Abstract
Background
The aim of this systematic review is to examine the literature for the risk of acute kidney injury (AKI) in patients who underwent transcatheter aortic valve replacement (TAVR) based on transapical (TA) versus transfemoral (TF) approaches.
Methods
A literature search was conducted utilizing Embase, Medline, Cochrane Database of Systematic Reviews and ClinicalTrials.gov from inception through December 2015. Studies that reported relative risk, odds ratio or hazard ratio comparing the AKI risk in patients who underwent TA-TAVR versus TF-TAVR were included. Pooled risk ratio (RR) and 95% confidence interval (CI) were calculated using a random effect, generic inverse variance method.
Results
Seventeen cohort studies with 5085 patients were enrolled in the analysis to assess the risk of AKI in patients undergoing TA-TAVR versus TF-TAVR. The pooled RR of AKI in patients who underwent TA-TAVR was 2.26 (95% CI 1.79–2.86) when compared with TF-TAVR. When meta-analysis was confined to the studies with adjusted analysis for confounders evaluating the risk of AKI following TAVR, the pooled RR of TA-TAVR was 2.89 (95% CI 2.12–3.94). The risk for moderate to severe AKI [RR 1.02 (95% CI 0.57–1.80)] in patients who underwent TA-TAVR compared with TF-TAVR was not significantly higher.
Conclusions
Our meta-analysis demonstrates an association between TA-TAVR and a higher risk of AKI. Future studies are required to assess the risks of moderate to severe AKI and mortality following TA-TAVR versus TF-TAVR.
Keywords: acute kidney injury, meta-analysis, transapical, transfemoral, transcatheter aortic valve replacement, mortality
Introduction
Transcatheter aortic valve replacement (TAVR), also known as transcatheter aortic valve implantation (TAVI), has now emerged as a viable treatment option for high-risk patients with severe aortic stenosis who are not suitable candidates for aortic valve replacement [1–5]. To date, >200 000 procedures have been performed worldwide [6]. Despite encouraging reports, AKI remains a common complication of TAVR, ranging from 15 to 57% [2, 7–9].
Transfemoral (TF) and transapical (TA) are the two most common approaches for TAVR procedures. TF-TAVR is considered the first choice at most centers, as it can be performed using moderate sedation and local anesthetics and also has shorter procedural and recovery times [8, 10–12]. The patients who underwent TA-TAVR usually had more comorbid conditions, in particular, peripheral vascular disease, which is a known risk factor for AKI following TAVR [6, 13]. On the other hand, compared with TA-TAVR, TF-TAVR generally requires a higher volume of contrast agent and a well-established cause for contrast-induced AKI. It is, therefore, not surprising that studies evaluating the risk of AKI in patients following TA-TAVR versus TF-TAVR are conflicting. A few studies have demonstrated higher AKI risk among symptomatic AS patients who underwent TA-TAVR [13–20]. Conversely, several studies have found no significantly greater incidence of AKI in patients who underwent TA-TAVR [21–28].
Thus, this systematic review and meta-analysis was conducted to compare the effects of TA-TAVR and TF-TAVR on the risk of AKI.
Materials and methods
Search strategy
Two investigators (C.T. and W.C.) independently searched published studies and conference abstracts indexed in Embase, Medline, Cochrane Database of Systematic Reviews and ClinicalTrials.gov from inception of the databases through December 2015 using the search strategy described in Supplementary data, Item S1. We also performed a manual search for additional relevant studies using the references from these retrieved articles.
Inclusion criteria
The inclusion criteria for this meta-analysis were (i) randomized controlled trials (RCTs) or observational studies published as original articles or conference abstracts that assessed the risk of AKI in patients who underwent TA-TAVR, (ii) available data on relative risk, odds ratio or hazard ratio with 95% confidence intervals (CIs) and (iii) a reference group comprising subjects who underwent TF-TAVR. No limits were implemented for language.
Study eligibility was individually determined by the two investigators noted as above. Differing decisions were solved by joint consensus. We appraised the quality of each included study by utilizing the Jadad quality assessment scale [29] for RCTs and the Newcastle–Ottawa quality assessment scale [30] for observational studies.
Data extraction
A standardized data collection form was used to extract the following information: last name of the first author, the title of the article, study design, year of study, country of origin, year of publication, sample size, AKI definition, mortality, confounder adjustment and adjusted effect estimate with 95% CI.
Statistical analysis
We performed data analysis using Review Manager software from the Cochrane Collaboration (Version 5.3, Copenhagen, Denmark). Point estimates and standard errors were obtained from each of the included studies and were united by the generic inverse variance method [31]. Given the high likelihood of between-study variances, a random effects model was used. Statistical heterogeneity was evaluated utilizing Cochran's Q test. The I2 statistic was computed to estimate the degree of variation across studies related to heterogeneity instead of chance. An I2 of 0‒25% renders insignificant heterogeneity, 26‒50% low heterogeneity, 51‒75% moderate heterogeneity and >75% high heterogeneity [32]. The presence of publication bias was appraised by funnel plots of the logarithm of odds ratios versus their standard errors [33].
Results
Our search strategy yielded 1327 relevant articles. Of these, 1169 were excluded based on the following measures: the abstract failing to indicate an appropriate type of article, study design, population or outcome of interest. The remaining 158 articles underwent full-length review and 141 of these were excluded for failing to meet criteria (113 articles did not report the outcomes of interest and 28 articles were not observational studies or RCTs). Seventeen cohort studies [13–28, 34] with 5085 patients were included in the meta-analysis to assess the risk of AKI in patients undergoing TA-TAVR versus TF-TAVR (Table 1).
Table 1.
Main characteristics of the studies included in this meta-analysis
| Aregger et al. [13] | Bagur et al. [23] | Elhmidi et al. [24] | Barbash et al. [34] | Kong et al. [16] | Nuis et al. [26] | |
|---|---|---|---|---|---|---|
| Country | Switzerland | Canada | Germany | USA | Australia | Netherlands, Canada, Germany, Belgium and Columbia |
| Study design | Cohort study | Cohort study | Cohort study | Cohort study | Cohort study | Cohort study |
| Year | 2009 | 2010 | 2011 | 2012 | 2012 | 2012 |
| Total number | 54 | 213 | 234 | 165 | 52 | 995 |
| AKI definition | Serum creatinine criteria of RIFLE classification at 7 days after procedure | A decrease of >25% in eGFR at 48 h following the procedure or the need for hemodialysis during index hospitalization | Serum creatinine criteria of RIFLE classification at 7 days after procedure | Increase in SCr of ≥0.3 mg/dL or ≥50% from baseline at 72 h after procedure | Serum creatinine criteria of RIFLE classification at 48 h after procedure | Increase in SCr of ≥0.3 mg/dL or ≥50% from baseline at 72 h after procedure |
| RR (95% CI) for AKI | 10.50 (2.22–49.69) | 2.11 (0.89–5.01) | 1.14 (0.53–2.47) | 2.92 (1.03–8.29) | 9.3 (4.3–23.7) | 1.38 (0.99–1.92) |
| RR (95% CI) for mortality | – | In-hospital mortality 2.36 (0.91–6.12) |
– | – | – | – |
| Confounder adjustment | None | None | None | Baseline GFR, sex, iodinated contrast per eGFR | RBC transfusion, hypertension | None |
| Quality assessment (Newcastle–Ottawa scale) | Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 3 Comparability: 1 Outcome: 3 |
Selection: 3 Comparability: 1 Outcome: 3 |
Selection: 3 Comparability: 0 Outcome: 3 |
| Khawaja et al. [25] | Genereux et al. [21] | Saia et al. [14] | Seiffert et al. [17] | Tanawuttiwat et al. [22] | Van der boon et al. [20] | |
| Country | UK | USA | Italy | Germany | USA | Italy, France, The Netherlands |
| Study design | Cohort study | Cohort study | Cohort study | Cohort study | Cohort study | Cohort study |
| Year | 2012 | 2013 | 2013 | 2013 | 2014 | 2014 |
| Total number | 248 | 218 | 102 | 281 | 64 | 882 |
| AKI definition | VARC-modified RIFLE classification stage 2 or 3 at 72 h after procedure | VARC-modified RIFLE classification stage 2 or 3 until hospital discharge | Increase in SCr of ≥0.3 mg/dL or ≥50% from baseline at 72 h after procedure | Increase in SCr of ≥0.3 mg/dL or ≥50% from baseline within 72 h after procedure | Increase in SCr of ≥0.3 mg/dL or ≥50% from baseline at 72 h after procedure | Increase in SCr of ≥0.3 mg/dL or ≥50% from baseline at 72 h after procedure |
| RR (95% CI) for AKI | 1.71 (0.95–3.06) | 2.56 (0.61–10.69) | 4.57 (1.53–13.59) | 1.90 (1.09–3.31) For stage 2 or 3 AKI 1.28 (0.51–3.26) |
2.93 (0.96–8.96) | 2.25 (1.42–3.56) For stage 3 AKI 1.92 (0.69–5.37) |
| RR for mortality | – | – | – | 1-year mortality 1.18 (0.71–1.96) |
– | In-hospital mortality 3.12 (1.43–6.82) 1-year mortality 1.88 (1.23–2.87) |
| Confounder adjustment | None | Age, sex, baseline creatinine, contrast volume, major vascular complication, life-threatening bleeding | Body surface area, logistic EuroScore, peripheral arterial disease, baseline GFR | None | None | Not specified |
| Quality assessment (Newcastle–Ottawa scale) | Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 3 Comparability: 2 Outcome: 3 |
Selection: 3 Comparability: 2 Outcome: 3 |
Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 3 Comparability: 1 Outcome: 3 |
| Murarka et al. [27] | Rouge et al. [28] | Van Rosendael et al. [15] | Thongprayoon et al. [18] | Schymik et al. [19] | ||
| Country | USA | France | The Netherlands | USA | German | |
| Study design | Cohort study | Cohort study | Cohort study | Cohort study | Cohort study | |
| Year | 2015 | 2015 | 2015 | 2015 | 2015 | |
| Total number | 123 | 150 | 210 | 386 | 708 | |
| AKI definition | Increase in SCr of ≥0.3 mg/dL or ≥50% from baseline at 7 days after procedure | Increase in SCr of ≥0.3 mg/dL or ≥50% from baseline at 7 days after procedure | Increase in SCr of ≥0.3 mg/dL or ≥50% from baseline at 7 days after procedure | An increase in SCr of ≥0.3 mg/dL within 48 h or ≥50% from the baseline at 7 days after procedure | Increase in SCr of ≥0.3 mg/dL or ≥50% from baseline at 30 days after procedure | |
| RR (95% CI) for AKI | 1.65 (0.66–4.13) For dialysis 1.16 (0.07–18.99) |
1.45 (0.51–4.12) | 2.76 (1.16–6.58) | 2.81 (1.72–4.65) | 2.09 (1.49–2.93) For stage 2 or 3 0.62 (0.33–1.19) |
|
| RR (95% CI) for mortality | 30-mortality mortality 1.17 (0.23–6.03) |
– | – | – | 30-day mortality: 0.68 (0.38–1.21) | |
| Confounder adjustment | None | None | Body surface area, heart rhythm, eGFR, logistic EuroScore, log-transformed calcium volume aortic valve, atherosclerosis burden | Baseline GFR, RBC transfusion, need for intra-aortic balloon pump | Propensity score matching | |
| Quality assessment (Newcastle–Ottawa scale) | Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 3 Comparability: 2 Outcome: 3 |
Selection: 3 Comparability: 2 Outcome: 3 |
Selection: 3 Comparability: 2 Outcome: 3 |
AKI, acute kidney injury; BMI, body mass index; CABG, coronary bypass grafting; CKD, chronic kidney disease; CPB, cardiopulmonary bypass; DM, diabetes mellitus; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; NR, not reported; RBC, red blood cell; RIFLE, Risk, Injury, Failure, Loss of kidney function and End-stage kidney disease; SCr, serum creatinine; TAVR, transcatheter aortic valve replacement.
Of the 17 cohort studies, 8 performed adjusted analysis for known risk factors for AKI [14–16, 18–21, 34]. Only four cohort studies assessed the risk of moderate to severe AKI in patients undergoing TA-TAVR versus TF-TAVR [17, 19, 20, 27]. Within selected studies, five were included in the post hoc analysis assessing mortality outcomes [17, 19, 20, 23, 27]. Supplementary data, Item S2 outlines our search methodology and selection process.
AKI definition
All included studies identified the AKI occurrence, based on the change in serum creatinine (SCr) or glomerular filtration rate (GFR) after TAVR. These studies had a heterogeneous definition of AKI as presented in Table 1. Most included studies [13–22, 24–28, 34] used standard AKI definitions [modified Risk, Injury, and Failure; and Loss; and End-stage kidney disease (RIFLE) [35], Acute Kidney Injury Network (AKIN) [36] or Kidney Disease: Improving Global Outcomes (KDIGO) criteria [37]]. AKI was diagnosed 48–72 h following/after a TAVR procedure in most included studies and only six studies [13, 15, 18, 24, 27, 28] identified AKI at 7 days following a TAVR procedure as suggested by Valve Academic Research Consortium-2 (VARC-2) consensus [38].
AKI risk
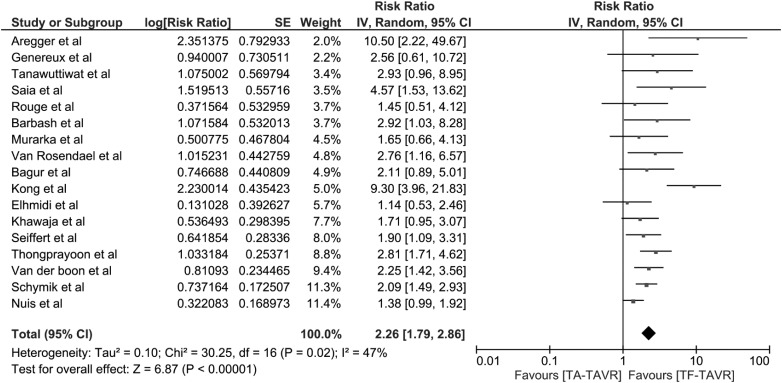
The pooled risk ratio (RR) of AKI in patients who underwent TA-TAVR was 2.26 (95% CI 1.79–2.86; I2 = 47%) (Figure 1). When meta-analysis was limited to the studies using standard AKI definitions, the pooled RR was 2.26 (95% CI 1.75–2.92; I2 = 53%). We also performed a meta-analysis of studies using VARC-2 consensus [13, 15, 18, 24, 27, 28]. The pooled RR of AKI in patients who underwent TA-TAVR was 2.19 (95% CI 1.37–3.49; I2 = 44%).
Fig. 1.
Forest plot of the included studies comparing AKI risk in patients who underwent TA-TAVR and those with TF-TAVR. Square data markers express RRs; horizontal lines are the 95% CIs with marker size indicating the statistical weight of the study using random effects meta-analysis. A diamond data marker denotes the overall RR and 95% CI for the outcome of interest.
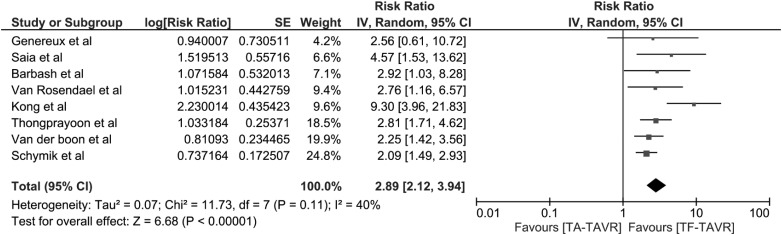
To minimize the effects of confounders, we performed a sensitivity analysis excluding the studies without adjusted analysis for known risk factors for AKI. The pooled RR of AKI remained significant in TA-TAVR [RR 2.89 (95% CI 2.12–3.94), I2 = 40%], (Figure 2).
Fig. 2.
Forest plot of the included studies with adjusted analysis comparing AKI risk in patients who underwent TA-TAVR and those with TF-TAVR. Square data markers express RRs; horizontal lines are the 95% CIs with marker size indicating the statistical weight of the study using random effects meta-analysis. A diamond data marker denotes the overall RR and 95% CI for the outcome of interest.
Moderate to severe AKI risk
Data regarding severe AKI requiring renal replacement therapy (RRT) were limited; four cohort studies evaluated the risk of moderate to severe AKI in patients undergoing TA-TAVR versus TF-TAVR. The pooled RR of moderate to severe AKI in patients who underwent TA-TAVR was 1.02 (95% CI 0.57–1.80; I2 = 24%).
Evaluation for publication bias
Funnel plots to evaluate publication bias for the risk of AKI in patients undergoing TA-TAVR versus TF-TAVR are summarized in Supplementary data, Figures S1 and S2. These graphs demonstrate no obvious asymmetry and thus suggest an insignificant publication bias.
Discussions
In this systematic review, we demonstrated a significant association between TA-TAVR and an overall 2.4-fold increased risk of AKI compared with those who underwent TF-TAVR. We were not able to show a significant difference in the incidence of moderate to severe AKI requiring RRT between patients in the two cohorts.
Although the mechanisms behind the higher frequency of AKI in TA-TAVR when compared with TF-TAVR are only speculative, there are several plausible explanations [6]. First, TF-TAVR has the advantage of implementation with local anesthesia and monitored anesthesia care rather than full general anesthesia. Second, procedure times for TF-TAVR are generally shorter [8, 10–12]. Both of these factors limit exposure to general anesthesia that may cause significant hemodynamic perturbations affecting renal perfusion and thereby cause a higher rate of AKI [12]. This potential risk confirms that TA-TAVR must be performed under general anesthesia. Third, there is a difference in the demographics of patient populations undergoing TA-TAVR and TF-TAVR. Patients who undergo TA-TAVR have more advanced atherosclerotic disease, which is a risk factor for AKI after TAVR [6, 13]. In our analysis of these studies, we adjusted for potential confounders and yet still demonstrated a significantly increased risk for AKI in patients who undergo TA-TAVR.
Despite a higher incidence of AKI in patients treated with TA-TAVR, our meta-analysis demonstrated that the risk of moderate to severe AKI was not significantly different. The data to analyze these particular outcomes, however, are limited, and additional studies will be required to delineate the relationship between TAVR approaches and AKI. Lastly, additional analyses are needed to ascertain whether this ultimately translates into a higher rate of RRT and mortality.
Although the selected studies were all of moderate to high quality, there are some limitations to the results. First, there are statistical heterogeneities in the final analysis. The potential sources of these heterogeneities include variations in the diagnosis methodology of AKI following TAVR and the differences in confounder adjusted methods. Second, as mentioned previously, data on severe AKI and subsequent mortality after TA-TAVR versus TF-TAVR are lacking. Therefore, we need studies on a larger scale to focus on the important outcomes, including the development of chronic kidney disease, the need for long-term dialysis and short and long-term mortality. Finally, this is a meta-analysis of observational studies with the inherent limitation of being able to confirm an association but not a causal relationship.
In summary, our meta-analysis demonstrates an association between TA-TAVR and a higher risk of AKI when compared with TF-TAVR. However, the risk of moderate to severe AKI following TA-TAVR and TF-TAVR is not significantly different.
Supplementary data
Supplementary data are available online at http://ndt.oxfordjournals.org.
Author contributions
All investigators had access to the data and played essential roles in writing the manuscript.
Conflict of interest statement
None declared.
Supplementary Material
References
- 1.O'Brien SM, Shahian DM, Filardo G et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg 2009; 88(1 Suppl): S23–S42 [DOI] [PubMed] [Google Scholar]
- 2.Adams DH, Popma JJ, Reardon MJ et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370: 1790–1798 [DOI] [PubMed] [Google Scholar]
- 3.Nishimura RA, Otto CM, Bonow RO et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: e521–e643 [DOI] [PubMed] [Google Scholar]
- 4.Reardon MJ, Adams DH, Kleiman NS et al. 2-Year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol 2015; 66: 113–121 [DOI] [PubMed] [Google Scholar]
- 5.Cheungpasitporn W, Thongprayoon C, Kashani K. Transcatheter aortic valve replacement: a kidney's perspective. J Renal Inj Prev 2016; 5: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Najjar M, Salna M, George I. Acute kidney injury after aortic valve replacement: incidence, risk factors and outcomes. Expert Rev Cardiovasc Ther 2015; 13: 301–316 [DOI] [PubMed] [Google Scholar]
- 7.Thongprayoon C, Cheungpasitporn W, Srivali N et al. Acute kidney injury after transcatheter aortic valve replacement: a systematic review and meta-analysis. Am J Nephrol 2015; 41: 372–382 [DOI] [PubMed] [Google Scholar]
- 8.Elhmidi Y, Bleiziffer S, Deutsch MA et al. Acute kidney injury after transcatheter aortic valve implantation: incidence, predictors and impact on mortality. Arch Cardiovasc Dis 2014; 107: 133–139 [DOI] [PubMed] [Google Scholar]
- 9.Thongprayoon C, Cheungpasitporn W, Srivali N et al. AKI after transcatheter or surgical aortic valve replacement. J Am Soc Nephrol 2016; 27: 1854–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherner M, Wahlers T. Acute kidney injury after transcatheter aortic valve implantation. J Thorac Dis 2015; 7: 1527–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheungpasitporn W, Thongprayoon C, Srivali N et al. Preoperative renin-angiotensin system inhibitors use linked to reduced acute kidney injury: a systematic review and meta-analysis. Nephrol Dial Transplant 2015; 30: 978–988 [DOI] [PubMed] [Google Scholar]
- 12.Ruparelia N, Prendergast BD. Transcatheter aortic valve implantation – what the general physician needs to know. Clin Med (Lond) 2015; 15: 420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aregger F, Wenaweser P, Hellige GJ et al. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant 2009; 24: 2175–2179 [DOI] [PubMed] [Google Scholar]
- 14.Saia F, Ciuca C, Taglieri N et al. Acute kidney injury following transcatheter aortic valve implantation: incidence, predictors and clinical outcome. Int J Cardiol 2013; 168: 1034–1040 [DOI] [PubMed] [Google Scholar]
- 15.van Rosendael PJ, Kamperidis V, van der Kley F et al. Atherosclerosis burden of the aortic valve and aorta and risk of acute kidney injury after transcatheter aortic valve implantation. J Cardiovasc Comput Tomogr 2015; 9: 129–138 [DOI] [PubMed] [Google Scholar]
- 16.Kong WY, Yong G, Irish A. Incidence, risk factors and prognosis of acute kidney injury after transcatheter aortic valve implantation. Nephrology 2012; 17: 445–451 [DOI] [PubMed] [Google Scholar]
- 17.Seiffert M, Schnabel R, Conradi L et al. Predictors and outcomes after transcatheter aortic valve implantation using different approaches according to the valve academic research consortium definitions. Catheter Cardiovasc Interv 2013; 82: 640–652 [DOI] [PubMed] [Google Scholar]
- 18.Thongprayoon C, Cheungpasitporn W, Srivali N et al. Incidence and risk factors of acute kidney injury following transcatheter aortic valve replacement. Nephrology 2015; doi:10.1111/nep.12704 [DOI] [PubMed] [Google Scholar]
- 19.Schymik G, Wurth A, Bramlage P et al. Long-term results of transapical versus transfemoral TAVI in a real world population of 1000 patients with severe symptomatic aortic stenosis. Circ Cardiovasc Interv 2015; 8: e000761 [DOI] [PubMed] [Google Scholar]
- 20.van der Boon RM, Marcheix B, Tchetche D et al. Transapical versus transfemoral aortic valve implantation: a multicenter collaborative study. Ann Thorac Surg 2014; 97: 22–28 [DOI] [PubMed] [Google Scholar]
- 21.Genereux P, Kodali SK, Green P et al. Incidence and effect of acute kidney injury after transcatheter aortic valve replacement using the new valve academic research consortium criteria. Am J Cardiol 2013; 111: 100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanawuttiwat T, O'Neill BP, Cohen MG et al. New-onset atrial fibrillation after aortic valve replacement: comparison of transfemoral, transapical, transaortic, and surgical approaches. J Am Coll Cardiol 2014; 63: 1510–1519 [DOI] [PubMed] [Google Scholar]
- 23.Bagur R, Webb JG, Nietlispach F et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 2010; 31: 865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elhmidi Y, Bleiziffer S, Piazza N et al. Incidence and predictors of acute kidney injury in patients undergoing transcatheter aortic valve implantation. Am Heart J 2011; 161: 735–739 [DOI] [PubMed] [Google Scholar]
- 25.Khawaja MZ, Thomas M, Joshi A et al. The effects of VARC-defined acute kidney injury after transcatheter aortic valve implantation (TAVI) using the Edwards bioprosthesis. EuroIntervention 2012; 8: 563–570 [DOI] [PubMed] [Google Scholar]
- 26.Nuis RJ, Rodes-Cabau J, Sinning JM et al. Blood transfusion and the risk of acute kidney injury after transcatheter aortic valve implantation. Circ Cardiovasc Interv 2012; 5: 680–688 [DOI] [PubMed] [Google Scholar]
- 27.Murarka S, Lazkani M, Neihaus M et al. Comparison of 30-day outcomes of transfemoral versus transapical approach for transcatheter aortic valve replacement: a single-center US experience. Ann Thorac Surg 2015; 99: 1539–1544 [DOI] [PubMed] [Google Scholar]
- 28.Rouge A, Huttin O, Aslam R et al. Mid-term results of 150 TAVI comparing apical versus femoral approaches. J Cardiothorac Surg 2015; 10: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jadad AR, Moore RA, Carroll D et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12 [DOI] [PubMed] [Google Scholar]
- 30.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605 [DOI] [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188 [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Easterbrook PJ, Berlin JA, Gopalan R et al. Publication bias in clinical research. Lancet 1991; 337: 867–872 [DOI] [PubMed] [Google Scholar]
- 34.Barbash IM, Ben-Dor I, Dvir D et al. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am Heart J 2012; 163: 1031–1036 [DOI] [PubMed] [Google Scholar]
- 35.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int 2008; 73: 538–546 [DOI] [PubMed] [Google Scholar]
- 36.Mehta RL, Kellum JA, Shah SV et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.KDIGO AKI Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl 2012; 2: 1–138 [Google Scholar]
- 38.Kappetein AP, Head SJ, Genereux P et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012; 33: 2403–2418 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.