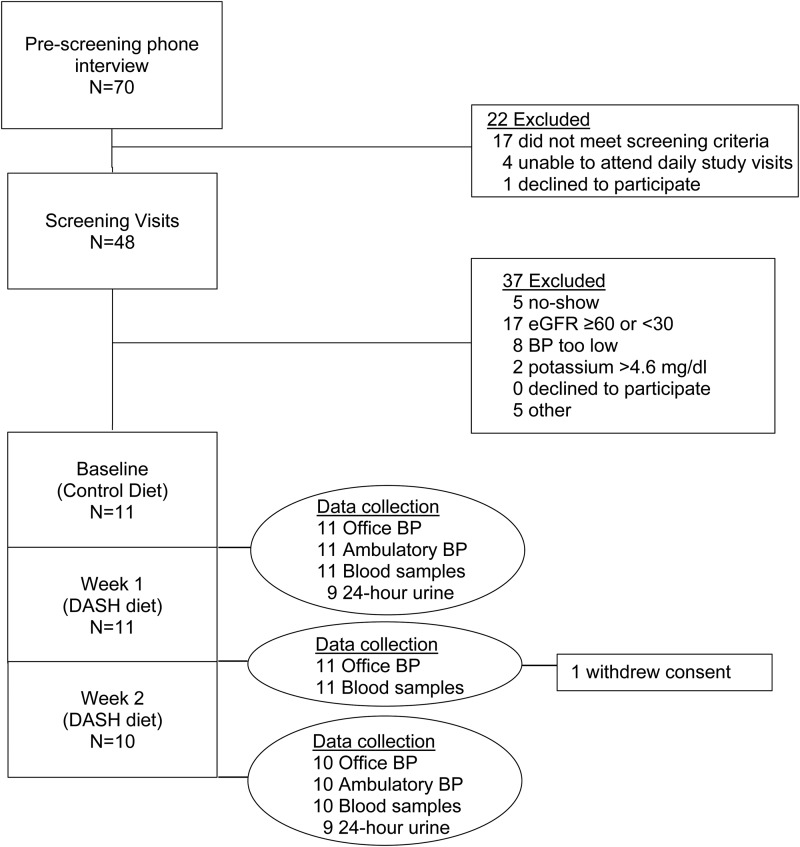
Study data were obtained by trained staff. Baseline data were collected on the last 2 days of the run-in diet and follow-up data were collected on the last 2 days of Weeks 1 and 2 of the DASH diet (Figure 1). Staff and study participants were not masked to study protocol or diet.
Fig. 1.
Participant flow and data collection schedule.
Body mass index
Height was measured in duplicate to the nearest 0.1 cm and averaged using a wall-mounted calibrated stadiometer at initial screening only. Weight was measured using a research quality calibrated digital scale in duplicate to the nearest 0.1 lb and averaged. Body mass index (BMI) was calculated as weight (kg)/height (m2).
Blood pressure
Participants did not smoke, eat, exercise or ingest caffeine 30 min prior to office BP measurements. After resting quietly for 5 min, seated BP was obtained from the right arm with a properly fitted cuff using an automated device (Omron HEM-907XL; OMRON Healthcare, Bannockburn, IL, USA). Three measurements were performed 1 min apart and averaged. Ambulatory BP was measured every 30 min between 07:00 and 21:59 h (daytime) and every hour between 22:00 and 06:59 h (nighttime) for a 24-h period using the SpaceLabs Model 90207 validated, automated device (Issaquah, WA, USA). Percent nocturnal BP decline was defined as: (mean daytime BP–mean nighttime BP)/mean daytime BP × 100 [11]. Dipping status was defined as nocturnal decline in systolic blood pressure (SBP) of 10% or more [11].
Laboratory studies
Fasting blood samples were assayed for glucose, sodium, potassium, bicarbonate, urea nitrogen, creatinine, calcium, phosphorus, albumin, creatinine, intact parathyroid hormone (PTH) and 25-hydroxy-vitamin-D. The CKD Epidemiology Collaboration creatinine (CKD-EPI) equation was used to determine estimated glomerular filtration rate (eGFR) [12]. The CKD-EPI equation was selected over other equations, such as MDRD, due to its greater accuracy in estimating kidney function in adults with preserved kidney function [12], which was characteristic of the majority of our screening population. Twenty-four hour urine was collected on Days 5 or 6 of the run-in period and for DASH week 2 and assayed for sodium, potassium, phosphorus, magnesium, bicarbonate, urea nitrogen, creatinine and albumin. To correct for variability in collection time, 24-h urine laboratory values were estimated using the following equation: (total urine concentration × 24 h)/total hours of self-reported urine collection. All laboratory studies were performed at LabCorp (Burlington, NC, USA).
Analytical strategy
When baseline and 1-week follow-up data are presented, all 11 enrollees are included; when 2-week data are presented, the 10 completers are included in the analyses. Because small studies are susceptible to the effect of outliers, individual-level data are also presented. Summary statistics were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). Continuous data are presented as mean ± standard deviation and categorical data as frequency counts and percentages. For continuous BP and laboratory outcomes, changes from baseline to post-intervention were compared. Shapiro–Wilk test was used to test for normality assumption. If this test was not significant, paired t-test was used in analyses; otherwise, the non-parametric method of Wilcoxon signed rank test was used to assess changes in BP and laboratory data. A P-value <0.05 was considered statistically significant.
Results
Eleven participants met the study criteria; one withdrew consent after completing DASH week 1 due to symptoms attributed to lactose intolerance and 10 completed the entire study (Figure 1). Of 11 participants, 6 were women, 10 were African American, mean age was 63.2 ± 12.0 years and mean eGFR was 52.0 ± 7.0 mL/min/1.73 m2 (range: 42.0–62.1 mL/min/1.73 m2). One participant (P7 who withdrew after DASH week 1) had eGFR >60 mL/min/1.73 m2 and was included in the study due to a rounding error. Individual participant data are shown in Table 3.
Table 3.
Demographic and baseline clinical characteristics
| P | Age (years) | Gender | Race | Diabetes (Y/N) | BMI (kg/m2) | Serum albumin (g/dL) | Serum creatinine (mg/dL) | eGFR (mL/min/1.73 m2) | Screening SBP (mmHg) | Screening DBP (mmHg) | Anti-hypertensive medication class |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 86 | M | W | N | 27.8 | 4.7 | 1.41 | 47.9 | 135.5 | 76.5 | ACEI |
| P2 | 56 | M | B | N | 31.9 | 4.2 | 1.98 | 41.9 | 144.5 | 89.5 | ARB, BB, loop |
| P3 | 68 | F | B | N | 31.7 | 4.2 | 1.08 | 59.6 | 143.5 | 86.5 | ACEI, thiazide |
| P4 | 56 | M | B | N | 31.2 | 4.7 | 1.49 | 59.3 | 138.0 | 76.0 | thiazide, alpha-p, |
| P5 | 45 | F | B | N | 24.7 | 3.9 | 1.30 | 57.3 | 150.5 | 102.0 | ACEI, thiazide |
| P6 | 71 | F | B | Y | 50.4 | 4.1 | 1.24 | 52.5 | 142.5 | 82.5 | ARB, BB, CCB, thiazide |
| P7 | 79 | F | B | N | 32.7 | 3.7 | 1.04 | 62.1 | 139.5 | 67.5 | BB, CCB, thiazide |
| P8 | 55 | F | B | N | 29.8 | 4 | 1.25 | 53.7 | 139.5 | 86.0 | ARB, thiazide, vasodilator |
| P9 | 64 | F | B | Y | 36.8 | 4.1 | 1.44 | 45.8 | 137.0 | 84.3 | ACEI, CCB, thiazide |
| P10 | 59 | M | B | N | 29.9 | 4.2 | 1.77 | 48.0 | 180.0 | 102.5 | ACEI, CCB, thiazide, alpha-c |
| P11 | 57 | M | B | N | 25.7 | 4.1 | 1.76 | 56.0 | 129.0 | 83.0 | ARB, BB, CCB, thiazide, loop |
Overall mean age was 63.2 ± 12.0 years, mean eGFR was 52.0 ± 7.0 mL/min/1.73 m2, mean screening SBP was 143.8 ± 13.2 mmHg and mean screening DBP was 85.5 ± 10.4 mmHg.
P, participant; M, male; F, female; W, White race; B, Black/African American race; BMI, body mass index; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta blocker; CCB, calcium channel blocker; thiazide, thiazide diuretic; loop, loop diuretic; alpha-p, peripheral alpha antagonist; alpha-c, central alpha agonist.
Adherence
Eight of 11 participants attended all scheduled on-site meals. Of the three participants who missed on-site meals, two (P4 and P10) received their meals ‘to go’ to prevent interrupting the study diet and one (P10) substituted non-study foods for 1 day. Weight remained stable from baseline to DASH week 2 (mean change −0.3 ± 1.4 kg; P = 0.56); six participants had <1% change and all had ≤2.5% change (see Supplementary data, Table S1).
Compared with baseline, 24-h urine excretion of potassium (P = 0.01), phosphorus (P = 0.03) and urea nitrogen (P = 0.04) were significantly higher at DASH week 2, likely reflecting increased consumption of fruits, vegetables and protein (Supplementary data, Table S2). There were no significant changes in urine excretion of sodium (P = 0.21), magnesium (P = 0.29) and calcium (P > 0.99).
Laboratory studies
At DASH week 2, there were no significant changes in mean serum phosphorus (P = 0.15), calcium (P = 0.48), urea nitrogen (P = 0.43), 25-hydroxy-vitamin-D (P = 0.44) or plasma PTH (P = 0.88) (Table 4). Incident hyperphosphatemia and hypercalcemia were not observed. Mean serum bicarbonate was significantly lower at DASH week 2 (−2.5 ± 3.0 mg/dL; P = 0.03). New onset metabolic acidosis was not observed. Serum potassium increased for 6 of 11 participants at DASH week 1; the largest was 1.1 mg/dL (from 3.6 to 4.7 mg/dL) and all others increased by 0.6 mg/dL or less. Serum potassium increased for 5 of 10 participants at DASH week 2; one increased by 0.7 mg/dL and all others by <0.5 mg/dL. Overall, mean serum potassium was significantly higher after DASH week 1 (+0.28 ± 0.4; P = 0.043) but was not significantly different from baseline after DASH week 2 (+0.15 ± 0.28; P = 0.13). Incident hyperkalemia was not observed.
Table 4.
Baseline laboratory studies and clinic blood pressure and change in results after 2 weeks of DASH diet
| Participant | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | Group mean ± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laboratory studies | ||||||||||||
| Potassium, mEq/L | 4.5 | 4.1 | 4.7 | 3.6 | 3.7 | 4.4 | 4.1 | 3.7 | 4.5 | 5.1 | 3.4 | 4.2 ± 0.6 |
| Δ | +0.4 | +0.3 | 0 | +0.7 | +0.3 | −0.3 | – | 0 | 0 | −0.1 | +0.2 | +0.2 ± 0.3 |
| Bicarbonate, mEq/L | 23 | 24 | 24 | 27 | 27 | 26 | 26 | 31 | 27 | 31 | 29 | 26.9 ± 2.8 |
| Δ | −2 | −1 | −1 | −4 | 0 | +2 | – | −8 | −1 | −5 | −5 | −2.5 ± 3.0* |
| Calcium, mg/dL | 9.1 | 9.2 | 9.7 | 9.5 | 9.7 | 9.4 | 9.5 | 9.8 | 9 | 9.5 | 9.3 | 9.4 ± 0.3 |
| Δ | −0.2 | +0.4 | −0.3 | −0.2 | −0.1 | +0.3 | – | −0.1 | +0.8 | +0.2 | 0 | +0.1 ± 0.3a |
| Creatinine, mg/dL | 1.4 | 2.1 | 1.1 | 1.5 | 1.4 | 1.4 | 1.0 | 1.5 | 1.5 | 1.8 | 1.7 | 1.5 ± 0.3 |
| Δ | +0.2 | 0 | −0.1 | 0 | −0.1 | −0.1 | – | −0.1 | 0 | +0.1 | +0.1 | 0 ± 0.1 |
| Phosphorus, mg/dL | 2.4 | 3.5 | 3.3 | 3.1 | 2.9 | 3.3 | 4.3 | 3.1 | 3.6 | 3.1 | 3.9 | 3.2 ± 0.4 |
| Δ | +0.5 | −0.1 | −0.1 | +0.6 | 0 | −0.3 | – | +0.2 | +0.3 | +0.2 | +0.1 | +0.1 ± 0.3 |
| Intact PTH, pg/mL | 56 | 48 | 35 | 37 | 30 | 41 | 40 | 56 | 55 | 103 | 41 | 50.2 ± 20.7 |
| Δ | +30 | −15 | +4 | +4 | +3 | −6 | – | +1 | −8 | −33 | +12 | +1.7 ± 3.5 |
| 25-vitamin D, ng/mL | 19.1 | 18.9 | 36.6 | 18.7 | 18.3 | 24.5 | 21.9 | 28.2 | 28 | 20.3 | 21.2 | 23.4 ± 6.0 |
| Δ | +6.1 | −0.4 | −7.7 | +5.5 | −1.9 | −1.5 | – | −5 | −9.3 | +1.2 | 0 | −1.3 ± 5.0 |
| Blood pressureb,c | ||||||||||||
| Clinic SBP, mmHg | 132.0 | 135.5 | 131.5 | 112.0 | 118.0 | 95.5 | 123.0 | 119.0 | 124.5 | 196.0 | 132.5 | 133.6 ± 24.0 |
| Δ | −14.5 | −5.0 | −11.5 | 4.5 | 12.0 | 27.5 | – | 2.5 | −4.5 | −20.0 | −8.0 | −1.6 ± 13.8 |
| Clinic DBP, mmHg | 77.0 | 84.5 | 81.0 | 74.5 | 80.5 | 60.0 | 65.5 | 76.5 | 79.5 | 112.5 | 79.5 | 79.5 ± 13.2 |
| Δ | −10.0 | −2.5 | −3.0 | −6.5 | 10.5 | 13.5 | – | 2.0 | −4.5 | −13.5 | −9.5 | −2.6 ± 8.9 |
| Ambulatory SBP, mmHg | 145 | 123 | 132 | 112 | 130 | 130 | – | 116 | 122 | 200 | 144 | 135.4 ± 25.1 |
| Δ | −17 | 3 | −4 | 7 | 2 | −8 | – | 3 | 1 | −14 | −19 | −4.6 ± 9.3 |
| Ambulatory DBP, mmHg | 81 | 74 | 74 | 69 | 90 | 69 | – | 69 | 75 | 113 | 76 | 79.0 ± 13.6 |
| Δ | −11 | 4 | −3 | −1 | 2 | −4 | – | 4 | −1 | −3 | −6 | −1.9 ± 4.6 |
P7 withdrew consent after completing 1 week of the DASH diet. Change (Δ) equals value at end of DASH week 2 minus value at baseline. DASH, Dietary Approaches to Stop Hypertension; SD, standard deviation; PTH, parathyroid hormone; SBP, systolic BP; DBP, diastolic BP.
*Statistically significant (P < 0.05).
aWilcoxon signed rank test; median change of −0.05 mg/dL and interquartile range of −0.20 and 0.30.
bP10 had increase in doses of angiotensin-converting enzyme inhibitor, central alpha agonist and loop diuretic during the feeding period for severe hypertension.
cP11 had reduction in dose of loop diuretic 2 days prior to the run-in period.
Blood pressure
Medication and screening BP data are shown in Table 3. Mean clinic SBP was 129.3 ± 24.9 mmHg at baseline, 133.6 ± 24.0 mmHg after DASH week 1 and 128.3 ± 17.5 after DASH week 2. SBP did not significantly change from baseline to DASH week 1 (4.4 ± 7.4 mmHg; P = 0.08) or baseline to DASH week 2 (−1.6 ± 13.8 mmHg; P = 0.72). Mean clinic DBP was 79.5 ± 13.2 mmHg at baseline, 82.5 ± 11.7 at DASH week 1, and 78.3 ± 10.2 at DASH week 2. DBP did not significantly change from baseline to DASH week 1 (2.9 ± 5.9 mmHg; P = 0.13) or baseline to DASH week 2 (−2.6 ± 8.9 mmHg; P = 0.38). After DASH week 2, clinic SBP was reduced in 6 of 10 participants (range: −4.5 to −20 mmHg) and clinic DBP was reduced in 7 of 10 participants (range: −2.5 to −13.5 mmHg); one participant had an increase in anti-hypertensive medications, one had a reduction and all others had no medication changes (Table 4).
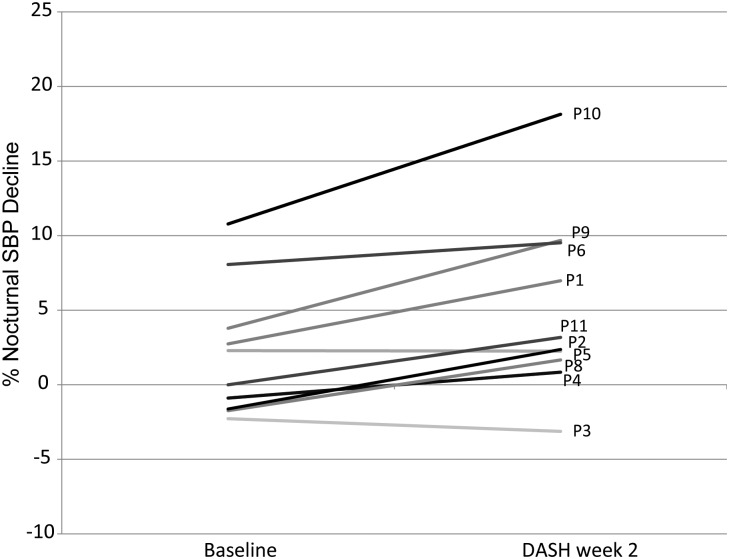
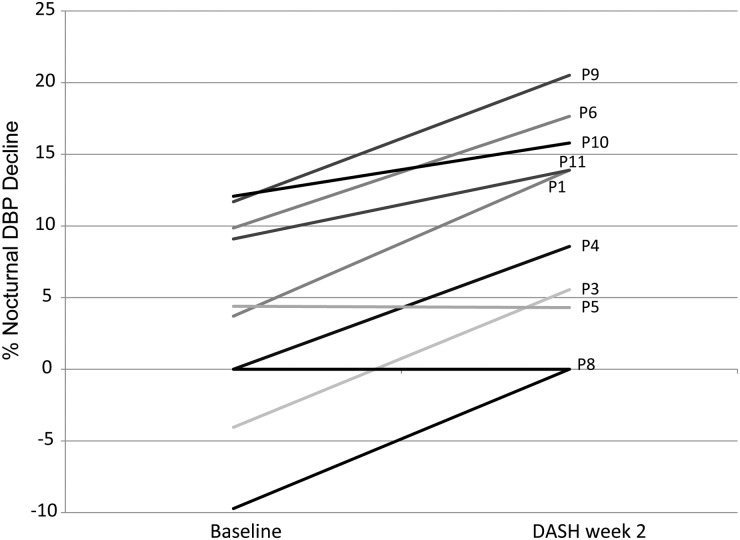
Mean ambulatory SBP was 135.4 ± 25.1 mmHg at baseline and 130.8 ± 19.8 mmHg at DASH week 2; net change was not significant (−4.6 ± 9.3 mmHg; P = 0.15). Mean ambulatory DBP was 79.0 ± 13.6 mmHg at baseline and 77.1 ± 13.7 at DASH week 2; net change was not significant (−1.9 ± 4.6 mmHg; P = 0.23). Daytime SBP, daytime DBP and nighttime SBP were also unchanged at DASH week 2 (−3.8 ± 9.2 mmHg, −0.7 ± 4.4 mmHg and −7.9 ± 11.5 mmHg, respectively; P > 0.05). Nighttime DBP was significantly lower (−5.3 ± 5.8 mmHg; P = 0.02) at DASH week 2. There were significant overall improvements in both percent nocturnal SBP decline (2.1 ± 4.4% to 5.1 ± 6.1%; P = 0.01) and percent nocturnal DBP decline (3.7 ± 7.2% to 10.0 ± 7.4%; P = 0.01) from baseline to DASH week 2, which means participants' BP trended towards that of a dipping pattern. Although all participants who were classified as non-dipper before the intervention remained non-dippers after the intervention, the degree to which daytime SBP and DBP was reduced improved in 8 of 10 and 9 of 10 participants, respectively. Individual-level nocturnal BP results are shown in Figures 2 and 3.
Fig. 2.
Percent nocturnal SBP decline at baseline and after DASH week 2 for 10 participants who completed the feeding protocol. Percent nocturnal decline in SBP was defined as: (mean daytime SBP − mean nighttime SBP)/mean daytime SBP × 100.
Fig. 3.
Percent nocturnal DBP decline at baseline and after DASH week 2 for 10 participants who completed the feeding protocol. Percent nocturnal decline in DBP was defined as: (mean daytime DBP − mean nighttime DBP)/mean daytime DBP × 100.
Discussion
Barriers to recommending the DASH diet for individuals with CKD are mainly related to concerns about safety. Our pilot data in adults with hypertension and moderate CKD demonstrated that a reduced-sodium DASH diet increased serum potassium and lowered serum bicarbonate modestly without causing incident hyperkalemia or new onset metabolic acidosis, suggesting that it may be safe for this patient population. In this small study, DASH significantly enhanced percent nocturnal BP decline, which is an ambulatory BP trait that has favorable cardiovascular benefits [11, 13]. Thus, our findings further suggest that a reduced-sodium DASH diet may be beneficial for BP in moderate CKD. To our knowledge, this is the first controlled feeding study of the DASH diet in moderate CKD.
Although our pilot data cannot provide definitive evidence of the safety and benefit of DASH in CKD, the individual-level data raise some matters that should be addressed in future research. For example, our participants consumed the DASH diet for 2 weeks without evidence of acute adverse effects; however, it is not known whether transient hyperkalemia and/or metabolic acidosis occurred between data collection visits. Serum potassium was highest for most participants (8 of 11) after receiving the DASH diet for 1 week but potassium was not significantly different from baseline after 2 weeks. This suggests that for individuals with stable, moderate CKD, the risk of developing acute hyperkalemia may be highest within the first week of initiating DASH, and in future studies early measurement will be essential. One participant (P1) whose serum potassium approached the upper limit of normal had several known risk factors for hyperkalemia, including age >65 years [3], mild metabolic acidosis at screening and use of angiotensin-converting enzyme inhibitor [3] without concomitant use of a loop or thiazide diuretic. In future studies, these risk factors should be considered in determining who may safely benefit from DASH. Nonetheless, our data are consistent with prior studies in mild and severe hypertensive kidney disease in which high fruit and vegetable intake (comparable to DASH) was well-tolerated [14, 15].
We observed the DASH diet to reduce serum bicarbonate concentrations in 8 of 11 participants. Reduced serum bicarbonate was unexpected because diets that are rich in fruits and vegetables tend to have base-producing properties [16, 17]. In four participants, the reduction was small (≤2 mg/dL), but in the others it was 4 mg/dL or more, and the largest reductions tended to occur in those with the highest baseline serum bicarbonate concentrations. Theoretically, differential adherence to the acid-producing foods in DASH (e.g. dairy, whole wheat, fish) could explain this finding, but statistically significant increases in 24-h urinary excretion of potassium, phosphorus and urea suggest dietary adherence to both base- and acid-producing foods. Due to our small sample size and lack of a control group to account for normal biological variability of serum bicarbonate, further studies are needed to confirm or negate this counterintuitive finding. If substantiated in a more definitive trial, it would have important implications for safety that perhaps may warrant modifying the DASH diet to more strongly emphasize base-producing foods and to de-emphasize acid-promoting foods.
We did not observe significant changes in serum calcium, phosphorus, vitamin D or plasma PTH concentrations, and individual-level data do not suggest a consistent trend. This suggests that the calcium, phosphorus and protein content of the DASH diet may not adversely affect mineral metabolism in individuals with moderate CKD. Similarly, PTH did not change in our study. In a prior study in individuals without CKD, DASH for up to 90 days did not increase PTH and was associated with reduced bone turnover [18]. Further studies are needed to determine whether the same potentially beneficial bone effects occur in individuals with CKD.
Overall, we did not observe significant reductions in mean clinic or ambulatory BP, despite prior evidence of BP-lowering with DASH within 1 week [19]. Several factors may account for this finding. First is the lack of statistical power in our pilot study. Secondly, 9 of 11 participants experienced a substantial reduction in BP after receiving the run-in diet, perhaps to an extent that any added effect of DASH on BP was masked. Improved medication adherence, regression to the mean, habituation and/or the low-sodium content of the run-in diet could have contributed to BP reductions that preceded and followed the DASH diet intervention. Lastly, although 2 weeks are sufficient for DASH to lower BP in individuals with normal kidney function [5–7, 20], it is possible that longer time is needed in adults with CKD. Despite these limitations, 6 of 10 participants experienced a reduction in SBP, suggesting an effect that would be evident in an adequately powered study and/or subsets with CKD that may be more responsive to DASH.
The observed reduction in nighttime DBP and enhanced percent nocturnal SBP and DBP decline may have important clinical implications. Non-dipping and a blunted nighttime BP decline are associated with increased cardiovascular events [13, 21]. Both phenomena are more common in African Americans [22–24] and increase in prevalence as kidney function worsens [25]. In a 4-month behavioral intervention trial, DASH significantly improved nocturnal dipping in African Americans with normal kidney function [22]. Whether improvements in nocturnal BP from a sodium-reduced DASH diet observed in the current study were related to our participants being primarily African American, their CKD status or both requires further determination. If substantiated in a more definitive trial, a favorable diurnal BP pattern could beneficially impact outcomes for African Americans and individuals with CKD, patient populations with high risk for cardiovascular disease.
Our study has several limitations. We used a single measurement of serum creatinine to determine eGFR. We believe the CKD status of our participants was appropriately defined because a qualifying eGFR was confirmed by medical record review for 9 of the 11 participants prior to study enrollment. Furthermore, little to no variability in serum creatinine (absolute change of 0–0.2 mg/dL from baseline) was detected for participants during the study, suggesting that baseline creatinine was an accurate reflection of their kidney function. Other limitations result primarily from our study being pilot in nature with a small sample size, which limits power, lack of a control group, and short duration of feeding, which limits conclusions about durability of effect. While the serendipitous enrollment of primarily African Americans limits generalizability of results, it also provides an opportunity to assess the effects of DASH in a patient population that shares a high burden on cardiovascular disease-related risk and could benefit substantially from non-pharmacologic intervention. Enrollment of only a few diabetics and few individuals with CKD stage 3B (eGFR of 30–44 mL/min/1.73 m2) also limits generalizability. Additionally, we could not assess DASH effects independent of sodium reduction and anti-hypertensive medications. However, this context likely represents the ‘real-world’ in which DASH would be prescribed as add-on therapy to anti-hypertensive medications and sodium reduction in this patient group.
Conclusion
Despite limitations, this pilot study lays important groundwork for assessing the safety and efficacy of DASH in CKD. There is need for further investigation focusing particularly on CKD stage 3B and more advanced stages of disease, as well as the potential for elevated serum potassium, reduced serum bicarbonate, improved nighttime BP and enhanced nocturnal dipping.
Supplementary data
Supplementary data are available online at http://ndt.oxfordjournals.org.
Conflict of interest statement
None declared.
Supplementary Material
Acknowledgements
The skilled work of the research team staff is greatly appreciated. Study data capture and management were supported by REDCap (Research Electronic Data Capture) a secure, web-based application [26]. This study was supported by the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health under Award Number P30DK096493 (Duke O'Brien Center for Kidney Research).
References
- 1.Kidney Disease Outcomes Quality Initiative. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43 (5 Suppl 1): S1–S290 [PubMed] [Google Scholar]
- 2.KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int Suppl 2012; 2: 343 [Google Scholar]
- 3.Moranne O, Froissart M, Rossert J et al. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol 2009; 20: 164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Einhorn LM, Zhan M, Hsu VD et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169: 1156–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel LJ, Moore TJ, Obarzanek E et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336: 1117–1124 [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Svetkey LP, Vollmer WM et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344: 3–10 [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ, Sacks FM, Carey VJ III et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005; 294: 2455–2464 [DOI] [PubMed] [Google Scholar]
- 8.Shirani F, Salehi-Abargouei A, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition 2013; 29: 939–947 [DOI] [PubMed] [Google Scholar]
- 9.Salehi-Abargouei A, Maghsoudi Z, Shirani F et al. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases-Incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition 2013; 29: 611–618 [DOI] [PubMed] [Google Scholar]
- 10.US Department of Agriculture, Agricultural Research Service. 2012. Total Nutrient Intakes: Percent Reporting and Mean Amounts of Selected Vitamins and Minerals from Food and Dietary Supplements, by Family Income (as % of Federal Poverty Threshold) and Age, What We Eat in America, NHANES 2009–2010 [Google Scholar]
- 11.Verdecchia P, Schillaci G, Guerrieri M et al. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation 1990; 81: 528–536 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metoki H, Ohkubo T, Kikuya M et al. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension 2006; 47: 149–154 [DOI] [PubMed] [Google Scholar]
- 14.Goraya N, Simoni J, Jo CH et al. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 2013; 8: 371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goraya N, Simoni J, Jo C et al. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int 2012; 81: 86–93 [DOI] [PubMed] [Google Scholar]
- 16.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 1995; 95: 791–797 [DOI] [PubMed] [Google Scholar]
- 17.Scialla JJ, Anderson CA. Dietary acid load: a novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis 2013; 20: 141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin PH, Ginty F, Appel LJ et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr 2003; 133: 3130–3136 [DOI] [PubMed] [Google Scholar]
- 19.Lin PH, Allen JD, Li YJ et al. Blood pressure-lowering mechanisms of the DASH dietary pattern. J Nutr Metab 2012; 2012: 472396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conlin PR, Erlinger TP, Bohannon A et al. The DASH diet enhances the blood pressure response to losartan in hypertensive patients. Am J Hypertens 2003; 16 (5 Pt 1): 337–342 [DOI] [PubMed] [Google Scholar]
- 21.Brotman DJ, Davidson MB, Boumitri M et al. Impaired diurnal blood pressure variation and all-cause mortality. Am J Hypertens 2008; 21: 92–97 [DOI] [PubMed] [Google Scholar]
- 22.Prather AA, Blumenthal JA, Hinderliter AL et al. Ethnic differences in the effects of the DASH diet on nocturnal blood pressure dipping in individuals with high blood pressure. Am J Hypertens 2011; 24: 1338–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muntner P, Lewis CE, Diaz KM et al. , Racial differences in abnormal ambulatory blood pressure monitoring measures: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Hypertens 2014; 28: 640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jehn ML, Brotman DJ, Appel LJ. Racial differences in diurnal blood pressure and heart rate patterns: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Arch Intern Med 2008; 168: 996–1002 [DOI] [PubMed] [Google Scholar]
- 25.Mojon A, Ayala DE, Pineiro L et al. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int 2013; 30: 145–158 [DOI] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.