Abstract
Background
Studies report variation in the incidence and outcomes of encapsulating peritoneal sclerosis (EPS). This study reports the incidence and outcome of EPS cases in a national cohort of peritoneal dialysis (PD) patients.
Methods
The incident cohort of adult patients who started PD between 1 January 2000 and 31 December 2007 in Scotland (n = 1238) was identified from the Scottish Renal Registry. All renal units in Scotland identified potential EPS cases diagnosed from 1 January 2000 to 31 December 2014, by which point all patients had a minimum of 7 years follow-up from start of PD.
Results
By 31 December 2014, 35 EPS cases were diagnosed in the 1238 patient cohort: an overall incidence of 2.8%. The incidence for subgroups with longer PD duration rises exponentially: 1.1% by 1 year, 3.4% by 3 years, 8.8% at 4 years, 9.4% at 5 years and 22.2% by 7 years. Outcomes are poor with mortality of 57.1% by 1 year after diagnosis. Survival analysis demonstrates an initial above-average survival in patients who later develop EPS, which plummets to well below average after EPS diagnosis.
Conclusions
The incidence of EPS is reassuringly low provided PD exposure is not prolonged and this supports ongoing use of PD. However, continuing PD beyond 3 years results in an exponential rise in the risk of developing EPS and deciding whether this risk is acceptable should be made on an individual patient basis.
Keywords: encapsulating peritoneal sclerosis, EPS, incidence, peritoneal dialysis
Introduction
Encapsulating peritoneal sclerosis (EPS) has been a major focus of the peritoneal dialysis (PD) literature in the past decade. Until the pathophysiology is fully understood, causative factors are identified, and reliable preventative or therapeutic strategies are available, it should remain a priority for research. We cannot yet predict who will succumb but we can advise patients that increasing duration of PD exposure is a clear risk factor for EPS [1–3]. Nephrologists advise and guide decisions about dialysis therapies, so it is easy to see why the potential for developing EPS may dissuade some clinicians from commencing PD at all. The decision whether to limit PD treatment duration, given the clear association of EPS with prolonged PD, is contentious, not least because of problems with incidence reporting [1, 2, 4–6]. Similarly, haemodialysis as the alternative option is not risk-free and we will never have a randomized trial to compare these modalities [7].
In 2009 we published our initial work, quantifying the incidence of EPS in an incident PD cohort [2]. The main motivation for carrying out this study was that no other published studies had reported a true incidence and were therefore potentially misleading. The intention of our work was to achieve this in the Scottish PD population, quantifying the incidence and recording the outcome of EPS cases using the ISPD diagnostic criteria [8]. There is a view that these criteria should be refined to improve consistency of diagnosis between studies and avoid potential over-reporting [8, 9].
Given that EPS is associated with duration of PD and many of our patients had short exposure to PD by 2009, it was necessary to follow our original cohort for several years to establish an accurate incidence. In this paper we present the results of further follow-up of the same PD cohort.
Materials and methods
Using the Scottish Renal Registry, we identified all adult patients who started PD between 1 January 2000 and 31 December 2007 in Scotland (n = 1238) and asked the 10 renal units to identify potential EPS cases diagnosed on or after 1 January 2000. We continued the data collection until 31 December 2014, by which point all patients had achieved a minimum of 7 years follow-up from start of PD.
Diagnosis
We examined medical records to make sure cases met the ISPD criteria for EPS diagnosis including clinical features and either radiological and/or histopathological confirmation [8]. Exclusion criteria included the presence of an alternative cause for EPS: tuberculosis, previous abdominal surgery (except PD catheter or transplant placement), active inflammatory bowel disease or previous bowel perforation, intraperitoneal chemotherapy, other cause of ascites including malignancy or liver cirrhosis. Ten potential cases were excluded because there was an alternative possible cause for their findings (n = 7) or there was insufficient radiological or pathological evidence to definitely diagnose EPS (n = 3). Clinical details relating to underlying primary renal disease, exposure to high-strength dextrose and/or Extraneal/Icodextrin dialysate, peritonitis episodes, radiological and/or laparotomy/oscopy findings and any treatment given were collected for all cases. Peritoneal equilibration tests (PET) are not regularly performed in stable PD patients and there were not enough PET results for meaningful analysis of any change in transporter status around the time of EPS diagnosis.
Statistical analysis
Statistical analyses were performed using SPSS® (version 19). The incidence of EPS was calculated using the number of EPS cases divided by number of patients on PD according to duration of PD exposure. The Kaplan–Meier analysis was used to calculate cumulative risk of EPS using the time from first PD to EPS diagnosis, without censoring for transplantation (intention-to-treat analysis). The Kaplan–Meier method was also used to compare patient survival from start of PD to death or study end.
Ethics
This study represents audit of usual clinical care, and was therefore exempt from requiring ethics submission.
Results
Clinical presentation and diagnosis
The major clinical characteristics of the EPS cases are shown in Table 1. Clinical presentation varied; overall 14/35 presented with acute bowel obstruction and had classical EPS findings at laparotomy. A further 15/35 had exploratory laparotomy or laparoscopies that revealed typical matted, adherent bowel classical of EPS. The remaining six cases were diagnosed on the basis of symptoms with computed tomography (CT) (n = 4) or ultrasound scan imaging (n = 2) confirming the diagnosis.
Table 1.
Clinical characteristics of EPS cases
| EPS cases characteristics | Number of cases |
|---|---|
| Age (median, IQR) | 53 (43–65) |
| Male, n (%) | 21 (60) |
| Primary renal diagnosis | |
| APKD | 8 |
| DM | 5 |
| Unknown | 10 |
| FSGS | 3 |
| IgA | 3 |
| VUR | 2 |
| Congenital/familial | 2 |
| Light chain deposition | 1 |
| Other | 1 |
| Duration PD | 1464 (1003–1934) days, |
| (median, IQR) | 4.0 (2.7–5.3) years |
| Peritonitis | |
| Never had peritonitis | 3 |
| Median number of peritonitis episodes (IQR) | 2 (1–3.5) |
| Number having fungal peritonitis | 3 |
| Number having staph aureus peritonitis | 11 |
| PD fluid prescription | |
| Icodextrin/Extraneal ever | 33 |
| High-strength dextrose (3.86% or equivalent) ever | 12 |
| Solely biocompatible fluid used | 0 |
| Betablocker or ACE/ARBa exposure, n (%) | |
| Betablocker use while on PD | 14 (40) |
| ACE or ARB use while on PD | 9 (26) |
| On PD at diagnosis? n (%) | |
| Yes | 14 (40) |
| Nob | 21 (60) |
| Functioning transplant at diagnosis | 4 (11) |
| Diagnosis confirmed by, n (%) | |
| CT scan appearance | 4 (11) |
| US scan appearance | 2 (6) |
| Laparotomy or laparoscopy | 29 (83) |
| Main presenting symptoms, n (%)c | |
| Abdominal pain | 21 (60) |
| Vomiting | 20 (57) |
| Acute or subacute bowel obstruction | 14 (40) |
| Ascites | 9 (26) |
| Bloody ascites | 3 (9) |
| Weight Loss | 23 (7) |
| Abdominal distension (but not obstruction) | 5 (1) |
| Unexplained elevated CRP | 11 (3) |
| CT scan (n = 27) or US scan (n = 2) findingsc | |
| Bowel obstruction | 9 |
| Dilated bowel (not overt obstruction) | 6 |
| Matted/tethered bowel loops | 18 |
| Thickened mesentery/peritoneum | 17 |
| Thickened bowel wall | 22 |
| Loculated ascites | 19 |
| Peritoneal calcification | 9 |
| Management (n and % 1 year mortality) | |
| No specific treatment | 11 (72) |
| Tamoxifen | 5 (20) |
| Tamoxifen and steroid | 6 (50) |
| Taxoxifen/azathioprine | 1 (0) |
| Sirolimus | 2 (100) |
| Prednisolone only | 4 (0) |
| Elective enterolysis/peritoneal stripping | 2 (50) |
| Attempted enterolysis during emergency laparotomy | At least 6d (83) |
APKD, adult polycystic kidney disease; DM, diabetes mellitus; FSGS, focal segmental glomerulosclerosis; VUR, vesicoureteral reflux; ACE/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; US, ultrasound; CRP, C-reactive protein.
aACE inhibitor or ARB drug treatment at some point while on PD.
bIncludes five diagnosed within 2 weeks of stopping PD when symptoms had been apparent (though not formally diagnosed) prior to transfer to haemodialysis (three patients) or transplant (two patients).
cNote most patients had more than one.
dOperation notes not available or unclear if attempted adhesiolysis in most cases.
Incidence and change in incidence over time
Between 1 January 2000 and 31 December 2007, 1238 patients were first exposed to PD. Table 2 shows the EPS incidence according to duration of PD exposure and the changes in the incidence. This gives an incidence of 8.7 per 1000 PD treatment years by 31 December 2007 and a rate of 13.6 per 1000 PD treatment years by 31 December 2014. There is a substantial increase in the proportion developing EPS after 5 years of PD (1 in 7.8 patients at risk).
Table 2.
PD exposure and number of EPS cases for the incident PD cohort at the three different data collection periods
| PD exposure (years) | 2008 analysis |
2009 analysis |
2015 analysis |
1 year mortality rate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PD cohort (n) | EPS cases (%) | Incidence (%) | PD cohort (n) | EPS cases (%) | Incidence (%) | PD cohort (n) | EPS cases (%) | Incidence (%) | 95% confidence intervals | ||
| ≤1 | 480 | 0 | 0 | 470 | 1 | 0.2 | 419 | 1 | 0.2 | 0.04–1.3 | 0 |
| >1–2 | 326 | 2 | 0.6 | 327 | 3 | 0.9 | 282 | 3 | 1.1 | 0.4–3.1 | 66% |
| >2–3 | 202 | 4 | 2.0 | 198 | 5 | 2.5 | 208 | 7 | 3.4 | 0.9–5.8 | 29% |
| >3–4 | 114 | 4 | 3.5 | 117 | 6 | 5.1 | 135 | 6 | 4.4 | 1.0–7.9 | 67% |
| >4–5 | 62 | 5 | 8.1 | 63 | 6 | 9.5 | 80 | 7 | 8.8 | 2.6–14.9 | 57% |
| >5–6 | 34 | 3 | 8.8 | 35 | 6 | 17.1 | 53 | 5 | 9.4 | 1.6–17.3 | 60% |
| >6–7 | 15 | 1 | 6.7 | 17 | 3 | 17.6 | 33 | 4 | 12.1 | 1.0–23.3 | 75% |
| >7–8 | 5 | 0 | 0 | 11 | 1 | 9.1 | 9 | 2 | 22.2 | 0–49.0 | 100% |
| >8 | – | – | – | – | – | – | 19 | 0 | – | – | 66% |
| Total | 1238 | 19 | 1.5 | 1238 | 31 | 2.5 | 1238 | 35 | 2.8 | 2.0–3.9 | 57.1% |
At the study end 753 patients had died (60.1%), 28 (2.3%) patients had been lost to follow-up by moving out of Scotland, 356 (28.8%) have a functioning renal transplant, 83 (6.7%) are on haemodialysis, 7 (0.6%) on home haemodialysis and 14 (1.1%) are on PD.
As-treated versus intention-to-treat analysis
If we plot the time from start of PD as total exposure, i.e. actual days spent on PD therapy, the EPS risk rises exponentially (Figure 1A). In practice, as shown in Table 2, 15.4% of patients on PD for 7–8 years, and none over 8 years so far have developed EPS. The survival analysis shows us the accumulated risk (as-treated) of EPS; if a patient manages to continue on PD for 8 years the risk of having developed EPS by that point is 40%. Figure 1B shows an intention-to-treat EPS-free survival curve from start of PD to EPS diagnosis only censoring at death or end of follow-up (31 December 2014). By 5 years, <3% and by 10 years just under 5% of patients will have developed EPS, reflecting the reality that few continue PD beyond 3 years because of death, technique failure or renal transplantation.
Fig. 1.
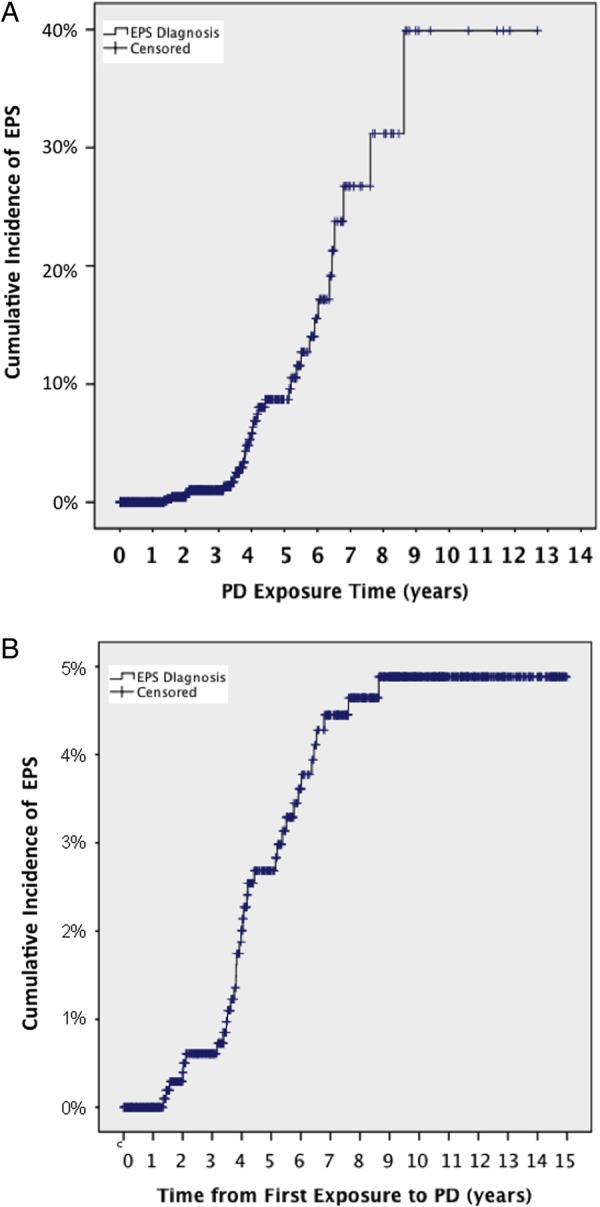
(A) Cumulative risk of EPS with increasing exposure to PD (as-treated) (censored at death or the end of study on 31 December 2014). (B) Incidence of EPS from start of PD regardless of whether patient continues on PD or not (intention-to-treat) (censored at death or the end of study on 31 December 2014).
Mortality
By the 31 December 2014, 29/35 (82.9%) of the patients who had developed EPS had died with a 1-year mortality of 57.1%, with no significant different between PD exposure categories (Table 1), although those with >6 years of PD exposure show a trend towards higher mortality (CHI square P = 0.08). The median survival was 288 days [range 4–2493, interquartile range (IQR) 50–1151 days] from EPS diagnosis. Of note, 11/14 (78.6%) of those presenting with acute bowel obstruction were dead by 2 years post-diagnosis compared with 13/21 (61.9%) of those with an alternative presentation.
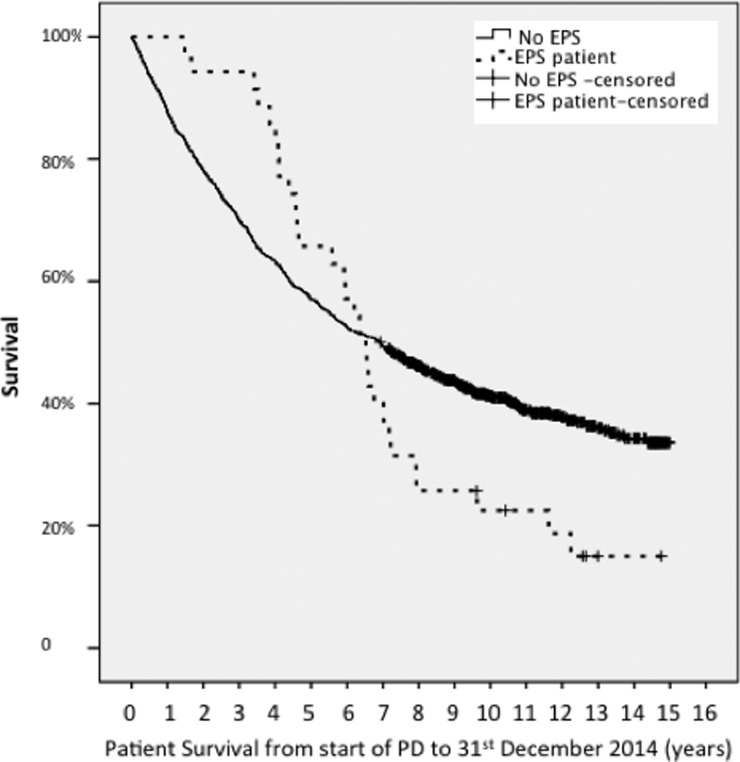
The survival from start of PD to death is a median of 2382 days for EPS cases (IQR 1599–3513 days) and 2482 days (IQR 880–3413 days) (P = not significant) for those who did not develop EPS. However, those who developed EPS had better survival in the first 3 years and thereafter, a steep fall in survival (Figure 2).
Fig. 2.
Survival of the 35 incident EPS cases compared with the 1203 incident PD cohort from time of starting PD to death. The data have not been censored for transplantation.
Discussion
We have accurately documented the incidence of EPS in a contemporary incident PD cohort in Scotland. The increase in incidence between the initial data analysis in 2008 and the last analysis in 2014 highlights the importance of length of follow-up, particularly when the complication being studied is related to duration of PD exposure [1, 2, 10, 11].
Overall incidence
By virtue of the longer follow-up we believe the 2015 data analysis provides the most accurate incidence; 2.8% overall. We can find no convincing evidence to suggest that the incidence is rising locally; it only appears to climb over the follow-up period because the cohort's PD exposure has increased. Indeed, fewer patients have been diagnosed in our most recent than earlier data collections. It is unlikely that more patients will develop EPS from our cohort given that so few patients are continuing PD beyond the last data collection.
Our incidence is identical (2.8%) to the incidence reported in a contemporary Italian study [3], similar to another UK series (3.3%) [12] but higher than an Australian study (0.7%) [1] and Japanese and Korean studies (0.8–2.5%) [13–16]. Kawanishi published a 2-year follow-up of a PD cohort in 2001, suggesting an incidence of 0.8% [13], and further analysis in 2004 reporting an incidence of 2.5%, likely reflecting longer follow-up inherent to the study design rather than a true incidence increase [14]. Several publications from North America confirm low overall EPS rates but comparable, if not higher, rates for patients on PD for 5 years or longer with one quoting an overall incidence of 1.2% but 15% after 6 years on PD [5, 17–19]. Overall incidence is only one part of the picture. Units with successful PD take-on rates and transplantation programmes will have a high turnover and a disproportionate number of patients on short-term PD, who will have lower risk of EPS.
Incidence according to PD exposure
Our data confirm the exponential rise in incidence with increasing PD exposure; approximately 1:13 with 4–5 years exposure, and 1:7.8 with 5–6 years exposure. A recent Italian study found rates of 3%, 6%, 18% and 75% at 2–4 years, 6–8 years, 8–10 years and 12–14 years respectively [16]. Rigby et al.'s study showed rates of 1.9%, 6.4%, 10.8% and 19.4% at 2, 4, 5 and 8 years respectively [3] which is similar to our own data. The rates are higher than in Japan: 3 years 0%, 5 years 0.7% and 8 years 2.1%, but in that 4-year study the denominator population was all prevalent patients on PD so it is not a true incidence, and may underestimate the rate [14]. Similarly, the retrospective nature of Johnson et al.'s case–control study increases the likelihood of missing cases (they found the incidence at 3 years was 0.3%, 5 years 0.8% and 8 years 3.9%) [11].
There are several potential reasons for differences in incidence between centres and countries. One possible difference is the use of ‘biocompatible’ or low GDP dialysis fluids, which may be associated with a lower EPS risk [20]. They were not used routinely in Scotland during this study period, which followed the UK Renal Association PD Guidelines' recommendation [21]. There has been a gradual move towards patients using ‘biocompatible’ fluid in recent years and it will be interesting to record whether the EPS incidence changes in future.
Diagnosis and timing of diagnosis
We used diagnostic criteria set out in the ISPD Guidance 2000 [8, 22]. A proportion of patients were highlighted to us for further consideration who had not been given an EPS diagnosis locally, but whom the nephrology team felt met criteria. It is relevant that most of our EPS cases were diagnosed prior to CT imaging diagnostic criteria being defined, and the scans were reported by ‘non-specialist’ radiologists in many cases [23–25]. Had we relied upon units to simply report confirmed EPS cases there would have been fewer cases.
We agree with the current belief that the diagnostic criteria need to be standardized to allow proper comparative studies to proceed [9]. One suggestion is to only include patients with overt bowel obstruction, but it may be difficult to define obstruction [22].
Of our cases, there were patients who had unimpressive imaging (e.g. ascites only), and no overt bowel obstruction but who undoubtedly had advanced EPS with a matted mass of bowel at laparotomy. Only two cases may be excluded if diagnostic criteria were modified as suggested above; both had classical symptoms and imaging showing loculated ascites with adherent, thickened bowel but without obstruction and did not undergo laparotomy/oscopy to confirm EPS, yet both died within 12 months of diagnosis. The significant mortality for those without overt obstruction does not equate to a more indolent disease course in our case series.
The clinical characteristics (Table 1) are similar to previously published case series. EPS was diagnosed after stopping PD in 60% of our cases, which is similar to Japanese data (69%) and higher than in an Australian study (21%) [11, 14]. No patients routinely receive peritoneal lavage after discontinuing PD in Scotland. Nine patients had been transplanted although only four (11%) cases were diagnosed with EPS with a functioning transplant, compared with 3% in an Japanese series, 8% in an Italian series, none in an Australian series and 25–29% in a Dutch study [3, 11, 14, 26, 27]. This wide variation may relate to differences in case ascertainment or PD duration pre-transplant.
Outcome
Patient survival with EPS is poor; the mortality rate for our cohort was 57.1% at 1 year after diagnosis and 67.7% by the study end. This finding compares with 37.5% [14] in Japan, and 56% [23] and 29.6% [12] in UK series. We have not found a relationship between mortality and duration of PD before EPS diagnosis despite Japanese data showing those diagnosed with EPS after longer PD exposure experienced exponentially higher mortality [14].
The finding that the median survival from start of PD to patient death is comparable for EPS cases and the PD population in general is perhaps misleading. Patients who develop EPS may have been among the fitter PD patients, having survived long enough on PD to get EPS. Figure 2 illustrates this whereby the survival curves initially diverge and the EPS patients have a better survival rate until around 4 years when the survival curves converge, and then cross as the EPS cases' survival dramatically falls off. This corresponds to the timing of EPS diagnosis and high mortality thereafter.
We have limited data regarding the dose and duration of medical therapies or details of exact surgical intervention at laparotomy for the majority of cases. The 1-year mortality (83%) for the six cases who had documented attempts at peritoneal stripping and/or division of adhesions during exploratory laparotomy demonstrates the potential poor outcome of non-specialist, unplanned surgical intervention for EPS. Only two cases had elective specialist enterolysis as this was not an option in the UK until recent years.
Implications of our results for clinical practice
When considering the risk of EPS for specific patients, there are two main scenarios: patients already established on treatment and patients considering starting PD. For patients already on PD, our incidence data quantify the risk of developing EPS for a given duration of PD (Table 2). By 2 years of PD the EPS risk is negligible, but after 5 years it is above 1 in 10. The risk of continuing PD for a patient awaiting renal transplantation after years of PD may be considered to be too great, whilst the same risk for a patient with no option for transplantation, no vascular access or no haemodialysis centre nearby may be acceptable.
The second scenario is more difficult, as when a patient commences PD it is not known how long they will continue PD or how long they will survive. Intention-to-treat analysis allows quantification of the predicted risk of developing EPS. In general this analysis should be reassuring, as the vast majority of patients stop PD for other reasons long before EPS is a significant risk.
Whether PD duration should be limited is controversial. We would argue that such decisions can only be made between an individual patient and his or her clinician in the context of local incidence and PD practice including assessment of peritoneal function. We hope that our data could help inform such decisions.
Limitations
If cases have been missed, the incidence we report is an underestimate. It was not the remit of our study to identify any predictors of EPS development, but we have contributed our data to a multinational study along with other large series in the hope that it may help address this question.
Conclusions
Our data show a minimal risk of EPS before 2 years of PD, but a significant risk (1 in 10) beyond 5 years of PD treatment in Scotland. Our data should reassure that the cumulative risk of developing EPS after starting PD and the risk of EPS after short-term PD are both low. This data may be used to inform long-term PD patients and help them decide whether continuing on PD is a risk worth taking.
Conflict of interest statement
None declared.
Acknowledgements
The authors would like to thank the Scottish renal units for their contribution to this work.
References
- 1.Rigby RJ, Hawley CM. Sclerosing peritonitis: the experience in Australia. Nephrol Dial Transplant 1998; 13: 154–159 [DOI] [PubMed] [Google Scholar]
- 2.Brown MC, Simpson K, Kerssens JJ et al. Encapsulating peritoneal sclerosis in the new millennium: a national cohort study. Clin J Am Soc Nephrol 2009; 4: 1222–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vizzardi V, Sandrini M, Zecchini S et al. Encapsulating peritoneal sclerosis in an Italian center: thirty year experience. J Nephrol 2016; 29: 259–267 [DOI] [PubMed] [Google Scholar]
- 4.Brown EA, Van Biesen W, Finkelstein FO et al. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: position paper for ISPD. Perit Dial Int 2009; 29: 595–600 [PubMed] [Google Scholar]
- 5.Gayomali C, Hussein U, Cameron SF et al. Incidence of encapsulating peritoneal sclerosis: a single-center experience with long-term peritoneal dialysis in the United States. Perit Dial Int 2011; 31: 279–286 [DOI] [PubMed] [Google Scholar]
- 6.Garosi G, Oreopoulos DG. No need for an ‘expiry date’ in chronic peritoneal dialysis to prevent encapsulating peritoneal sclerosis. Int Urol Nephrol 2009; 41: 903–907 [DOI] [PubMed] [Google Scholar]
- 7.Korevaar JC, Feith GW, Dekker FW et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int 2003; 64: 2222–2228 [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi Y, Kawanishi H, Mujais S et al. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. Perit Dial Int 2000; 20 (Suppl 4): S43–S55 [PubMed] [Google Scholar]
- 9.Lambie M, Braun N, Davies SJ. Towards standardized reporting in studies of encapsulating peritoneal sclerosis. Perit Dial Int 2013; 33: 482–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakao M, Yokoyama K, Yamamoto I et al. Risk factors for encapsulating peritoneal sclerosis in long-term peritoneal dialysis: a retrospective observational study. Ther Apher Dial 2014; 18: 68–73 [DOI] [PubMed] [Google Scholar]
- 11.Johnson DW, Cho Y, Livingston BE et al. Encapsulating peritoneal sclerosis: incidence, predictors, and outcomes. Kidney Int 2010; 77: 904–912 [DOI] [PubMed] [Google Scholar]
- 12.Summers AM, Clancy MJ, Syed F et al. Single-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end-stage renal failure. Kidney Int 2005; 68: 2381–2388 [DOI] [PubMed] [Google Scholar]
- 13.Kawanishi H. Encapsulating peritoneal sclerosis in Japan: prospective multicenter controlled study. Perit Dial Int 2001; 21 (Suppl 3): S67–S71 [PubMed] [Google Scholar]
- 14.Kawanishi H, Kawaguchi Y, Fukui H et al. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis 2004; 44: 729–737 [PubMed] [Google Scholar]
- 15.Lee HY, Kim BS, Choi HY et al. Sclerosing encapsulating peritonitis as a complication of long-term continuous ambulatory peritoneal dialysis in Korea. Nephrology (Carlton) 2003; 8: S33–S39 [DOI] [PubMed] [Google Scholar]
- 16.Nomoto Y, Kawaguchi Y, Kubo H et al. Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of the Japanese Sclerosing Encapsulating Peritonitis Study Group. Am J Kidney Dis 1996; 28: 420–427 [DOI] [PubMed] [Google Scholar]
- 17.Blake PG. Is encapsulating peritoneal sclerosis rare in North America? Perit Dial Int 2011; 31: 263–264 [DOI] [PubMed] [Google Scholar]
- 18.Trigka K, Dousdampanis P, Chu M et al. Encapsulating peritoneal sclerosis: a single-center experience and review of the literature. Int Urol Nephrol 2011; 43: 519–526 [DOI] [PubMed] [Google Scholar]
- 19.Bansal S, Sheth H, Siddiqui N et al. Incidence of encapsulating peritoneal sclerosis at a single U.S. university center. Adv Perit Dial 2010; 26: 75–81 [PubMed] [Google Scholar]
- 20.Kawanishi H, Shintaku S, Banshodani M et al. Past and present perspectives on encapsulating peritoneal sclerosis. Contrib Nephrol 2015; 185: 87–97 [DOI] [PubMed] [Google Scholar]
- 21.Woodrow G, Davies SJ. Renal Association Clinical Practice Guidelines on Peritoneal Dialysis, 2010. Available at: http://www.renal.org/guidelines/modules/peritoneal-dialysis-in-ckd#sthash.iB63Gwyb.dpbs
- 22.Kawaguchi Y, Saito A, Kawanishi H et al. Recommendations on the management of encapsulating peritoneal sclerosis in Japan, 2005: diagnosis, predictive markers, treatment, and preventive measures. Perit Dial Int 2005; 25: S83–S95 [PubMed] [Google Scholar]
- 23.Balasubramaniam G, Brown EA, Davenport A et al. The Pan-Thames EPS study: treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol Dial Transplant 2009; 24: 3209–3215 [DOI] [PubMed] [Google Scholar]
- 24.Tarzi RM, Lim A, Moser S et al. Assessing the validity of an abdominal CT scoring system in the diagnosis of encapsulating peritoneal sclerosis. Clin J Am Soc Nephrol 2008; 3: 1702–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlijm A, Stoker J, Bipat S et al. Computed tomographic findings characteristic for encapsulating peritoneal sclerosis: a case-control study. Perit Dial Int 2009; 29: 517–522 [PubMed] [Google Scholar]
- 26.Korte MR, Sampimon DE, Lingsma HF et al. Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS study. Perit Dial Int 2011; 31: 269–278 [DOI] [PubMed] [Google Scholar]
- 27.Hendriks PM, Ho-dac-Pannekeet MM, van Gulik TM et al. Peritoneal sclerosis in chronic peritoneal dialysis patients: analysis of clinical presentation, risk factors, and peritoneal transport kinetics. Perit Dial Int 1997; 17: 136–143 [PubMed] [Google Scholar]