Abstract
Background
Intradialytic hypertension (IDH) increases morbidity and mortality. The prevalence in South Africa is unknown. The pathogenesis is unclear, but it has been suggested that IDH may be due to subclinical fluid overload. The objective of this study was to determine the prevalence of IDH and to evaluate its association with fluid overload using bioimpedance spectroscopy (BIS).
Methods
A cross-sectional study involving 190 chronic haemodialysis patients in the Western Cape province of South Africa was conducted between January 2013 and May 2014. IDH was defined as a >10 mmHg increase in systolic blood pressure in at least four of six prior consecutive haemodialysis sessions.
Results
The prevalence of IDH was 28.4% (n = 54). There was a trend towards pre-dialysis overhydration in the IDH group when compared with controls {2.6 L [95% confidence interval (CI) 1.7–3.4] versus 1.8 L [95% CI 1.4–2.1], respectively; P = 0.06} as measured by BIS, but no difference in mean ultrafiltration (UF) volume (2.4 versus 2.6 L; P = 0.30). A trend towards greater use of antihypertensive drugs was noted in the IDH group [2.5 drugs (95% CI 2.15–2.87) versus 2.1 (95% CI 1.82–2.30); P = 0.05]. More participants in the IDH group received calcium channel blockers (54 versus 36; P = 0.03).
Conclusions
The prevalence of IDH in our treatment centres is much higher than previously reported. Subclinical fluid overload may be a major contributing factor to the mechanism of this condition. The use of BIS identifies patients who may benefit from additional UF.
Keywords: bioimpedance monitoring, haemodialysis, intradialytic hypertension
Background
A paradoxical increase in blood pressure (BP) during chronic haemodialysis sessions, also known as intradialytic hypertension (IDH), is a well-known but uncommon complication. Various definitions of IDH exist, but, to date, there is no standard definition. As a result, the prevalence ranges from 5 to 15%, depending on the definition used [1, 2]. A common definition is that by Inrig et al. [3], who define IDH as a systolic blood pressure (SBP) increase ≥10 mmHg from pre- to post-hemodialysis in at least four of six treatments.
IDH increases the incidence of cardiovascular morbidity and mortality. A secondary analysis of 443 patients in the Crit-Line Intradialytic Monitoring Benefit Study (CLIMB) reported that patients with an intradialytic increase in SBP had twice the risk for hospitalization or death at 6 months [3]. Analysis of 1748 incident haemodialysis patients in the United States Renal Data System (USRDS) found that the adjusted hazard for death at 2 years for haemodialysis patients was 6% for every 10 mmHg increase in SBP [4].
The pathogenesis of IDH is likely to be multifactorial. Several studies have identified extracellular fluid overload as a primary driver of this process [5–7]. Fluid overload increases stroke volume, cardiac output and, subsequently, BP. In these studies, patients with IDH not responsive to antihypertensive medication became normotensive after intensified ultrafiltration (UF) [6–8]. Correction of fluid status is labour intensive and often requires extended dialysis sessions or aggressive UF. It may take weeks to optimize the fluid status of these patients, and BP may only respond after a month of aggressive lowering in dry weight.
Other mechanisms thought to be involved in the pathogenesis include increased activity of the renin–angiotensin–aldosterone system (RAAS) and overactivity of the sympathetic nervous system. Dialysate-related factors such as high dialysate sodium and calcium concentrations as well as removal of dialysable antihypertensive drugs may contribute [2, 9]. Erythropoietin-stimulating agents (ESAs) have also been associated with the development of hypertension in haemodialysis patients. ESAs administered intravenously at the latter stage of a dialysis session have been shown to increase mean arterial pressure (MAP) by >10 mmHg during the interdialytic period [10]. Endothelial dysfunction has also been implicated. The dialysis-related increase in endothelin-1 (ET-1) concentrations and decrease in nitric oxide (NO) have been documented in several studies [11, 12].
Owing to the paucity of randomized trials on the management of IDH, treatment options have been largely driven by expert opinion. Management is directed at all of the aforementioned pathogenic mechanisms, but normalizing fluid overload and dietary sodium is recommended as the first step in management [2]. Defining the fluid status of chronic haemodialysis patients is difficult. Most dialysis units adopt the traditional ‘trial and error’ method for determining dry weight. This is considered the point during dialysis at which the reduction in BP is regarded by the clinician as too low after a specific volume has been removed. However, this method relies heavily on clinical judgement and is fraught with danger. Excessive fluid removal may result in intradialytic hypotension, whereas underestimation of dry weight may cause fluid overload with hypertension and a subsequent increase in cardiovascular morbidity and mortality.
Recently published randomized controlled trials advocate the use of bioimpedance spectroscopy (BIS) to accurately determine fluid status in chronic haemodialysis patients [13, 14]. The volume of separate body fluid compartments can be determined using a body composition monitor (BCM). This device has been validated against various gold standard methods [15]. Patients with ‘subclinical fluid overload’ may be identified using a BCM—a group often missed when traditional clinical methods are used to determine dry weight. In particular, patients with post-dialysis overhydration (OH) of the extracellular space [extracellular water (ECW)] have been identified as being hypertensive. Gradual dry weight adjustment using a BCM has been shown to promote regression of left ventricular mass, reduced arterial stiffness and reduced BP in these patients [14, 16].
Intradialytic hypotension is frequently encountered in chronic haemodialysis patients. Nephrologists often focus their attention on prevention and management of this complication, neglecting optimal management of patients with IDH. Early identification and management of IDH may reduce the burden of cardiovascular complications.
To the best of our knowledge, there is no data on the prevalence of IDH in South Africa. Therefore, we conducted a cross-sectional study to determine the prevalence of IDH in dialysis units in the Western Cape province of South Africa. The secondary objective was to investigate any association between IDH and interdialytic weight gain (IDWG), degree of fluid overload as assessed clinically and by BIS, quantity and timing of antihypertensives, ESA dose and route of administration, time-averaged sodium concentration, dialysate calcium concentration and haemodialysis modality.
Methods
This study was registered at ClinicalTrials.gov (NCT01916668) on 1 February 2013. The study was conducted in accordance with the ethical principles set out by the Declaration of Helsinki and approval for the study was granted by the Human Research Ethics Committee of Stellenbosch University (study number: S12/10/264).
Study design, setting and participants
This multicentre, cross-sectional study of IDH in chronic haemodialysis patients was conducted between January 2013 and May 2014 in the Western Cape province of South Africa.
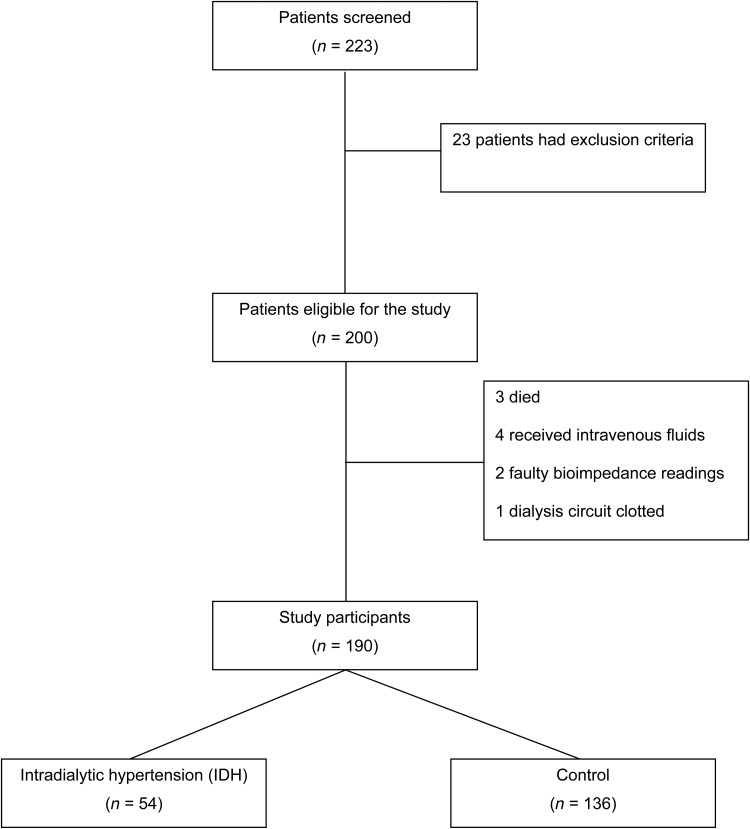
Four haemodialysis units in the Western Cape were used. One unit was Tygerberg Academic Hospital in the public sector, with the remaining units being Panorama, Athlone and Winelands Kidney and Dialysis Centres in the private sector. All chronic haemodialysis patients ≥18 years of age with the ability to give informed consent were eligible. Patients who were unable to give informed consent and had contraindications to BIS measurement (limb amputations, pre-existing implanted cardiac devices such as pacemakers or implantable cardioverter defibrillators) received >200 mL of intravenous fluid or antibiotics for acute illness during the dialysis session; those for whom BP could not be measured by routine methods in the upper limbs were excluded (Figure 1). All patients were studied during their midweek dialysis session.
Fig. 1.
Number of participants screened and included in the final analysis.
Participants in the public sector were younger than those in the private sector (Table 1). This is due to criteria used by the Western Cape public sector when selecting patients for renal replacement therapy (RRT). The age difference is also a reflection of national differences in the age of those accessing public and private sector treatment facilities [17]. Nearly half of the participants were of mixed race. This reflects the racial composition of the population of the Western Cape [18]. Data regarding the cause of end-stage renal disease (ESRD) were not collected.
Table 1.
Comparison of clinical baseline characteristics
| All patients (n = 190) |
IDH (n = 54) |
Control (n = 136) |
P-value | ||||
|---|---|---|---|---|---|---|---|
| Overall mean age (years) | 55.7 | (53.6–57.7) | 57.1 | (52.8–61.2) | 55.1 | (52.8–57.5) | 0.42 |
| Mean age by unit (years) | |||||||
| TBH (public) | 43.9 | (40.9–46.9) | 38.0 | (32.1–43.9) | 46.0 | (42.9–49.0) | 0.02 |
| AKDC | 56.2 | (51.7–60.8) | 62.1 | (54.6–69.7) | 54.8 | (49.7–59.9) | 0.20 |
| WKDC | 57.3 | (53.1–61.6) | 58.2 | (49.6–66.8) | 56.9 | (52.1–61.8) | 0.78 |
| PKDC | 60.9 | (57.6–64.2) | 63.0 | (57.8–68.2) | 59.9 | (55.7–64.1) | 0.39 |
| Males, n (%) | 110.0 | (58) | 29.0 | (53.7) | 81.0 | (59.5) | 0.40 |
| Race, n (%) | |||||||
| Black | 26.0 | (14.0) | 6.0 | (11.1) | 20.0 | (14.7) | 0.23 |
| Mixed | 94.0 | (49.5) | 23.0 | (42.6) | 71.0 | (52.2) | 0.23 |
| White | 70.0 | (37.0) | 25.0 | (46.3) | 45.0 | (33.1) | 0.23 |
| BP meds, n (%) | |||||||
| ACEI | 63.0 | (33.0) | 22.0 | (35.0) | 41.0 | (65.0) | 0.16 |
| ARB | 29.0 | (15.0) | 8.0 | (27.0) | 21.0 | (73.0) | 0.91 |
| α-blocker | 33.0 | (17.0) | 13.0 | (39.0) | 20.0 | (61.0) | 0.12 |
| β-blocker | 91.0 | (48.0) | 29.0 | (32.0) | 62.0 | (68.0) | 0.31 |
| CCB | 104.0 | (55.0) | 36.0 | (67.0) | 68.0 | (50.0) | 0.03 |
| Diuretic | 91.0 | (48.0) | 25.0 | (27.0) | 66.0 | (73.0) | 0.78 |
| Hydralazine | 5.0 | (0.03) | 2.0 | (40.0) | 3.0 | (60.0) | 0.56 |
| Minoxidil | 2.0 | (0.01) | 1.0 | (50.0) | 1.0 | (50.0) | 0.50 |
| BP meds | 2.2 | (1.9–2.4) | 2.5 | (2.2–2.9) | 2.1 | (1.8–2.3) | 0.05 |
| ESA dose (IU) | 6532 | (5890–7175) | 6896 | (5679–8293) | 6352 | (5611–7094) | 0.62 |
| Dialysis mode, n (%) | |||||||
| HD | 39.0 | (20) | 10.0 | (18) | 29.0 | (21) | 0.67 |
| HDF | 151.0 | (80) | 44.0 | (82) | 107.0 | (79) | 0.67 |
| Dialysis time (min/wk) | 660.0 | (648–672) | 648.0 | (618–678) | 667.0 | (648–678) | 0.30 |
| Pre-dialysis weight (kg) | 77.0 | (74.2–79.7) | 74.5 | (69.4–79.7) | 77.9 | (74.7–81.3) | 0.29 |
| BMI (kg/m2) | 26.8 | (25.9–27.6) | 26.4 | (24.7–28.0) | 27.0 | (26.0–28.0) | 0.55 |
| Pre-dialysis BP (mmHg) | |||||||
| Systolic | 153.1 | (149.5–157.1) | 159.0 | (152.7–165.5) | 150.8 | (146.3–155.3) | 0.05 |
| MAP | 100.5 | (98.2–103.0) | 103.5 | (99.6–107.3) | 99.3 | (96.4–102.2) | 0.11 |
| Pre-dialysis OH (L) | 2.0 | (1.7–2.4) | 2.6 | (1.7–3.5) | 1.8 | (1.4–2.2) | 0.06 |
| Pre-dialysis ECW (%) | 10.4 | (8.7–12.1) | 10.4 | (8.7–12.1) | 9.6 | (7.8–11.5) | 0.12 |
Values expressed in a range in parentheses refer to 95% CIs; single values refer to percentage of total population.
IDH, intradialytic hypertension; TBH, Tygerberg Hospital; AKDC, Athlone Kidney and Dialysis Centre; WKDC, Winelands Kidney and Dialysis Centre; PKDC, Panorama Kidney and Dialysis Centre; BP, blood pressure; meds, medication; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; ESA, erythropoietin-stimulating agent; IU, international units; HD, haemodialysis; HDF, haemodiafiltration; BMI, body mass index; MAP, mean arterial pressure; OH, overhydration; ECW, extracellular water.
Dialysis procedure
We defined IDH as a ≥10 mmHg rise in SBP between pre- and post-dialysis in at least four of six previous consecutive dialysis sessions. BP, MAP and pulse rate were measured using standardized online electronic BP monitors before dialysis, hourly during dialysis and up to 30 min after dialysis. Dialysate calcium levels and time-averaged sodium concentrations were measured during the procedure.
Bioimpedance monitoring
Patient weight was determined using an electronic standardized weight scale. IDWG was calculated according to the following formula: IDWG (kg) = pre-dialysis weight (kg) – post-dialysis weight (kg) of the previous session. This IDWG was used to determine the target total UF volume for the dialysis session.
Fluid status was estimated using BIS. Four electrodes were attached to the ipsilateral arm and leg: two to the anterior aspect of the arm [one at the wrist and one on the hand (4 cm apart)] and two to the anterior aspect of the leg [one at the ankle and one on the foot (4 cm apart)]. All participants had two BIS measurements using a BCM before dialysis and ∼30 min after dialysis on a midweek dialysis day. ECW, intracellular water (ICW), total body water (TBW), lean tissue mass/weight and volume of the fat compartment were subsequently determined.
Subclinical fluid overload was defined as normovolaemia as judged by clinical assessment, but with positive OH as measured by BIS.
Medication charts
Medication charts were reviewed for data regarding ESA dose, as well as antihypertensive drug use and timing to the dialysis procedure.
Statistical analysis
The primary aim of the study was to determine the prevalence of IDH. A total of 190 participants were enrolled and were included in the final statistical analysis. Data were analysed using Statistica 12 (2014) and SAS 9.1.3 (SAS Institute, Cary, NC, USA). Continuous data were analysed using mean and standard deviation (where normally distributed) and median and interquartile range (where not normally distributed). Dichotomous data were presented as frequencies and proportions and nominal data were analysed with frequency distributions. To estimate population parameters, 95% confidence intervals (CIs) for means and proportions were calculated.
For all continuous data other than BP, comparisons between the IDH and control patients were performed using t-tests if data were normally distributed, whereas non-normal distributed data were analysed using the Mann–Whitney U test. Comparisons of nominal data were performed using χ2 tests of independence. Box plots were presented for these analyses, where appropriate.
BP data were measured at set time points during a dialysis session. An analysis of variance (ANOVA) was performed to compare group changes over time. Adjusted and unadjusted analyses were also performed, where results were adjusted for the UF rate. Mean and plot CIs were presented for these analyses. A significant P-value was regarded as ≤0.05.
Results
Baseline characteristics
Two hundred and twenty-three patients were screened. Two hundred patients met inclusion criteria and were recruited. However, three patients died before data collection, four patients received intravenous fluids on dialysis, two patients had faulty BCM readings and the dialysis circuit of one of the patients clotted.
One hundred and ninety patients were included in the final analysis. Fifty-four patients met the study definition of IDH. The remaining patients (n = 136) were assigned to the control group (Figure 1). The mean age of IDH patients was 57.1 years (95% CI 52.8–61.2) and was similar to the control group (P = 0.42). The proportion of males in the control group was greater than in the IDH group; however, this difference was not statistically significant (P = 0.42). Similarly, there were no significant racial differences between groups (Table 1).
Primary outcome
Fifty-four of the 190 patients were found to have IDH, resulting in a prevalence of 28.4% (95% CI 26–31.4).
Secondary outcomes
Pre-dialysis
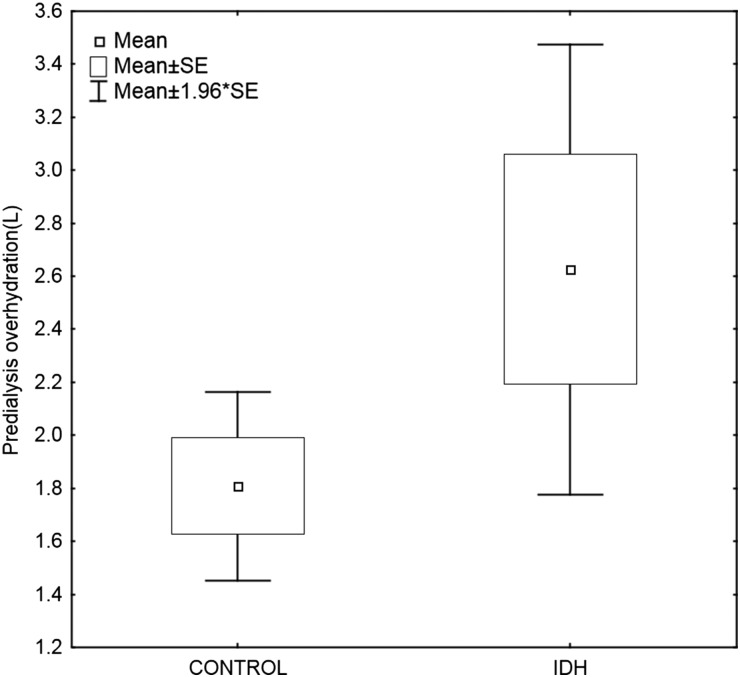
The groups were well matched at baseline regarding pre-dialysis weight, BMI, mode of dialysis, time on dialysis, pre-dialysis BP, ESA dose and route of administration (Table 1). The proportion of patients on calcium channel blockers (CCBs) was greater in the IDH group. Thirty-six of 54 (67%) IDH patients were using CCBs, compared with 68 of 136 (50%) control patients (P = 0.03). There was a trend towards greater antihypertensive drug use in the IDH group (2.5 versus 2.1; P = 0.05). There was no significant intergroup difference with regard to the use of other antihypertensive medication or the timing of drug administration (pre- versus post-dialysis). A trend towards pre-dialysis OH in the IDH group [2.6 L (95% CI 1.7–3.4) versus the control group 1.8 L (95% CI 1.4–2.1); P = 0.06] was noted (Figure 2).
Fig. 2.
Pre-dialysis OH (in litres) in the IDH versus control group. IDH, intradialytic hypertension.
Intra-dialysis
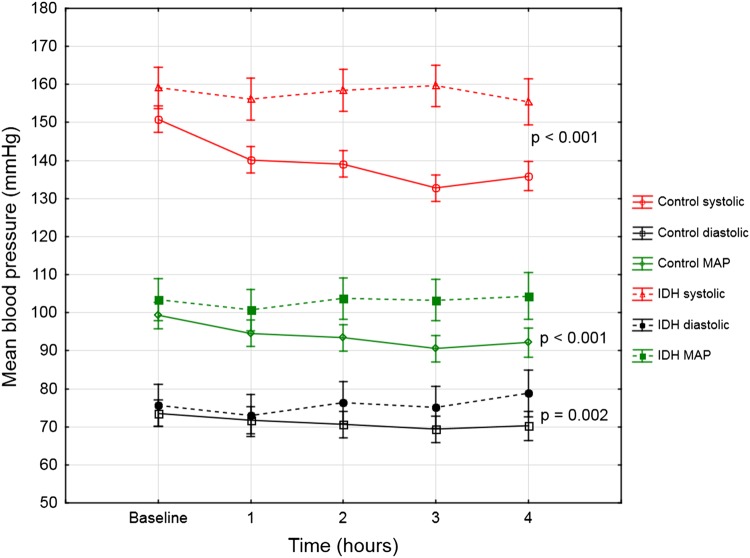
A statistically significant difference in BP (systolic, diastolic and MAP) was evident at all time points during the dialysis session (P < 0.001) (Figure 3). The mean UF rate was marginally higher in the control group (707.0 versus 609.4 mL/h); however, this difference was not statistically significant (P = 0.52). Time-averaged sodium concentration and dialysate calcium concentration were similar between groups (Table 2). The IDH group had a mean weekly dialysis time of 648 min compared with the control group's 667 min (P = 0.26).
Fig. 3.
Mean intradialytic BP trend (in mmHg) during the 4 h of haemodialysis in those with IDH versus the control group. Note: Vertical lines represent 95% CIs. IDH, intradialytic hypertension.
Table 2.
Comparison of intradialytic and post-dialysis clinical parameters
| All patients (n = 190) |
IDH (n = 54) |
Control (n = 136) |
P-value | ||||
|---|---|---|---|---|---|---|---|
| Overall prevalence, n (%) | 54 | (28.4) | 136 | (71.6) | |||
| Prevalence by unit, n (%) | |||||||
| TBH | 39 | (20.5) | 10 | (25.6) | 29 | (74.4) | |
| AKDC | 36 | (19.0) | 7 | (19.4) | 29 | (81.0) | |
| WKDC | 45 | (23.7) | 14 | (31.1) | 31 | (68.9) | |
| PKDC | 70 | (36.8) | 23 | (32.9) | 47 | (67.1) | |
| Dialysate Ca2+(mmol/L) | 1.36 | (1.33–1.39) | 1.34 | (1.29–1.39) | 1.36 | (1.33–1.40) | 0.45 |
| Time avg. Na+ (mmol/L) | 138.4 | (138.1–138.7) | 138.3 | (137.7–139) | 138.4 | (138.1–138.8) | 0.72 |
| UF rate (mL/h) | 679.0 | (573.5–784.5) | 609.4 | (525.3–693.5) | 707.0 | (562.6–851.5) | 0.52 |
| UF volume (L) | 2.4 | (2.2–2.5) | 2.2 | (1.9–2.5) | 2.5 | (2.3–2.7) | 0.30 |
| Post-dialysis OH (L) | 0.082 | (−0.26–0.42) | 0.79 | (−0.04–1.62) | −0.17 | (−0.52–0.18) | 0.06 |
| Post-dialysis ECW (%) | −0.001 | (−0.02–0.02) | 3.50 | (−1.40–8.50) | −1.40 | (−3.70–0.80) | 0.04 |
| Post-dialysis weight (kg) | 75.1 | (72.4–77.8) | 72.9 | (67.7–78.1) | 76.0 | (72.8–79.2) | 0.31 |
Values expressed in a range in parentheses refer to 95% CIs; single values refer to percentage of total population.
TBH, Tygerberg Hospital; AKDC, Athlone Kidney and Dialysis Centre; WKDC, Winelands Kidney and Dialysis Centre; PKDC, Panorama Kidney and Dialysis Centre; avg., averaged; UF, ultrafiltration; OH, overhydration; ECW, extracellular water.
Post-dialysis
There was a trend towards post-dialysis OH in the IDH group (+0.79 versus −0.17 L; P = 0.06). However, the proportion of ECW post-dialysis was greater in the IDH group (3.5 versus 1.4%; P = 0.04). There was no significant difference between groups regarding total UF volume [2.2 L (95% CI 1.9–2.5) versus 2.5 L (95% CI 2.3–2.7); P = 0.30] in the IDH versus control group, respectively. Post-dialysis weight was similar [72.9 kg (95% CI 67.7–78.0) versus 76.0 kg (95% CI 72.7–79.2); P = 0.29] in the IDH versus control group, respectively (Table 2).
Discussion
The IDH prevalence of 28.4% found in this study is high. Van Buren et al. [19] reviewed 22 955 haemodialysis treatments and found a prevalence of 21.3 per 100 treatments and also reported a persistent prevalence of 8% over an 8-month period. This latter finding refutes the view that IDH may be a transient phenomenon. Nongnuch et al. [16] did a prospective audit of 531 patients and found a prevalence of 18%. Prevalence may differ owing to variations in defining IDH. The aforementioned studies defined IDH as a ≥10 mmHg increase in SBP during the haemodialysis session [16, 19]. However, the definition for the purposes of this study included that this increase must have occurred in at least four of six prior dialysis sessions. Despite this stricter definition, the prevalence remained high. Another factor that may have contributed to the higher prevalence we observed was the inclusion of participants who had recently commenced haemodialysis. Other researchers excluded patients recently placed on haemodialysis who had not reached clinically determined target dry weights [16, 19]. Prevalence also differed within the four units, with the highest prevalence found in the units with participants who were of older age. Older age has previously been associated with IDH. The reason for this remains unknown, but it may be related to arterial stiffness. Mourad et al. [20] compared aortic pulse wave velocity (PWV) in those with haemodialysis-responsive BP (defined as a MAP decrease of >5% during dialysis) to those with haemodialysis-unresponsive BP (defined as an inability to decrease MAP >5% during dialysis). PWV was greater in those who were unresponsive, suggesting that arterial stiffness may play a role in IDH. Of interest was that the unresponsive group was older (mean age 58 years) compared with the responsive group (mean age 54 years).
South Africa offers RRT in two sectors—private and public. According to the 2012 South African Renal Registry, the overall RRT rate was 164 per million population (pmp) [17]. This is considerably lower than first-world countries and other countries with similar gross national products. However, in recent years the private sector has grown exponentially, with current treatment rates of 620 pmp. In contrast, the public sector RRT rate has remained virtually unchanged over the past two decades at only 73 pmp, mainly due to cost constraints. The Western Cape provincial government instituted selection criteria for patients with ESRD who are dependent on the public sector for RRT. The resultant differences in participant characteristics in the public sector may have affected its prevalence, as participants were younger (Table 1) and none had underlying diabetes mellitus at enrolment (personal communication with South African Renal Registry).
Despite our definition of IDH, the high mean SBP remained relatively constant in the IDH group throughout the dialysis procedure, which suggests resistance to UF (Figure 3). However, this could further be explained by the retrospective division of participants into groups based on the prior six consecutive dialysis sessions and subsequent prospective collection of BIS measurements and dialysis data. Therefore, it is possible that some of the participants classified as having IDH may not have had a significant increase in SBP on the day of data collection, as treatment for IDH may already have been instituted at the time of data collection.
Post-dialysis BIS measurements for OH and percentage ECW revealed that the IDH group remained in a positive fluid balance whereas the control group achieved a negative balance (Table 2). Participants were allowed to consume fluids during dialysis, and this may have affected post-dialysis BIS measurements. Interestingly, the difference in mean reduction in weight correlated with the difference in mean reduction in OH as measured by BIS; however, target dry weights, and therefore UF volumes, which were determined clinically, failed to identify this subclinical fluid overload. Similar findings using BIS measurements have been reported previously, where it was found that the ECW:TBW ratio before and after haemodialysis was greater in those with IDH when compared with those who developed intradialytic hypotension [16]. Therefore, BIS may provide a more objective measure of hydration status when compared with clinical examination, particularly when no overt signs of fluid overload are present.
Both the IDH group and control group did not receive their full 720 min per week of dialysis. The IDH group had a mean weekly dialysis time of 648 min, compared with 667 min in the control group. Despite the fact that these intergroup differences were not statistically significant, they may be clinically relevant. The IDH group received 60 min less dialysis per month, which could have contributed to the subclinical fluid overload. The Kidney Disease Outcomes Quality Initiative clinical practice guidelines recommend short, frequent dialysis sessions on a weekly basis, which is defined as <3 h/session for 5–7 sessions/week [21]. In three randomized controlled trials, more frequent dialysis was associated with improved BP control when compared with conventional haemodialysis [22–24].
A sodium gradient favouring the net absorption of sodium during the haemodialysis session may contribute to the pathogenesis of IDH [25]. A shortcoming of this study was determination of the time-averaged sodium concentration rather than the sodium gradient. A low calcium dialysate bath has been shown to mitigate post-dialysis hypertension [26]. However, there was no association between IDH and dialysate calcium concentration in this study.
Recently, several studies have implicated endothelial dysfunction as the main factor in the pathogenesis of IDH. It has been reported that serum ET-1 concentrations increase in those with a rise in BP during haemodialysis, while changes in nitrate/nitrite concentrations are comparable in those without an increase in BP [27].
The IDH group were using marginally more antihypertensives, and in particular more CCBs. CCBs cause pre-capillary vasodilatation resulting in high capillary hydrostatic pressure and subsequent oedema [28]. This may partly explain the trend towards pre-dialysis OH in the IDH group. BP medication thought to mitigate the intradialytic rise in BP is mainly angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs) and beta blockers. ACEI and ARBs reduce RAAS activity, whereas β-blockers (especially carvedilol) have shown to reduce endothelial dysfunction [11]. It is interesting that in this study, the proportion of IDH patients on these agents was lower than in the control group. However, this difference was not significant. Chou et al. [12] reported that RAAS and sympathetic overactivity were unlikely to play a role in the genesis of IDH. It has been suggested that removal of water-soluble antihypertensives during haemodialysis may contribute to IDH; however, this could not be verified.
ESAs administered in high doses are believed to cause hypertension by increasing vascular stiffness as well as increasing blood viscosity, leading to endothelial damage [2]. There was no significant difference between groups with regard to ESA dose. ESAs were administered intravenously in the private units and subcutaneously in the public unit. We could not establish any association between the route of ESA administration and IDH.
Management of this condition is focussed on fluid removal over a sustained period of time, as demonstrated in the dry-weight reduction in hypertensive hemodialysis patients trial [29]. This is achieved by dialysing patients longer and/or more frequently, which increases dialysis costs. In addition, treatment of hypertension and endothelial dysfunction necessitates the use of non-dialysable, antihypertensive drugs such as ARBs and carvedilol. These management strategies may not be practical in busy units already functioning at full capacity.
BIS is used sparingly in the private sector and not at all in the public sector. The cost of consumables is the major reason for this. Clinical assessment is notorious for providing a poor estimate of hydration status, but the routine use of BIS for determining pre-dialysis hydration status and setting more accurate UF targets may assist in mitigating IDH and its deleterious consequences.
Limitations
The prevalence may have differed had we used an alternative definition. The inclusion of younger participants from the public sector may have affected the overall prevalence, as IDH has previously been reported to occur more frequently in older patients. As this was a cross-sectional study, a cause-and-effect relationship between IDH and subclinical fluid overload cannot be made. The primary aim of this study was the estimation of prevalence. A larger cohort may be required to find a statistical significance regarding the association between subclinical fluid overload and IDH.
Conclusion
This study has indicated that the prevalence of IDH is higher than that reported in the literature. Subclinical fluid overload may be a major contributing factor to the mechanism of this condition. As clinical assessment of hydration status is poor, BIS may be used to assist in identifying IDH patients with subclinical fluid overload who may benefit from additional UF.
Conflict of interest statement
C.F. is an employee of FMC. S.S. and M.-Y.C. have consulting roles at FMC dialysis units.
Acknowledgements
The authors acknowledge Fresenius Medical Care (FMC) for providing the BCM device and allowing staff to assist with data collection, and Prof Razeen Davids and Dr Firdows Noor for proofreading the abstract and final article. FMC lent a BCM device to the researchers and donated the electrodes used for bioimpedance measurements.
References
- 1.Chazot C, Jean G. Intradialytic hypertension: it is time to act. Nephron Clin Pract 2010; 115: c182–c188 [DOI] [PubMed] [Google Scholar]
- 2.Locatelli F, Cavalli A, Tucci B. The growing problem of intradialytic hypertension. Nat Rev Nephrol 2010; 6: 41–48 [DOI] [PubMed] [Google Scholar]
- 3.Inrig JK, Oddone EZ, Hasselblad V et al. . Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int 2007; 71: 454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inrig JK, Patel UD, Toto RD et al. . Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the dialysis morbidity and mortality wave 2 study. Am J Kidney Dis 2009; 54: 881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal R, Light RP. Intradialytic hypertension is a marker of volume excess. Nephrol Dial Transplant 2010; 25: 3355–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirit M, Akcicek F, Terzioglu E et al. . ‘Paradoxical’ rise in blood pressure during ultrafiltration in dialysis patients. Nephrol Dial Transplant 1995; 10: 1417–1420 [PubMed] [Google Scholar]
- 7.Gunal AI, Karaca I, Celiker H et al. . Paradoxical rise in blood pressure during ultrafiltration is caused by increased cardiac output. J Nephrol 2002; 15: 42–47 [PubMed] [Google Scholar]
- 8.Fourtounas C. “Malignant” intradialytic hypertension: a severe form of intradialytic hypertension. Am J Kidney Dis 2010; 56: 418; author reply 418–419 [DOI] [PubMed] [Google Scholar]
- 9.Inrig JK. Intradialytic hypertension: a less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis 2010; 55: 580–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner FS, Eschbach JW, Haley NR et al. . Hypertension following erythropoietin therapy in anemic hemodialysis patients. Am J Hypertens 1990; 3(12 Pt 1): 947–955 [DOI] [PubMed] [Google Scholar]
- 11.Inrig JK, Van Buren P, Kim C et al. . Intradialytic hypertension and its association with endothelial cell dysfunction. Clin J Am Soc Nephrol 2011; 6: 2016–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou KJ, Lee PT, Chen CL et al. . Physiological changes during hemodialysis in patients with intradialysis hypertension. Kidney Int 2006; 69: 1833–1838 [DOI] [PubMed] [Google Scholar]
- 13.Hur E, Usta M, Toz H et al. . Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis 2013; 61: 957–965 [DOI] [PubMed] [Google Scholar]
- 14.Onofriescu M, Hogas S, Voroneanu L et al. . Bioimpedance-guided fluid management in maintenance hemodialysis: a pilot randomized controlled trial. Am J Kidney Dis 2014; 64: 111–118 [DOI] [PubMed] [Google Scholar]
- 15.Wabel P, Chamney P, Moissl U et al. . Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif 2009; 27: 75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nongnuch A, Campbell N, Stern E et al. . Increased postdialysis systolic blood pressure is associated with extracellular overhydration in hemodialysis outpatients. Kidney Int 2015; 87: 452–457 [DOI] [PubMed] [Google Scholar]
- 17.Davids M, Marais N, Jacobs J. South African Renal Registry Annual Report 2012. South African Renal Society, Cape Town 2014. [Google Scholar]
- 18.Statistics South Africa. Census 2011. Pretoria: Statistics South Africa, 2012
- 19.Van Buren PN, Kim C, Toto RD et al. . The prevalence of persistent intradialytic hypertension in a hemodialysis population with extended follow-up. Int J Artif Organs 2012; 35: 1031–1038 [DOI] [PubMed] [Google Scholar]
- 20.Mourad A, Khoshdel A, Carney S et al. . Haemodialysis-unresponsive blood pressure: cardiovascular mortality predictor? Nephrology (Carlton) 2005; 10: 438–441 [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 2015; 66: 884–930 [DOI] [PubMed] [Google Scholar]
- 22.Culleton BF, Walsh M, Klarenbach SW et al. . Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA 2007; 298: 1291–1299 [DOI] [PubMed] [Google Scholar]
- 23.FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med 2010; 363: 2287–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocco MV, Lockridge RS Jr, Beck GJ et al. . The effects of frequent nocturnal home hemodialysis: the frequent hemodialysis network nocturnal trial. Kidney Int 2011; 80: 1080–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Movilli E, Camerini C, Gaggia P et al. . Role of dialysis sodium gradient on intradialytic hypertension: an observational study. Am J Nephrol 2013; 38: 413–419 [DOI] [PubMed] [Google Scholar]
- 26.Katzir Z, Michlin A, Boaz M et al. . Antihypertensive effect of low calcium dialysis. Isr Med Assoc J 2005; 7: 704–707 [PubMed] [Google Scholar]
- 27.Raj DS, Vincent B, Simpson K et al. . Hemodynamic changes during hemodialysis: role of nitric oxide and endothelin. Kidney Int 2002; 61: 697–704 [DOI] [PubMed] [Google Scholar]
- 28.Sica DA. Calcium channel blocker-related peripheral edema: can it be resolved? Clin Hypertens (Greenwich) 2003; 5: 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal R, Alborzi P, Satyan S et al. . Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension 2009; 53: 500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]