Abstract
The neurobiological substrates that cause people with dyslexia to experience difficulty in acquiring accurate and fluent reading skills are still largely unknown. Although structural and functional brain anomalies associated with dyslexia have been reported in adults and school-age children, these anomalies may represent differences in reading experience rather than the etiology of dyslexia. Conducting MRI studies of pre-readers at risk for dyslexia is one approach that enables us to identify brain alterations that exist before differences in reading experience emerge. The current review summarizes MRI studies that examine brain differences associated with risk for dyslexia in children before reading instruction and meta-analyzes these studies. In order to link these findings with current etiological theories of dyslexia, we focus on studies that take a modular perspective rather than a network approach. Although some of the observed differences in pre-readers at risk for dyslexia may still be shaped by language experiences during the first years of life, such studies underscore the existence of reading-related brain anomalies prior to reading onset and could eventually lead to earlier and more precise diagnosis and treatment of dyslexia.
Introduction
Developmental dyslexia is the most common learning disability, but we do not yet fully understand its core neurobiological cause(s) [1]. MRI brain imaging studies, when performed at an early point in children’s reading development, have the potential to validate theories about the etiology of dyslexia by precisely localizing early neurobiological anomalies. Although an increasing number of studies take a network and/or a multivariate pattern analytical approach in identifying differences in dyslexia [e.g., 2], here, we deliberately take a modular perspective and focus on the location of neurobiological anomalies related to dyslexia in order to link findings with etiological theories of dyslexia. We therefore do not include electrophysiological studies, which have higher temporal resolution but typically have lower spatial resolution [for an overview of other behavioral and electroencephalography (EEG) studies see 3,4].
Much of our understanding of the brain basis of dyslexia comes from MRI studies of adults and older school-aged children, which limits the conclusions that can be drawn regarding the origin of the neurobiological differences in dyslexia. Indeed, learning to read is a dynamic process that depends on brain plasticity as well as implicit and explicit learning. Such learning-induced brain plasticity has been demonstrated in studies of non-literate adults who show both functional and structural changes in reading-related brain regions after learning to read [5]. In addition, findings from MRI studies comparing individuals with dyslexia to both age-matched and younger reading level-matched control groups suggest that some of the brain differences observed in dyslexia can be explained by differences in the amount and quality of reading experience [6]. Hence, neurobiological anomalies in dyslexia observed at later stages of reading development (mainly in left temporo-parietal (TP) and occipito-temporal (OT) regions, [7]) may reflect impoverished or reduced reading experience among those with dyslexia [8] rather than a true biological cause of dyslexia.
Recently, advances in MRI technology and the refinement of child-friendly scanning protocols have made it feasible to study the brain in young children prior to reading onset. At least 16 published studies have used MRI to examine cognitive processes associated with reading in pre-readers, including those who are at risk for dyslexia, in several independent cohorts [9–24]. In this review, we discuss and, for the first time, quantify the location and function of the reported differences in reading-related regions and pathways observed in pre-readers. We then interpret these findings vis-à-vis existing etiological theories of dyslexia. These theories vary in their conceptualization of the core deficit in dyslexia, including cognitive-linguistic, perceptual, and meta-cognitive deficit perspectives [for detailed discussion see 25,26,27]. We focus here on neurobiological findings that relate to theories for which behavioral studies have found differences in preliterate children at risk compared to low risk for developing dyslexia: specifically theories associated with phonological, orthographic, and lower-level perceptual/attention deficits.
Dyslexia-related differences in pre-readers
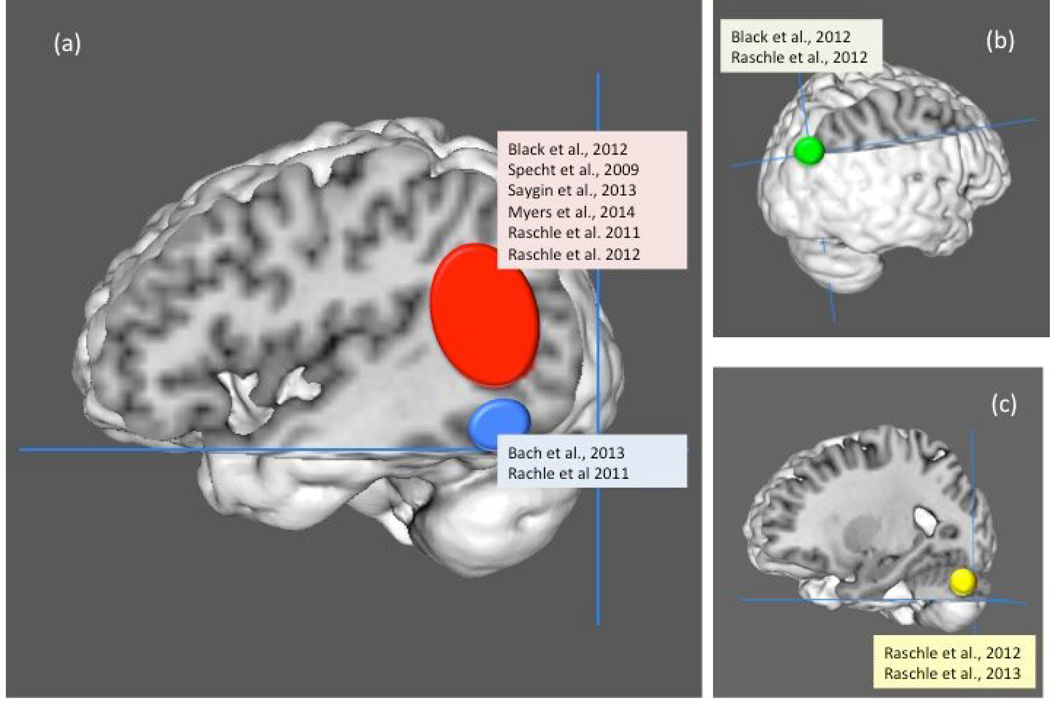
Several studies to date have focused on pre-readers who are at risk for developing dyslexia because of a family member with dyslexia or because of low performance on standardized behavioral measures that are strongly associated with later reading. Though it would be informative to follow these pre-readers until they could be classified as having dyslexia or typical reading abilities, very few studies have taken this approach. Differences between pre-readers with and without risk for dyslexia have been found across a wide variety of different structural and functional MRI measures [10,12–18,21–24]. Differences associated with dyslexia risk are consistently found across studies in four main brain regions (left TP, OT, and cerebellum as well as right parietal, see meta-analysis of prereading studies in Figure 1). These findings of similar anomalies in at risk pre-readers and dyslexic individuals compared to their respective controls provide the first evidence that neurobiological differences observed in adults and children with dyslexia are not purely reading experience-driven, but are more likely related to etiological differences. Other brain regions have also been indicated, though less consistently, across studies. We now turn to examining these findings in greater detail as they relate to theories of the etiology of dyslexia.
Figure 1. Neurobiological differences in pre-readers associated with risk for dyslexia.
Regional differences in pre-readers that are related to familial or behavioral risk for dyslexia, or to later reading outcome revealed by meta-analysis are shown. Only coordinate-based studies are included in this figure (no coordinates are available from references 10, 18, 23, 24). Studies contributing to each cluster are listed in the figure. Analyses were performed in Ginger ALE 2.3. A cluster is identified when reported by multiple studies with a cluster-level significance threshold of p < .01 (10,000 permutations). The red cluster represents the left temporo-parietal (TP) region (center at Montreal Neurological Institute (MNI) coordinates −46, −61, 14, and containing 5 sub-peaks within the cluster), the blue cluster is the left fusiform gyrus in the occipito-temporal (OT) region (−44, −57, −15), the green cluster is in the right parietal lobe (45, −71, 33), and the yellow cluster in the left cerebellum (−26, −79, −27). The circles are drawn proportional to the size of the cluster, which reflects the variability in location within the cluster and not the strength of the effect.
Phonological deficit theory
A deficit in phonological processing, and especially in phonological awareness (i.e. the ability to process and manipulate the sound structure of words), is widely recognized as an underlying cause of dyslexia [25,28]. The phonological deficit theory is supported by behavioral evidence from longitudinal studies showing pre-reading phonological deficits [29], and from phonological training studies yielding improved reading [30].
In adults and school-age children, phonological processing has repeatedly been associated with a left-lateralized network including the TP region (including the supramarginal gyrus, planum temporale, and superior temporal gyrus) as well as inferior and superior frontal regions [31,32, for a meta-analysis see 33]. Anomalies in the left TP region (Figure 1a; shown in red) are consistently observed in dyslexia, including decreased functional activation [34,35] as well as atypical gray matter volume [36] and white matter organization [37]. These robust differences in the left TP region are often interpreted as neural evidence for the phonological deficit theory, yet such a causal interpretation needs validation in pre-readers, especially given the fact that some studies comparing dyslexic readers with reading-matched and age-matched controls have not found differences in this area [38] whereas others have [6].
Several MRI studies in pre-readers at risk for dyslexia also observe different neural organization in left TP regions in these children [10,12–14,16,21,22,24]. Further, gray and white matter volume in left TP regions predict later reading skills [11,39]. Importantly for validation of the phonological deficit theory, left TP anomalies in at-risk pre-readers have been associated with participants’ phonological processing difficulties; functional MRI (fMRI) studies in both English and Norwegian children show that left TP is hypoactivated in at-risk compared to typically-developing pre-readers during a phonological processing task [13] and in beginning readers during an alphabetic decoding reading task [21]. In addition, two independent diffusion MRI studies found that phonological awareness scores were correlated with organization of the left arcuate fasciculus, a white matter tract that connects TP and frontal regions [16,18]. Thus, it may not be only a local anomaly in TP cortex per se, but different functional and structural connectivity between TP and other reading-related regions that give rise to phonological difficulties [40–42].
Together with evidence from fMRI studies of skilled readers [33], these data suggest that the link between phonological processing and left TP seems to be established prior to reading and might remain present throughout reading development. Yet, in contrast to adults, pre-readers seem to recruit a more distributed network of regions for phonological processing which includes left OT and cerebellar as well as right hemisphere areas [14,18,21] (see also Figure 1 panel b and c), although these additional regions seem less consistently impaired in pre-readers at risk for dyslexia. Further, based on the few studies to date that followed at-risk pre-readers and examined whether these differences are markers of which children develop dyslexia, the involvement of TP regions are not fully clear. One study found no significant differences in left TP cortical thickness among pre-readers who later developed dyslexia versus those who did not; significant group differences were observed in other more sensory regions [17]. The number of pre-readers who later developed dyslexia in this study was small (N=7), however [43] Another study found that children with family history of dyslexia differed from controls in the organization of the left arcuate fasciculus; further, the trajectory of left arcuate development over time predicted their later reading ability [24]. In sum, though our meta-analysis identified left TP abnormalities in pre-readers at risk for dyslexia, future studies should further investigate the precise role of this region in reading development.
Orthographic deficit theory
Fluent reading also depends on orthographic processing, i.e. the ability to identify written letter patterns and words as whole units (rather than letter by letter). As orthographic knowledge is chiefly acquired by repeated and successful phonological decoding of words [44], orthographic problems in dyslexics are often considered secondary to a primary phonological deficit. However, some studies show that orthographic processing might be an independent cause of dyslexia, as it predicts word reading ability after controlling for phonological processing [45,46]. In parallel to the left TP region’s involvement in phonological processing, the left ventral OT area, including the fusiform gyrus, plays a key role in orthographic processing in skilled readers [47,48]. In a subsection of the OT region often referred to as the visual word form area (VWFA), activation to words is reduced among individuals with dyslexia relative to controls [49].
In pre-readers and early readers at risk for dyslexia, the left OT region shows reduced gray matter volume [14], different patterns of anatomical folding [23], reduced functional activation [21], and reduced connectivity to frontal regions [18]. Furthermore, among a small sample of pre-readers, activation in the OT region to words and symbols predicted later reading ability beyond the contribution of behavioral and EEG measures [19]. Among children who could read beginning words, this area showed activation for words that must be remembered as wholes because of their irregular spelling pattern, but not for words that could be decoded based on grapheme-phoneme correspondences [21].
Although many studies suggest involvement of the left OT region in orthographic processing, it is not yet clear how specialized this region is for orthographic processing early in reading development. Indeed, studies of pre-readers have found that phonological awareness skills relate to left OT structure [14], function [13] and connectivity patterns [18]. In addition, performing an orthographic task activates regions beyond left OT including bilateral TP, frontal and parietal regions in pre- and early readers [10, 13, 17, 21]. More longitudinal studies, starting at the pre-reading stage, should be conducted to validate whether neural specialization for reading-related cognitive functions is something that is only established after reading acquisition.
Theories based on perceptual and other deficits
Theories based on perceptual deficits generally do not deny that cognitive-linguistic problems characterize dyslexia; however, they assert that these phonological and orthographic deficits are caused, in turn, by lower-level deficits. In pre-readers, perceptual deficit theories have not been frequently investigated using MRI methods. One study [15] found that pre-readers at risk for dyslexia showed hypoactivation in pre-frontal regions during processing of nonlinguistic auditory stimuli with rapid frequency transitions. Activation during the rapid auditory processing task was correlated with phonological awareness, suggesting a link to reading ability through that process. Indirect support for perceptual theories also comes from the aforementioned study that showed reduced cortical thickness in primary auditory and visual areas in a small sample of pre-readers who were later diagnosed with dyslexia [17]; however, no behavioral or functional MRI data were provided in that study to support a direct link with perceptual abilities. Therefore, further investigation of perceptual theories by means of MRI studies in pre-readers is needed in order to confirm whether atypical neural processing of perceptual information is a precursor and cause of dyslexia. Other perceptual theories including deficits in visual-spatial attention [50] and neural coding of auditory stimuli [51] have been validated in pre-readers, but have not yet been investigated using MRI.
In contrast, theories based on other deficits have been tested using MRI in older children and adults, but not yet in pre-readers. Deficits in auditory/temporal sampling, which may account for deficits in reading speed and accuracy in dyslexia, have been observed [52,53]. There is also evidence for the double-deficit hypothesis [54], which suggests that weaknesses in either rapid automatized naming (RAN) or phonological awareness can cause dyslexia, and that those with both deficits are the most severely impaired readers. Functional activation and connectivity patterns in these groups are consistent with this hypothesis [55]. In terms of brain structure, white matter tracts relating to PA have been identified, but no measures of tract organization have yet consistently been associated with RAN [16,18].
Limitations of current evidence in pre-readers
Although the MRI studies of pre-readers reviewed here provide hints about the validity of the different etiological models, interpretation in terms of causal effects is limited by a few shortcomings. First, prenatal and early developmental and environmental effects also shape many of the processes underlying reading and the brain. Efforts to scan infants, for whom these differences are more minimal, are already underway and may provide new insights into these questions. Second, a more comprehensive assessment of parental influences might lead to a better understanding of the genetic and environmental mechanisms underlying dyslexia [56]. For example, it may be the case that parents with dyslexia provide different quality or quantity of reading-related input to their child, though behavioral studies suggest that such differences have a modest effect on behavioral indices of reading and language development [57]. Third, the modulating role of orthographic depth is not examined in the current review due to a small number of MRI studies in pre-readers per language. Although a recent meta-analysis of cognitive studies indicated that differences in phonemic awareness between pre-readers with and without familial risk were not dependent on orthographic depth [57], a meta-analysis on fMRI studies in school-aged children and adults with dyslexia did show a modulating effect in phonological-related regions such as left TP, with dyslexic hypo-activation only present in shallow orthographies [58].
Finally, and most importantly, most MRI studies to date have examined pre-readers at-risk for dyslexia but have not followed up to establish which of these children actually develop dyslexia [but see 17, 24]. (Some longitudinal studies using EEG, which is less expensive and easier to acquire than MRI, have taken this approach [59,60].) It remains therefore to be determined whether the pattern of brain anomalies observed in at-risk pre-readers will be the same in the subset who actually develop dyslexia. At-risk children who become typical readers display subtle problems in phonemic awareness, but these problems are less severe than the deficits among at-risk pre-readers who do develop dyslexia [57]. Therefore, it might be that left TP anomalies, which are typically associated with phonemic awareness, will be more severe in the at-risk pre-readers who eventually develop dyslexia. Longitudinal follow-up of the at-risk pre-readers, allowing classification according to later reading outcome, may significantly improve our understanding of neural risk factors related to dyslexia.
Conclusion
The purpose of this review was to examine whether dyslexia-related anomalies can be detected with MRI prior to reading onset, and whether taking a modular perspective of brain function and identification of the anomalous locations can inform theories on the etiology of dyslexia. The most consistent findings across studies are differences in left TP brain regions between at-risk pre-readers and controls. Because left TP is associated with phonological processing, this provides substantial support for the phonological deficit theory. Differences in left ventral OT regions are also observed prior to reading onset, though less consistently. Finally, some evidence is also provided for an early deficit in perceptual regions, especially in the auditory domain [15,17].
It is important to highlight that several studies indicate early connectivity differences in at-risk pre-readers [10,16,18]. One possibility is that structural connectivity differences give rise to differences in function; a recent study found that even before the VWFA is selective for letters and words in pre-readers, structural connectivity “fingerprints” in kindergarten children of varying reading ability predicted location of the VWFA 2.5 years later [61]. This finding suggests that inefficient communication and coordination between cortical regions prior to reading instruction could shape neural activation during reading. Insufficient functional and structural connectivity among the regions of the reading network may give rise to the complex behavioral deficits seen in dyslexia, such as difficulty with rapid naming and reading fluency.
Given the complexity of reading and variety of profiles observed in dyslexia, considering each etiological theory in isolation may be a too simplistic a view. As multi-componential models suggest, it is very unlikely that a single underlying causal factor drives the heterogeneous patterns of reading difficulties across individuals with dyslexia [62]. Dyslexia is perhaps more accurately conceptualized as a complex interaction of different risk and protective factors, and the weighting of each of these factors can vary across different individuals with dyslexia. It may be that inefficient auditory and phonological neural systems cause reading difficulties in one individual with dyslexia, but another individual may struggle as a result of predominant visual-orthographic integration problems. In addition, genetic, environmental, and meta-cognitive factors can modulate these risks [57].
Educators and clinicians should consider that brain differences in dyslexia are present before children learn to read, and thus that waiting for these problems to resolve on their own is inefficient. However, at the moment, brain measures are insufficiently sensitive and specific at the individual level to aid in early diagnosis. Future studies using MRI and other brain imaging technologies may reveal early, reliable and cost-effective biomarkers of dyslexia. Until then, the most efficient approach is still examination of a combination of early, comprehensive behavioral assessments and demographic information such as family history, followed by intervention that is designed to ameliorate the individual’s particular areas of difficulty.
Highlights.
The core neurobiological cause of dyslexia is still not fully understood.
Brain anomalies in adults with dyslexia might be either a consequence or cause.
This qualitative and quantitative review focuses on MRI studies in pre-readers.
We link the location of brain anomalies in at-risk pre-readers to dyslexia theories.
At-risk pre-readers display reliable left temporo-parietal and occipito-temporal differences.
Early connectivity problems fit with a multifactorial theory of dyslexia.
Acknowledgments
Maaike Vandermosten is a postdoctoral fellow of the Research Foundation Flanders. Fumiko Hoeft was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grants K23HD054720 (PI: F. Hoeft), R01HD078351 (PI: F. Hoeft), R01HD044073 (PI: L. Cutting, Vanderbilt U), R01HD065794 (PI: K. Pugh, Haskins Labs), P01HD001994 (PI: J. Rueckl, Haskins Labs), the National Institute of Mental Health (NIMH) Grants R01MH104438 (PI: C. Wu Nordahl, UC Davis MIND Institute), R01MH103371 (PI: D. Amaral, UC Davis MIND Institute), the National Science Foundation (NSF) Grant NSF1540854 SL-CN (PI: A. Gazzaley), and Oak Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: nothing declared
Contributor Information
Maaike Vandermosten, Email: maaike.vandermosten@ppw.kuleuven.be.
Fumiko Hoeft, Email: fumiko.hoeft@ucsf.edu.
References
- 1.Peterson RL, Pennington BF. Developmental dyslexia. Annu. Rev. Clin. Psychol. 2015;11:283–307. doi: 10.1146/annurev-clinpsy-032814-112842. [DOI] [PubMed] [Google Scholar]
- 2.Cui Z, Xia Z, Su M, Shu H, Gong G. Disrupted white matter connectivity underlying developmental dyslexia: A machine learning approach. Hum. Brain Mapp. 2016;37:1443–1458. doi: 10.1002/hbm.23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozernov-Palchik O, Gaab N. Tackling the “dyslexia paradox”: reading brain and behavior for early markers of developmental dyslexia. WIREs Cogn. Sci. 2016;7:156–176. doi: 10.1002/wcs.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leppänen PHT, Hämäläinen JA, Guttorm TK, Eklund KM, Salminen H, Tanskanen A, Torppa M, Puolakanaho A, Richardson U, Pennala R, et al. Infant brain responses associated with reading-related skills before school and at school age. Neurophysiol. Clin. 2012;42:35–41. doi: 10.1016/j.neucli.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Dehaene S, Cohen L, Morais J, Kolinsky R. Illiterate to literate: behavioural and cerebral changes induced by reading acquisition. Nat. Rev. Neurosci. 2015;16:234–244. doi: 10.1038/nrn3924. [DOI] [PubMed] [Google Scholar]
- 6.Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc. Natl. Acad. Sci. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton ES, Beach SD, Gabrieli JDE. Neurobiology of dyslexia. Curr. Opin. Neurobiol. 2015;30:73–78. doi: 10.1016/j.conb.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham A, Stanovich K. What reading does for the mind. Am. Educ. 1998;22:137–149. [Google Scholar]
- 9.Gimenez P, Bugescu N, Black JM, Hancock R, Pugh K, Nagamine M, Kutner E, Mazaika P, Hendren R, McCandliss BD, et al. Neuroimaging correlates of handwriting quality as children learn to read and write. Front. Hum. Neurosci. 2014;8:155. doi: 10.3389/fnhum.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosseini SMH, Black JM, Soriano T, Bugescu N, Martinez R, Raman MM, Kesler SR, Hoeft F. Topological properties of large-scale structural brain networks in children with familial risk for reading difficulties. Neuroimage. 2013;71:260–274. doi: 10.1016/j.neuroimage.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers CA, Vandermosten M, Farris EA, Hancock R, Gimenez P, Black JM, Casto B, Drahos M, Tumber M, Hendren RL, et al. White matter morphometric changes uniquely predict children’s reading acquisition. Psychol. Sci. 2014;25:1870–1883. doi: 10.1177/0956797614544511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black JM, Tanaka H, Stanley L, Nagamine M, Zakerani N, Thurston A, Kesler S, Hulme C, Lyytinen H, Glover GH, et al. Maternal history of reading difficulty is associated with reduced language-related gray matter in beginning readers. Neuroimage. 2012;59:3021–3032. doi: 10.1016/j.neuroimage.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raschle NM, Zuk J, Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc. Natl. Acad. Sci. U. S. A. 2012;109:2156–2161. doi: 10.1073/pnas.1107721109. By investigating functional networks during phonological processing in pre-readers at risk for dyslexia, the authors demonstrated that dyslexia-related differences in the activation maps during phonological processing are not just a consequence of reading problems, but are present before reading onset.
- 14.Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 2011;57:742–749. doi: 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raschle NM, Stering PL, Meissner SN, Gaab N. Altered neuronal response during rapid auditory processing and its relation to phonological processing in prereading children at familial risk for dyslexia. Cereb. Cortex. 2014;24:2489–2501. doi: 10.1093/cercor/bht104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saygin ZM, Norton ES, Osher DE, Beach SD, Cyr AB, Ozernov-Palchik O, Yendiki A, Fischl B, Gaab N, Gabrieli JDE. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J. Neurosci. 2013;33:13251–13258. doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clark KA, Helland T, Specht K, Narr KL, Manis FR, Toga AW, Hugdahl K. Neuroanatomical precursors of dyslexia identified from pre-reading through to age 11. Brain. 2014;137:3136–3141. doi: 10.1093/brain/awu229. This is one of the only studies that followed pre-readers longitudinally in order to differentiate which children developed dyslexia. Although results should be interpreted with caution due to the small samle size (n=7 pre-readers who developed dyslexia), neuroanatomical anomalies were observed. More specifically, MRI measures of cortical thickness at pre-reading age were significantly different among those who did versus did not develop dyslexia, not in the traditional reading network, but in regions that support lower-level visual and cognitive processing. Differences in the reading network between the groups were only evident after reading acquistion at age 11, suggesting that such differences are driven by differential reading experience.
- 18. Vandermosten M, Vanderauwera J, Theys C, De Vos A, Vanvooren S, Sunaert S, Wouters J, Ghesquière P. A DTI tractography study in pre-readers at risk for dyslexia. Dev. Cogn. Neurosci. 2015;14:8–15. doi: 10.1016/j.dcn.2015.05.006. In a large group of pre-readers (N = 71), diffusion MRI was used to study the links between white matter organization and behavioral and familial markers of dyslexia. The study confirmed associations between the bilateral arcuate fasciculus and phonological processing, and additionally showed early involvement of ventral tracts related to familial risk.
- 19.Bach S, Richardson U, Brandeis D, Martin E, Brem S. Print-specific multimodal brain activation in kindergarten improves prediction of reading skills in second grade. Neuroimage. 2013;82:605–615. doi: 10.1016/j.neuroimage.2013.05.062. [DOI] [PubMed] [Google Scholar]
- 20.Brem S, Bach S, Kucian K, Kujala JV, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U. Brain sensitivity to print emerges when children learn letter–speech sound correspondences. Proc. Natl. Acad. Sci. 2010;107:7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Specht K, Hugdahl K, Ofte S, Nygård M, Bjørnerud A, Plante E, Helland T. Brain activation on pre-reading tasks reveals at-risk status for dyslexia in 6-year-old children. Scand. J. Psychol. 2009;50:79–91. doi: 10.1111/j.1467-9450.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- 22.Yamada Y, Stevens C, Dow M, Harn BA, Chard DJ, Neville HJ. Emergence of the neural network for reading in five-year-old beginning readers of different levels of pre-literacy abilities: an fMRI study. Neuroimage. 2011;57:704–713. doi: 10.1016/j.neuroimage.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Im K, Pienaar R, Paldino MJ, Gaab N, Galaburda AM, Grant PE. QAtypical sulcal pattern in children with developmental dyslexia and at-risk kindergartners. Cereb. Cortex. 2016;26:1138–1148. doi: 10.1093/cercor/bhu305. Here, authors examined the sulcal folding patterns of the brain, which are thought to reflect very early, genetically-based influences on brain development. This has been a less common technique employed in studies of reading. Nonetheless, the authors found that children with dyslexia and at-risk preschoolers had more sulcal folds that were smaller in size. These anatomical differences may result in different patterns of activation or connectivity.
- 24. Wang Y, Mauer MV, Raney T, Peysakhovich B, Becker BLC, Sliva DD, Gaab N. Development of tract-specific white matter pathways during early reading development in at-risk children and typical controls. Cereb. Cortex. 2016 doi: 10.1093/cercor/bhw095. This cross-sectional and longitudinal study examined relations between white matter structure in the reading network and reading ability. At the pre-reading stage, the arcuate fasciculus differed between children with versus without family history of dyslexia. The trajectory of white matter development also helped predict children's eventual reading outcome, providing further evidence for the important role of white matter in reading.
- 25.Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): what have we learned in the past four decades? J. Child Psychol. Psychiatry. 2004;45:2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- 26.Giraud A-L, Ramus F. Neurogenetics and auditory processing in developmental dyslexia. Curr. Opin. Neurobiol. 2013;23:37–42. doi: 10.1016/j.conb.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Goswami U. Sensory theories of developmental dyslexia: three challenges for research. Nat. Rev. Neurosci. 2015;16:43–54. doi: 10.1038/nrn3836. [DOI] [PubMed] [Google Scholar]
- 28.Hulme C, Snowling MJ. Learning to read: What we know and what we need to understand better. Child Dev. Perspect. 2015;7:1–5. doi: 10.1111/cdep.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dandache S, Wouters J, Ghesquière P. Development of reading and phonological skills of children at family risk for dyslexia: a longitudinal analysis from kindergarten to sixth grade. Dyslexia. 2014;20:305–329. doi: 10.1002/dys.1482. [DOI] [PubMed] [Google Scholar]
- 30.Duff FJ, Hayiou-Thomas ME, Hulme C. Evaluating the effectiveness of a phonologically based reading intervention for struggling readers with varying language profiles. Read. Writ. 2011;25:621–640. [Google Scholar]
- 31.Price CJ, Moore CJ, Humphreys GW, Wise RJ. Segregating semantic from phonological processes during reading. J. Cogn. Neurosci. 1997;9:727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- 32.Kovelman I, Norton ES, Christodoulou JA, Gaab N, Lieberman DA, Triantafyllou C, Wolf M, Whitfield-Gabrieli S, Gabrieli JDE. Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cereb. Cortex. 2012;22:754–764. doi: 10.1093/cercor/bhr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Ann. N. Y. Acad. Sci. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- 36.Richlan F, Kronbichler M, Wimmer H. Structural abnormalities in the dyslexic brain: a meta-analysis of voxel-based morphometry studies. Hum. Brain Mapp. 2013;34:3055–3065. doi: 10.1002/hbm.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandermosten M, Boets B, Wouters J, Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci. Biobehav. Rev. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Krafnick AJ, Flowers DL, Luetje MM, Napoliello EM, Eden GF. An investigation into the origin of anatomical differences in dyslexia. J. Neurosci. 2014;34:901–908. doi: 10.1523/JNEUROSCI.2092-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linkersdörfer J, Jurcoane A, Lindberg S, Kaiser J, Hasselhorn M, Fiebach CJ, Lonnemann J. The association between gray matter volume and reading proficiency: a longitudinal study of beginning readers. J. Cogn. Neurosci. 2015;27:308–318. doi: 10.1162/jocn_a_00710. [DOI] [PubMed] [Google Scholar]
- 40.Ramus F. Neuroimaging sheds new light on the phonological deficit in dyslexia. Trends Cogn. Sci. 2014;18:274–275. doi: 10.1016/j.tics.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 41. Boets B, Op de Beeck HP, Vandermosten M, Scott SK, Gillebert CR, Mantini D, Bulthé J, Sunaert S, Wouters J, Ghesquière P. Intact but less accessible phonetic representations in adults with dyslexia. Science. 2013;342:1251–1254. doi: 10.1126/science.1244333. The authors used machine-learning analyses of fMRI data to identify brain regions where phonetic representations are less distinctively represented in adults with dyslexia. Functional alterations were not found in the regions hosting these representations, but structural and functional connectivity analyses showed that they were less well connected. This emphasizes the need to consider dyslexia-related brain anomalies using multimodal and connectivity approaches.
- 42.Boets B. Dyslexia: reconciling controversies within an integrative developmental perspective. Trends Cogn. Sci. 2014;18:501–503. doi: 10.1016/j.tics.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Kraft I, Cafiero R, Schaadt G, Brauer J, Neef NE, Müller B, Kirsten H, Wilcke A, Boltze J, Friederici AD, et al. Cortical differences in preliterate children at familiar risk of dyslexia are similar to those observed in dyslexic readers. Brain. 2015;138:e378. doi: 10.1093/brain/awv036. [DOI] [PubMed] [Google Scholar]
- 44.Share DL. Phonological recoding and self-teaching: sine qua non of reading acquisition. Cognition. 1995;55:151–218. doi: 10.1016/0010-0277(94)00645-2. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham A, Perry K, Stanovich K. Converging evidence for the concept of orthographic processing. Read. Writ. 2001;14:549–568. [Google Scholar]
- 46.Morfidi E, van der Leij A, de Jong PF, Scheltinga F, Bekebrede J. Reading in two orthographies: A cross-linguistic study of Dutch average and poor readers who learn English as a second language. Read. Writ. 2006;20:753–784. [Google Scholar]
- 47.McCandliss BD, Noble KG. The development of reading impairment: a cognitive neuroscience model. Ment. Retard. Dev. Disabil. Res. Rev. 2003;9:196–204. doi: 10.1002/mrdd.10080. [DOI] [PubMed] [Google Scholar]
- 48.Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annu. Rev. Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- 49.Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends Cogn. Sci. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Franceschini S, Gori S, Ruffino M, Pedrolli K, Facoetti A. A causal link between visual spatial attention and reading acquisition. Curr. Biol. 2012;22:814–819. doi: 10.1016/j.cub.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 51.White-Schwoch T, Kraus N. Physiologic discrimination of stop consonants relates to phonological skills in pre-readers: a biomarker for subsequent reading ability? Front. Hum. Neurosci. 2013;7:899. doi: 10.3389/fnhum.2013.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehongre K, Morillon B, Giraud A-L, Ramus F. Impaired auditory sampling in dyslexia: further evidence from combined fMRI and EEG. Front. Hum. Neurosci. 2013;7:454. doi: 10.3389/fnhum.2013.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goswami U, Power AJ, Lallier M, Facoetti A. Oscillatory “temporal sampling” and developmental dyslexia: toward an over-arching theoretical framework. Front. Hum. Neurosci. 2014;8:904. doi: 10.3389/fnhum.2014.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolf M, Bowers PG. The double-deficit hypothesis for the developmental dyslexias. J. Educ. Psychol. 1999;91:415–438. [Google Scholar]
- 55.Norton ES, Black JM, Stanley LM, Tanaka H, Gabrieli JDE, Sawyer C, Hoeft F. Functional neuroanatomical evidence for the double-deficit hypothesis of developmental dyslexia. Neuropsychologia. 2014;61:235–246. doi: 10.1016/j.neuropsychologia.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Bergen E, van der Leij A, de Jong PF. The intergenerational multiple deficit model and the case of dyslexia. Front. Hum. Neurosci. 2014;8:346. doi: 10.3389/fnhum.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snowling MJ, Melby-Lervåg M. Oral language deficits in familial dyslexia: a meta-analysis and review. Psychol. Bull. 2016 doi: 10.1037/bul0000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin A, Kronbichler M, Richlan F. Dyslexic brain activation abnormalities in deep and shallow orthographies: A meta-analysis of 28 functional neuroimaging studies. Hum. Brain Mapp. 2016 doi: 10.1002/hbm.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Zuijen TL, Plakas A, Maassen BAM, Maurits NM, van der Leij A. Infant ERPs separate children at risk of dyslexia who become good readers from those who become poor readers. Dev. Sci. 2013;16:554–563. doi: 10.1111/desc.12049. [DOI] [PubMed] [Google Scholar]
- 60.Leppänen PHT, Hämäläinen JA, Salminen HK, Eklund KM, Guttorm TK, Lohvansuu K, Puolakanaho A, Lyytinen H. Newborn brain event-related potentials revealing atypical processing of sound frequency and the subsequent association with later literacy skills in children with familial dyslexia. Cortex. 2010;46:1362–1376. doi: 10.1016/j.cortex.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Saygin ZM, Osher D, Norton ES, Youssoufian D, Beach SD, Feather J, Gaab N, Gabrieli JDE, Kanwisher N. Connectivity precedes function in the development of the visual word form area. Nat. Neurosci. 2016 doi: 10.1038/nn.4354. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pennington BF, Santerre-Lemmon L, Rosenberg J, MacDonald B, Boada R, Friend A, Leopold DR, Samuelsson S, Byrne B, Willcutt EG, Olson RK. Individual prediction of dyslexia by single versus multiple deficit models. J Abnorm Psychol. 2012;121(1):212–224. doi: 10.1037/a0025823. Here, authors examined behavioral deficits in two large samples of twins. Analyses revealed that approximately equal proportions of individuals with dyslexia were best characterized as fitting with existing single deficit versus multiple deficit models. A large group, approximately 40%, did not fit with any existing models, suggesting the need for further refinement of our theories of dyslexia.