Abstract
Background
Low-molecular weight heparin (LMWH) is commonly used as an anticoagulant for haemodialysis by a single-bolus injection. However, its application in extended haemodialysis has been infrequently studied. In particular, for nocturnal home haemodialysis patients sleeping throughout treatment, the need for additional intradialytic bolus might render the use of LMWH impractical. To overcome this limitation, we changed traditional bolus injections to continuous infusion. We first tested our method among in-centre 4-h haemodialysis patients to establish a feasible and safe infusion regimen before utilizing it in extended dialyses at home.
Methods
Recruited patients were given nadroparin (standardized at 65 IU/kg) as an anticoagulant for haemodialysis. They were first randomized to receive nadroparin either by bolus injection or infusion. Afterwards, the patients underwent crossover to receive the alternate method of LMWH anticoagulation. The degrees of anticoagulation and bleeding complications were compared.
Results
Sixteen haemodialysis patients were recruited. After nadroparin administration, anti-Xa levels at the first hour were significantly higher by the bolus than the infusion methods (0.68 ± 0.10 versus 0.49 ± 0.10 IU/mL, P < 0.001) and were similar by the second hour (0.56 ± 0.10 versus 0.55 ± 0.11 IU/mL, P = 0.64). At the sixth hour, anti-Xa levels by the infusion method were significantly higher (0.35 ± 0.13 versus 0.25 ± 0.10 IU/mL, P < 0.001), suggesting the infusion approach required a dosage reduction. There were no bleeding events reported in either method.
Conclusions
LMWH infusion is feasible and safe. The method avoids early excessive anticoagulation caused by bolus injection and reduces the LMWH dose. Future studies should be conducted to evaluate LMWH infusion in extended haemodialysis treatment.
Keywords: anticoagulation, continuous infusion, haemodialysis, low-molecular weight heparin, nadroparin
Introduction
Anticoagulation is an essential element of haemodialysis therapy for patients with end-stage renal disease (ESRD). An extracorporeal circuit is a prerequisite for the delivery of adequate dialysis. In a majority of patients with low bleeding risk, systemic heparin is utilized for anticoagulation. Given its better bioavailability at a lower dose, longer half-life and more predictable anticoagulant responses, low-molecular weight heparin (LMWH) has been increasingly preferred over unfractionated heparin (UFH) in conventional intermittent haemodialysis [1]. Moreover, potential superior effects on lipid profiles, reduced risks for osteoporosis [2–4] and lower risks of heparin-induced thrombocytopenia (HIT) [5] favour LMWH over UFH.
LMWH is typically given as a single intravenous bolus into the arterial limb when starting dialysis. While such drug administration convenience is advantageous, the single bolus injection limits the efficacy of LMWH in haemodialysis to conventional 4-h treatments. In extended haemodialysis lasting 6–8 h, additional intradialytic boluses of LMWH are often required to ensure circuit patency [6]. However, this approach may not be practical for all patients, especially for those receiving nocturnal haemodialysis at home when they are sleeping during treatment. As a result, UFH by continuous infusion, which requires no additional intradialytic manoeuvres, is more commonly employed in this group of patients [7].
LMWH administration by continuous infusion has been less studied in the literature. Enoxaparin and nadroparin infusion have been evaluated for continuous haemofiltration in critical care settings [8, 9]. For intermittent haemodialysis, while an infusion regimen has been recommended for dalteparin [1], there is a paucity of data on LMWH infusion, let alone direct comparisons against the traditional bolus method. As a result, we performed a pilot study to first evaluate the feasibility and safety of LMWH infusion as an anticoagulant for conventional intermittent haemodialysis, to construct an appropriate infusion regimen for future use in extended haemodialysis.
Materials and methods
All patients ≥18 years old with ESRD receiving thrice-weekly 4-h haemodialysis at our institution were screened. Patients who had an underlying bleeding disorder, a history of intolerance to LMWH, had been receiving oral anticoagulants or other drugs that could affect heparin activity (e.g. tetracycline, digitalis, and antihistamines, etc.), or were unable to give informed consent were excluded from our study. Nadroparin (Fraxiparine, GlaxoSmithKline) was thoroughly mixed with normal saline into a 20-mL syringe before use. Such a dilution allowed more precise individual dosing of nadroparin, standardized at 65 IU/kg. For the bolus method, nadroparin was injected into the arterial limb of a haemodialysis circuit. For the infusion method, a loading dose at 35 IU/kg was given, followed by 10 IU/kg infusion per hour for 3 h (i.e. stopping 1 h before the end of dialysis). The study protocol was approved by the Ethics Committee of the hospital, and we fully adhered to the principles of Good Clinical Practice and Declaration of Helsinki. Written informed consent was obtained from all participants in the study.
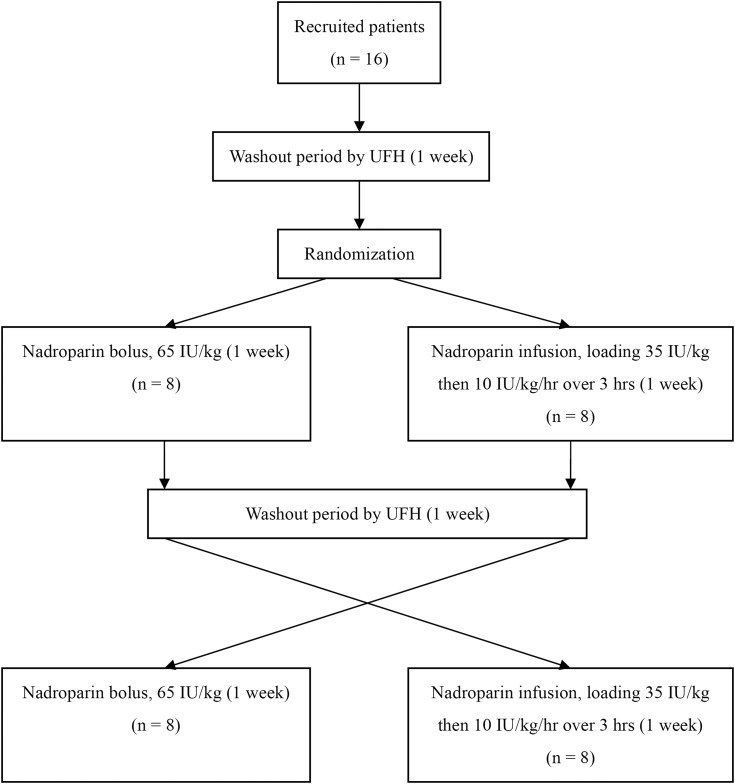
Each recruited patient underwent a 4-week haemodialysis treatment at our institution. The first week was a washout period using UFH as the anticoagulant. In the second week, the patients were randomized to receive nadroparin for dialysis by either bolus or infusion method in a 1:1 manner. The third week was another washout period with UFH. In the fourth week, the patients underwent crossover to receive the alternate method of LMWH anticoagulation (Figure 1). All haemodialyses were delivered by Fresenius 4008 devices, using synthetic hollow fibre dialysers (polysulfone membrane FX80M capillary middle-flux dialysers; Fresenius Medical Care, Bad Homburg, Germany). A new dialyser was utilized for each dialysis treatment and was pre-rinsed with 1000-mL normal saline. Bicarbonate haemodialysate was used. The dialysate flow rate was maintained at 500 mL/min and the blood flow was kept between 200 and 300 mL/min depending on vascular access conditions of the patients. Ultrafiltration was performed as clinically indicated.
Fig. 1.
Study flowchart. UFH, unfractionated heparin.
The evaluations of each patient, including blood sampling and thrombus assessment, were carried out during mid-week haemodialysis treatments for both methods (i.e. during the second session, in the second and fourth weeks of the study). Degrees of thrombus formation in the dialyser and arterial and venous air traps were assessed by two dialysis nurses, one of whom was blinded to the study. We employed a 5-grade scale: Grade 0, no detectable clot; Grade 1, minimal clot; Grade 2, moderate clot; Grade 3, major clot formation but dialysis still feasible; Grade 4, complete occlusion by thrombus rendering dialysis impossible. Compressions of the arterial and venous cannulation sites post-dialysis were performed sequentially by the patients themselves. Total haemostasis times required were noted, and reported as the summation of haemostasis times required for both cannulation sites. Any bleeding events during the study were also recorded.
For blood sampling, pre-dialysis blood collection was performed before nadroparin administration (Time 0), which included complete blood picture, prothrombin time (PT), activated thromboplastin time (APTT) and measurements of anti-Xa, urea and creatinine levels. Immediate post-dialysis urea and creatinine levels were also collected for the calculation of single-pool Kt/V (spKt/V) to evaluate dialysis clearance [10]. At the first, second and sixth hours after the administration of nadroparin, blood samplings for PT, APTT and anti-Xa were also performed. All blood collections during dialysis were performed in accordance with the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines [11].
Anti-Xa assay
Doubly spun (3750 rpm × 10 min) platelet-poor plasma was prepared from citrated peripheral blood. Platelet counts were verified to be <10 × 109/L. Plasma samples were frozen at −70°C before testing. The plasma level of LMWH was measured by anti-Xa activity with an amidolytic method using a Stachrom Heparin Kit (Diagnostica Stago, France) on a CA-7000 coagulometer (Sysmex, Japan). Testing procedures followed the instructions of the manufacturer, except a 5-point calibration curve was used for a more accurate quantitation instead of the 3-point calibration curve recommended by the manufacturer.
Statistical methods
Statistical evaluations were performed using the SPSS version 13.0 software package (SPSS, Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation or median (interquartile range), whereas categorical variables were expressed as proportions. Comparisons of data were made by paired Student's t-test, Wilcoxon signed-rank test or Fisher's exact test as appropriate. Analysis of variance (ANOVA) with Bonferroni post hoc test was used if three or more groups of data were involved for a comparison. Two-sided P-values were reported, with P-values <0.05 considered significant.
Results
A total of 16 patients were recruited and all completed our study. Their baseline characteristics are shown in Table 1. The mean age was 58.9 years, with a median haemodialysis vintage of 3.6 years. Most patients underwent dialysis via native arteriovenous fistulae. Individual nadroparin dosages were prescribed in accordance with body weight at 3928.5 ± 422.2 IU. All patients underwent thrice-weekly 4-h haemodialysis treatments uneventfully during our study. During LMWH administration via the bolus method, two patients terminated dialysis 16 and 8 min early, whereas one patient terminated 5 min early during administration via infusion. These early termination cases were due to significant muscle cramps. None of the patients encountered premature termination of dialysis due to clotting of the extracorporeal circuit.
Table 1.
Baseline demographics of patients (n = 16)
| Age, years | 58.9 ± 7.6 |
| Male gender, n (%) | 8 (50) |
| Body mass index, kg/m2 | 24.2 ± 2.0 |
| Aetiology of ESRD, n (%) | |
| Hypertension | 2 (12.5) |
| Diabetes mellitus | 3 (18.8) |
| Glomerulonephritis | 2 (12.5) |
| Polycystic kidney disease | 4 (25) |
| Others | 5 (31.2) |
| Comorbidities, n (%) | |
| Hypertension | 15 (93.8) |
| Diabetes mellitus | 4 (25) |
| Ischemic heart disease | 3 (18.8) |
| Cerebrovascular disease | 1 (6.3) |
| History of malignancy | 5 (31.3) |
| Hemodialysis vintage, years | 3.6 (2.6–7.3) |
| Dialysis access, n (%) | |
| Native fistula | 14 (87.5) |
| Synthetic graft | 2 (12.5) |
Continuous variables are expressed as mean ± standard deviation or median (interquartile range).
ESRD, end-stage renal disease.
The baseline anti-Xa levels of recruited patients upon study entry were confirmed to be undetectable. The comparisons of various parameters between the bolus and infusion methods are shown in Table 2. At Time 0, PT was slightly prolonged during the infusion method, likely with little clinical relevance (10.68 ± 0.52 versus 10.45 ± 0.50, P = 0.005). Other parameters at Time 0 were similar between the two methods, including negligible anti-Xa levels. After nadroparin administration at the start of dialysis, a typical early peak was avoided with the infusion method, as shown by the lower anti-Xa level at the first hour (0.49 ± 0.10 versus 0.68 ± 0.10 IU/mL, P < 0.001). At the second hour, the anti-Xa level in the infusion method was similar to that in the bolus approach (0.55 ± 0.11 versus 0.56 ± 0.10 IU/mL, P = 0.64). At the sixth hour (i.e. 2 h after the end of dialysis), the anti-Xa level was higher in the infusion method (0.35 ± 0.13 versus 0.25 ± 0.10 IU/mL, P < 0.001). When comparing with Time 0, the anti-Xa levels at all time points were significantly elevated in both methods (P < 0.001). Apart from the anti-Xa level, nadroparin also affected PT and APTT. PT was significantly prolonged at the first hour in both methods and was similar to that at Time 0 at subsequent time points. APTT was also significantly prolonged at the first and second hours in both approaches, and the effect of APTT prolongation was still detectable in the infusion method at the sixth hour.
Table 2.
Comparison of blood parameters between the bolus and infusion methods
| Parameters | Bolus method (n = 16) | Infusion method (n = 16) | P-value |
|---|---|---|---|
| Time 0 | |||
| Haemoglobin, g/dL | 10.21 ± 1.48 | 10.16 ± 1.57 | 0.67 |
| Platelet, × 109/L | 207.25 ± 77.91 | 199.19 ± 87.42 | 0.28 |
| PT, sec | 10.45 ± 0.50 | 10.68 ± 0.52 | 0.005 |
| APTT, sec | 35.81 ± 2.82 | 35.50 ± 3.63 | 0.63 |
| Anti-Xa level, IU/mL | 0.00 ± 0.00 | 0.00 ± 0.02 | 0.33 |
| Time first houra | |||
| PT, sec | 11.26 ± 0.60* | 11.28 ± 0.63* | 0.90 |
| APTT, sec | 57.59 ± 18.00* | 53.40 ± 5.93* | 0.31 |
| Anti-Xa level, IU/mL | 0.68 ± 0.10* | 0.49 ± 0.10* | <0.001 |
| Time second houra | |||
| PT, sec | 10.95 ± 0.67 | 11.16 ± 0.70 | 0.13 |
| APTT, sec | 52.09 ± 6.50* | 53.38 ± 6.08* | 0.33 |
| Anti-Xa level, IU/mL | 0.56 ± 0.10* | 0.55 ± 0.11* | 0.64 |
| Time sixth houra | |||
| PT, sec | 10.43 ± 0.54 | 10.78 ± 0.50 | 0.04 |
| APTT, sec | 40.51 ± 3.37 | 42.95 ± 4.01* | 0.02 |
| Anti-Xa level, IU/mL | 0.25 ± 0.10* | 0.35 ± 0.13* | <0.001 |
Data expressed as mean ± standard deviation.
APTT, activated partial thromboplastin time; PT, prothrombin time.
aHours post-administration of nadroparin.
*P < 0.05 versus Time 0, by analysis of variance with Bonferonni post hoc test.
There was no significant difference in the spKt/V between the bolus and infusion methods (1.38 ± 0.06 versus 1.38 ± 0.07, P = 0.64). Post-dialysis vascular access haemostasis times (40.7 ± 14.6 versus 41.0 ± 14.2 mins, P = 0.82) and other thrombus assessments were similar (Table 3). No bleeding events were reported.
Table 3.
Comparison of average thrombus scoring assessment between the bolus and infusion methods
| Bolus method (n = 16) | Infusion method (n = 16) | P-value | |
|---|---|---|---|
| Arterial header | 0.5 (0.1–1.4) | 0.8 (0.1–1.8) | 0.60 |
| Venous header | 0.3 (0.0–1.0) | 0.0 (0.0–0.9) | 0.44 |
| Dialyser | 0.0 (0.0–0.5) | 0.0 (0.0–0.9) | 0.53 |
| Arterial air trap | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.32 |
| Venous air trap | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 1.0 |
Data expressed as median (interquartile range).
Discussion
Our study provides the first clinical data on the safety and feasibility of LMWH infusions compared with bolus injections for haemodialysis. Conventionally, LMWH action has been assessed based on anti-Xa activity, for which 0.5 IU/mL has been recommended as the target level [12]. An ideal anticoagulant should be safe to use, without causing excessive anticoagulation leading to potential haemorrhage. For LMWH, one drawback of the traditional method is an unavoidable overanticoagulation effect early after injection. Within the initial 2 h, high anti-Xa levels of >1.0 IU/mL were reported after the administration of enoxaparin, nadroparin or tinzaparin [13–15]. These results have been summarized in a meta-analysis of randomized trials on LMWH [16]. In our study, similar early peaks of the anticoagulant effect were observed among patients treated with the bolus method, with a mean anti-Xa level of ∼0.7 IU/mL during the first hour. For the infusion method, sudden spikes in LMWH action were avoided, and the anti-Xa levels were stably maintained at 0.5 IU/mL during the first and second hours. This highly suggested that our proposed infusion approach could be a safer option within the first 2 h of dialysis. Given that overanticoagulation is uncommon beyond 2 h of LMWH administration in previous trials [16], bleeding risks related to our infusion approach are probably of less concern despite not recording anti-Xa levels at the third and fourth hours. Additionally, the uneventful completion of dialysis treatment and the similar dialytic clearances and thrombus scores observed for the infusion method compared with those in the bolus method supported the feasibility of our proposed infusion regimen to maintain extracorporeal circuit patency during haemodialysis.
Higher levels of anti-Xa activity in the infusion group were observed at the sixth hour (i.e. 2 h post-dialysis) when identical dosages of nadroparin were utilized for both methods. This may indicate higher post-dialytic haemorrhagic risk by the infusion method due to a more residual anticoagulant effect. Such a drawback could be easily resolved by reducing the LMWH dosage, such as a reduction in the hourly infusion rate or an earlier cessation of infusion, to alleviate post-dialysis anticoagulant activity. Further clinical study is necessary to validate our postulates and to define optimal infusion regimens. Blood sampling at mid-week haemodialysis in our study also allowed us to identify any potential LMWH accumulation with the infusion method. Anti-Xa levels at Time 0 (i.e. 48 h after nadroparin administration in preceding haemodialysis treatment) were negligible, implying no significant LMWH accumulation with the infusion method for conventional thrice-weekly haemodialysis.
As mentioned earlier, one major aim of our proposed LMWH infusion method was for application in dialysis beyond conventional 4-h treatments, especially for nocturnal home haemodialysis patients. Our favourable results show that the infusion approach could be a safe and feasible option for LMWH administration. In the literature, studies on anticoagulation in extended haemodialysis therapy have been relatively scarce. LMWH administration has been limited to the bolus method and has principally been applied to nocturnal dialysis delivered in-centre only [5, 17]. The need for intradialytic boluses of LMWH prohibits its use for patients on nocturnal haemodialysis at home. The potential superior effects on lipid and bone metabolism and lower risk of HIT may make LMWH a preferred choice over UFH. The latter is particularly relevant because the development of HIT would render nocturnal home haemodialysis problematic, if not impossible [18]. Certainly, an extrapolation of our results into extended haemodialysis treatment requires additional clinical trials that apply infusion approaches of LMWH to this particular group of patients.
There were several limitations in our study. The sample size was relatively small, and we lacked more data on intradialytic anti-Xa levels. Therefore, our study was inevitably underpowered to report potential significant differences between the two anticoagulation methods. Additionally, the extrapolation of our results to other ethnic groups may not be necessarily applicable because all recruited subjects were Chinese. Furthermore, the markers of coagulation activation, such as thrombin–antithrombin complex and prothrombin fragments 1 and 2, were not monitored. This restricted our circuit patency evaluation only to any visible clots. However, with dialysis prescriptions kept separate from the anticoagulation method, the comparable dialytic clearance achieved by the infusion approach likely suggested a similar efficacy in preventing clinically relevant clot formation. Finally, anti-Xa levels were used in our study to evaluate the degree of anticoagulation; however, the correlation between anti-Xa levels with clinical bleeding and thrombosis during LMWH use has been challenged [19].
To conclude, we have modified the administration method of LMWH for anticoagulation in intermittent haemodialysis. Compared with the traditional single-bolus approach, our infusion regimen was found to be equally safe and feasible, with additional merits of avoiding excessive anticoagulation early after bolus injection and possible LMWH dose reduction. Further clinical trials are needed to determine whether the LMWH infusion method could be applied in extended haemodialysis treatment.
Conflict of interest statement
There are no conflicts of interest to declare for all authors.
Acknowledgements
We would like to thank our dialysis nurses Wing-Ho Chau and Pui-Lai Cheng, who were responsible for the haemodialysis treatment and blood collection during the study. Additionally, we would like to thank Dr Jason So and colleagues of the Haematology Laboratory at the Queen Mary Hospital, Hong Kong SAR, who provided us support for testing anti-Xa activity in the blood samples.
References
- 1.Davenport A. Review article: Low-molecular-weight heparin as an alternative anticoagulant to unfractionated heparin for routine outpatient haemodialysis treatments. Nephrology (Carlton) 2009; 14: 455–461 [DOI] [PubMed] [Google Scholar]
- 2.Deuber HJ, Schulz W. Reduced lipid concentrations during four years of dialysis with low molecular weight heparin. Kidney Int 1991; 40: 496–500 [DOI] [PubMed] [Google Scholar]
- 3.Wiemer J, Winkler K, Baumstark M et al. , Influence of low molecular weight heparin compared to conventional heparin for anticoagulation during haemodialysis on low density lipoprotein subclasses. Nephrol Dial Transplant 2002; 17: 2231–2238 [DOI] [PubMed] [Google Scholar]
- 4.Lai KN, Ho K, Cheung RC et al. Effect of low molecular weight heparin on bone metabolism and hyperlipidemia in patients on maintenance hemodialysis. Int J Artif Organs 2001; 24: 447–455 [PubMed] [Google Scholar]
- 5.Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106: 2710–2715 [DOI] [PubMed] [Google Scholar]
- 6.Buitenwerf E, Risselada A, van Roon EN et al. , Effect of nadroparin on anti-Xa activity during nocturnal hemodialysis. BBA Clin 2015; 3: 276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breen C. Anticoagulation and dialysis access practice in home haemodialysis in the UK. NDT Plus 2011; 4 (Suppl 3): iii19–iii20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joannidis M, Kountchev J, Rauchenzauner M et al. Enoxaparin vs. unfractionated heparin for anticoagulation during continuous veno-venous hemofiltration: a randomized controlled crossover study. Intensive Care Med 2007; 33: 1571–1579 [DOI] [PubMed] [Google Scholar]
- 9.Oudemans-van Straaten HM, van Schilfgaarde M, Molenaar PJ et al. , Hemostasis during low molecular weight heparin anticoagulation for continuous venovenous hemofiltration: a randomized cross-over trial comparing two hemofiltration rates. Crit Care 2009; 13: R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugirdas JT. Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv Ren Replace Ther 1995; 2: 295–304 [DOI] [PubMed] [Google Scholar]
- 11.Hemodialysis Adequacy 2006 Work Group. Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 2006; 48 (Suppl 1): S2–S90 [DOI] [PubMed] [Google Scholar]
- 12.Schrader J, Stibbe W, Armstrong VW et al. Comparison of low molecular weight heparin to standard heparin in hemodialysis/hemofiltration. Kidney Int 1988; 33: 890–896 [DOI] [PubMed] [Google Scholar]
- 13.Guillet B, Simon N, Sampol JJ et al. Pharmacokinetics of the low molecular weight heparin enoxaparin during 48 h after bolus administration as an anticoagulant in haemodialysis. Nephrol Dial Transplant 2003; 18: 2348–2353 [DOI] [PubMed] [Google Scholar]
- 14.Stefoni S, Cianciolo G, Donati G et al. Standard heparin versus low-molecular-weight heparin. A medium-term comparison in hemodialysis. Nephron 2002; 92: 589–600 [DOI] [PubMed] [Google Scholar]
- 15.Hainer JW, Sherrard DJ, Swan SK et al. Intravenous and subcutaneous weight-based dosing of the low molecular weight heparin tinzaparin (Innohep) in end-stage renal disease patients undergoing chronic hemodialysis. Am J Kidney Dis 2002; 40: 531–538 [DOI] [PubMed] [Google Scholar]
- 16.Lim W, Cook DJ, Crowther MA. Safety and efficacy of low molecular weight heparins for hemodialysis in patients with end-stage renal failure: a meta-analysis of randomized trials. J Am Soc Nephrol 2004; 15: 3192–3206 [DOI] [PubMed] [Google Scholar]
- 17.Verhave G, Weijmer MC, van Jaarsveld BC. Anticoagulation with dalteparin and nadroparin in nocturnal haemodialysis. Neth J Med 2015; 73: 270–275 [PubMed] [Google Scholar]
- 18.Faratro R, D'Gama C, Chan C. The use of alternative anti-coagulation strategies for a nocturnal home hemodialysis patient with heparin-induced thrombocytopenia. CANNT J 2008; 18: 32–35 [PubMed] [Google Scholar]
- 19.Bara L, Leizorovicz A, Picolet H et al. , Correlation between anti-Xa and occurrence of thrombosis and haemorrhage in post-surgical patients treated with either Logiparin (LMWH) or unfractionated heparin. Postsurgery Logiparin Study Group. Thromb Res 1992; 65: 641–650 [DOI] [PubMed] [Google Scholar]