Abstract
Background
Oridonin (ORI) can inhibit proliferation and migration in various types of cancer cell lines. However, the exact mechanism remains unclear. We investigated the migration inhibitory effect of ORI on human pancreatic cancer SW1990 cells and dissected the possible molecular mechanism(s).
Methods
CCK-8 assay was used to observe the cell viability. Wound healing assay, transwell assay and spontaneous metastasis model were used to observe the migration activities. Real-time PCR, immunofluorescence, western blot analysis and immunohistochemistry methods were used to observe the expression of genes or proteins.
Results
ORI inhibited the migration of SW1990 cells. Real-time PCR and immuno-fluorescence analyses of epithelial-to-mesenchymal transition (EMT) markers were compared between control group and ORI group. The expression of mesenchymal molecular markers, such as vimentin, snail and slug decreased. The expression of epithelial-related marker E-cadherin increased. Wnt/β-catenin signalling was inhibited by ORI using luciferase reporter assay. ORI can decrease the β-catenin protein level not only in the nucleus, but also in the cytoplasm and the whole cell after the treatment with ORI and glycogen synthase kinase 3β (GSK3β) was increased in the ORI-treated group. CHIR could attenuate the effects of ORI in SW1990 cells. We established a mice model by injecting 1 × 106 SW1990 cells into nude mice intraperitoneally to test whether ORI affects tumour metastasis. Metastatic formation was inhibited by ORI (5 and 10 mg/kg) compared with the control group. Tumour sections stained with anti-E-cadherin, anti-vimentin and anti-β-catenin antibodies revealed that ORI inhibited EMT, as well as the Wnt/β-catenin pathway in vivo.
Conclusions
ORI can inhibit pancreatic cancer cell SW1990 migration and EMT by down-regulating Wnt/β-catenin signal transduction in vitro and in vivo. Therefore, it can be potentially and effectively used in the clinical management of pancreatic cancer.
Keywords: Oridonin, Pancreatic cancer, Metastasis, Epithelial mesenchymal transition, Wnt/β-catenin pathway
Background
Pancreatic cancer, which is one of the most common cancers of the digestive system, is a leading cause of morbidity and mortality in both developed and developing countries. Pancreatic cancer is characterised by a covert anatomical structure, aggressive biology, resistance to conventional therapeutic agents, and no early detectable biomarkers were observed [1]. Consequently, pancreatic cancer presents a great challenge in oncology, with a 5-year survival rate of only 5 % [2]. This is true for metastatic diseases, wherein the average life expectancy is just three to 6 months. Treatment options for pancreatic cancers are limited. Less than 20 % of patients with pancreatic cancer are subjected to surgical removal, whereas other patients are typically treated with chemotherapy or chemotherapy with radiation. Pancreatic cancer is one of the few cancers in which survival has not improved substantially in 40 years. Therefore, searching for new strategies to prevent and treat pancreatic cancer is essential.
The epithelial-to-mesenchymal transition (EMT) is among the most critical processes that occur during the progression of tumour metastasis. EMT-induced adenocarcinoma cells can acquire malignant features, such as invasion, metastatic capabilities and chemo-resistance [3, 4]. In human pancreatic tumour samples, fibronectin and vimentin are increased in high-grade tumours, with a corresponding decrease in E-cadherin expression. These patients have worse prognoses than those who demonstrate less evidence of EMT. Primary tumours with mesenchymal features (75 % of the total number of tumours) developed metastatic lesions to the liver and lungs [5]. Numerous signalling pathways that are involved in the regulation of EMT, such as transforming growth factor-beta, notch and Wnt signalling pathways, are highly activated in metastatic pancreatic cancers and appear to be associated with prognosis [6–8].
Oridonin, a tetracycline diterpenoid compound extracted from the traditional Chinese medicine Rabdosia rubescens, has anti-inflammatory, antibacterial and antitumour effects [9]. ORI inhibits the development of various types of cancers and is an effective and low-toxic antitumour medicine [10–12]. However, mechanisms underlying the antitumour activities of ORI, and whether or not it can suppress the migration of pancreatic cancer remain largely unknown.
We investigated the effects of ORI on pancreatic cancer cells. ORI inhibits pancreatic cancer cell migration and EMT in vitro and in vivo by suppressing the Wnt/β-catenin signalling pathway.
Methods
Chemicals and reagents
ORI was purchased from the Yuanye Biological Institutions (Shanghai, China). Its purity was measured by HPLC and determined to be 99 %. ORI was dissolved in dimethyl sulfoxide (DMSO) to make a stock solution (10 mM) and stored at −20 °C. The DMSO concentration was kept below 0.1 % in all experiments and did not exert any detectable effect on cell growth or cell death. Fetal bovine serum (FBS), 0.25 % trypsin containing EDTA and high glucose DMEM were obtained from Gibco (USA). Cell Counting Kit-8 (CCK-8) was purchased from DoJinDo (Japan). RNA extraction kit was purchased from Life Technologies Co. CHIR99021, a GSK3β inhibitor (R&D), was added into medium at a concentration of 2 μM 1 h before administration of ORI.
Cell culture
Pancreatic cancer cell lines (Aspc1, Bxpc3, Panc1, SW1990) was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). The cells were cultured in DMEM supplemented with 10 % FBS, 100U/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified atmosphere of 5 % CO2, and subcultured when confluency reached 70–80 % by 0.25 % trypsin.
Cytotoxicity assay
The cytotoxic effect of ORI on pancreatic cancer cells was determined by using CCK-8 kit. Briefly, the logarithmic phase cells were plated in 96-well culture plates (5 × 103 cells per well). After 24 h of incubation, cells were treated with vehicle alone (0.1 % DMSO) or various concentrations (7, 15, 31, 62, 125, 250 μM) of ORI, followed by a 48 h-culture. Each group had 6 wells, and CCK-8 (100 μl) was added to each well 1 h before the end of incubation. The absorbance at 450 nm was read using Bio-Tek, ELX800. Experiments were repeated three times. The cytotoxic effect was expressed as a relative percentage of cell death calculated as follows: Cell death (%) = 100 −(dosing absorbance − blank absorbance)/(control absorbance − blank absorbance) × 100.
Cell migration assay
A wound scratch assay was performed in order to study the migration of SW1990 cells. A 6-well plate was seeded with cells at 60 % confluence. After overnight incubation, a scratch was made at the centre of each well using a 200 μl pipette tip. Cells were further incubated with 0, 7 or 15 μM ORI. Three parallel lines were drawn at the border of the wound, and the distance between the lines were measured 24 h post-scratching. The microscopic magnification (100×) of this area was photographed. The wound healing effect was determined by measuring the percentage of the migrating area compared with the area of the initial wound. Each experiment was conducted in triplicate.
Transwell migration assay
SW1990 cells were subjected to a transwell migration assay. 1 × 105 cells were added to the upper chamber, and the lower chamber was filled with 7 or 15 μM ORI of culture medium containing no FBS. After incubation for 24 h, cells in the upper chamber were carefully removed with a cotton swab, and cells that passed through the membrane to the lower chamber surface were fixed and stained with 0.5 % crystal violet. Cells from each well were counted in 3 random high-power fields under a microscope (100×). Each experiment was repeated in triplicate.
Real-time PCR
Total cellular RNA was extracted using TRIzol (Invitrogen) reagent. The reverse transcription was performed with 2 μg of total RNA, using an oligo (dT) primer and other reagents, and cDNA was synthesized using the Revert Aid First-Strand cDNA Synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. Real-time PCR was carried out using the miScript SYBR Green PCR kit (Qiagen, Venlo, Netherlands). The cycle conditions for the PCR reaction were as follows: 95 °C for 15 min followed by 40 cycles of 60 °C for 20 s, and 72 °C for 40 s. The expression of each RNA was normalized to that of the internal control glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The specific primer pairs used in this PCR reaction were: E-cadherin (NM_004360.3), forward: 5′-ACAGCCCCGCCTTATGATTCTC-3′, reverse: 5′-AAGCGATTGCCCCATTCG-TT-3′; vimentin (NM_003380.3), forward: 5′-AGATGGCCCTTGACATTGAG-3′, reverse: 5′-CCAGAGGGAGTGAATCCAGA-3′; snail (NM_005985.3), forward: 5′-AATCGGAAGCCTAACTACAGCG-3′, reverse: 5′-GTCCCAGATGAGCATT-GGCA-3′; slug (NM_003068.4), forward: 5′-GTCCGCTCTGCCGCACCTGA-3′, reverse:5′-GCTTGAGGGTCTGAATCTTGCT-3′; GAPDH (BC026907.1), forward: 5′-CTGCACCACCAACTGCTTAG-3′, reverse: 5′-GTCTTCTGGGTGGCAGTGAT-3′. The changes of expression were calculated using the (2 [−ΔΔCT]) method. Each plate was run in triplicate.
Immunofluorescence
After treated with or without ORI, SW1990 grown on 6-well plate were fixed in 4 % (w/v) paraformaldehyde and permeabilized with 0.1 % Triton-X 100 in PBS. E-cadherin (CST, 1:200), vimentin (CST, 1:200) and slug (CST, 1:200) were visualized by sequential incubation with indicated antibody overnight at 4 °C. Pictures were acquired by using a fluorescence microscope (Olympus).
Western blot
40 μg proteins were separated by 10 % sodium dodecyl sulfate–polyacrylamide gel (SDS-PAGE), and blotted onto polyvinylidene difluoride (PVDF) membranes. The membranes were incubated with the primary antibodies against Histone H1 (Millipore, 1:1000), Non-phospho (Active) β-catenin (CST, 1:1000), β-catenin (CST, 1:1000), GSK-3β(CST, 1:1000), GAPDH (KangChen Bio-tech, 1:1000) at 4 °C overnight separately, and further incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:10,000; keyGEN-BIO) for 1 h. Bands were visualized by Western Blotting Reagents (EMD Millipore, Billerica, MA, USA).
Luciferase reporter assay
Sub-confluent SW1990 cells were seeded in 24-well plate and transfected with 0.5 μg β-catenin/Tcf4 luciferase reporter (pTop-luc) per well with Lipofectamine (Invitrogen). After incubating for 12 h, cells were treated with 0.1 % DMSO or 15 μM ORI. 24 h later, cells were lysed and subjected to luciferase assays using luciferase assay kit (Promega). Each assay was done in triplicate.
Spontaneous metastasis model and Hematoxylin & Eosin (H&E) staining
SW1990 cells (1 × 106) in 50 μl PBS were injected into the nude mice intraperitoneally. Based on the different treatments, mice were segregated into 3 groups (each group has 5 mice). ORI (5 mg/kg and 10 mg/kg) was injected intraperitoneally every day for 10 days starting from the second day of SW1990 injection. Lungs were dissected and fixed in 10 % formaldehyde after 1 month. Paraffin-embedded lungs were cut for H & E staining.
Immunohistochemistry (IHC)
Immunohistochemistry analysis was performed on 5-mm sections of formalin-fixed paraffin-embedded lungs derived from mice. Serial sections were cut from each tissue, and further stained to evaluate the expression of EMT markers (E-cadherin and vimentin) and β-catenin (1:200).
Statistical analysis
Quantitative results were expressed as mean ± standard deviation (SD). The statistical analysis was performed by using two-tailed unpaired t test (between two groups) or one-way analysis of variance (ANOVA) (among three or more groups) under the computer software SAS 9.2 (SAS Institute Inc., Cary, NC, USA). A two-tailed P value <0.05 was considered to be statistically significant.
Results
ORI inhibits cell migration in SW1990 pancreatic cell line
After treatment with different concentrations (7, 15, 31, 62, 125 or 250 μM) of ORI for 12, 24, 36 and 48 h, the proliferation rates of four human pancreatic cancer cell lines (Axpc1, Bxpc3, Panc1, SW1990) were assessed via CCK-8 kit. As shown in Fig. 1a, ORI exhibited anti-proliferative effects on all four cell lines in a concentration-dependent manner, with SW1990 being the least sensitive to ORI. Therefore, we decided to use this cell line, which was derived from a spleen metastasis of a grade II pancreatic adenocarcinoma [13], to evaluate the role of ORI in pancreatic cancer metastasis and EMT.
Fig. 1.
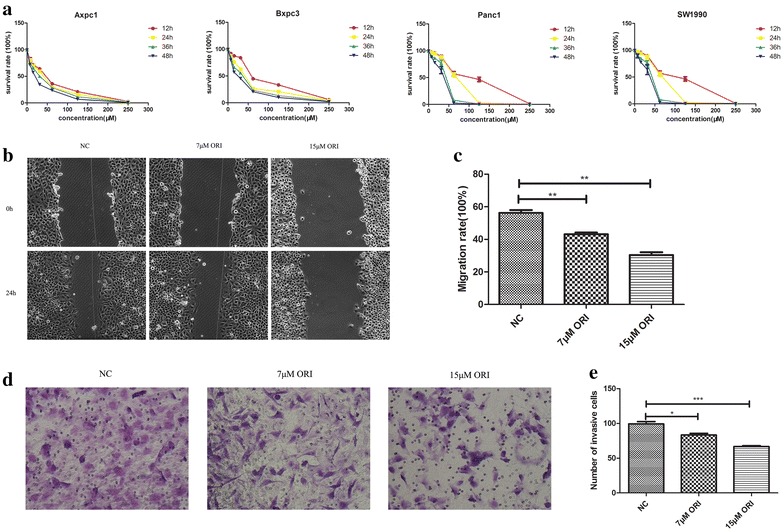
ORI inhibits SW1990 cell migration. a Cell viability was determined by using a CCK-8 assay. b–e Cell migration was determined by using wound healing and transwell assay. The migrative ability of ORI group (7 and 15 μM) was compared with that in the control group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group
IC50 of ORI in SW1990 of 24 h was 63.47 μM (Table 1). ORI at doses higher than 30 μM inhibited cell growth. ORI concentrations of 7 and 15 μM were used to treat the cells for 24 h to evaluate the role of ORI in pancreatic cancer metastasis. The effect of ORI on cell migration was evaluated via wound healing and transwell migration assays. As shown in Fig. 1c and e, the migrative ability of SW1990 was significantly decreased after ORI treatment, compared with that in the control. Treatment with the 15 μM ORI concentration resulted in 46 % reduction in cell migrative ability (Fig. 1c; P < 0.05).
Table 1.
IC50 of ORI
| IC50 (μM) | 12 h | 24 h | 36 h | 48 h |
|---|---|---|---|---|
| Axpc1 | 38.68 | 32.25 | 26.27 | 17.99 |
| Bxpc3 | 65.41 | 37.51 | 29.30 | 21.66 |
| Panc1 | 86.83 | 63.46 | 38.76 | 32.49 |
| SW1990 | 87.25 | 63.47 | 38.95 | 32.57 |
EMT of SW1990 is affected by ORI
EMT is an early event in tumour migration. It is related to the structural changes in the intercellular junction, which is essential for migration [14]. We hypothesised whether the inhibition of tumour cell migration by ORI is associated with EMT. SW1990 were treated with 15 μM ORI for 24 h and evaluated for the expression of EMT markers by real-time PCR and immunofluorescent staining. Real-time PCR showed that the expression of mesenchymal markers, such as vimentin and transcription factors snail and slug, decreased, whereas the expression of epithelial-related genes E-cadherin was increased (Fig. 2a, b). However, immunofluorescent staining did not show significant changes of E-cadherin and slug.
Fig. 2.
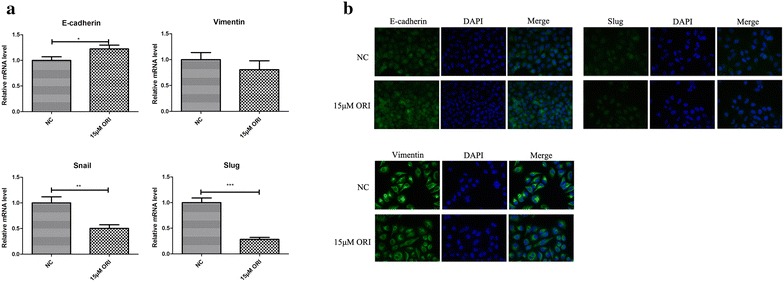
Impact of ORI on EMT of tumor cells. a Real-time PCR analysis of EMT markers were compared between control group and ORI group (15 μM). Data are presented as the mean ± SD, n = 3. b Immunofluorescence of EMT markers were compared between control group and ORI group (15 μM). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group
ORI inhibits β-catenin signalling in human pancreatic cancer cells
Given that stabilization and nucleus translocation of β-catenin are key events in transduction of the canonical Wnt/β-catenin signalling, which is highly involved in cancer metastasis, we conducted western blot analysis to explore whether oridonin can suppress β-catenin activity. The results indicated that ORI can decrease the β-catenin protein level not only in the nucleus, but also in the cytoplasm and the whole cell after the treatment with ORI for 24 h (Fig. 3b). Luciferase assays demonstrated that ORI dramatically suppressed the transcriptional activity of β-catenin (Fig. 3a). The stability of β-catenin in the cytoplasm is tightly regulated by the Axin/APC/GSK3β complex. Phosphorylation of β-catenin by GSK3β resulted in its degradation, which led to the inactivation of Wnt/β-catenin signalling [15–18].Thus, we investigated whether ORI could also affect the expression of GSK-3β, which is the negative regulator of Wnt/β-catenin signalling. As shown in Fig. 3b, the total expression level of GSK3β increased after ORI treatment. ORI may regulate Wnt/β-catenin signalling by enhancing the function of GSK3β in SW1990 cells.
Fig. 3.
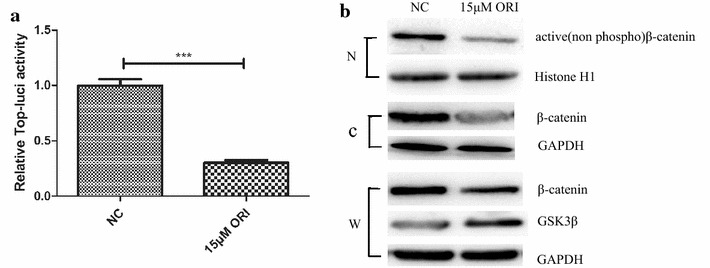
Functions of ORI relied on β-catenin and GSK3β. a Luciferase assays. b Representative western blots for active β-catenin, β-catenin(total) and GSK3β levels in control or ORI group. Histone H1 and GAPDH were used as internal control. (N, nucleus; C, cytoplasm; W, whole cell)
CHIR attenuates the inhibitory function of ORI
To further validate the role of β-catenin and GSK3β in the migration inhibitory effect of ORI on SW1990 cells, SW1990 was tested with CHIR, a GSK-3-specific inhibitor. CHIR attenuated the inhibitory function of ORI. WB results indicated that CHIR can reverse the decrease of the β-catenin in the cytoplasm, nucleus and the whole cell (Fig. 4d). As shown in Fig. 4a and b, after simulation with CHIR, the inhibited migration of SW1990 in the ORI group (15 μM) was markedly improved. Furthermore, the expression of E-cadherin decreased, whereas, the expression of vimentin, snail and slug increased after CHIR treatment (Fig. 4c, e, P < 0.05). These results suggest that down-regulation of β-catenin plays a critical role in the function of ORI in SW1990 cells.
Fig. 4.
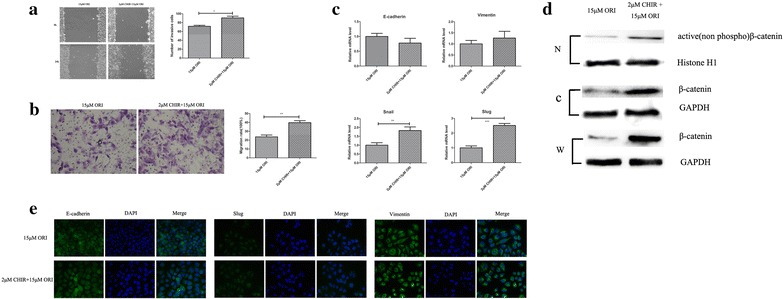
CHIR attenuated the inhibitory function of ORI. a and b Cell migration was determined by using wound healing and transwell assay. The migrative ability of CHIR group (2 μM CHIR + 15 μM ORI) was compared with that seen in the ORI group (15 μM). c Real-time PCR analysis of EMT markers were compared between CHIR group(2 μM CHIR + 15 μM ORI) and ORI group (15 μM). d Representative western blots for active β-catenin and β-catenin(total) levels in ORI or CHIR group. Histone H1 and GAPDH were used as internal control. (N, nucleus; C, cytoplasm; W, whole cell). e Immunofluorescence of EMT markers were compared between CHIR group (2 μM CHIR + 15 μM ORI) and ORI group (15 μM). Data are presented as the mean ± SD, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 vs. ORI group (15 μM)
ORI inhibits tumour metastasis in vivo
We injected 1 × 106 SW1990 cells into nude mice to test whether ORI affects tumour metastasis. As shown in Fig. 5a, ORI treatment significantly decreased the body weight loss compared with the control. In addition, on the fourth week, two of the five mice in the control group died. By contrast, no death was recorded in the ORI group (5 and 10 mg/kg). Furthermore, lung metastasis was significantly inhibited compared with the control group, even though no metastatic nodules could be observed by the naked eye. A representative lung from each group was shown in Fig. 5b. IHC analysis of tumour sections stained with anti-E-cadherin, anti-vimentin and anti-β-catenin antibodies revealed that ORI inhibited EMT as well as Wnt/β-catenin pathway in vivo (Fig. 5c).
Fig. 5.
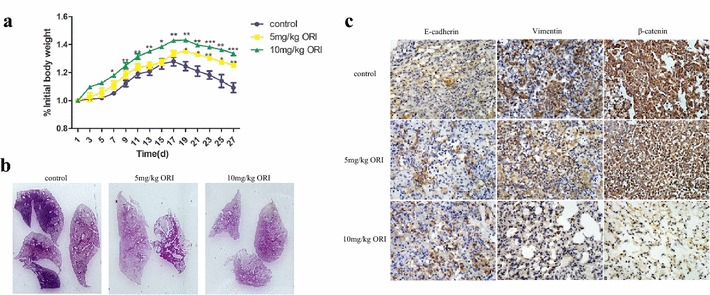
ORI inhibited tumor metastasis in vivo. a The change of body weight after injection of SW1990 and ORI. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group. b Photographs of H&E staining of lung tissues from control or treatment (ORI) group were shown. c IHC images of lungs stained with E-cadherin, vimentin and β-catenin
Discussion
ORI is a tetracycline diterpenoid compound extracted from the traditional Chinese medicinal plant Rabdosia rubescens. It exerts immune protective effects in severe inflammatory diseases. ORI has shown remarkable anti-proliferative and pro-apoptotic effects against leukemia and some types of solid tumours [19–23]. It also inhibits migration in various cancer cells [24–26]. ORI has been reported to significantly inhibit lung tumour metastasis through anti-angiogenesis by blocking Notch signalling; it also inhibits tumour invasion and metastasis in vitro possibly by decreasing the expression of MMPs and regulating the Integrin β1/FAK pathway in human breast cancer MDA-MB-231 cells. However, the effect of ORI on the migration of pancreatic cancer cells remains unclear. We investigated these mechanisms of ORI on pancreatic cancer cells, and ORI can inhibit pancreatic cancer cell SW1990 migration and EMT by down-regulating Wnt/β-catenin signal transduction.
EMT is defined by epithelial cells taking on a mesenchymal phenotype, characterised by loss of apical-basal polarity, increased cellular motility and reorganization of cytoskeleton [27]. There are three major types of EMT: type 1 refers to embryogenesis; type 2 refers to wound healing; and type 3 refers to cancer and metastases [28]. During the process of type 3 EMT, epithelial cells lose contact with their neighbours and gain mesenchymal properties, which enable them to break through the basement membrane for their metastatic dissemination. One of the hallmarks of EMT is the functional loss of E-cadherin (encoded by CDH1), which is thought to be a metastasis suppressor during tumour progression [29]. Vimentin and the transcription factors, snail and slug, are prominent inducers of EMT and strongly repress E-cadherin expression [30, 31]. Highly invasive breast cancer cells have been studied and selected, and these cells displayed EMT characteristics and dramatically enhanced invasive abilities with decreased levels of E-cadherin and increased vimentin, fibronectin, Twist and AKT2 [32]. Metastasis may be largely dependent on the ability of cancer cells to acquire EMT characteristics [33, 34]. Researchers found that paeoniflorin blocked the migration and invasion of breast cancer cells by repressing EMT under hypoxic conditions [35]. Cui speculated that asparaginyl endopeptidase could promote the invasion and metastasis of gastric cancer via EMT through AKT and MAPK signalling pathways [36]. Our results showed that the expression of mesenchymal molecular markers (vimentin, snail and slug) were decreased by ORI, whereas the expression of E-cadherin increased, indicating that ORI affects the EMT process in pancreatic cancer cells.
Wnt/β-catenin signalling pathway plays a critical role in various cancers [37, 38]. In the canonical Wnt/β-catenin signalling, Wnt ligands bind to the dual receptor complex comprised of frizzled and low-density lipoprotein receptor-related protein 5/6 (LRP5/6). This leads to inactivation of the β-catenin destruction complex and Axin/APC/GSK-3β; the critical mediator β-catenin is relieved from its constitutive proteosomal degradation. β-catenin subsequently accumulates in the cytoplasm and translocates into the nucleus, where it associates with transcription factors to regulate the downstream target genes [39]. Increased cytoplasmic and/or nuclear accumulation of β-catenin protein is a common feature in human pancreatic cancers and it is involved in its pathogenesis [40]. The compounds from Chinese herbal medicine targeting Wnt/β-catenin signalling can be considered as suitable candidates for pancreatic cancer treatment. Given that ORI could activate GSK3β activity and up-regulate the expression of DKK1 in human osteosarcoma [41], the anti–migratory effect of ORI on pancreatic cancer cells might result from targeting Wnt/β–catenin signalling. Our data showed that ORI can reduce the protein levels of active β-catenin and its transcriptional activity in the nucleus. Moreover, inhibiting phosphorylation of GSK3β could attenuate the function of ORI on migration and EMT in SW1990. In addition, ORI-treated tumour xenografts express much lower levels of β-catenin compared with control tumours. It is then very likely that the effect of ORI on pancreatic cancer metastasis is due to its suppressive effect on β-catenin signalling.
Conclusions
Our data suggest that ORI can be effective for human pancreatic cancer. The anticancer effect of ORI in SW1990 cells may result from the suppression of EMT by increasing GSK-3β activity and inactivating Wnt/β-catenin signal transduction. Future studies should be directed to the identification of more ORI target proteins, which are essential to elucidate the molecular mechanisms underlying the anti-cancer capacity of ORI.
Authors’ contributions
QQL was the main experimental investigator and had drafted the manuscript. QY participated the data analysis. KC helped to complete the molecular experiments. XHJ and YWS supervised the study and the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Professor Tingjun Ye for his excellent academic assistance.
Competing interests
The authors declare that they have no competing interests.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Abbreviations
- ORI
oridonin
- EMT
epithelial-to-mesenchymal transition
- GSK3β
glycogen synthase kinase 3β
- DKK-1
dickkopf-1
Contributor Information
Qian-Qian Liu, Email: lqqsj13@163.com.
Ke Chen, Email: 814866188@qq.com.
Qiao Ye, Email: 997105035@qq.com.
Xiao-Hua Jiang, Email: xjiang@cuhk.edu.hk.
Yun-Wei Sun, Email: sun_yunwei@qq.com.
References
- 1.Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 4.Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Rasheed ZA, Yang J, Wang Q, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102(5):340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 2009;15(14):4674–4679. doi: 10.1158/1078-0432.CCR-09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang MH, Chen CL, Chau GY, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50(5):1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- 8.Araki K, Shimura T, Suzuki H, et al. E/N-cadherin switch mediates cancer progression via TGF-beta-induced epithelial-to mesenchymal transition in extrahepatic cholangiocarcinoma. Br J Cancer. 2011;105(12):1885–1893. doi: 10.1038/bjc.2011.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun HD, Huang SX, Han QB. Diterpenoids from Isodon species and their biological activities. Nat Prod Rep. 2006;23:673–698. doi: 10.1039/b604174d. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Wu L, Tashiro S, et al. Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK and mitochondrial signaling pathways. J Pharmacol Sci. 2008;107:370–379. doi: 10.1254/jphs.08044FP. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y, Qiu F, Ye YC, et al. Oridonin induces G2/M arrest and apoptosis via activating ERK-p53 apoptotic pathway and inhibiting PTK-RAS-RAFJNK survival pathway in murine fibrosarcoma L929 cells. Arch Biochem Biophys. 2009;490:70–75. doi: 10.1016/j.abb.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Gao FH, Hu XH, Li W, et al. Oridonin induces apoptosis and senescence in colorectal cancer cells by increasing histone hyperacetylation and regulation of p16, p21, p27 and c-myc. BMC Cancer. 2010;10:610. doi: 10.1186/1471-2407-10-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyriazis AP, McCombs WB, 3rd, Sandberg AA, et al. Establishment and characterization of human pancreatic adenocarcinoma cell line SW-1990 in tissue culture and the nude mouse. Cancer Res. 1983;43(9):4393–4401. [PubMed] [Google Scholar]
- 14.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Hamada F, Ishidate T, et al. Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3 beta and APC and reduces the beta-catenin level. Genes Cells. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- 17.Cross DA, Alessi DR, Cohen P, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 18.Bikkavilli RK, Feigin ME, Malbon CC. p38 mitogen-activated protein kinase regulates canonical Wnt/beta-catenin signaling by inactivation of GSK3beta. J Cell Sci. 2008;121:3598–3607. doi: 10.1242/jcs.032854. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y, Shan Q, Gong Y, et al. Oridonin in combination with imatinib exerts synergetic anti-leukemia effect in Ph+ acute lymphoblastic leukemia cells in vitro by inhibiting activation of LYN/mTOR signaling pathway. Cancer Biol Ther. 2012;13:1244–1254. doi: 10.4161/cbt.21460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Jiang H, Wang C, et al. Oridonin triggers apoptosis in colorectal carcinoma cells and suppression of microRNA-32 expression augments oridonin-mediated apoptotic effects. Biomed Pharmacother. 2015;72:125–134. doi: 10.1016/j.biopha.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Xu B, Shen W, Liu X, et al. Oridonin inhibits BxPC-3 cell growth through cell apoptosis. Acta Biochim Biophys Sin (Shanghai) 2015;47:164–173. doi: 10.1093/abbs/gmu134. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XH, Liu YX, Jia M, et al. Oridonin inhibits tumor growth in glioma by inducing cell cycle arrest and apoptosis. Cell Mol Biol (Noisy-le-grand) 2014;60:29–36. [PubMed] [Google Scholar]
- 23.Dong Y, Zhang T, Li J, et al. Oridonin inhibits tumor growth and metastasis through anti-angiogenesis by blocking the Notch signaling. PLoS One. 2014;9:e113830. doi: 10.1371/journal.pone.0113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li YC, Sun MR, Zhao YH, et al. Oridonin suppress cell migration via regulation of nonmuscle myosin IIA. Cytotechnology. 2016;68(3):389–397. doi: 10.1007/s10616-014-9790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Zhong Z, Wan J, et al. Oridonin induces apoptosis, inhibits migration and invasion on highly-metastatic human breast cancer cells. Am J Chin Med. 2013;41(1):177–196. doi: 10.1142/S0192415X13500134. [DOI] [PubMed] [Google Scholar]
- 26.Ren KK, Wang HZ, Xie LP, et al. The effects of oridonin on cell growth, cell cycle, cell migration and differentiation in melanoma cells. J Ethnopharmacol. 2006;103(2):176–180. doi: 10.1016/j.jep.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Investig. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Wang Yifan, Shi Jian, Chai Kequn, et al. The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13(9):963–972. doi: 10.2174/15680096113136660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kokkinos MI, Wafai R, Wong MK, et al. Vimentin and epithelial-mesenchymal transition in human breast cancer-observations in vitro and in vivo. Cells Tissues Organs. 2007;185(1–3):191–203. doi: 10.1159/000101320. [DOI] [PubMed] [Google Scholar]
- 32.Cheng GZ, Chan J, Wang Q, et al. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 33.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 34.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Z, Wang S, Song C, et al. Paeoniflorin prevents hypoxia-induced epithelial-mesenchymal transition in human breast cancer cells. Onco Targets Ther. 2016;9:2511–2518. doi: 10.2147/OTT.S102422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui Y, Wang Y, Li H, et al. Asparaginyl endopeptidase promotes the invasion and metastasis of gastric cancer through modulating epithelial-to-mesenchymal transition and analysis of their phosphorylation signaling pathways. Oncotarget. 2016 doi: 10.18632/oncotarget.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nusse R. The Wnt gene family in tumorigenesis and in normal development. J Steroid Biochem Mol Biol. 1992;43(1–3):9–12. doi: 10.1016/0960-0760(92)90181-H. [DOI] [PubMed] [Google Scholar]
- 38.Bullions LC, Levine AJ. The role of beta-catenin in cell adhesion, signal transduction and cancer. Curr Opin Oncol. 1998;10(1):81–87. doi: 10.1097/00001622-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka Y, Kato K, Notohara K, et al. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61(23):8401–8404. [PubMed] [Google Scholar]
- 41.Liu Y, Liu YZ, Zhang RX, et al. Oridonin inhibits the proliferation of human osteosarcoma cells by suppressing Wnt/β-catenin signaling. Int J Oncol. 2014;45(2):795–803. doi: 10.3892/ijo.2014.2456. [DOI] [PubMed] [Google Scholar]


