Abstract
BACKGROUND
Current treatment guidelines recommend adjuvant mitotane after resection of adrenocortical carcinoma with high-risk features (eg, tumor rupture, positive margins, positive lymph nodes, high grade, elevated mitotic index, and advanced stage). Limited data exist on the outcomes associated with these practice guidelines.
STUDY DESIGN
Patients who underwent resection of adrenocortical carcinoma from 1993 to 2014 at the 13 academic institutions of the US Adrenocortical Carcinoma Group were included. Factors associated with mitotane administration were determined. Primary end points were recurrence-free survival (RFS) and overall survival (OS).
RESULTS
Of 207 patients, 88 (43%) received adjuvant mitotane. Receipt of mitotane was associated with hormonal secretion (58% vs 32%; p = 0.001), advanced TNM stage (stage IV: 42% vs 23%; p = 0.021), adjuvant chemotherapy (37% vs 5%; p < 0.001), and adjuvant radiation (17% vs 5%; p = 0.01), but was not associated with tumor rupture, margin status, or N-stage. Median follow-up was 44 months. Adjuvant mitotane was associated with decreased RFS (10.0 vs 27.9 months; p = 0.007) and OS (31.7 vs 58.9 months; p = 0.006). On multivariable analysis, mitotane was not independently associated with RFS or OS, and margin status, advanced TNM stage, and receipt of chemotherapy were associated with survival. After excluding all patients who received chemotherapy, adjuvant mitotane remained associated with decreased RFS and similar OS; multivariable analyses again showed no association with recurrence or survival. Stage-specific analyses in both cohorts revealed no association between adjuvant mitotane and improved RFS or OS.
CONCLUSIONS
When accounting for stage and adverse tumor and treatment-related factors, adjuvant mitotane after resection of adrenocortical carcinoma is not associated with improved RFS or OS. Current guidelines should be revisited and prospective trials are needed.
Adrenocortical carcinoma (ACC) is an uncommon malignancy with an estimated incidence of only 0.72 cases per million people per year in the United States.1 Complete resection represents the only potential for cure, with a 5-year survival rate of only 5% in patients not undergoing curative resection.2,3 Yet even after resection of ACC, 5-year survival rates remain poor, ranging from 39% to 55%.2,4 During the span of 2 decades, these bleak outcomes have not improved.4,5 There are limited data suggesting a role for radiation therapy or cytotoxic chemotherapy in the treatment of resectable ACC; however, there is undoubtedly a need for effective adjuvant therapy in select surgical patients.6,7
One such potential therapy is mitotane (also known as dichlorodiphenildichloroethane or o,p’DDD), a close relative of the pesticide dichlorodiphenyltrichloroethane (DDT). The potential therapeutic effects of mitotane were first appreciated in 1949, when Nelson and colleagues8 reported that mitotane caused cytotoxicity and atrophy of the adrenal cortex in a canine model. In 1960, Bergenstal and colleagues9 were the first to apply these findings clinically in a patient with metastatic ACC, reporting regression of metastatic disease. Subsequent reports have supported the role of mitotane in the treatment of unresectable ACC10; however, data on the use of mitotane in the adjuvant setting have been conflicting.3,11–13 Given the rarity of ACC, randomized prospective trials evaluating adjuvant mitotane are nonexistent, and most retrospective studies are limited by small sample size and/or single-institution bias.
The 2015 National Comprehensive Cancer Network guidelines14 recommend consideration of the use of adjuvant mitotane in the setting of high-risk disease: increased tumor size, positive margins, high grade, and capsular rupture. The guidelines themselves, however, specify that this recommendation is based on category 3 evidence only, suggesting that the role of mitotane in this setting might only be palliative through control of hormonal symptoms rather than preventative of tumor recurrence.
The data supporting these guidelines are limited, and treatment with mitotane does not come without risk. Toxicities are common and include lethargy, somnolence, vertigo, parasthesias, anorexia, nausea, vomiting, hormonal dysregulation, and skin changes.15–18 Additionally, mitotane affects hepatic metabolism of other drugs.19 As this treatment is not benign, additional understanding of its value is needed. Therefore, we sought to determine the relationship of the use of adjuvant mitotane with recurrence-free survival (RFS) and overall survival (OS) in a multi-institutional study of a US population.
METHODS
Patient population
Thirteen academic institutions comprise the US Adrenocortical Carcinoma Group: Emory University, Stanford University, The Johns Hopkins University, Medical College of Wisconsin, New York University, The Ohio State University, Washington University in St Louis, University of Wisconsin, University of California San Diego, University of Texas Southwestern, University of California San Francisco, Vanderbilt University, and Wake Forest University. The IRBs at all participating centers approved this study. This collaboration retrospectively identified all patients who underwent resection of ACC from 1993 to 2014 at each institution. Demographic, pathologic, and clinical data were collected through review of the medical record. The TNM pathologic staging was based on the 7th edition of the American Joint Committee on Cancer guidelines.20 Postoperative complications were defined and scored by the Clavien-Dindo criteria.21 Survival data were determined by chart review and confirmed through review of the Social Security Death Index database.
From this population (n = 265), only patients with data on receipt of mitotane were included (n = 211). From these, 30-day mortalities (n = 1) and patients who received neoadjuvant mitotane were excluded (n = 3) resulting in a study population of 207 patients. For analyses of recurrence, patients with a grossly positive margin (R2) or unknown margin status were excluded (n = 33), leaving only patients who had a curative-intent resection. Adjuvant mitotane therapy was defined as receipt of mitotane in the postoperative period as a planned postsurgical therapy, not including delivery of mitotane to treat known recurrence or progression of disease.
Statistical analysis
Statistical analyses were conducted using SPSS Statistics 21.0 (IBM Corp). A p value <0.05 was considered statistically significant. Patients who did and did not receive adjuvant mitotane were compared using chi-square or Fisher’s exact tests and independent t-tests for categorical and continuous variables, respectively. Univariate binary logistic regression was conducted to determine factors associated with delivery of adjuvant mitotane. Variables that had a significant relationship on univariate analysis were included in the multivariable model. Univariate survival analyses were conducted by Kaplan-Meier log-rank tests and Cox regression. Multivariable Cox proportional hazard models were constructed to include mitotane therapy and variables that were significantly associated with survival on univariate analysis. Survival analyses were conducted in all patients and in a subgroup of patients that excluded patients who received cytotoxic chemotherapy instead of or in combination with mitotane. Additional subgroup survival analyses were conducted stratified by TNM stage, T stage, N stage, M stage, tumor hormonal secretion, resection margin, and intraoperative tumor rupture. The primary aim was to determine the relationship of receipt of adjuvant mitotane with RFS and OS.
RESULTS
Patient population and adjuvant mitotane therapy
Two hundred and seven patients were included and are described in Table 1. Of these, 88 (43%) patients received adjuvant mitotane. Mitotane plasma levels were available in 32 of these patients; 15 had serum levels 14 to 20 mg/L, and in 17 patients, the level did not reach 14 mg/L. Median treatment course was 6 months (range 1 to 48 months). Receipt of mitotane was associated with tumor hormonal secretion (58% vs 32%; p = 0.001), advanced TNM stage (stage IV: 42% vs 23%; p = 0.02), delivery of adjuvant chemotherapy (37% vs 5%; p < 0.001), and adjuvant radiation therapy (17% vs 5%; p = 0.01). Adjuvant mitotane was not associated with tumor rupture, margin status, or N-stage; detailed description and comparison of these 2 cohorts are available in Table 1. On univariate binary logistic regression, tumor hormone secretion (odds ratio = 2.9; 95% CI, 1.6–5.3; p < 0.001) and advanced TNM stage (stage IV: odds ratio = 1.6; 95% CI, 1.3–31.7; p = 0.03) were associated with delivery of adjuvant mitotane therapy, and in multivariable analysis, tumor hormone secretion persisted as an independent factor for receiving mitotane treatment (odds ratio = 2.4; 95% CI, 1.29–4.54; p = 0.01; Table 2).
Table 1.
Clinicopathologic and Treatment Factors of Patients Stratified by Receipt of Adjuvant Mitotane
| Variable | All patients (n = 207) | No mitotane (n = 119) | Mitotane (n = 88) | p Value |
|---|---|---|---|---|
| Male | 80 (39) | 45 (38) | 35 (39) | 0.89 |
| Age, y, mean ± SD | 51.3 ± 15.2 | 52.8 ± 14.9 | 49.3 ± 15.5 | 0.10 |
| BMI, kg/m2, mean ± SD | 29.0 ± 8.2 | 29.6 ± 9.2 | 28.3 ± 7.0 | 0.34 |
| ASA class | ||||
| 1 | 30 (21) | 16 (21) | 14 (21) | |
| 2 | 33 (23) | 17 (22) | 16 (24) | |
| 3 | 68 (48) | 36 (47) | 32 (49) | |
| 4 | 12 (8) | 8 (10) | 4 (6) | 0.83 |
| Hormone secretion | 83 (43) | 35 (32) | 48 (58) | 0.001* |
| Familial syndrome | 4 (2) | 2 (2) | 2 (3) | 1.00 |
| Minimally invasive procedure | 38 (19) | 22 (19) | 16 (19) | 0.93 |
| Additional organ resection | 94 (48) | 47 (42) | 47 (55) | 0.08 |
| Intraoperative tumor rupture | 19 (11) | 11 (11) | 8 (10) | 1.00 |
| Tumor size, cm, mean ± SD | 12.1 ± 5.7 | 11.7 ± 5.3 | 12.7 ± 6.1 | 0.24 |
| T1 | 11 (6) | 9 (8) | 2 (3) | 0.17 |
| T2 | 77 (41) | 48 (44) | 29 (36) | |
| T3 | 74 (39) | 39 (36) | 35 (42) | |
| T4 | 27 (14) | 13 (12) | 14 (18) | |
| N1 | 22 (35) | 9 (26) | 13 (46) | 0.15 |
| M1 | 35 (17) | 11 (9) | 24 (28) | 0.001* |
| Stage | ||||
| I | 11 (6) | 9 (8) | 2 (2) | |
| II | 63 (33) | 41 (37) | 22 (27) | |
| III | 59 (31) | 35 (32) | 24 (29) | |
| IV | 59 (31) | 25 (23) | 34 (42) | 0.02* |
| R0 | 127 (69) | 76 (72) | 51 (65) | 0.58 |
| R1 | 47 (26) | 24 (23) | 23 (30) | |
| R2 | 10 (5) | 6 (6) | 4 (5) | |
| Postoperative adrenal insufficiency | 43 (25) | 17 (18) | 26 (34) | 0.02* |
| Complication | 91 (56) | 47 (52) | 44 (60) | 0.44 |
| Adjuvant radiation | 18 (10) | 5 (5) | 13 (17) | 0.01* |
| Adjuvant chemotherapy | 38 (19) | 6 (5) | 32 (37) | <0.001* |
| Death | 94 (46) | 53 (45) | 41 (47) | 0.86 |
| Recurrence | 97 (59) | 53 (55) | 44 (64) | 0.43 |
| Local recurrence | 45 (28) | 24 (26) | 21 (30) | 0.61 |
| Distant recurrence | 69 (42) | 35 (37) | 34 (49) | 0.17 |
Values are n (%) unless otherwise noted.
Significant.
ASA, American Society of Anesthesiologists; BMI, body mass index; R0, microscopically negative margin; R1, microscopically positive margin; R2, grossly positive margin.
Table 2.
Predictors of Administration of Adjuvant Mitotane: Binary Logistic Regression
| Variable | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Male | 1.1 | 0.6–1.9 | 0.78 | |||
| Age, y | 1.0 | 1.0–1.0 | 0.10 | |||
| BMI, kg/m2 | 1.0 | 0.9–1.0 | 0.34 | |||
| ASA class | ||||||
| 1 | 1.0 | Ref | Ref | |||
| 2 | 1.1 | 0.4–2.9 | 0.89 | |||
| 3 | 1.0 | 0.4–2.4 | 0.97 | |||
| 4 | 0.6 | 0.1–2.3 | 0.43 | |||
| Hormone secretion | 2.9 | 1.6–5.3 | <0.001 | 2.4 | 1.3–4.5 | 0.01* |
| Familial syndrome | 1.5 | 0.2–10.6 | 0.71 | |||
| Minimally invasive procedure | 1.0 | 0.5–2.0 | 0.93 | |||
| Additional organ resection | 1.7 | 1.0–3.1 | 0.06 | |||
| Intraoperative tumor rupture | 1.0 | 0.4–2.6 | 0.97 | |||
| Tumor size, cm | 1.0 | 1.0–1.1 | 0.24 | |||
| Stage | ||||||
| I | 1.0 | Ref | Ref | 1.0 | Ref | Ref |
| II | 2.4 | 0.5–12.2 | 0.29 | 2.2 | 0.4–11.7 | 0.35 |
| III | 3.1 | 0.6–15.6 | 0.17 | 2.7 | 0.5–14.4 | 0.24 |
| IV | 6.3 | 1.3–31.7 | 0.03 | 4.8 | 0.9–25.3 | 0.07 |
| R0 | 1.0 | Ref | Ref | |||
| R1 | 1.4 | 0.7–2.8 | 0.30 | |||
| R2 | 1.0 | 0.3–3.7 | 0.99 | |||
| Complication | 1.3 | 0.7–2.5 | 0.35 | |||
Significant.
ASA, American Society of Anesthesiologists; BMI, body mass index; OR, odds ratio; R0, microscopically negative margin; R1, microscopically positive margin; R2, grossly positive margin; Ref, reference.
Survival, recurrence, and adjuvant mitotane therapy
Median follow-up for survivors was 44 months. For patients who underwent curative-intent resections with recurrence data available (n = 164), there were 97 (59%) patients who had a recurrence, which included 45 (28%) local recurrences and 69 (42%) distant recurrences. There was no difference in recurrence rates or patterns of recurrence between patients who did and did not receive mitotane (Table 1). In the entire cohort during the follow-up period, there were 94 (46%) deaths.
Recurrence-free survival and overall survival: all patients
Delivery of adjuvant mitotane was associated with decreased RFS (10.0 vs 27.9 months; p = 0.01; Fig. 1A); however, on multivariable analysis accounting for other factors associated with RFS, only advanced TNM stage remained independently associated with decreased RFS, and mitotane did not (Table 3). Similarly, on univariate analysis, adjuvant mitotane was associated with decreased OS (31.7 vs 58.9 months; p = 0.01; Fig. 1B); however, on multivariable analysis, mitotane was not independently associated with OS. Factors that were independently associated with OS included margin status, advanced TNM stage, and receipt of chemotherapy (Table 3).
Figure 1.
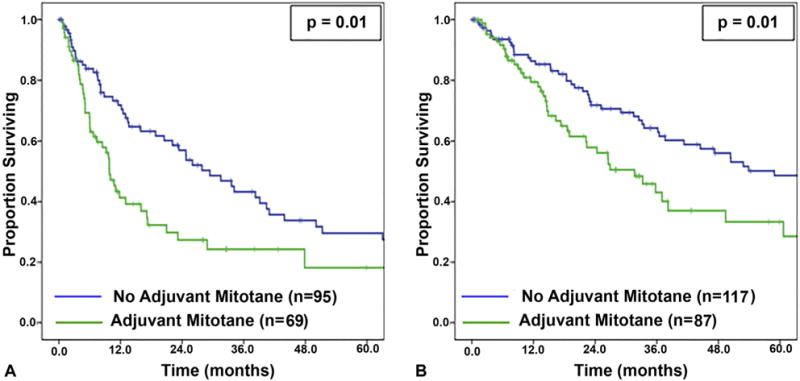
(A) Recurrence-free survival and (B) overall survival stratified by receipt of mitotane therapy for all patients.
Table 3.
Factors Associated with Recurrence-Free and Overall Survival in All Patients
| Variable | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Recurrence-free survival | ||||||
| Age, y | 1.0 | 1.0–1.0 | 0.22 | |||
| Hormone secretion | 1.6 | 1.1–2.4 | 0.03* | 1.3 | 0.8–2.0 | 0.31 |
| Familial syndrome | 0.4 | 0.1–3.0 | 0.39 | |||
| Intraoperative tumor rupture | 1.6 | 0.8–3.0 | 0.16 | |||
| Stage | ||||||
| I | 1.0 | Ref | Ref | 1.0 | Ref | Ref |
| II | 1.4 | 0.5–4.2 | 0.50 | 1.5 | 0.4–5.0 | 0.53 |
| III | 2.8 | 1.0–7.8 | 0.06 | 2.7 | 0.8–9.1 | 0.10 |
| IV | 4.8 | 1.7–13.9 | 0.004* | 4.0 | 1.2–14.0 | 0.03* |
| R1 | 0.7 | 0.4–1.2 | 0.14 | |||
| Postoperative adrenal insufficiency | 1.3 | 0.8–2.1 | 0.34 | |||
| Complication | 1.1 | 0.7–1.7 | 0.65 | |||
| Adjuvant radiation | 1.1 | 0.6–2.2 | 0.78 | |||
| Adjuvant chemotherapy | 1.4 | 0.8–2.4 | 0.19 | |||
| Mitotane | 1.8 | 1.2–2.7 | 0.01* | 1.4 | 0.9–2.2 | 0.21 |
| Overall survival | ||||||
| Age, y | 1.0 | 1.0–1.0 | 0.79 | |||
| Hormone secretion | 1.7 | 1.1–2.6 | 0.01* | 2.3 | 1.2–4.3 | 0.01* |
| Familial syndrome | 0.9 | 0.2–3.8 | 0.91 | |||
| Intraoperative tumor rupture | 1.6 | 0.8–3.0 | 0.20 | |||
| Stage | ||||||
| I | 1.0 | Ref | Ref | 1.0 | Ref | Ref |
| II | 3.7 | 0.5–21.8 | 0.21 | 1.8 | 0.2–14.3 | 0.59 |
| III | 6.4 | 0.8–49.5 | 0.08 | 1.6 | 0.2–13.2 | 0.65 |
| IV | 17.3 | 2.3–132.3 | 0.01 | 7.9 | 1.0–63.9 | 0.05 |
| R0 | 1.0 | Ref | Ref | 1.0 | Ref | Ref |
| R1 | 2.8 | 1.8–4.6 | <0.001* | 2.8 | 1.5–5.4 | 0.002* |
| R2 | 4.3 | 2.0–9.3 | <0.001* | 4.4 | 1.3–14.5 | 0.02* |
| Postoperative adrenal insufficiency | 1.5 | 0.9–2.5 | 0.17 | |||
| Complication | 1.8 | 1.1–2.9 | 0.03* | 0.9 | 0.5–1.7 | 0.70 |
| Adjuvant radiation | 1.1 | 0.5–2.5 | 0.76 | |||
| Adjuvant chemotherapy | 1.7 | 1.0–2.8 | 0.048* | 0.4 | 0.2–0.9 | 0.03* |
| Mitotane | 1.8 | 1.2–2.8 | 0.01* | 0.88 | 0.5–1.6 | 0.68 |
Significant.
HR, hazard ratio; R0, microscopically negative margin; R1, microscopically positive margin; R2, grossly positive margin; Ref, reference.
Recurrence-free survival and overall survival: subgroup analyses
When the 38 patients who received adjuvant systemic therapy instead of or in addition to mitotane were excluded, adjuvant mitotane remained associated with decreased RFS (9.8 vs 29.4 months; p = 0.01; Fig. 2A), but this association again did not persist on multivariable analysis (Table 4). In this cohort, mitotane was not associated with OS on univariate analyses (38.2 vs 58.9 months; p = 0.12; Fig. 2B) or on multivariable (Table 4) analyses. Similarly, after also excluding patients who received radiation therapy from this subgroup (n = 15), such that mitotane was the only adjuvant therapy given, mitotane again was associated with decreased RFS (9.8 vs 31.5 months; p = 0.002) and was not associated with OS (33.2 vs 58.9 months; p = 0.06).
Figure 2.
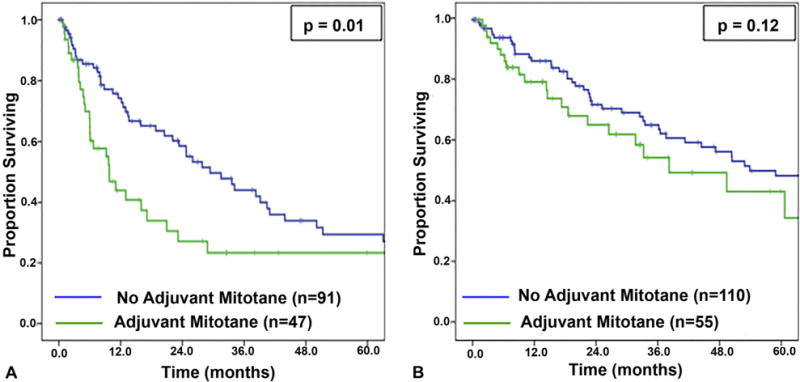
(A) Recurrence-free survival and (B) overall survival stratified by receipt of mitotane therapy, excluding patients who received systemic therapies other than mitotane.
Table 4.
Factors Associated with Recurrence-Free and Overall Survival in Patients Who Did Not Receive Adjuvant Cytotoxic Chemotherapy
| Variable | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | Variable | HR | 95% CI | Variable | |
| Recurrence-free survival | ||||||
| Age, y | 1.0 | 1.0–1.0 | 0.34 | |||
| Hormone secretion | 1.8 | 1.2–2.9 | 0.01* | 1.5 | 0.9–2.5 | 0.10 |
| Familial syndrome | 0.5 | 0.6–3.3 | 0.43 | |||
| Intraoperative tumor rupture | 1.1 | 0.6–2.7 | 0.53 | |||
| Stage | ||||||
| I | 1.0 | Ref | Ref | 1.0 | Ref | Ref |
| II | 1.4 | 0.5–4.1 | 0.55 | 1.4 | 0.4–4.6 | 0.64 |
| III | 2.7 | 0.9–7.8 | 0.07 | 2.6 | 0.8–8.6 | 0.13 |
| IV | 11.2 | 3.6–35.3 | <0.001* | 9.2 | 2.5–34.2 | 0.001* |
| R1 | 1.6 | 0.9–2.7 | 0.11 | |||
| Postoperative adrenal insufficiency | 1.6 | 0.9–2.8 | 0.09 | |||
| Complication | 1.4 | 0.8–2.3 | 0.23 | |||
| Adjuvant radiation | 1.3 | 0.6–2.8 | 0.56 | |||
| Adjuvant chemotherapy | 1.9 | 1.2–3.0 | 0.01* | 1.4 | 0.8–2.4 | 0.25 |
| Overall survival | ||||||
| Age, y | 1.0 | 1.0–1.0 | 0.50 | |||
| Hormone secretion | 1.8 | 1.1–2.9 | 0.02* | 2.2 | 1.1–4.4 | 0.02* |
| Familial syndrome | 0.6 | 0.1–4.0 | 0.56 | |||
| Intraoperative tumor rupture | 1.7 | 0.8–3.7 | 0.15 | |||
| Stage | ||||||
| I | 1.0 | Ref | Ref | 1.0 | Ref | Ref |
| II | 3.7 | 0.5–29.0 | 0.21 | 1.9 | 0.2–15.2 | 0.56 |
| III | 6.1 | 0.8–47.8 | 0.08 | 1.5 | 0.2–12.6 | 0.70 |
| IV | 29.8 | 3.8–234.1 | 0.001* | 10.6 | 1.3–86.7 | 0.03* |
| R0 | 1.0 | Ref | Ref | 1.0 | Ref | Ref |
| R1 | 3.6 | 2.0–6.0 | <0.001* | 3.8 | 1.8–8.2 | 0.001* |
| R2 | 6.0 | 2.5–14.5 | <0.001* | 4.5 | 1.3–15.4 | 0.001* |
| Postoperative adrenal insufficiency | 1.6 | 0.8–2.9 | 0.16 | |||
| Complication | 2.1 | 1.2–3.7 | 0.01* | 1.1 | 0.5–2.2 | 0.91 |
| Adjuvant radiation | 1.1 | 0.5–2.9 | 0.79 | |||
| Adjuvant chemotherapy | 1.5 | 0.9–2.6 | 0.12 | 0.7 | 0.3–1.5 | 0.71 |
Significant.
HR, hazard ratio; R0, microscopically negative margin; R1, microscopically positive margin; R2, grossly positive margin; Ref, reference.
Subgroup analysis of patients stratified by TNM stage, T stage, N stage, M stage, tumor hormonal secretion, resection margin, and tumor grade revealed that adjuvant mitotane was not associated with improved RFS or OS in either high-risk or low-risk subgroups. This finding persisted when excluding patients who received cytotoxic chemotherapy (Table 5).
Table 5.
Relationship Between Mitotane and Survival in Subset Analyses
| Subgroup | All patients | Patients who did not receive chemotherapy | ||||
|---|---|---|---|---|---|---|
| No mitotane, mo | Mitotane, mo | p Value | No mitotane, mo | Mitotane, mo | p Value | |
| Recurrence-free survival | ||||||
| Stage | ||||||
| I* | — | — | — | — | — | — |
| II | 41.0 | 11.8 | 0.08 | 41.0 | 28.9 | 0.17 |
| II | 24.7 | 10.0 | 0.08 | 24.7 | 16.0 | 0.27 |
| IV | 7.6 | 7.4 | 0.80 | 8.1 | 5.1 | 0.87 |
| T1* | — | — | — | — | — | — |
| T2 | 40.4 | 28.9 | 0.33 | 41.0 | 28.9 | 0.15 |
| T3 | 20.5 | 9.8 | 0.10y | 24.7 | 11.0 | 0.03† |
| T4 | 8.1 | 8.4 | 0.84 | 3.1 | 6.0 | 0.79 |
| N0 | 39.1 | 8.4 | 0.004† | 39.1 | 16.0 | 0.11 |
| N1 | 7.9 | 13.0 | 0.48 | 7.9 | 13.0 | 0.80 |
| M0 | 29.4 | 11.8 | 0.03† | 29.4 | 13.0 | 0.11 |
| M1* | 7.6 | 7.4 | 0.33 | — | — | — |
| No hormone secretion | 34.1 | 17.2 | 0.25 | 34.1 | MNR | 0.91 |
| Hormone secretion | 22.1 | 7.4 | 0.07 | 24.7 | 5.1 | 0.01† |
| R0 | 33.6 | 9.8 | 0.01y | 33.6 | 9.8 | 0.02† |
| R1 | 13.0 | 9.8 | 0.64 | 23.4 | 6.0 | 0.13 |
| No capsular rupture | 33.6 | 9.8 | 0.002† | 33.6 | 9.8 | 0.01† |
| Capsular rupture | 5.2 | 4.7 | 0.81 | 5.2 | 4.7 | 0.81 |
| Overall survival | ||||||
| Stage | ||||||
| I* | — | — | — | — | — | — |
| II | 207.4 | 86.3 | 0.26 | 207.4 | 86.3 | 0.26 |
| III | 65.5 | 38.2 | 0.11 | 65.5 | 38.2 | 0.32 |
| IV | 11.4 | 14.9 | 0.63 | 8.2 | 14.4 | 0.21 |
| T1* | — | — | — | — | — | — |
| T2 | 207.4 | 86.3 | 0.36 | 207.4 | 86.3 | 0.45 |
| T3 | 50.4 | 22.3 | 0.06 | 50.4 | 38.2 | 0.40 |
| T4 | 11.4 | 14.9 | 0.72 | 11.0 | 14.4 | 0.63 |
| N0 | MNR | 26.9 | 0.09 | MNR | 33.2 | 0.24 |
| N1 | 18.4 | 24.3 | 0.86 | 16.9 | MNR | 0.44 |
| M0 | 76.1 | 38.2 | 0.16 | 65.5 | 60.7 | 0.50 |
| M1 | 7.4 | 14.6 | 0.51 | 4.1 | 6.5 | 0.54 |
| No hormone secretion | 52.9 | 49.4 | 0.92 | 53.9 | MNR | 0.34 |
| Hormone secretion | 47.3 | 19.0 | 0.04† | 47.3 | 26.5 | 0.11 |
| R0 | 96.3 | 38.2 | 0.02† | 36.3 | 23.1 | 0.20 |
| R1 | 23.2 | 11.5 | 0.45 | 23.2 | 9.0 | 0.32 |
| R2 | 11.0 | 24.3 | 0.60 | 11.0 | 10.1 | 0.77 |
| No capsular rupture | 76.1 | 33.2 | 0.02y | 65.5 | 60.7 | 0.32 |
| Capsular rupture | 41.3 | 14.5 | 0.30 | 41.3 | 9.4 | 0.50 |
No event in one or both groups.
Significant.
MNR, median not reached; R0, microscopically negative margin; R1, microscopically positive margin; R2, grossly positive margin.
DISCUSSION
This study evaluated the relationship of adjuvant mitotane therapy with RFS and OS after resection of ACC. Delivery of mitotane therapy was not associated with improved patient outcomes on either univariate or multi-variable analysis. These results persisted in subgroup analysis of patients after excluding those who received chemotherapy. In addition, in subgroup analyses of patients for whom National Comprehensive Cancer Network guidelines recommend adjuvant mitotane therapy (advanced stage, positive margins, lymph node involvement, and tumor rupture), it still was not associated with improved RFS and OS. To the authors’ knowledge, this report represents the largest multi-institutional US study on adjuvant mitotane for ACC to date.
The rationale for use of mitotane in the adjuvant setting after resection of ACC has been extrapolated from studies of its use in patients with advanced and metastatic disease, where tumor response rates ranged from 5% to 49%.10,15,17,22–24 The highest response rates, however, were in patients treated concurrently with etoposide, doxorubicin, cisplatin, and mitotane combination therapy.23,24 Given the rarity of this disease, these studies were consistently limited by small sample size. In addition, mitotane was usually given in combination with other cytotoxic chemotherapies, making it impossible to discern whether tumor response represented the effect of mitotane, chemotherapeutic agents, or the combination thereof.25 As such, based on these studies in advanced disease, administration of mitotane as adjuvant therapy for resected disease is debatable.
The few prospective studies of patients receiving mitotane in the adjuvant setting have been similarly limited by sample size and by the undefined role of cytotoxic chemotherapy. Baudin and colleagues16 conducted a single-arm study of 11 patients who received mitotane as adjuvant therapy in which 8 (72%) patients had disease recurrence, questioning its utility in the adjuvant setting. Another nonrandomized prospective study of 19 patients who were all offered mitotane treatment revealed that patients who received adjuvant mitotane did not have improved disease-free survival or OS compared with those who did not receive adjuvant mitotane.26 In contrast, Khan and colleagues7 compared 17 patients who received adjuvant streptozotocin plus mitotane with 11 patients who underwent surgery alone. Patients who received streptozotocin and mitotane combination therapy had improved OS and RFS. It is not possible, however, to generalize the outcomes from such few patients to the population as a whole, and the role of mitotane alone, apart from that of streptozotocin, cannot be surmised from this study.7 The current study attempted to account for this interaction between chemotherapy and mitotane by assessing the group as a whole, including patients who received mitotane and chemotherapy, but also performing subgroup analysis excluding patients who received chemotherapy to assess the association of mitotane alone compared with no adjuvant therapy. In both cases, adjuvant mitotane was not associated with improved RFS or OS.
Similar to the prospective studies, retrospective studies of adjuvant mitotane have yielded discordant results with similar limitations.3,13,27–33 The landmark study that supports adjuvant mitotane was conducted in 2007 by Terzolo and colleagues11 and included 177 patients treated at 55 European centers comparing 47 Italian patients treated with mitotane with 2 control groups that received no adjuvant therapy: Italian (n = 55) and German (n = 75). Patients who received adjuvant mitotane had improved RFS compared with both control groups (mitotane: 42 months vs Italian control: 10 months; p < 0.001; mitotane: 42 months vs German control: 25 months, p = 0.005), and the OS was only improved when compared with the Italian cohort, but not the German control group (mitotane: 110 months vs Italian control: 52 months; p = 0.01; mitotane: 110 months vs German control: 67 months; p = 0.10).11 This improvement in RFS but not OS associated with adjuvant mitotane has been observed in other studies6,12,34 and given the side-effect profile of mitotane, it brings into question the value of this therapy that delays recurrence but ultimately does not prolong life. The validity and generalizability of the Terzolo study11 has also been questioned with regard to the quality control of surgery, or lack thereof, as the patients were treated at 55 different centers. The recurrence rates in the control arms were 73% and 91% compared with 49% in the mitotane group. In contrast, Grubbs and colleagues12 found that patients who underwent ACC resection at a high-volume oncologic referral center had a recurrence rate of 50%, the majority did not receive adjuvant mitotane therapy. Similarly, in the current study conducted at 13 US academic centers, the recurrence rate was only 59%. These high recurrence rates in the Terzolo study could suggest inadequate surgery in the control groups, thereby confounding the results.
As current data for adjuvant mitotane are conflicting, the potential benefits of this therapy must be weighed against its risks. Mitotane can be associated with severe toxicity and side effects including lethargy, somnolence, vertigo, nausea, vomiting, diarrhea, anorexia, hematologic changes, and endocrine abnormalities.7,10,15,17,18 Given this side-effect profile and the fact that some studies have suggested that mitotane therapy is associated with improved outcomes only in certain subgroups of patients,35 current National Comprehensive Cancer Network and European guidelines recommend consideration of treatment with adjuvant mitotane in select patients with high-risk features.14,36 In a subgroup analysis of these high-risk patients for whom mitotane is recommended, however, mitotane still was not associated with improved RFS or OS in the current study. Similarly, in low-risk subgroups, receipt of mitotane was not associated with improved outcomes (Table 5).
Undoubtedly, additional studies to determine when, or if, mitotane should be used and to explore other potential therapeutic options for ACC are merited. Historically, the anti-tumor mechanism of mitotane has not been well understood. Recently, however, this complex pathway has been better elucidated.37 Understanding this pathway creates the opportunity for identification of potential new drug targets for this disease with limited current pharmacologic therapeutic options. Additionally, exploring this pathway could lead to identification of potential biomarkers that could be predictive of response to mitotane therapy. For example, Volante and colleagues38 have found improved outcomes after mitotane therapy in patients with increased tumor expression of the Ribonucleotide Reductase Large Subunit. Beyond targeting the tumor and the tumor environment, assessing the metabolic profile of patients with ACC could guide therapy, as drug metabolism could be intimately related to response to therapy.39
The rarity of ACC has historically been a barrier to conducting large, randomized clinical trials for patients with resectable disease; however, an international collaboration has recently opened the Efficacy of Adjuvant Mitotane Treatment (ADIUVO) trial that is currently underway.40 This phase III clinical trial, randomized to adjuvant mitotane after surgery vs surgery alone, was designed to prospectively evaluate the effects of mitotane on patient outcomes. Results of this trial will shed much needed light on this topic.
This study was limited by its retrospective nature where interpretation of results is restricted to the determination of associations of mitotane therapy and outcomes, and causality cannot be inferred. In addition, studies have suggested that mitotane levels <14 mg/L can be subtherapeutic.16,34,41 Mitotane levels were not available for the entire patient population that spans 2 decades, and some of the levels that were reported were <14 mg/L. Notably, studies have cautioned that interpretation of this level can be misleading, as the serum level fluctuates greatly, depending on the timing of the blood draw,42 and predicting the blood level based on the dosage is complex.43 Additionally, many European studies have used the proposed European Network for the Study of Adrenal Tumors staging in analyses rather than the American Joint Committee on Cancer staging, as a SEER study has previously shown that European Network for the Study of Adrenal Tumors staging better discriminates between stage II and III patients.44 The current study used American Joint Committee on Cancer staging criteria, but also stratified patients by T, N, and M stage, attempting to account for any potential bias introduced by choice of staging system. Recent literature has suggested that increased Ki-67 represents an important marker for risk of recurrence in patients after resection of ACC.45 Ki-67 was not routinely tested in the patient population of the current study, and in this study of 13 institutions spanning 20 years, it was not feasible to attain these data. As this study does span a 20-year period, some would question the relevance of outcomes to the current population being treated for ACC. Yet, recent studies have confirmed that in the last 2 decades, outcomes and management strategies of patients being treated for ACC have not improved or changed substantially, thereby justifying the inclusion of patients over this timespan.4,5
CONCLUSIONS
When accounting for stage and adverse tumor and treatment-related factors, adjuvant mitotane therapy after resection of ACC is not associated with improved RFS or OS. Current guidelines should be revisited, and prospective trials are needed. Future efforts should be directed toward genetic profiling of individual tumors to identify specific pathways to target with novel therapies.
Acknowledgments
This study was supported in part by the Katz Foundation.
Footnotes
Disclosure Information: Nothing to disclose.
Presented at the Southern Surgical Association 127th Annual Meeting, Hot Springs, VA, December 2015.
Author Contributions
Study conception and design: Postlewait, Ethun, Tran, Prescott, Pawlik, Wang, Glenn, Hatzaras, Shenoy, Phay, Keplinger, Fields, Jin, Weber, Salem, Sicklick, Gad, Yopp, Mansour, Duh, Seiser, Solorzano, Kiernan, Votanopoulos, Levine, Staley, Poultsides, Maithel
Acquisition of data: Postlewait, Ethun, Tran, Prescott, Pawlik, Wang, Glenn, Hatzaras, Shenoy, Phay, Keplinger, Fields, Jin, Weber, Salem, Sicklick, Gad, Yopp, Mansour, Duh, Seiser, Solorzano, Kiernan, Votanopoulos, Levine, Staley, Poultsides, Maithel
Analysis and interpretation of data: Postlewait, Maithel
Drafting of manuscript: Postlewait, Maithel
Critical revision: Postlewait, Ethun, Tran, Prescott, Pawlik, Wang, Glenn, Hatzaras, Shenoy, Phay, Keplinger, Fields, Jin, Weber, Salem, Sicklick, Gad, Yopp, Mansour, Duh, Seiser, Solorzano, Kiernan, Votanopoulos, Levine, Staley, Poultsides, Maithel
References
- 1.Kebebew E, Reiff E, Duh QY, et al. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30:872–878. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 2.Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol. 1999;6:719–726. doi: 10.1007/s10434-999-0719-7. [DOI] [PubMed] [Google Scholar]
- 3.Haak HR, Hermans J, van de Velde CJ, et al. Optimal treatment of adrenocortical carcinoma with mitotane: results in a consecutive series of 96 patients. Br J Cancer. 1994;69:947–951. doi: 10.1038/bjc.1994.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 5.Kutikov A, Mallin K, Canter D, et al. Effects of increased cross-sectional imaging on the diagnosis and prognosis of adrenocortical carcinoma: analysis of the National Cancer Database. J Urology. 2011;186:805–810. doi: 10.1016/j.juro.2011.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Else T, Williams AR, Sabolch A, et al. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2014;99:455–461. doi: 10.1210/jc.2013-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan TS, Imam H, Juhlin C, et al. Streptozocin and o,p’DDD in the treatment of adrenocortical cancer patients: long-term survival in its adjuvant use. Ann Oncol. 2000;11:1281–1287. doi: 10.1023/a:1008377915129. [DOI] [PubMed] [Google Scholar]
- 8.Nelson AA, Woodard G. Severe adrenal cortical atrophy (cytotoxic) and hepatic damage produced in dogs by feeding 2,2-bis(parachlorophenyl)-1,1-dichloroethane (DDD or TDE) Arch Pathol. 1949;48:387–394. [PubMed] [Google Scholar]
- 9.Bergenstal DM, Hertz R, Lipsett MB, Moy RH. Chemotherapy of adrenocortical cancer with O,P’ddd. Ann Intern Med. 1960;53:672–682. [Google Scholar]
- 10.Decker RA, Elson P, Hogan TF, et al. Eastern Cooperative Oncology Group study 1879: mitotane and adriamycin in patients with advanced adrenocortical carcinoma. Surgery. 1991;110:1006–1013. [PubMed] [Google Scholar]
- 11.Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 12.Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol. 2010;17:263–270. doi: 10.1245/s10434-009-0716-x. [DOI] [PubMed] [Google Scholar]
- 13.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25:891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 14.NCCN clinical practice guidelines in oncology neuroendocrine tumors. Version 1. National Comprehensive Cancer Network website; 2015. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp-site. Accessed September 11, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Williamson SK, Lew D, Miller GJ, et al. Phase II evaluation of cisplatin and etoposide followed by mitotane at disease progression in patients with locally advanced or metastatic adrenocortical carcinoma: a Southwest Oncology Group Study. Cancer. 2000;88:1159–1165. [PubMed] [Google Scholar]
- 16.Baudin E, Pellegriti G, Bonnay M, et al. Impact of monitoring plasma 1,1-dichlorodiphenildichloroethane (o,p’DDD) levels on the treatment of patients with adrenocortical carcinoma. Cancer. 2001;92:1385–1392. doi: 10.1002/1097-0142(20010915)92:6<1385::aid-cncr1461>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Abraham J, Bakke S, Rutt A, et al. A phase II trial of combination chemotherapy and surgical resection for the treatment of metastatic adrenocortical carcinoma: continuous infusion doxorubicin, vincristine, and etoposide with daily mitotane as a P-glycoprotein antagonist. Cancer. 2002;94:2333–2343. doi: 10.1002/cncr.10487. [DOI] [PubMed] [Google Scholar]
- 18.Daffara F, De Francia S, Reimondo G, et al. Prospective evaluation of mitotane toxicity in adrenocortical cancer patients treated adjuvantly. Endocr Relat Cancer. 2008;15:1043–1053. doi: 10.1677/ERC-08-0103. [DOI] [PubMed] [Google Scholar]
- 19.Kroiss M, Quinkler M, Lutz WK, et al. Drug interactions with mitotane by induction of CYP3A4 metabolism in the clinical management of adrenocortical carcinoma. Clin Endocrinol. 2011;75:585–591. doi: 10.1111/j.1365-2265.2011.04214.x. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerario AM, Worden FP, Ramm CA, et al. The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: a multi-institutional NCI-sponsored trial. Hormones Cancer. 2014;5:232–239. doi: 10.1007/s12672-014-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berruti A, Terzolo M, Sperone P, et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocr Relat Cancer. 2005;12:657–666. doi: 10.1677/erc.1.01025. [DOI] [PubMed] [Google Scholar]
- 24.Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 25.Gagliano T, Gentilin E, Benfini K, et al. Mitotane enhances doxorubicin cytotoxic activity by inhibiting P-gp in human adrenocortical carcinoma cells. Endocrine. 2014;47:943–951. doi: 10.1007/s12020-014-0374-z. [DOI] [PubMed] [Google Scholar]
- 26.Vassilopoulou-Sellin R, Guinee VF, Klein MJ, et al. Impact of adjuvant mitotane on the clinical course of patients with adrenocortical cancer. Cancer. 1993;71:3119–3123. doi: 10.1002/1097-0142(19930515)71:10<3119::aid-cncr2820711037>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Kasperlik-Zaluska AA, Migdalska BM, Zgliczynski S, Makowska AM. Adrenocortical carcinoma. A clinical study and treatment results of 52 patients. Cancer. 1995;75:2587–2591. doi: 10.1002/1097-0142(19950515)75:10<2587::aid-cncr2820751028>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Dickstein G, Shechner C, Arad E, et al. Is there a role for low doses of mitotane (o,p’-DDD) as adjuvant therapy in adrenocortical carcinoma? J Clin Endocrinol Metab. 1998;83:3100–3103. doi: 10.1210/jcem.83.9.5113. [DOI] [PubMed] [Google Scholar]
- 29.Wangberg B, Khorram-Manesh A, Jansson S, et al. The long-term survival in adrenocortical carcinoma with active surgical management and use of monitored mitotane. Endocr Relat Cancer. 2010;17:265–272. doi: 10.1677/ERC-09-0190. [DOI] [PubMed] [Google Scholar]
- 30.Abdel-Aziz TE, Rajeev P, Sadler G, et al. Risk of adrenocortical carcinoma in adrenal tumours greater than 8 cm. World J Surg. 2015;39:1268–1273. doi: 10.1007/s00268-014-2912-5. [DOI] [PubMed] [Google Scholar]
- 31.Loncar Z, Djukic V, Zivaljevic V, et al. Survival and prognostic factors for adrenocortical carcinoma: a single institution experience. BMC Urol. 2015;15:43. doi: 10.1186/s12894-015-0038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luton JP, Cerdas S, Billaud L, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med. 1990;322:1195–1201. doi: 10.1056/NEJM199004263221705. [DOI] [PubMed] [Google Scholar]
- 33.Gratian L, Pura J, Dinan M, et al. Treatment patterns and outcomes for patients with adrenocortical carcinoma associated with hospital case volume in the United States. Ann Surg Oncol. 2014;21:3509–3514. doi: 10.1245/s10434-014-3931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terzolo M, Baudin AE, Ardito A, et al. Mitotane levels predict the outcome of patients with adrenocortical carcinoma treated adjuvantly following radical resection. Eur J Endocrinol. 2013;169:263–270. doi: 10.1530/EJE-13-0242. [DOI] [PubMed] [Google Scholar]
- 35.Abiven G, Coste J, Groussin L, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metabolism. 2006;91:2650–2655. doi: 10.1210/jc.2005-2730. [DOI] [PubMed] [Google Scholar]
- 36.Berruti A, Fassnacht M, Baudin E, et al. Adjuvant therapy in patients with adrenocortical carcinoma: a position of an international panel. J Clin Oncol. 2010;28:e401–e402. doi: 10.1200/JCO.2009.27.5958. author reply e403. [DOI] [PubMed] [Google Scholar]
- 37.Sbiera S, Leich E, Liebisch G, et al. Mitotane inhibits Sterol-O-Acyl Transferase 1 triggering lipid-mediated endoplasmic reticulum stress and apoptosis in adrenocortical carcinoma cells. Endocrinology. 2015;156:3895–3990. doi: 10.1210/en.2015-1367. [DOI] [PubMed] [Google Scholar]
- 38.Volante M, Terzolo M, Fassnacht M, et al. Ribonucleotide reductase large subunit (RRM1) gene expression may predict efficacy of adjuvant mitotane in adrenocortical cancer. Clin Cancer Res. 2012;18:3452–3461. doi: 10.1158/1078-0432.CCR-11-2692. [DOI] [PubMed] [Google Scholar]
- 39.Ronchi CL, Sbiera S, Volante M, et al. CYP2W1 is highly expressed in adrenal glands and is positively associated with the response to mitotane in adrenocortical carcinoma. PLoS One. 2014;9:e105855. doi: 10.1371/journal.pone.0105855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berruti A. Efficacy of Adjuvant Mitotane Treatment (ADIUVO) Available at: https://clinicaltrials.gov/ct2/show/NCT00777244. Accessed December 28, 2015.
- 41.Redlich A, Boxberger N, Strugala D, et al. Systemic treatment of adrenocortical carcinoma in children: data from the German GPOH-MET 97 trial. Klin Padiatr. 2012;224:366–371. doi: 10.1055/s-0032-1327579. [DOI] [PubMed] [Google Scholar]
- 42.Kerkhofs TM, Derijks LJ, Ettaieb MH, et al. Short-term variation in plasma mitotane levels confirms the importance of trough level monitoring. Eur J Endocrinol. 2014;171:677–683. doi: 10.1530/EJE-14-0388. [DOI] [PubMed] [Google Scholar]
- 43.Kerkhofs TM, Derijks LJ, Ettaieb H, et al. Development of a pharmacokinetic model of mitotane: toward personalized dosing in adrenocortical carcinoma. Ther Drug Monit. 2015;37:58–65. doi: 10.1097/FTD.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 44.Lughezzani G, Sun M, Perrotte P, et al. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the international union against cancer-staging system: a North American validation. Eur J Cancer. 2010;46:713–719. doi: 10.1016/j.ejca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Beuschlein F, Weigel J, Saeger W, et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab. 2015;100:841–849. doi: 10.1210/jc.2014-3182. [DOI] [PubMed] [Google Scholar]


