Abstract
The cellular prion protein (PrPC) has been proposed to play an important role in the pathogenesis of Alzheimer’s disease. In cellular models PrPC inhibited the action of the β-secretase BACE1 on wild type amyloid precursor protein resulting in a reduction in amyloid-β (Aβ) peptides. Here we have assessed the effect of genetic ablation of PrPC in transgenic mice expressing human wild type amyloid precursor protein (line I5). Deletion of PrPC had no effect on the α- and β-secretase proteolysis of the amyloid precursor protein (APP) nor on the amount of Aβ38, Aβ40 or Aβ42 in the brains of the mice. In addition, ablation of PrPC did not alter Aβ deposition or histopathology phenotype in this transgenic model. Thus using this transgenic model we could not provide evidence to support the hypothesis that PrPC regulates Aβ production.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia affecting 30 million individuals world-wide [1,2]. Age is the greatest risk factor for AD, with the incidence doubling every 5 years after age 65. Therefore, with our ageing population, AD is placing immense financial and social pressure on society. Currently there are no treatments that either cure or halt the progression of this neurodegenerative disease [3]. The majority (>95%) of AD cases have no underlying genetic mutation and are referred to as sporadic or late-onset AD [4]. In a small proportion of cases, mutations in the genes encoding the amyloid precursor protein (APP) or presenilin (PS) 1 or PS2 give rise to early onset, familial AD [4]. The disease is characterised by the deposition in the brain of extracellular plaques of amyloid-β (Aβ) which is derived from the proteolytic processing of APP [5], along with intracellular neurofibrillary tangles of hyperphosphorylated tau protein [6]. APP is cleaved first by the β-secretase, β-site APP cleaving enzyme-1 (BACE1), and then by the PS-containing γ-secretase complex to release Aβ, the predominant forms of which are 40 or 42 amino acids in length (Aβ40 and Aβ42, respectively) [5].
Cleavage of APP by BACE1 is the rate-limiting step in Aβ production [7] and various cellular proteins have been reported to influence this step, including the cellular form of the prion protein (PrPC) [8]. PrPC inhibited the action of BACE1 on wild type APP (APPWT) in various cellular models, in part through glycosaminoglycan-mediated interaction at the cell surface and in part through retaining the pro-domain containing form of BACE1 in the early secretory pathway [8,9]. In the brains of PrPC null mice there was a significant increase in the amount of endogenous murine Aβ [8], consistent with PrPC having a role in regulating the production of Aβ from APP in vivo. Together with the observation that PrPC was decreased in the brains from sporadic AD individuals and that the amount of PrPC inversely correlated with BACE1 activity, soluble and insoluble Aβ and Braak stage in the human brain [10,11], led us to propose that PrPC may function to protect against AD and that loss of PrPC would lead to the earlier onset of AD [12,13].
The inhibitory effect of PrPC on the BACE1 cleavage of APP was only apparent on APPWT and was lost on APP with the Swedish double point mutation adjacent to the BACE1 cleavage site (APPSwe) [9]. APPSwe is subject to BACE1 cleavage in the secretory pathway [14,15] rather than in the endosomal pathway as for APPWT [16,17]. As PrPC interacted directly with the prodomain of the immature Golgi-localised form of BACE1, decreasing the amount of BACE1 at the cell surface and in endosomes, this provided a mechanism to explain the differential inhibitory effect of PrPC towards APPWT and APPSwe [9]. In transgenic mice expressing human APPSwe.Ind we [9] and others [18–20] have reported that upon genetic ablation of PrPC there is no alteration in APP processing, Aβ levels or plaque pathology, consistent with this differential inhibitory mechanism. Therefore, in this study we aimed to determine whether ablation of PrPC in transgenic mice expressing human APPWT results in increased Aβ and subsequently causes the premature appearance of plaque pathology.
Materials and Methods
Transgenic Animals
Transgenic APPWT mice over expressing human wild-type APP (line I5) or APPSwe,Ind mice over expressing human APP with the Swedish (K670N/M671L) and Indiana (V717F) familial AD mutations (line J20) [21] were obtained from The Jackson Laboratory, (Line B6.Cg-Tg(PDGFB-APP)5Lms/J, stock number 004662 and B6.Cg-Tg(PDGFB-APPSwe,Ind)20Lms/2J, stock number 006293, respectively) and The J. David Gladstone Institutes, San Francisco, CA 94158) and crossed with inbred PrP knockout mice (129Ola PrP-/-) [22]. The genetic background of all mice used in this study was mixed B6/129Ola and only female mice were used. Operators were blinded to genotype and animals were randomly assigned to cages of n = 4 and given access to food and water ad libitum. During housing, animals were monitored daily for health status and no adverse effects were noted. All the transgenic mice used in this study were genotyped. DNA was prepared from ear punch tissue using a DNeasy Kit (Qiagen). PCR was performed using the protocol specific for these mice from The Jackson Laboratory. At end point, animals were culled by cervical dislocation and brain hemispheres were either frozen at -80°C for biochemical analysis or fixed in 10% formol saline for histopathological analysis. These experiments were approved by The Roslin Institute’s Animal Welfare and Ethical Review Board and were conducted according to the regulations of the UK Home Office Animals (Scientific Procedures) Act 1986. All efforts were made to minimise suffering.
Homogenisation
Brain hemispheres were homogenised using a two-step extraction protocol [23]. Briefly, initial homogenisation (120 mg/ml wet weight) was carried out using an electrical homogeniser in 2% (w/v) SDS containing protease inhibitor cocktail (Roche Diagnostics GMbH, Germany) and PhosSTOP phosphatase inhibitor (Roche Diagnostics GMbH, Germany), followed by centrifugation at 100,000 g for 1 h at 4°C. The resultant supernatant (containing ‘soluble’ Aβ) was collected and analysed as described below. The pellet was extracted in 70% (v/v) formic acid in dH2O followed by centrifugation at 100,000 g for 1 h at 4°C. The supernatant (containing ‘insoluble’ Aβ) was collected and analysed as described below.
SDS-PAGE and Immunoblot Analysis
Samples were mixed with an equal volume of SDS dissociation buffer (125 mM Tris/HCl, pH 6.8, 2% (w/v) SDS, 20% (v/v) glycerol, 100 mM dithiothreitol, 0.002% (w/v) bromophenol blue) and boiled for 5 min. Proteins from mouse brain homogenate (30 μg) were resolved by SDS-PAGE using 7–17% polyacrylamide gradient gels. Resolved proteins were then transferred to Immobilon P polyvinylidene difluoride membrane (Amersham, Little Chalfont, UK). The membrane was blocked by incubation for 1 h with PBS containing 0.1% (v/v) Tween-20 and 5% (w/v) dried milk powder. Antibody incubations were performed in PBS-Tween containing 2% (w/v) BSA. Antibody 6D11 (Eurogentec Ltd., Liege, Belgium) recognises PrPC, antibody Y188 (Abcam, Cambridge, UK) was used to detect full length APP and antibody AC15 (Sigma, Dorset, UK) was used to detect actin. Horseradish peroxidase (HRP)-conjugated secondary antibodies were used in the same buffer. Bound antibody was detected using the enhanced chemiluminescence detection method (Amersham Biosciences, Amersham, UK).
Measurement of Aβ and Soluble APP Fragments by Mesoscale Discovery Analysis
Biochemical analysis was performed on APPWT/PrP+/+ (n = 3), APPWT/PrP-/- (n = 6) mice at 32 and 75 weeks of age, and APPSwe,Ind/PrP+/+ (n = 5, 7 and 4 at 5, 10 and 40 weeks, respectively) Aβ peptides (Aβ38, Aβ40 and Aβ42) and soluble APP fragments (sAPPα and sAPPβ) contained in the soluble (SDS extracted) fraction and Aβ peptides in the insoluble (formic acid extracted) fraction were assessed using the Mesoscale Discovery (MSD) platform. Aβ38, Aβ40 and Aβ42 were measured using the V-Plex Aβ peptide panel (6E10) kit and sAPPα and sAPPβ using the sAPPα/sAPPβ multiplex kit according to the manufacturer’s instructions (MSD, Maryland US). SDS samples were diluted 1:40 and formic acid samples 1:500 in diluent 35.
Histology
Pathological analysis was performed on APPWT/PrP+/+ (n = 4), APPWT/PrP-/- (n = 4) and APPSwe,Ind/PrP+/+ (n = 4) mice at 32 and 75 weeks of age (the group size dropped to an n = 1 in the 75 week APPWT/PrP+/+ group due to intercurrent losses). Sections from a 129Ola (WT) mouse at 75 weeks of age were also analysed. Neuropathologic evaluation was performed blind by two operators. Fixed brain tissue was processed and tissue sections were prepared as described [24]. Paraffin sections (6 μm) were stained with hematoxylin-eosin and immunostained with the following antibodies: 4G8 (1/300) raised against Aβ17–24 (Covance; SIG-39200); OC (1/500) that recognises a conformational-dependent epitope, specific to fibrils and soluble fibrillar oligomers (a gift from Professor C. Glabe, Dept. Neurology, University of California at Irvine School of Medicine, Irvine, CA, USA); anti-synaptophysin (1/150) that recognises a synaptic vesicle protein (Synaptic systems, 101002); anti-glial fibrillary acidic protein (GFAP) (1/400) (DAKO Z0334); anti-Iba1 (1/1000) a calcium-binding protein specifically expressed in macrophages and microglia (Wako 019–9741). Primary antibody binding was detected with biotinylated goat anti-species specific IgG (Stratech Scientific Ltd.) and the Vectastain Elite ABC Kit (Vector Laboratories), visualized with diaminobenzidine. For synaptophysin immunolabelling antigen retrieval (DAKO S1699) and enhancement visualization system (EnVision™ K5007) were used. Antigen retrieval using 0.1M citrate buffer was performed with the Iba1 labelling.
Statistical Analysis
Densitometric analysis was performed using the advanced image data analyser (AIDA) programme (Raytest Scientific Ltd). The Kolmogorov-Smirnov test was used to determine that the data in each group was normally distributed. Following this the Levene’s test was used to ensure that the data sets were of equal variance. In samples where the data met the criteria of a normal distribution and equal variance, the parametric independent t-test was used to calculate significance. In the samples which were not normally distributed the non-parametric two-tailed Mann-Whitney U test was used to compare two independent samples. The data were analysed using the Statistical Package for Social Sciences (SPSS 19) program (Chicago, USA).
Results and Discussion
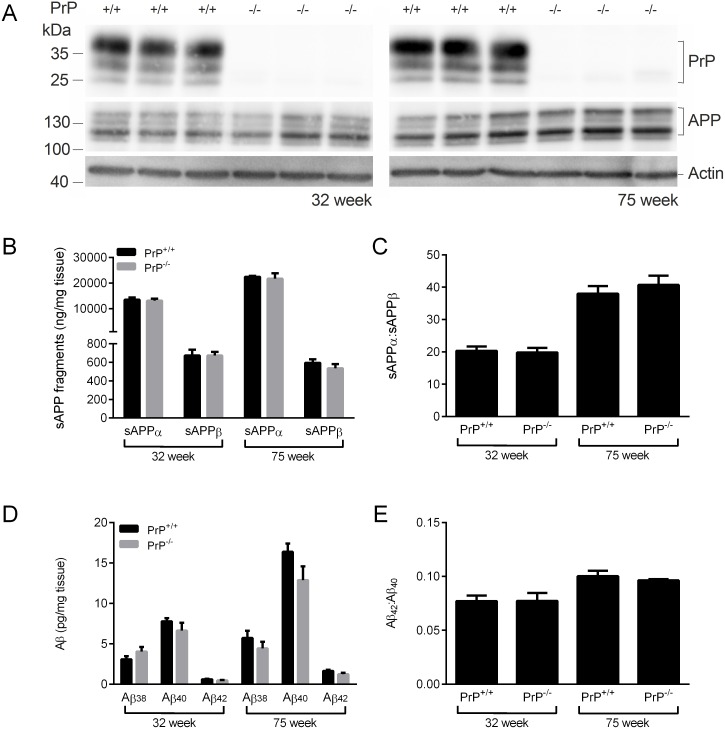
In order to investigate whether ablation of PrPC in transgenic mice expressing human APPWT results in increased Aβ, mice expressing human APPWT [21] were crossed with PrPC-null 129/Ola mice [22]. Immunoblot analysis of mice at 32 and 75 weeks of age confirmed the absence of PrPC in the APPWT/PrP-/- mice and that the lack of PrPC had no effect on APP expression (Fig 1A). The amounts of the soluble APP species, sAPPα and sAPPβ, were no different between the APPWT/PrP-/- and APPWT/PrP+/+ mice at either 32 or 75 weeks of age (Fig 1B), indicating that deletion of PrPC had no effect on either the α- or β-secretase cleavage of APP. There was a significant increase in sAPPα in both genotypes at 75 weeks of age compared to 32 weeks of age (Fig 1B) which resulted in an increased sAPPα:sAPPβ ratio (Fig 1C). The levels of soluble (SDS-extracted) Aβ38, Aβ40 and Aβ42 were investigated. At both 32 and 75 weeks of age there was no difference in the amount of soluble Aβ38, Aβ40 or Aβ42 in the brain homogenates of the APPWT/PrP-/- mice compared to the APPWT/PrP+/+ mice (Fig 1D). The amount of Aβ40 and Aβ42 was higher at 75 weeks of age than at 32 weeks of age regardless of Prnp genotype (Fig 1D), with a proportionately larger increase in Aβ42 such that the Aβ42: Aβ40 ratio increased in both the APPWT/PrP-/- and APPWT/PrP+/+ mice with age (Fig 1E). The levels of Aβ peptides in the insoluble (formic acid extracted) fraction from either the APPWT/PrP-/- or the APPWT/PrP+/+ mice were below the limit of detection in the assay. These data indicate that lack of PrPC in transgenic mice expressing human APPWT does not alter the proteolysis of APP or the levels of Aβ.
Fig 1. Ablation of PrPC does not affect APP proteolysis or Aβ peptide levels in APPWT mice.
Brain hemispheres from 32 week and 75 week old APPWT/PrP+/+ and APPWT/PrP-/- mice were subjected to two-step homogenisation, and the soluble proteins (SDS extracted) were subjected to SDS-PAGE and immunoblotting. (A) representative immunoblots of PrPC and APP, with actin as a loading control. (B) Soluble (SDS extracted) sAPPα and sAPPβ detected by MSD ELISA and (C) the ratio of sAPPα to sAPPβ. (D) Soluble (SDS extracted) Aβ38, Aβ40 and Aβ42 detected by MSD ELISA and (E) the ratio of Aβ42 to Aβ40. Data shown as mean ± SEM (n = 3–6). There was no significant difference between the APPWT/PrP+/+ and APPWT/PrP-/- at the same time point for any of the APP or Aβ peptides.
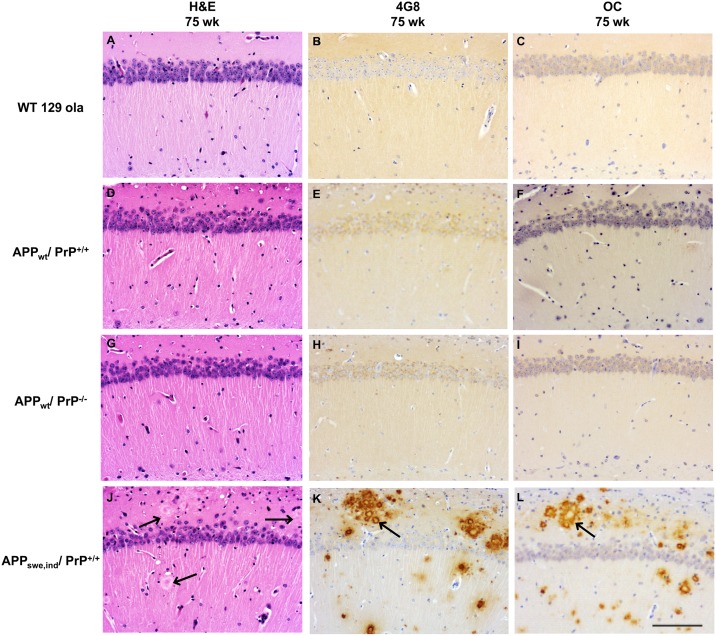
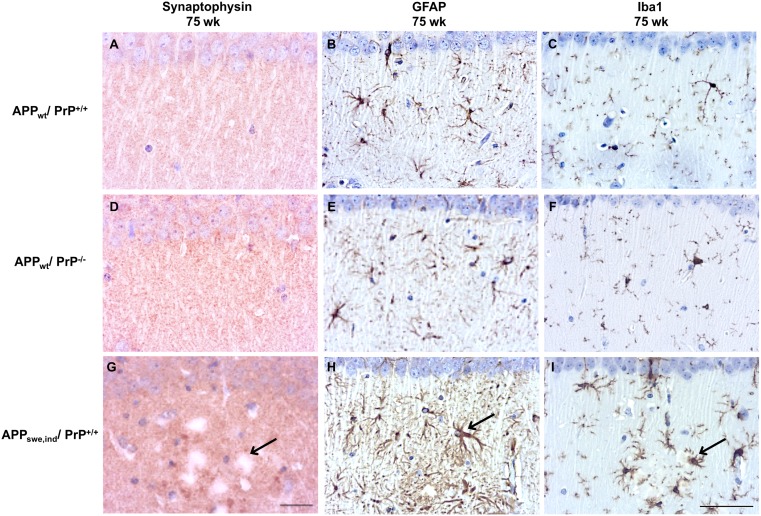
To investigate whether lack of PrPC in the APPWT/PrP-/- mice affected the deposition of Aβ in the brain, histopathological analysis of the hippocampus was carried out at 32 weeks of age and compared to APPWT/PrP+/+ mice. APPSwe,Ind/PrP+/+ mice, which are known to accumulate limited Aβ-positive plaques at this age, were also analysed [9,21]. APPWT/PrP+/+ and APPWT/PrP-/- mice at 32 weeks of age showed no obvious neuropathologic alterations (data not shown). To explore whether histopathologic alterations were detected in older animals, we also examined mice at 75 weeks of age. Histopathological analysis of WT, APPWT/PrP+/+ and APPWT/PrP-/- mice at 75 weeks of age showed similar histopathologic features; i.e. absence of obvious neuronal loss or other obvious alterations in the hippocampus proper or dentate gyrus (Fig 2A, 2D and 2G) or 4G8 positive Aβ plaque formation (Fig 2B, 2E and 2H) in these mice. We investigated whether the ablation of PrPC affected the level of Aβ fibrils and fibrillar oligomers by using the OC antibody [25]. We observed that WT, APPWT/PrP+/+ and APPWT/PrP-/- mice did not accumulate OC immunopositive deposits as shown by immunohistochemistry (Fig 2C, 2F and 2I). As expected, in the APPSwe,Ind/PrP+/+ mice at 75 weeks of age there was disruption of the hippocampal structure with accumulation of 4G8 and OC positive Aβ deposition forming numerous uni and multicentric plaques (Fig 2J, 2K and 2L), as seen previously [9]. Synapses are particularly vulnerable to the toxic effect of protein oligomers. Thus, we performed immunostaining for the detection of the presynaptic protein synaptophysin and observed disruption of immunoreactivity in 75 week old APPSwe,Ind/PrP+/+ mice but not in APPWT/PrP+/+ or APPWT/PrP-/- mice (Fig 3A, 3D and 3G). Glial markers (GFAP and Iba1) showed similar intensity and pattern of reactivity in APPWT/PrP+/+ and APPWT/PrP-/- mice (Fig 3B, 3C, 3E and 3F). In contrast, gliosis was observed particularly in the vicinity of amyloid deposits in APPSwe,Ind/PrP+/+ mice (Fig 3H and 3I). Thus, we did not detect any effect of PrPC ablation on Aβ deposition using immunohistochemistry.
Fig 2. Ablation of PrPC does not affect Aβ deposition in APPWT mice.
Histopathologic analysis of the hippocampus of hematoxylin-eosin stained sections of WT, APPWT/PrP++ and APPWT/PrP-- mice show absence of obvious alterations in the pyramidal cell layer, stratum oriens and lacunosum-molecular (A, D and G). No Aβ deposits are seen in sections probed with antibody 4G8 (B, E and H) or antibody OC (C, F and I). In contrast, APPSwe,Ind/PrP+/+ mice show plaques (arrows) in sections stained with hematoxylin-eosin (J) and abundant Aβ-reactive deposits in sections probed with antibody 4G8 (K) and OC (L). All images x20 magnification and scale bar = 100μm.
Fig 3. Ablation of PrPC does not affect synaptophysin immunoreactivity and does not induce gliosis in APPWT mice.
APPWT/PrP+/+ and APPWT/PrP-/- mice show retention of the pre-synaptic marker synaptophysin (A, D) while APPSwe.Ind/PrP+/+ mice reveal disorganization of synaptophysin immunoreactivity (arrow) (G). Astrogliosis and microgliosis are observed in 75 week old APPSwe.Ind/PrP+/+ mice, (arrows) (H, I) but not in mice expressing APPWT/PrP+/+ and APPWT/PrP-/- (B, C, E, F). Section were probed with anti-synaptophysin (A, D, G), anti-GFAP (B, E, H), and anti-Iba1 (C, F, I) antibodies. Figures A,D,G (synaptophysin) x60 magnification scale bar = 20μm, figures B,E,H (GFAP) and C,F,I (Iba1) x40 magnification scale bar = 50μm.
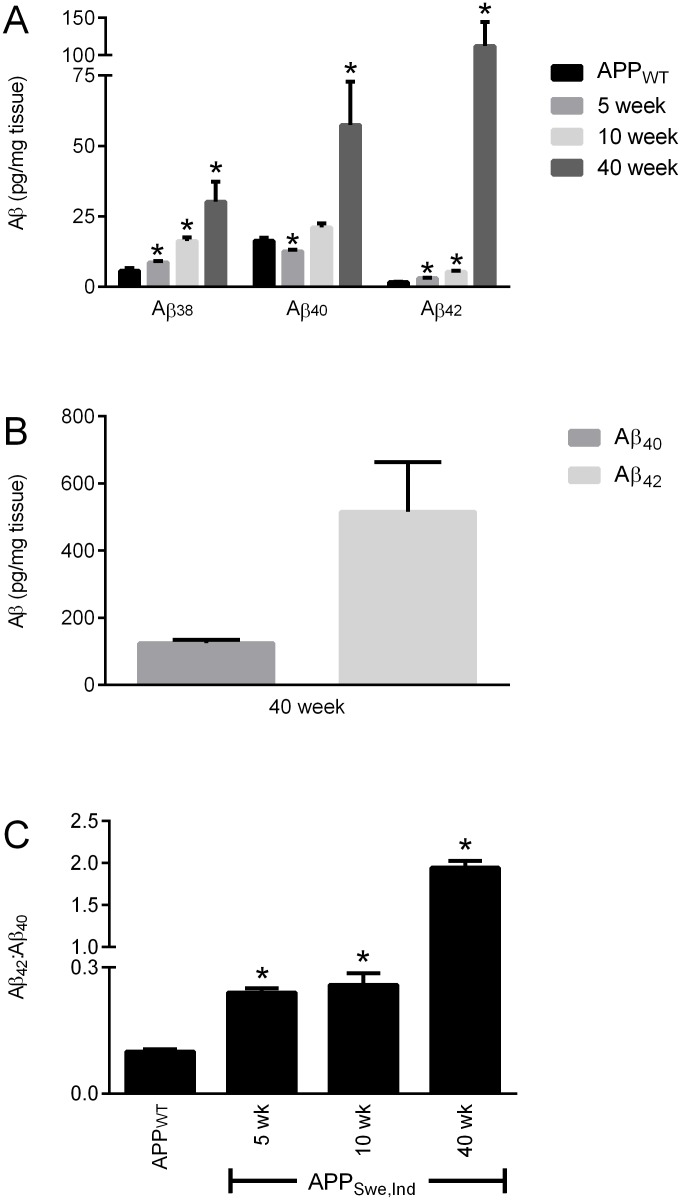
A comparison of the Aβ levels between the APPWT/PrP+/+ mice at 75 weeks of age with APPSwe,Ind/PrP+/+ mice at 5 and 10 weeks (before significant Aβ deposition occurs) and at 40 weeks (after Aβ deposition in plaques appears) revealed that at 75 weeks of age the amount of Aβ peptides in the soluble (SDS extracted) fraction of the APPWT/PrP+/+ mice was comparable to the amount of each peptide in the 5 week old APPSwe,Ind/PrP+/+ mice (Fig 4A). Although Aβ40 and Aβ42 were readily detectable in the insoluble (formic acid extracted) fraction from the APPSwe,Ind/PrP+/+ mice (Fig 4B), no Aβ was detected in the insoluble fraction from the APPWT/PrP+/+ mice. The soluble Aβ42:Aβ40 ratio increased with age in both APPWT/PrP+/+ and APPSwe,Ind/PrP+/+ mice (Fig 4C) but was significantly higher in the APPSwe,Ind/PrP+/+ mice than in the APPWT/PrP+/+ mice consistent with the presence of the Indiana mutation increasing production of Aβ42 as observed previously [21]. Even at 75 weeks of age the amount of Aβ42 in the APPWT/PrP+/+ mice is significantly lower than that in the APPSwe,Ind/PrP+/+ mice at 5, 10 and 40 weeks of age (Fig 4A). Thus in the APPWT/PrP+/+ mice even at 75 weeks of age the low level of Aβ42 is likely below the threshold required for deposition to occur.
Fig 4. Comparison of Aβ levels in APPWT and APPSwe.Ind mice.
Brain hemispheres from 75 week old APPWT/PrP+/+ and from 5, 10 and 40 week old APPSwe,Ind/PrP+/+ mice were subjected to two-step homogenisation. (A) Soluble (SDS extracted) and (B) insoluble (formic acid extracted) Aβ38, Aβ40 and Aβ42 detected by MSD ELISA and (C) the ratio of Aβ42:Aβ40. Data shown as mean ± SEM (n = 3–6), * p<0.05 compared to the 75 week old APPWT/PrP+/+ group.
Conclusion
In cellular models PrPC differentially affected the activity of the β-secretase BACE1 towards APPWT and APPSwe, inhibiting the processing of the former but having no effect on the cleavage of the latter [8,9]. Thus we undertook this study in order to investigate the effect of genetic ablation of PrPC on human APPWT processing in vivo. In contrast to the cellular models, lack of PrPC had no effect on the α- and β-secretase proteolysis of APP or on the amount of Aβ peptides in the brains of mice expressing human APPWT. Also we did not detect any Aβ deposition as shown by immunohistochemistry in the mice lacking PrPC as compared to those with a normal level of the protein. In the human brain the amount of PrPC inversely correlated with BACE1 activity, soluble and insoluble Aβ and Braak stage [11], consistent with levels of PrPC affecting APP processing and Aβ production. However, we could find no evidence that loss of PrPC affected APP proteolysis, Aβ levels or plaque pathology in vivo in transgenic mice expressing human APPWT. Whether this reflects a difference in the role of PrPC in regulating APP processing and Aβ production between this transgenic mouse model and the situation in the human brain will require further study.
Abbreviations
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- BACE1
β-site APP cleaving enzyme-1
- PrPC
prion protein
Data Availability
All relevant data are within the paper.
Funding Statement
The authors gratefully acknowledge the financial support of Alzheimer’s Research UK (ART-PPG2009B-8), and the Medical Research Council of Great Britain (G0802189) and the University of Manchester. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Burns A, Iliffe S. Alzheimer's disease. BMJ. 2009;338: 467–471. [DOI] [PubMed] [Google Scholar]
- 2.Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Science translational medicine. 2011;3: 77sr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selkoe D, Mandelkow E, Holtzman D. Deciphering Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2: a011460 10.1101/cshperspect.a011460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh DM, Teplow DB. Alzheimer's disease and the amyloid beta-protein. Progress in molecular biology and translational science. 2012;107: 101–124. 10.1016/B978-0-12-385883-2.00012-6 [DOI] [PubMed] [Google Scholar]
- 5.Vardy ER, Catto AJ, Hooper NM. Proteolytic mechanisms in amyloid-beta metabolism: therapeutic implications for Alzheimer's disease. Trends Mol Med. 2005;11: 464–472. [DOI] [PubMed] [Google Scholar]
- 6.Lee VM, Brunden KR, Hutton M, Trojanowski JQ. Developing therapeutic approaches to tau, selected kinases, and related neuronal protein targets. Cold Spring Harb Perspect Med. 2011;1: a006437 10.1101/cshperspect.a006437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole SL, Vassar R. The Alzheimer's disease Beta-secretase enzyme, BACE1. Mol Neurodegener. 2007;2: 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkin ET, Watt NT, Hussain I, Eckman EA, Eckman CB, Manson JC, et al. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer's amyloid precursor protein. Proc Natl Acad Sci USA. 2007;104: 11062–11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths HH, Whitehouse IJ, Baybutt H, Brown D, Kellett KAB, Jackson CD, et al. Prion Protein Interacts with BACE1 Protein and Differentially Regulates Its Activity toward Wild Type and Swedish Mutant Amyloid Precursor Protein. J Biol Chem. 2011;286: 33489–33500. 10.1074/jbc.M111.278556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitehouse IJ, Jackson CD, Turner AJ, Hooper NM. Prion protein is reduced in aging and in sporadic but not in familial Alzheimer's disease. J Alzheimers Dis. 2010;22: 1023–1031. 10.3233/JAD-2010-101071 [DOI] [PubMed] [Google Scholar]
- 11.Whitehouse IJ, Miners JS, Glennon EB, Kehoe PG, Love S, Kellett KA, et al. Prion protein is decreased in Alzheimer's brain and inversely correlates with BACE1 activity, amyloid-beta levels and Braak stage. PLoS One. 2013;8: e59554 10.1371/journal.pone.0059554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooper NM, Turner AJ. A new take on prions: preventing Alzheimer's disease. Trends Biochem Sci. 2008;33: 151–155. 10.1016/j.tibs.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 13.Griffiths HH, Whitehouse IJ, Hooper NM. Regulation of amyloid-beta production by the prion protein. Prion. 2012;6: 217–222. 10.4161/pri.18988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haass C, Lemere CA, Capell A, Citron M, Seubert P, Schenk D, et al. The Swedish mutation causes early-onset Alzheimer's disease by b-secretase cleavage within the secretory pathway. Nature Med. 1995;1: 1291–1296. [DOI] [PubMed] [Google Scholar]
- 15.Thinakaran G, Teplow DB, Siman R, Greenberg B, Sisodia SS. Metabolism of the 'Swedish' amyloid precursor protein variant in Neuro2a (N2a) cells. Evidence that cleavage at the 'b-secretase' site occurs in the Golgi apparatus. J Biol Chem. 1996;271: 9390–9397. [DOI] [PubMed] [Google Scholar]
- 16.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. b-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286: 735–741. [DOI] [PubMed] [Google Scholar]
- 17.Huse JT, Pijak DS, Leslie GJ, Lee VM, Doms RW. Maturation and endosomal targeting of beta -site amyloid precursor protein-cleaving enzyme. THE ALZHEIMER'S DISEASE beta -SECRETASE. J Biol Chem. 2000;275: 33729–33737. [DOI] [PubMed] [Google Scholar]
- 18.Calella AM, Farinelli M, Nuvolone M, Mirante O, Moos R, Falsig J, et al. Prion protein and Abeta-related synaptic toxicity impairment. EMBO Mol Med. 2010;2: 306–314. 10.1002/emmm.201000082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, et al. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30: 6367–6374. 10.1523/JNEUROSCI.0395-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cisse M, Sanchez PE, Kim DH, Ho K, Yu GQ, Mucke L. Ablation of cellular prion protein does not ameliorate abnormal neural network activity or cognitive dysfunction in the J20 line of human amyloid precursor protein transgenic mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31: 10427–10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mucke L, Masliah E, Yu G-Q, Mallory M, Rockenstein EM, Tatsuno G, et al. High-Level Neuronal Expression of Abeta 1–42 in Wild-Type Human Amyloid Protein Precursor Transgenic Mice: Synaptotoxicity without Plaque Formation. J Neurosci. 2000;20: 4050–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manson JC, Clarke AR, Hooper ML, Aitchison L, McConnell I, Hope J. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol. 1994;8: 121–127. [DOI] [PubMed] [Google Scholar]
- 23.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21: 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccardo P, Manson JC, King D, Ghetti B, Barron RM. Accumulation of prion protein in the brain that is not associated with transmissible disease. Proc Natl Acad Sci U S A. 2007;104: 4712–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007;2: 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.